Published online Sep 28, 2025. doi: 10.35712/aig.v6.i2.110109
Revised: June 16, 2025
Accepted: September 10, 2025
Published online: September 28, 2025
Processing time: 122 Days and 13.5 Hours
Artificial intelligence (AI) is transforming gastroenterology by enhancing dia
Core Tip: Artificial intelligence (AI) is being applied across a range of gastrointestinal conditions from cancer and liver disease to functional disorders and is driving the development of new tools in digital health. It has demonstrated equal or superior efficiency compared with humans in diagnostic accuracy, treatment planning, and healthcare delivery. However, several important challenges remain, including data privacy concerns, limited transparency of algorithms, inherent biases, and difficulties integrating AI into traditional clinical workflows. Addressing these issues is essential for clinicians to fully benefit from AI and for its continued development in the field.
- Citation: De Silva AP, Prabagar K. Artificial intelligence in gastroenterology: Enhancing clinical practice, managing challenges and exploring future directions. Artif Intell Gastroenterol 2025; 6(2): 110109
- URL: https://www.wjgnet.com/2644-3236/full/v6/i2/110109.htm
- DOI: https://dx.doi.org/10.35712/aig.v6.i2.110109
Artificial intelligence (AI) refers to the capability of machines or computer systems to perform tasks that typically require human intelligence. These tasks range from simple activities, such as driving a car, to complex processes like formulating medical solutions by analyzing clinical data[1].
The foundational idea of machine intelligence was first proposed by Alan Turing, who introduced the Turing Test, a benchmark to determine whether a machine can exhibit human-like intelligent behavior. The term “Artificial Intelligence” was officially termed during the Dartmouth Workshop in the 1950s, marking the formal recognition of AI as an academic discipline[2].
Over time AI has undergone significant evolution in the medical field. Early developments focused on rule-based expert systems, primarily used to diagnose infectious diseases and recommend antibiotic therapies[3]. These systems evolved into Clinical Decision Support Systems, which were integrated with electronic medical records to provide clinicians with alerts, reminders, and guideline-based recommendations to support clinical decision-making[2,4].
Substantial progress occurred in the early 2000s with the introduction of machine learning (ML), enabling AI to interpret complex imaging modalities such as X-rays, magnetic resonance imaging (MRI), and CT scans to assist in diagnostic processes[5]. The development of deep learning (DL), particularly convolutional neural networks (CNNs), further revolutionized the role of AI in healthcare by allowing it to perform complex diagnostic tasks, formulate personalized treatment plans, and support follow-up care across multiple specialties. Currently, AI is advancing towards multimodal integration, a system that combines text, images, and structured clinical data to deliver real-time clinical support[1,6].
In gastroenterology the need for AI arises from several clinical demands. These include continuous patient monitoring, execution of repetitive procedures, delivery of personalized treatment, early detection of disease, and accurate prediction of disease flares. Chronic conditions such as inflammatory bowel disease often generate vast volumes of data requiring efficient analysis while the high stakes of misdiagnosis emphasize the need to reduce human error. AI meets these demands by enhancing diagnostic accuracy and improving clinical outcomes by providing automated support.
This review aimed to provide an overview of the key AI technologies used in medicine with a particular focus on their application in gastroenterology. It examined how AI contributes to both diagnosis and treatment, explored the challenges associated with its implementation, and outlined future directions.
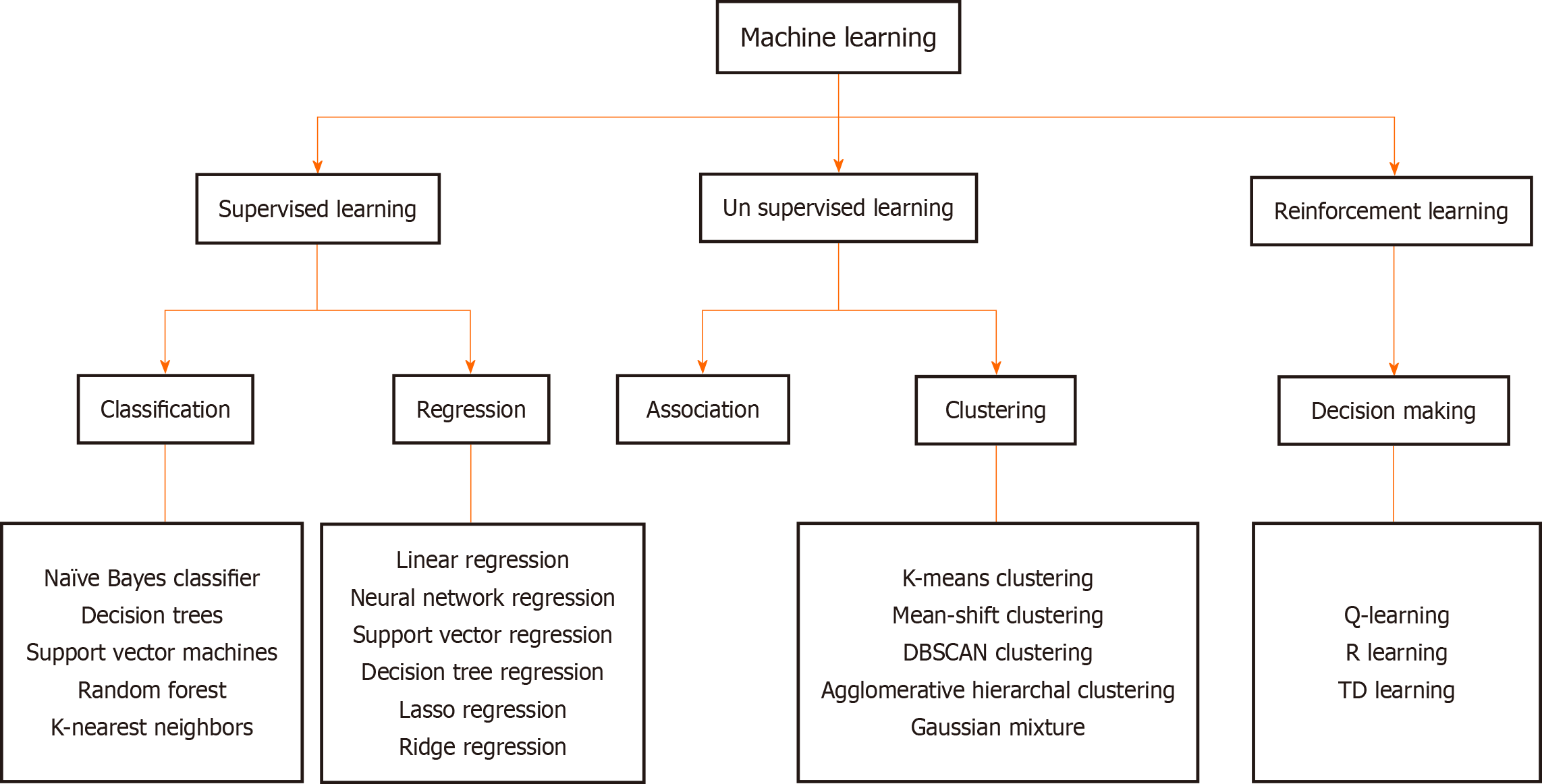
AI systems fundamentally rely on data, which they process using various models to generate clinically meaningful outputs. Two key approaches in this domain are ML and its specialized subset, DL. ML involves developing algorithms that learn from data and make independent predictions or decisions. These models improve over time as they are exposed to more data[6]. They are broadly categorized into three main types: Supervised learning (SL); unsupervised learning; and reinforcement learning (RL) (Figure 1).
In SL, models are trained on labeled datasets containing both inputs and corresponding outputs. Once trained these models can predict or classify new, unseen data[7]. For example, a model trained on endoscopic videos labeled with the presence or absence of polyps can subsequently diagnose similar lesions in new patients[8]. SL can be applied through two main approaches: Classification and regression. Classification models predict discrete outcomes, such as determining whether a patient has a particular pathology. Regression models on the other hand estimate continuous output values, such as predicting liver stiffness based on variables like age, body mass index, and blood test results[9].
Unsupervised learning in contrast uses unlabeled data to detect hidden patterns or groupings within it. A common technique is clustering in which the model groups similar data together. This can lead to the identification of new tumor subtypes with distinct prognoses and therapeutic responses[10]. Another technique, association rule learning, identifies frequent new relationships within data, such as the common co-occurrence of hypertension with chronic kidney disease[11].
In RL, a system learns to make decisions by interacting with the environment and receiving feedback in the form of rewards or penalties based on its actions. RL focuses on learning optimal responses through trial and error to maximize cumulative rewards over time. It has been applied to develop personalized treatment strategies. For example RL models have been used to optimize dosing strategies in sepsis management by continuously adjusting drug levels based on a patient’s changing physiological state[12].
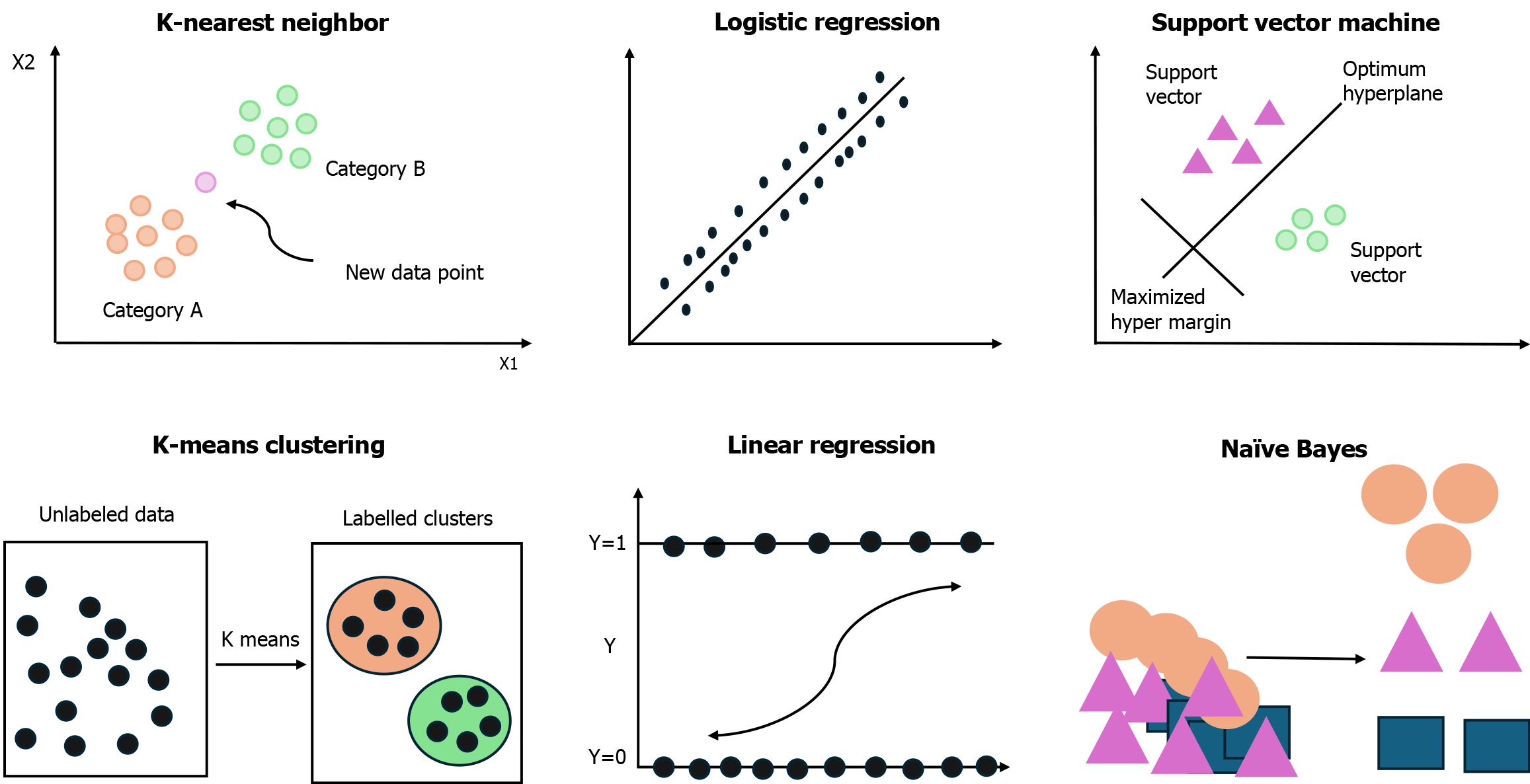
Several classical ML algorithms are routinely used in clinical settings as illustrated in Figure 2. The k-nearest neighbors algorithm classifies data based on their similarity to previously labeled examples and is useful in tasks like skin lesion classification and distinguishing between benign and malignant breast tumors[13]. Linear regression is used to explore the relationship between dependent and independent variables and is often applied to predict outcomes such as blood pressure based on demographic and clinical factors[14]. Logistic regression, designed for binary outcomes, is widely used to estimate the probability of events such as sepsis or hospital readmission[15].
In addition other advanced algorithms, such as Naive Bayes, Support Vector Machines, AdaBoost, and XGBoost, are becoming increasingly prominent. These models have proven effective in tasks like disease prediction, tumor classification, and patient outcome forecasting, particularly when dealing with large, complex datasets[16-18].
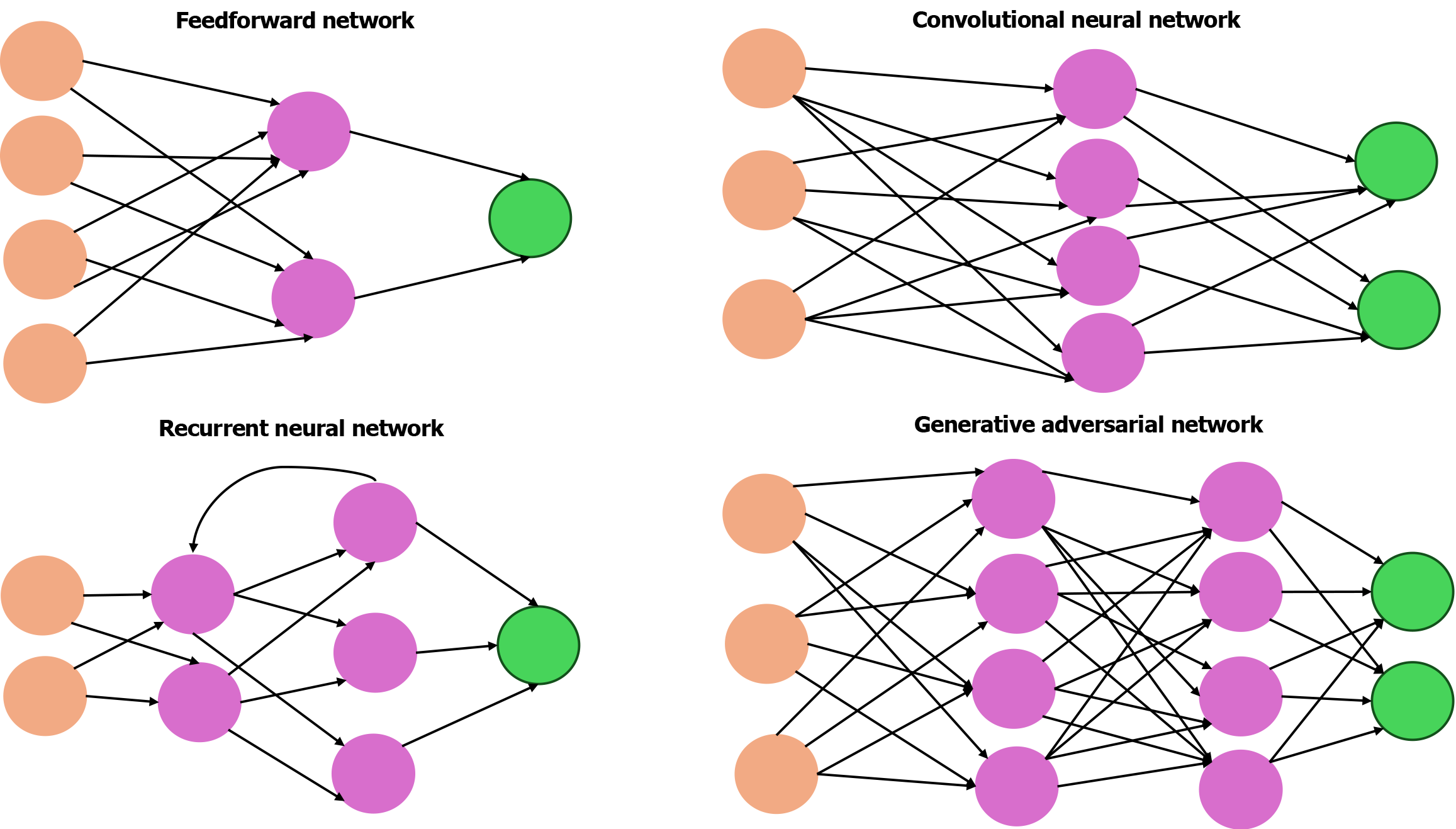
DL, a specialized subset of ML, primarily relies on artificial neural networks (ANNs). ANNs are computational models inspired by the structure of the human brain, consisting of interconnected layers of artificial neurons as illustrated in Figure 3. The most basic form is the feedforward network in which data passes in one direction through multiple layers. These networks are used in tasks such as pattern recognition and classification[19].
Another important type is the recurrent neural network (RNN), which is specifically designed to process sequential data. Unlike traditional feedforward networks RNNs incorporate loops in their architecture, allowing them to maintain a form of memory. This capability enables them to use information from previous inputs to influence current predictions, making them effective in analyzing time-series medical data such as heart rate variability in electrocardiogram signals, respiratory patterns, and fluctuations in blood glucose levels, thereby predicting flare ups in chronic conditions[20-22]. RNNs are widely used in natural language processing tasks such as analyzing clinical notes, patient histories, and symptom timelines.
However, their limitations in capturing long-range temporal dependencies led to the development of transformer neural networks. Transformers utilize self-attention mechanisms to identify and prioritize the most relevant features within input data. These models are applied across clinical document analysis, speech recognition, and even image-based diagnosis. The attention mechanisms can also highlight critical regions in medical images, aiding clinicians in making more accurate diagnostic decisions[23]. Transformers form the foundation of large language models like GPT and BERT, which excel at understanding and generating human-like medical text[24]. These models support advanced clinical decision making by enabling rapid retrieval of medical knowledge, summarizing vast medical literature, and providing context-aware recommendations. This also significantly improves clinician-patient communication.
Deep neural networks, which consist of multiple hidden layers, represent a more advanced form of ANNs that are more accurate. Within this category CNNs are a specialized type tailored for image analysis. They extract features directly from raw pixels and use them in tasks such as detecting pulmonary nodules and coronary artery calcifications on CT scans[25]. CNNs are extensively used for semantic segmentation in which each pixel in an image is classified into predefined categories, such as normal tissue, malignant lesion, or inflamed area, allowing precise identification of pathological regions. This pixel-level classification enables accurate mapping of lesion boundaries aiding targeted interventions[26]. CNNs also perform instance segmentation, which not only labels each pixel but also differentiates between distinct instances of the same class (e.g., multiple polyps within a single colonoscopy image). This capability is critical in clinical scenarios where distinguishing individual lesions impacts treatment decisions. Algorithms such as U-Net and Mask R-CNN have been widely used for these segmentation tasks, facilitating enhanced visualization, quantification, and monitoring of disease progression[27].
Other notable types of deep neural networks include autoencoders, which are unsupervised models used to reconstruct normal anatomy (e.g., in brain MRI) and detect anomalies[28]. Generative adversarial networks, composed of a generator and a discriminator, are capable of producing synthetic medical data such as realistic electrocardiogram signals or medical images, which can be used to train data models[29]. In addition advanced DL strategies such as transfer learning and few-shot learning further enhance these models. Transfer learning allows models to apply knowledge gained from one task to another, providing a benefit when labeled data are limited[30]. Few-shot learning enables generalization from very few examples, making it especially valuable for diagnosing rare diseases[31]. Deep RL, which integrates neural networks with reward-based learning frameworks, extends the capabilities of traditional DL by enabling dynamic decision-making in complex environments with promising applications in robotic surgery, clinical decision support, and the optimization of clinical trial design[32,33].
Certain ML algorithms are inherently scalable to DL architectures and serve as effective bridges between traditional ML and DL approaches. Notably, semi-SL and self-SL exemplify such strategies. Semi-SL leverages a small amount of labeled data alongside a large volume of unlabeled data to enhance model performance, making it particularly valuable in domains where annotated data is often limited[34]. In contrast self-SL generates supervisory signals directly from the input data by formulating pretext tasks, allowing models to learn from vast unlabeled datasets. For example in medical imaging, models can be trained to reconstruct randomly masked regions of chest X-rays. Through this process the algorithm learns general anatomical features and pathological patterns by predicting missing portions of the image based on the surrounding visible context[35].
AI has significantly enhanced the diagnosis and management of a wide range of gastroenterological conditions from acute presentations such as gastrointestinal bleeding (GIB) to chronic disorders like irritable bowel syndrome (IBS). A summary of AI applications across these conditions is provided in Table 1.
| Condition | AI application |
| Malignant and premalignant lesions | Enhances WLE, NBI, WATS, VLE, and I-scan imaging for detection; assists targeted biopsies; real-time feedback during endoscopy for quality control |
| Colorectal carcinoma | Automated histopathological image analysis & tumor grading; early metastases detection; personalizes treatment; assesses bowel prep quality |
| H. pylori infection | Detects H. pylori infection; “H. pylori AI-Clinician”; assists in novel eradication technique identification |
| Gastrointestinal bleeding | Predicts acute GIB episodes, outcomes, bleeding severity; predicts need for transfusion and mortality in ICU; identifies bleeding sources endoscopically |
| Irritable bowel syndrome | Diagnoses via bowel sound/acoustic analysis; differentiates subtypes; detects subtle mucosal changes; personalizes diet; uses adaptive feedback and symptom monitoring apps |
| Liver conditions | Detects fibrosis and steatosis via imaging; classifies focal lesions; predicts NASH/HCC progression and outcomes; identifies varices; predicts survival and transplant-free survival |
| Pancreatic conditions | Predicts severity and outcomes of acute pancreatitis; differentiates chronic/autoimmune pancreatitis; differentiates pancreatic cystic neoplasms; early detection and staging of pancreatic ductal adenocarcinoma |
| Pediatric conditions | Early diagnosis of biliary atresia, intussusception, eosinophilic esophagitis |
Substantial advancements have been made in the assessment of precancerous and cancerous esophageal lesions, particularly in the early and accurate detection of adenocarcinoma and squamous cell carcinoma. These developments are especially critical given the increasing incidence and poor prognosis associated with esophageal adenocarcinoma[36].
In Barrett’s esophagus, a well-known precursor to adenocarcinoma, AI-driven computer-assisted diagnosis systems have improved endoscopic surveillance and enabled more accurate diagnosis. These systems have demonstrated sensitivities as high as 84.0% and specificities up to 90.7%, outperforming conventional, non-AI-guided methods, which report a sensitivity and specificity of 77% and 86%, respectively[37].
Beyond Barrett’s esophagus AI integration has improved various endoscopic modalities. Techniques such as white-light endoscopy, narrow-band imaging, and wide-area transepithelial sampling when combined with DL algorithms have shown increased detection rates[38,39]. Similarly, volumetric laser endomicroscopy and I-scan imaging augmented by AI improve diagnostic accuracy by analyzing complex imaging data and assisting in targeted biopsies[40]. These AI-enhanced tools not only outperform traditional methods in efficiency but also serve as effective alternatives to labor-intensive approaches[41].
AI also contributes to real-time quality control during endoscopic procedures by providing immediate feedback on technique and helping to standardize practices across operators. This reduces interoperator variability and supports consistent diagnostic performance[41].
Furthermore, AI significantly improves polyp detection during colonoscopy, a key factor in identifying premalignant lesions for colorectal cancer prevention. AI facilitates the accurate identification of polyps, particularly small or flat lesions that are often missed using conventional techniques[42]. Studies have shown that AI-assisted colonoscopy increases the adenoma detection rate from 20.3% to 29.1% and the polyp detection rate from 29.8% to 45.0%[43,44]. These systems, especially those employing DL algorithms, analyze real-time endoscopic video to highlight suspicious lesions, thereby assisting endoscopists in timely and accurate detection.
The integration of AI has revolutionized the diagnosis, early detection of metastasis, and treatment of colorectal carcinoma. In diagnostic applications automated histopathological image analysis provides more objective and rapid tumor grading, biomarker assessment, and evaluation of tumor growth patterns[45]. Additionally, AI algorithms aid in radiological interpretation, facilitating earlier detection of tumors and metastases through imaging modalities such as MRI, CT, and ultrasonography.
Liver metastasis is a common complication of colorectal cancer. Radiomics combined with CNNs, which integrate imaging data with clinical information, have been used to predict the development of colorectal liver metastases[46,47]. AI has shown an ability comparable with radiologists in detecting liver metastases via imaging, and even through noninvasive methods like breath analysis[48].
In treatment planning AI supports clinical decision-making by predicting responses to therapies such as ablation and chemotherapy and by identifying optimal treatment regimens. For example ML models have been developed to predict chemotherapy outcomes and potential complications, enabling oncologists to tailor treatment strategies effectively[49]. Moreover, AI tools analyze radiomic and clinical data to forecast disease progression, recurrence risk, and patient survival, thereby guiding personalized follow-up and management strategies[50,51].
Significant progress has also been made in assessing bowel preparation quality for colonoscopy, an essential factor in both the diagnosis and surveillance of colorectal carcinoma. Traditional scoring systems, like the Boston Bowel Preparation Scale, are often inaccurate due to interobserver variability. To address this a study developed two CNN models trained on large colonoscopy video datasets. These AI systems demonstrated an accuracy of 85.3% in detecting inadequate bowel cleanliness and achieved 100% sensitivity in certain testing scenarios[52]. These findings suggest that AI can offer real-time, objective assessments of bowel preparation quality, potentially enhancing colonoscopy effectiveness and standardizing evaluation practices across operators.
The management of Helicobacter pylori (H. pylori) infections is undergoing significant advancement, particularly in the areas of diagnosis, personalized treatment, and eradication strategies. AI-aided diagnostic tools, especially those utilizing CNNs, have demonstrated high accuracy in detecting H. pylori infection through endoscopic images. Some models have achieved sensitivities of up to 100% and specificities of approximately 81%, facilitating early and accurate diagnosis[53].
Moreover, AI is contributing to personalized treatment planning. Systems such as “H. pylori AI-Clinician” utilize patient-specific data to customize therapeutic regimens, predicting the most effective treatment combinations[54]. These AI-driven platforms employ advanced ML models to generate individualized recommendations. This personalized approach has been shown to enhance treatment success, increase eradication rates, and potentially reduce the long-term risk of H. pylori-associated gastric cancer[54,55].
Additionally, AI is being applied to the discovery of novel eradication strategies. It assists in screening herbal compounds and alternative therapies with potential anti-H. pylori properties, thereby accelerating the identification and development of new treatment options[55].
The application of AI addresses several challenges in the management of GIB, including the prediction of bleeding episodes, risk assessment, and outcome forecasting. For the prediction of bleeding episodes, ML algorithms have been integrated into electronic medical records. These models are trained on retrospective datasets and are applied to real-time clinical data to identify patients with acute GIB. This approach has demonstrated superiority over traditional systems such as SNOMED, enabling timely risk stratification by automatically activating ML models when predefined clinical criteria are met[56].
In outcome prediction ML models have outperformed conventional scoring systems. Traditional tools such as the Glasgow-Blatchford Score, admission Rockall score, and AIMS65 are limited in their ability to predict key outcomes including rebleeding, the need for intervention, and mortality[57]. In contrast ML models, particularly those utilizing algorithms like XGBoost, have demonstrated improved predictive accuracy[58,59]. For instance one study showed that an ML model surpassed traditional scores in predicting the composite endpoint of intervention or death within 30 days, enhancing the identification of patients who are low risk and may be managed as outpatients[60]. In another study involving 5691 patients in the intensive care unit with acute GIB, an ML model predicted mortality more accurately than the widely used APACHE IVa score[61]. AI has also been employed to forecast antithrombotic-associated GIB and to predict transfusion requirements, providing timely insights to support clinical decision-making[62].
Furthermore, AI has enhanced diagnostic precision during endoscopic procedures by analyzing video footage to detect bleeding sources such as ulcers or varices. These tools can also assess bleeding severity and guide therapeutic decisions, ultimately contributing to improved patient outcomes[63].
AI is increasingly being used in the management of IBS, particularly in improving diagnosis, personalizing treatment, and enhancing symptom monitoring. An important area of research involves the analysis of bowel sounds, which offers a noninvasive and cost-effective diagnostic modality. One study demonstrated significant differences in bowel sound intervals between patients with IBS and healthy individuals, achieving a sensitivity of 89% and a specificity of 100%[64]. Subsequently, various ML algorithms have been applied to refine this approach. The development of the IBS Acoustic Index, which achieved 87% sensitivity and specificity, has provided an objective and practical tool for diagnosis[65]. Similarly, in a 2023 study, ML models were applied to clinical datasets to accurately differentiate between IBS subtypes, thereby improving diagnostic precision and enabling better-targeted therapeutic strategies[66].
Another emerging application is the AI-assisted colonoscopic imaging. While IBS has traditionally been considered a functional disorder without endoscopic abnormalities, researchers utilized Google Cloud AutoML Vision to analyze colonoscopy images from patients with IBS and healthy controls. Remarkably, the model demonstrated high specificity (97.6%) in distinguishing patients with IBS from controls, suggesting that AI can detect subtle mucosal changes which are not visible to the human eye[67].
In terms of treatment ENBIOSIS, an AI-driven dietary recommendation platform, has emerged as a personalized intervention tool. This system employs microbiota profiling in conjunction with XGBoost algorithms to tailor dietary interventions. In a comparative study, patients with IBS who received AI-personalized diets showed significantly greater improvements in symptom severity and gut microbiota composition compared with those on a standard low-FODMAP diet[68].
Second-generation AI systems further enhance treatment efficacy through the use of closed-loop, adaptive feedback mechanisms. These systems integrate real-time data, including clinical symptoms, genomic and microbiome profiles, heart rate variability, and gastrointestinal motility to continuously deliver personalized care[69]. Another emerging approach is the AI-enabled digital pill system, which adjusts medication regimens based on clinician input and patient-reported outcomes. This system extends from basic symptom tracking to biologically informed dosing protocols, utilizing metrics such as cytokine levels and heart rate variability to individualize therapy[70].
In symptom monitoring AI also demonstrates significant potential. The Dieta mobile application uses AI to classify stool images according to the Bristol Stool Scale. In a pilot study the application outperformed patient self-reporting, achieving an accuracy of 95%, thereby highlighting its utility in real-time symptom tracking and treatment monitoring[71].
AI has brought significant advancements to the noninvasive diagnosis, risk stratification, and treatment planning of liver diseases through prognosis-based predictions. In liver fibrosis and steatosis, AI-powered tools utilizing ultrasound, CT, MRI, and elastography combined with DL and CNNs have demonstrated high sensitivity and specificity in detecting and staging disease severity[72,73]. CNNs applied to imaging data enable noninvasive assessment of liver stiffness and fat content, thereby reducing reliance on liver biopsies[72]. For example in patients with chronic hepatitis C, ANNs trained on biopsy data accurately predicted significant fibrosis[74,75]. ML models incorporating parameters such as age, AST, albumin, and platelet count have also been effective in identifying advanced fibrosis . Similarly, in chronic hepatitis B ANN-based models have outperformed traditional scoring systems such as Fib-4[76].
In the diagnosis of focal liver lesions, ML models have shown high accuracy in classifying hepatic nodules, such as cysts, hemangiomas, and hepatocellular carcinoma (HCC), especially when integrated with clinical data[77].
In nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, ML models have aided in predicting disease progression and clinical outcomes by analyzing a combination of laboratory data, demographic variables, and imaging biomarkers. These models have also been effective in forecasting complications such as portal hypertension and HCC[78-80].
In HCC specifically AI contributes to early diagnosis, risk stratification, treatment selection, and post-treatment monitoring. ML-based diagnostic models have outperformed traditional tumor markers like alpha-fetoprotein while DL algorithms have enhanced the accuracy of risk stratification in patients with compensated cirrhosis[81,82]. Multi-modal AI models using RNA, mRNA, and methylation data have successfully identified molecular features associated with tumor aggressiveness and prognosis[83,84].
AI is also being employed to predict prognosis in chronic liver disease. Radiomics extracted from CT images can identify high-risk varices, and DL models have demonstrated utility in predicting post-transplant survival[85,86]. Furthermore, AI algorithms trained on histopathological slides can predict survival outcomes in patients with HCC undergoing surgical resection[87].
AI has demonstrated significant progress in differentiating various pancreatic pathologies and in facilitating personalized treatment planning through risk prediction. In both acute and chronic pancreatitis, ML algorithms and ANNs have outperformed traditional scoring systems such as APACHE-II in predicting disease severity, complications, and mortality with some models achieving accuracies up to 97.5%[88,89]. AI applications have also demonstrated high specificity in distinguishing chronic from autoimmune pancreatitis and identifying functional abdominal pain, particularly when using imaging techniques such as endoscopic ultrasound and radiomics[90].
In the assessment of pancreatic cystic neoplasms, DL and radiomics-based models have surpassed radiologists and conventional clinical guidelines in accurately classifying cyst types and estimating malignancy risk[91,92]. Tools like CompCyst, which integrate clinical, imaging, and biochemical data, have significantly reduced the rate of unnecessary surgical interventions[93].
For pancreatic ductal adenocarcinoma, DL models applied to CT and positron emission tomography CT imaging have demonstrated a superior tumor detection rate compared with conventional methods[94]. AI has also been effective in distinguishing pancreatic ductal adenocarcinoma from benign pancreatic conditions and in improving survival prediction through the analysis of complex, multidimensional datasets. These capabilities support earlier diagnosis and enable more personalized therapeutic strategies[95-97].
AI techniques have been applied to enhance early diagnosis and management of pediatric gastrointestinal and hepatobiliary diseases. In neonatal liver disease, particularly biliary atresia, ML algorithms have significantly improved diagnostic accuracy. A study employing the XGBoost algorithm integrated clinical data, laboratory indices, and imaging findings from ultrasound and hepatobiliary scintigraphy to distinguish biliary atresia in neonates with cholestasis. This model outperformed traditional diagnostic methods and enabled earlier intervention, leading to better clinical outcomes[98].
In eosinophilic esophagitis an AI platform using semantic segmentation of biopsy slides achieved a histological classification accuracy of 86.7%[99]. The model effectively identified, quantified, and graded key histopathologic features across the eosinophilic esophagitis spectrum with performance comparable with that of gastrointestinal pathologists, highlighting its potential in both diagnosis and treatment planning.
Similarly, AI has facilitated the noninvasive diagnosis of pediatric intussusception. A DL algorithm applied to abdominal ultrasound images demonstrated potential in improving diagnostic speed and accuracy. Continued advancements of AI systems for real-time detection of hallmark sonographic signs, such as the “concentric circle” sign, may further enhance clinical decision-making in children presenting with suspected intussusception[100].
The integration of AI in gastroenterology has led to transformative advancements, significantly improving diagnostic accuracy, detection rates, and clinical decision-making. DL and ML algorithms have demonstrated superior sensitivity and specificity compared to conventional clinician-based assessments across a wide spectrum of gastrointestinal diseases[37,41,42,53].
Unlike traditional risk prediction models, which typically rely on a limited set of variables, AI-based models can integrate large volumes of clinical, imaging, and histopathological data to forecast disease progression and personalize treatment strategies. Key advantages of AI include its ability to recognize complex patterns, function without fatigue, continuously learn and adapt, and remain unaffected by human error. These capabilities enable accurate risk prediction, early identification of complications or treatment failure, and the development of personalized therapies, ultimately contributing to improved patient outcomes[50,60,78,79].
AI also addresses long-standing limitations in conventional diagnostic and therapeutic approaches, such as the invasive nature of certain procedures, interoperator variability, and the challenges of integrating patient-specific data and preferences into treatment plans. Moreover, it accelerates research efforts by identifying novel hypotheses, streamlining data analysis, and opening new avenues for exploration[41,69].
Beyond diagnostics AI enhances overall healthcare delivery by improving cost-effectiveness, reducing unnecessary procedures, automating administrative tasks, and optimizing resource allocation[101,102]. It also contributes to lower hospital readmission rates through predictive analytics that facilitate timely interventions[103]. Additionally, AI supports remote monitoring and telemedicine, expanding access to care in underserved areas while alleviating the burden on healthcare systems[104].
Despite the transformative potential of AI, its integration into clinical practice is accompanied by several significant challenges. A primary concern involves data privacy and cybersecurity. As AI systems routinely handle sensitive patient information, they are inherently vulnerable to data breaches and cyberattacks. Addressing these risks necessitates the enforcement of stringent data protection regulations, such as the General Data Protection Regulation and the Health Insurance Portability and Accountability Act. Additionally, the adoption of privacy-preserving techniques in ML, such as federated learning, differential privacy, and encrypted computation, is essential to ensure secure data storage and transmission, thereby enabling responsible and ethical data sharing for AI applications[105].
Ethical considerations also represent a major obstacle, particularly regarding algorithmic bias, transparency, and accountability. AI tools trained on non-diverse or unrepresentative datasets may yield biased outputs that can lead to underdiagnosis or misdiagnosis in specific demographic groups, perpetuating health disparities[106]. Compounding this issue is the lack of interpretability in many AI models, especially DL architectures. This “black box” nature renders it difficult for clinicians to comprehend or validate the rationale behind AI-generated recommendations, thereby eroding trust and impeding clinical adoption. Moreover, when such opaque systems make erroneous decisions, attributing responsibility or determining legal liability becomes problematic[107]. Another emerging concern is the phenomenon of AI hallucinations, particularly observed in large language models. These models can generate outputs that are syntactically plausible yet factually incorrect or misleading. In a clinical context such hallucinations pose a serious risk to patient safety if not rigorously vetted by medical professionals[108].
These issues highlight the urgent need for well-defined ethical guidelines and regulatory frameworks. However, formulating such frameworks is particularly challenging due to the rapid pace of AI development and the heterogeneous and fragmented nature of global regulatory standards.
Additionally, most existing AI systems represent “narrow AI” designed for specific tasks without general reasoning abilities. Consequently, they cannot replicate the nuanced clinical judgment or contextual understanding that human physicians possess. This limitation undermines their generalizability and adaptability: While an AI model may perform well in the environment in which it was trained, its performance often deteriorates when applied to new clinical settings or slightly varied data inputs. Furthermore, narrow AI models typically lack the capacity for transfer learning, necessitating retraining for each new application. They also remain fragile with small perturbations in input data potentially resulting in significant output errors, raising concerns about their robustness in dynamic, real-world clinical environments[109].
Finally, the technical and practical integration of AI into existing healthcare systems presents logistical hurdles. AI systems must be interoperable with electronic health records, which often differ between institutions and were not originally designed for AI compatibility. Successful implementation also demands clinician training, workflow restructuring, and institutional support, which may disrupt established practices and encounter resistance from healthcare personnel[110]. Overcoming these multifaceted challenges is crucial to ensure the safe, ethical, and effective adoption of AI in gastroenterology and broader clinical practice.
There remains a substantial scope for further innovation and application of AI in gastroenterology. One key area of advancement lies in the use of AI models for predicting disease progression through the analysis of longitudinal data and early detection of clinical deterioration[111]. Such predictive tools are particularly valuable in managing chronic gastrointestinal conditions prone to exacerbations, including inflammatory bowel disease, liver cirrhosis, and pancreatic insufficiency.
Another promising frontier is the development of real-time AI assistance embedded within electronic health records and deployed via edge-computing-enabled wearable devices. These systems can locally process physiological signals, laboratory results, and vital signs to generate immediate clinical alerts, thereby supporting clinicians in real-time at the point of care[112].
Advanced risk stratification algorithms offer the potential to refine patient classification by integrating multi-modal data, including genomic, biochemical, and lifestyle information, to predict complications or therapeutic responsiveness. This would enable truly personalized management strategies, particularly for diseases where reliable risk models are currently lacking[113].
In the surgical domain AI is poised to support automated or semi-automated minimally invasive procedures, enhancing precision, safety, and efficiency while reducing patient recovery times[114]. AI models can also forecast postoperative complications such as surgical site infections, facilitating early interventions and improved postoperative care[115]. Furthermore, intraoperative AI guidance can assist surgeons by providing real-time decision support and optimizing outcomes.
Beyond clinical care, AI holds transformative potential in drug discovery and development. By accelerating target identification, optimizing compound screening, and predicting therapeutic responses, AI can drastically shorten drug development timelines. It can also refine clinical trial design and forecast adverse events, thereby enhancing safety and efficacy in pharmacological interventions[116]. While there are emerging efforts to develop unified guidelines for the use of AI in gastroenterology, these frameworks still require substantial refinement to ensure comprehensive, standardized, and ethically sound implementation across clinical and research settings[117,118].
AI has already begun to revolutionize the field of gastroenterology by significantly improving diagnostic accuracy, therapeutic planning, and long-term patient monitoring. Its ability to process and learn from vast and complex datasets enables earlier disease detection, personalized care, and improved clinical outcomes.
Nevertheless, several challenges must be addressed to ensure the responsible and effective integration of AI into clinical practice. Concerns surrounding data privacy, regulatory compliance, ethical use, and the seamless incorporation of AI tools into existing clinical workflows remain pressing issues. Overcoming these barriers will require coordinated efforts involving technologists, clinicians, ethicists, and policymakers.
Looking ahead, the future of AI in gastroenterology is promising. Continued research and development are likely to enhance real-time procedural support, improve prediction of disease flares, enable safer and more effective surgical interventions, and accelerate the development of novel therapeutics. As these technologies mature, they have the potential to redefine standards of care, improve access, and ultimately transform the landscape of gastrointestinal healthcare.
| 1. | Sheikh H, Prins C, Schrijvers E. Artificial Intelligence: Definition and Background. In: Mission AI. Research for Policy. Cham: Springer, 2023. [DOI] [Full Text] |
| 2. | Muthukrishnan N, Maleki F, Ovens K, Reinhold C, Forghani B, Forghani R. Brief History of Artificial Intelligence. Neuroimaging Clin N Am. 2020;30:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Yu VL, Buchanan BG, Shortliffe EH, Wraith SM, Davis R, Scott AC, Cohen SN. Evaluating the performance of a computer-based consultant. Comput Programs Biomed. 1979;9:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Moja L, Kwag KH, Lytras T, Bertizzolo L, Brandt L, Pecoraro V, Rigon G, Vaona A, Ruggiero F, Mangia M, Iorio A, Kunnamo I, Bonovas S. Effectiveness of computerized decision support systems linked to electronic health records: a systematic review and meta-analysis. Am J Public Health. 2014;104:e12-e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Wang S, Summers RM. Machine learning and radiology. Med Image Anal. 2012;16:933-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl Vis Sci Technol. 2020;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 317] [Reference Citation Analysis (5)] |
| 7. | Kufel J, Bargieł-Łączek K, Kocot S, Koźlik M, Bartnikowska W, Janik M, Czogalik Ł, Dudek P, Magiera M, Lis A, Paszkiewicz I, Nawrat Z, Cebula M, Gruszczyńska K. What Is Machine Learning, Artificial Neural Networks and Deep Learning?-Examples of Practical Applications in Medicine. Diagnostics (Basel). 2023;13:2582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 133] [Reference Citation Analysis (0)] |
| 8. | Cao H, Wang Y, Chen J, Jiang D, Zhang X, Tian Q, Wang M. In: Karlinsky L, Michaeli T, Nishino K (eds). Swin-Unet: Unet-Like Pure Transformer for Medical Image Segmentation. Lecture Notes in Computer Science. Cham: Springer, 2023. [DOI] [Full Text] |
| 9. | Dave M, Patel N. Artificial intelligence in healthcare and education. Br Dent J. 2023;234:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 10. | Syed AB, Zoga AC. Artificial Intelligence in Radiology: Current Technology and Future Directions. Semin Musculoskelet Radiol. 2018;22:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Ramezankhani A, Pournik O, Shahrabi J, Azizi F, Hadaegh F. An application of association rule mining to extract risk pattern for type 2 diabetes using tehran lipid and glucose study database. Int J Endocrinol Metab. 2015;13:e25389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bologheanu R, Kapral L, Laxar D, Maleczek M, Dibiasi C, Zeiner S, Agibetov A, Ercole A, Thoral P, Elbers P, Heitzinger C, Kimberger O. Development of a Reinforcement Learning Algorithm to Optimize Corticosteroid Therapy in Critically Ill Patients with Sepsis. J Clin Med. 2023;12:1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Takemura A, Shimizu A, Hamamoto K. Discrimination of breast tumors in ultrasonic images using an ensemble classifier based on the AdaBoost algorithm with feature selection. IEEE Trans Med Imaging. 2010;29:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Park D, Cho SJ, Kim K, Woo H, Kim JE, Lee JY, Koh J, Lee J, Choi JS, Chang DK, Choi YH, Chung JI, Cha WC, Jeong OS, Jekal SY, Kang M. Prediction Algorithms for Blood Pressure Based on Pulse Wave Velocity Using Health Checkup Data in Healthy Korean Men: Algorithm Development and Validation. JMIR Med Inform. 2021;9:e29212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Amrollahi F, Kennis BD, Shashikumar SP, Malhotra A, Taylor SP, Ford J, Rodriguez A, Weston J, Maheshwary R, Nemati S, Wardi G, Meier A. Prediction of Readmission Following Sepsis Using Social Determinants of Health. Crit Care Explor. 2024;6:e1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Li S, Zeng Y, Chapman WC Jr, Erfanzadeh M, Nandy S, Mutch M, Zhu Q. Adaptive Boosting (AdaBoost)-based multiwavelength spatial frequency domain imaging and characterization for ex vivo human colorectal tissue assessment. J Biophotonics. 2020;13:e201960241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Zhang J, Xu J, Hu X, Chen Q, Tu L, Huang J, Cui J. Diagnostic Method of Diabetes Based on Support Vector Machine and Tongue Images. Biomed Res Int. 2017;2017:7961494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genomics Proteomics. 2018;15:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 466] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 19. | Bishop C. Pattern Recognition and Feedforward Neural Networks. MIT Encyclopedia Cogn Sci. 1999;4:21-25. |
| 20. | Minic A, Jovanovic L, Bacanin N, Stoean C, Zivkovic M, Spalevic P, Petrovic A, Dobrojevic M, Stoean R. Applying Recurrent Neural Networks for Anomaly Detection in Electrocardiogram Sensor Data. Sensors (Basel). 2023;23:9878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Fitzgerald O, Perez-Concha O, Gallego-Luxan B, Metke-Jimenez A, Rudd L, Jorm L. Continuous time recurrent neural networks: Overview and benchmarking at forecasting blood glucose in the intensive care unit. J Biomed Inform. 2023;146:104498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Kumar AK, Ritam M, Han L, Guo S, Chandra R. Deep learning for predicting respiratory rate from biosignals. Comput Biol Med. 2022;144:105338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Azad R, Kazerouni A, Heidari M, Aghdam EK, Molaei A, Jia Y, Jose A, Roy R, Merhof D. Advances in medical image analysis with vision Transformers: A comprehensive review. Med Image Anal. 2024;91:103000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 109] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 24. | Nazi ZA, Peng W. Large Language Models in Healthcare and Medical Domain: A Review. Informatics. 2024;11:57. [DOI] [Full Text] |
| 25. | Chamberlin J, Kocher MR, Waltz J, Snoddy M, Stringer NFC, Stephenson J, Sahbaee P, Sharma P, Rapaka S, Schoepf UJ, Abadia AF, Sperl J, Hoelzer P, Mercer M, Somayaji N, Aquino G, Burt JR. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for lung cancer screening: accuracy and prognostic value. BMC Med. 2021;19:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Asgari Taghanaki S, Abhishek K, Cohen JP, Cohen-adad J, Hamarneh G. Deep semantic segmentation of natural and medical images: a review. Artif Intell Rev. 2021;54:137-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 27. | Pal A, Rai HM, Frej MBH, Razaque A. Advanced Segmentation of Gastrointestinal (GI) Cancer Disease Using a Novel U-MaskNet Model. Life (Basel). 2024;14:1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Baur C, Denner S, Wiestler B, Navab N, Albarqouni S. Autoencoders for unsupervised anomaly segmentation in brain MR images: A comparative study. Med Image Anal. 2021;69:101952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 29. | Zhang YH, Babaeizadeh S. Synthesis of standard 12lead electrocardiograms using two-dimensional generative adversarial networks. J Electrocardiol. 2021;69:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Rios A, Kavuluru R. Neural transfer learning for assigning diagnosis codes to EMRs. Artif Intell Med. 2019;96:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Guo X, Pan Y, Xia Y, Yuan Y. Dynamic feature splicing for few-shot rare disease diagnosis. Med Image Anal. 2023;90:102959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Liu SQ, Ngiam KY, Feng ML. Deep Reinforcement Learning for Clinical Decision Support: A Brief Survey. Available from: arXiv:1907.09475. [DOI] [Full Text] |
| 33. | Loftus TJ, Filiberto AC, Li Y, Balch J, Cook AC, Tighe PJ, Efron PA, Upchurch GR Jr, Rashidi P, Li X, Bihorac A. Decision analysis and reinforcement learning in surgical decision-making. Surgery. 2020;168:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Jiao R, Zhang Y, Ding L, Xue B, Zhang J, Cai R, Jin C. Learning with limited annotations: A survey on deep semi-supervised learning for medical image segmentation. Comput Biol Med. 2024;169:107840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 35. | Zhou L, Liu H, Bae J, He J, Samaras D, Prasanna P. Self Pre-Training with Masked Autoencoders for Medical Image Classification and Segmentation. 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI), Cartagena, Colombia, 2023, 1-6,. [DOI] [Full Text] |
| 36. | Sheikh M, Roshandel G, McCormack V, Malekzadeh R. Current Status and Future Prospects for Esophageal Cancer. Cancers (Basel). 2023;15:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 162] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 37. | Tan JL, Chinnaratha MA, Woodman R, Martin R, Chen HT, Carneiro G, Singh R. Diagnostic Accuracy of Artificial Intelligence (AI) to Detect Early Neoplasia in Barrett's Esophagus: A Non-comparative Systematic Review and Meta-Analysis. Front Med (Lausanne). 2022;9:890720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Tsai MC, Yen HH, Tsai HY, Huang YK, Luo YS, Kornelius E, Sung WW, Lin CC, Tseng MH, Wang CC. Artificial intelligence system for the detection of Barrett's esophagus. World J Gastroenterol. 2023;29:6198-6207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Visaggi P, de Bortoli N, Barberio B, Savarino V, Oleas R, Rosi EM, Marchi S, Ribolsi M, Savarino E. Artificial Intelligence in the Diagnosis of Upper Gastrointestinal Diseases. J Clin Gastroenterol. 2022;56:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Kahn A, McKinley MJ, Stewart M, Wang KK, Iyer PG, Leggett CL, Trindade AJ. Artificial intelligence-enhanced volumetric laser endomicroscopy improves dysplasia detection in Barrett's esophagus in a randomized cross-over study. Sci Rep. 2022;12:16314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Fukuda H, Ishihara R, Kato Y, Matsunaga T, Nishida T, Yamada T, Ogiyama H, Horie M, Kinoshita K, Tada T. Comparison of performances of artificial intelligence versus expert endoscopists for real-time assisted diagnosis of esophageal squamous cell carcinoma (with video). Gastrointest Endosc. 2020;92:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 42. | Zhang Y, Zhang X, Wu Q, Gu C, Wang Z. Artificial Intelligence-Aided Colonoscopy for Polyp Detection: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Laparoendosc Adv Surg Tech A. 2021;31:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 43. | Koh FH, Ladlad J; SKH Endoscopy Centre, Teo EK, Lin CL, Foo FJ. Real-time artificial intelligence (AI)-aided endoscopy improves adenoma detection rates even in experienced endoscopists: a cohort study in Singapore. Surg Endosc. 2023;37:165-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 44. | Barua I, Vinsard DG, Jodal HC, Løberg M, Kalager M, Holme Ø, Misawa M, Bretthauer M, Mori Y. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 45. | Wang KS, Yu G, Xu C, Meng XH, Zhou J, Zheng C, Deng Z, Shang L, Liu R, Su S, Zhou X, Li Q, Li J, Wang J, Ma K, Qi J, Hu Z, Tang P, Deng J, Qiu X, Li BY, Shen WD, Quan RP, Yang JT, Huang LY, Xiao Y, Yang ZC, Li Z, Wang SC, Ren H, Liang C, Guo W, Li Y, Xiao H, Gu Y, Yun JP, Huang D, Song Z, Fan X, Chen L, Yan X, Li Z, Huang ZC, Huang J, Luttrell J, Zhang CY, Zhou W, Zhang K, Yi C, Wu C, Shen H, Wang YP, Xiao HM, Deng HW. Accurate diagnosis of colorectal cancer based on histopathology images using artificial intelligence. BMC Med. 2021;19:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 46. | Alshohoumi F, Al-Hamdani A, Hedjam R, AlAbdulsalam A, Al Zaabi A. A Review of Radiomics in Predicting Therapeutic Response in Colorectal Liver Metastases: From Traditional to Artificial Intelligence Techniques. Healthcare (Basel). 2022;10:2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Han T, Zhu J, Chen X, Chen R, Jiang Y, Wang S, Xu D, Shen G, Zheng J, Xu C. Application of artificial intelligence in a real-world research for predicting the risk of liver metastasis in T1 colorectal cancer. Cancer Cell Int. 2022;22:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Rompianesi G, Pegoraro F, Ceresa CD, Montalti R, Troisi RI. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J Gastroenterol. 2022;28:108-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (3)] |
| 49. | Russo V, Lallo E, Munnia A, Spedicato M, Messerini L, D'Aurizio R, Ceroni EG, Brunelli G, Galvano A, Russo A, Landini I, Nobili S, Ceppi M, Bruzzone M, Cianchi F, Staderini F, Roselli M, Riondino S, Ferroni P, Guadagni F, Mini E, Peluso M. Artificial Intelligence Predictive Models of Response to Cytotoxic Chemotherapy Alone or Combined to Targeted Therapy for Metastatic Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancers (Basel). 2022;14:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Aikemu B, Xue P, Hong H, Jia H, Wang C, Li S, Huang L, Ding X, Zhang H, Cai G, Lu A, Xie L, Li H, Zheng M, Sun J. Artificial Intelligence in Decision-Making for Colorectal Cancer Treatment Strategy: An Observational Study of Implementing Watson for Oncology in a 250-Case Cohort. Front Oncol. 2020;10:594182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Yin Z, Yao C, Zhang L, Qi S. Application of artificial intelligence in diagnosis and treatment of colorectal cancer: A novel Prospect. Front Med (Lausanne). 2023;10:1128084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 52. | Lee JY, Calderwood AH, Karnes W, Requa J, Jacobson BC, Wallace MB. Artificial intelligence for the assessment of bowel preparation. Gastrointest Endosc. 2022;95:512-518.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 53. | Lin CH, Hsu PI, Tseng CD, Chao PJ, Wu IT, Ghose S, Shih CA, Lee SH, Ren JH, Shie CB, Lee TF. Application of artificial intelligence in endoscopic image analysis for the diagnosis of a gastric cancer pathogen-Helicobacter pylori infection. Sci Rep. 2023;13:13380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Higgins K, Nyssen OP, Southern J, Laponogov1 I, Aida Consortitum, Veselkov D, Gisbert JP, Kanonnikoff TF, Veselkov K. The Helicobacter pylori AI-Clinician: Harnessing Artificial Intelligence to Personalize H. pylori Treatment Recommendations. Available from: arXiv:2412.06841. [DOI] [Full Text] |
| 55. | Addissouky TA, Wang Y, El Sayed IET, Baz AE, Ali MMA, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef Univ J Basic Appl Sci. 2023;12:80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Nigam GB, Murphy MF, Travis SPL, Stanley AJ. Machine learning in the assessment and management of acute gastrointestinal bleeding. BMJ Med. 2024;3:e000699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Budimir I, Gradišer M, Nikolić M, Baršić N, Ljubičić N, Kralj D, Budimir I Jr. Glasgow Blatchford, pre-endoscopic Rockall and AIMS65 scores show no difference in predicting rebleeding rate and mortality in variceal bleeding. Scand J Gastroenterol. 2016;51:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Boros E, Pintér J, Molontay R, Prószéky KG, Vörhendi N, Simon OA, Teutsch B, Pálinkás D, Frim L, Tari E, Gagyi EB, Szabó I, Hágendorn R, Vincze Á, Izbéki F, Abonyi-Tóth Z, Szentesi A, Vass V, Hegyi P, Erőss B. New machine-learning models outperform conventional risk assessment tools in Gastrointestinal bleeding. Sci Rep. 2025;15:6371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Tsai SC, Lin CH, Chu CJ, Lo HY, Ng CJ, Hsu CC, Chen SY. Machine Learning Models for Predicting Mortality in Patients with Cirrhosis and Acute Upper Gastrointestinal Bleeding at an Emergency Department: A Retrospective Cohort Study. Diagnostics (Basel). 2024;14:1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 60. | Shung DL, Au B, Taylor RA, Tay JK, Laursen SB, Stanley AJ, Dalton HR, Ngu J, Schultz M, Laine L. Validation of a Machine Learning Model That Outperforms Clinical Risk Scoring Systems for Upper Gastrointestinal Bleeding. Gastroenterology. 2020;158:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 61. | Deshmukh F, Merchant S. Explainable machine learning models for predicting mortality from gastrointestinal bleeding in intensive care units. Chest. 2020;158:A656. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Maulahela H, Annisa NG. Current advancements in application of artificial intelligence in clinical decision-making by gastroenterologists in gastrointestinal bleeding. Artif Intell Gastroenterol. 2022;3:13-20. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 63. | He XJ, Wang XL, Su TK, Yao LJ, Zheng J, Wen XD, Xu QW, Huang QR, Chen LB, Chen CX, Lin HF, Chen YQ, Hu YX, Zhang KH, Jiang CS, Liu G, Li DZ, Li DL, Wen W. Artificial intelligence-assisted system for the assessment of Forrest classification of peptic ulcer bleeding: a multicenter diagnostic study. Endoscopy. 2024;56:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Craine BL, Silpa M, O'Toole CJ. Computerized auscultation applied to irritable bowel syndrome. Dig Dis Sci. 1999;44:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Nowak JK, Nowak R, Radzikowski K, Grulkowski I, Walkowiak J. Automated Bowel Sound Analysis: An Overview. Sensors (Basel). 2021;21:5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Mousavi E, Hassanzadeh Keshteli A, Sehhati M, Vaez A, Adibi P. Exploring new subgroups for irritable bowel syndrome using a machine learning algorithm. Sci Rep. 2023;13:18483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 67. | Tabata K, Mihara H, Nanjo S, Motoo I, Ando T, Teramoto A, Fujinami H, Yasuda I. Artificial intelligence model for analyzing colonic endoscopy images to detect changes associated with irritable bowel syndrome. PLOS Digit Health. 2023;2:e0000058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Tunali V, Arslan NÇ, Ermiş BH, Derviş Hakim G, Gündoğdu A, Hora M, Nalbantoğlu ÖU. A Multicenter Randomized Controlled Trial of Microbiome-Based Artificial Intelligence-Assisted Personalized Diet vs Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet: A Novel Approach for the Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2024;119:1901-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 69. | Nakata R, Tanaka F, Sugawara N, Kojima Y, Takeuchi T, Shiba M, Higuchi K, Fujiwara Y. Analysis of autonomic function during natural defecation in patients with irritable bowel syndrome using real-time recording with a wearable device. PLoS One. 2022;17:e0278922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Avey S, Chatterjee M, Manyakov NV, Cooper P, Sabins N, Mosca K, Mori S, Baribaud F, Morris M, Lehar J, Deiteren A, Cossu M, Smets S, Huizer T, Lamousé-Smith E, Campbell K, Pandis I. Using a wearable patch to develop a digital monitoring biomarker of inflammation in response to LPS challenge. Clin Transl Sci. 2024;17:e13734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 71. | Pimentel M, Mathur R, Wang J, Chang C, Hosseini A, Fiorentino A, Rashid M, Pichetshote N, Basseri B, Treyzon L, Chang B, Leite G, Morales W, Weitsman S, Kraus A, Rezaie A. A Smartphone Application Using Artificial Intelligence Is Superior To Subject Self-Reporting When Assessing Stool Form. Am J Gastroenterol. 2022;117:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Jeon SK, Lee JM, Joo I, Yoon JH, Lee G. Two-dimensional Convolutional Neural Network Using Quantitative US for Noninvasive Assessment of Hepatic Steatosis in NAFLD. Radiology. 2023;307:e221510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 73. | Zhang ZY, Li GX, Wang ZQ, Xia F, Zhao N, Nie HB, Ye ZZ, Lin JS, Hui YY, Liu XC. Fully Automated Deep Learning-enabled Detection for Hepatic Steatosis on Computed Tomography: A Multicenter International Validation Study. Available from: arXiv: 2210.15149. [DOI] [Full Text] |
| 74. | Piscaglia F, Cucchetti A, Benlloch S, Vivarelli M, Berenguer J, Bolondi L, Pinna AD, Berenguer M. Prediction of significant fibrosis in hepatitis C virus infected liver transplant recipients by artificial neural network analysis of clinical factors. Eur J Gastroenterol Hepatol. 2006;18:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Cai J, Chen T, Qi Y, Liu S, Chen R. Fibrosis and inflammatory activity diagnosis of chronic hepatitis C based on extreme learning machine. Sci Rep. 2025;15:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Wei R, Wang J, Wang X, Xie G, Wang Y, Zhang H, Peng CY, Rajani C, Kwee S, Liu P, Jia W. Clinical prediction of HBV and HCV related hepatic fibrosis using machine learning. EBioMedicine. 2018;35:124-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Zhou J, Wang W, Lei B, Ge W, Huang Y, Zhang L, Yan Y, Zhou D, Ding Y, Wu J, Wang W. Automatic Detection and Classification of Focal Liver Lesions Based on Deep Convolutional Neural Networks: A Preliminary Study. Front Oncol. 2020;10:581210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 78. | Schattenberg JM, Balp MM, Reinhart B, Porwal S, Tietz A, Pedrosa MC, Docherty M. Identification of Fast Progressors Among Patients With Nonalcoholic Steatohepatitis Using Machine Learning. Gastro Hep Adv. 2024;3:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 79. | Bosch J, Chung C, Carrasco-Zevallos OM, Harrison SA, Abdelmalek MF, Shiffman ML, Rockey DC, Shanis Z, Juyal D, Pokkalla H, Le QH, Resnick M, Montalto M, Beck AH, Wapinski I, Han L, Jia C, Goodman Z, Afdhal N, Myers RP, Sanyal AJ. A Machine Learning Approach to Liver Histological Evaluation Predicts Clinically Significant Portal Hypertension in NASH Cirrhosis. Hepatology. 2021;74:3146-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 80. | Sarkar S, Alurwar A, Ly C, Piao C, Donde R, Wang CJ, Meyers FJ. A Machine Learning Model to Predict Risk for Hepatocellular Carcinoma in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Adv. 2024;3:498-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 81. | Li X, Bao H, Shi Y, Zhu W, Peng Z, Yan L, Chen J, Shu X. Machine learning methods for accurately predicting survival and guiding treatment in stage I and II hepatocellular carcinoma. Medicine (Baltimore). 2023;102:e35892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Xu Y, Zhang B, Zhou F, Yi YP, Yang XL, Ouyang X, Hu H. Development of machine learning-based personalized predictive models for risk evaluation of hepatocellular carcinoma in hepatitis B virus-related cirrhosis patients with low levels of serum alpha-fetoprotein. Ann Hepatol. 2024;29:101540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 83. | Liu S, Yang Z, Li G, Li C, Luo Y, Gong Q, Wu X, Li T, Zhang Z, Xing B, Xu X, Lu X. Multi-omics Analysis of Primary Cell Culture Models Reveals Genetic and Epigenetic Basis of Intratumoral Phenotypic Diversity. Genomics Proteomics Bioinformatics. 2019;17:576-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 1125] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 85. | Yan C, Li M, Liu C, Zhang Z, Zhang J, Gao M, Han J, Zhang M, Zhao L. Development of a non-invasive diagnostic model for high-risk esophageal varices based on radiomics of spleen CT. Abdom Radiol (NY). 2024;49:4373-4382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 86. | Yu YD, Lee KS, Man Kim J, Ryu JH, Lee JG, Lee KW, Kim BW, Kim DS; Korean Organ Transplantation Registry Study Group. Artificial intelligence for predicting survival following deceased donor liver transplantation: Retrospective multi-center study. Int J Surg. 2022;105:106838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 87. | Saillard C, Schmauch B, Laifa O, Moarii M, Toldo S, Zaslavskiy M, Pronier E, Laurent A, Amaddeo G, Regnault H, Sommacale D, Ziol M, Pawlotsky JM, Mulé S, Luciani A, Wainrib G, Clozel T, Courtiol P, Calderaro J. Predicting Survival After Hepatocellular Carcinoma Resection Using Deep Learning on Histological Slides. Hepatology. 2020;72:2000-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 88. | Mofidi R, Duff MD, Madhavan KK, Garden OJ, Parks RW. Identification of severe acute pancreatitis using an artificial neural network. Surgery. 2007;141:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Hong WD, Chen XR, Jin SQ, Huang QK, Zhu QH, Pan JY. Use of an artificial neural network to predict persistent organ failure in patients with acute pancreatitis. Clinics (Sao Paulo). 2013;68:27-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Mashayekhi R, Parekh VS, Faghih M, Singh VK, Jacobs MA, Zaheer A. Radiomic features of the pancreas on CT imaging accurately differentiate functional abdominal pain, recurrent acute pancreatitis, and chronic pancreatitis. Eur J Radiol. 2020;123:108778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Li H, Shi K, Reichert M, Lin K, Tselousov N, Braren R, Fu D, Schmid R, Li J, Menze B. Differential Diagnosis for Pancreatic Cysts in CT Scans Using Densely-Connected Convolutional Networks. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:2095-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Kurita Y, Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Koda H, Tajika M, Shimizu Y, Nakajima A, Kubota K, Niwa Y. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci Rep. 2019;9:6893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Springer S, Masica DL, Dal Molin M, Douville C, Thoburn CJ, Afsari B, Li L, Cohen JD, Thompson E, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Simpson RE, Fernandez-Del Castillo C, Mino-Kenudson M, Brugge W, Brand RE, Singhi AD, Scarpa A, Lawlor R, Salvia R, Zamboni G, Hong SM, Hwang DW, Jang JY, Kwon W, Swan N, Geoghegan J, Falconi M, Crippa S, Doglioni C, Paulino J, Schulick RD, Edil BH, Park W, Yachida S, Hijioka S, van Hooft J, He J, Weiss MJ, Burkhart R, Makary M, Canto MI, Goggins MG, Ptak J, Dobbyn L, Schaefer J, Sillman N, Popoli M, Klein AP, Tomasetti C, Karchin R, Papadopoulos N, Kinzler KW, Vogelstein B, Wolfgang CL, Hruban RH, Lennon AM. A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med. 2019;11:eaav4772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 94. | Liu SL, Li S, Guo YT, Zhou YP, Zhang ZD, Li S, Lu Y. Establishment and application of an artificial intelligence diagnosis system for pancreatic cancer with a faster region-based convolutional neural network. Chin Med J (Engl). 2019;132:2795-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 95. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M, Dietrich CF, Havre R, Gheorghe C, McKay C, Gheonea DI, Ciurea T; European EUS Elastography Multicentric Study Group. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol. 2012;10:84-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 96. | Zhang Y, Lobo-Mueller EM, Karanicolas P, Gallinger S, Haider MA, Khalvati F. CNN-based survival model for pancreatic ductal adenocarcinoma in medical imaging. BMC Med Imaging. 2020;20:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 97. | Katzman JL, Shaham U, Cloninger A, Bates J, Jiang T, Kluger Y. DeepSurv: personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC Med Res Methodol. 2018;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 861] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 98. | Choi HJ, Kim YE, Namgoong JM, Kim I, Park JS, Baek WI, Lee BS, Yoon HM, Cho YA, Lee JS, Shim JO, Oh SH, Moon JS, Ko JS, Kim DY, Kim KM. Development and Validation of a Machine Learning-Based Prediction Model for Detection of Biliary Atresia. Gastro Hep Adv. 2023;2:778-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 99. | Larey A, Aknin E, Daniel N, Osswald GA, Caldwell JM, Rochman M, Wasserman T, Collins MH, Arva NC, Yang GY, Rothenberg ME, Savir Y. Harnessing artificial intelligence to infer novel spatial biomarkers for the diagnosis of eosinophilic esophagitis. Front Med (Lausanne). 2022;9:950728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 100. | Li Z, Song C, Huang J, Li J, Huang S, Qian B, Chen X, Hu S, Shu T, Yu G. Performance of Deep Learning-Based Algorithm for Detection of Pediatric Intussusception on Abdominal Ultrasound Images. Gastroenterol Res Pract. 2022;2022:9285238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 101. | Wang ZY, Wei LJ, Xue L. Overcoming Medical Overuse with AI Assistance: An Experimental Investigation. Available from: arXiv:2405.10539. [DOI] [Full Text] |
| 102. | Lavoie-Gagne O, Woo JJ, Williams RJ 3rd, Nwachukwu BU, Kunze KN, Ramkumar PN. Artificial Intelligence as a Tool to Mitigate Administrative Burden, Optimize Billing, Reduce Insurance- and Credentialing-Related Expenses, and Improve Quality Assurance Within Health Care Systems. Arthroscopy. 2025;S0749-8063(25)00216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 103. | Davis S, Zhang J, Lee I, Rezaei M, Greiner R, McAlister FA, Padwal R. Effective hospital readmission prediction models using machine-learned features. BMC Health Serv Res. 2022;22:1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 104. | Perez K, Wisniewski D, Ari A, Lee K, Lieneck C, Ramamonjiarivelo Z. Investigation into Application of AI and Telemedicine in Rural Communities: A Systematic Literature Review. Healthcare (Basel). 2025;13:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 105. | Yadav N, Pandey S, Gupta A, Dudani P, Gupta S, Rangarajan K. Data Privacy in Healthcare: In the Era of Artificial Intelligence. Indian Dermatol Online J. 2023;14:788-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 106. | Seyyed-Kalantari L, Zhang H, McDermott MBA, Chen IY, Ghassemi M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat Med. 2021;27:2176-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 107. | Contaldo MT, Pasceri G, Vignati G, Bracchi L, Triggiani S, Carrafiello G. AI in Radiology: Navigating Medical Responsibility. Diagnostics (Basel). 2024;14:1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 108. | Roustan D, Bastardot F. The Clinicians' Guide to Large Language Models: A General Perspective With a Focus on Hallucinations. Interact J Med Res. 2025;14:e59823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 109. | Yang J, Soltan AAS, Clifton DA. Machine learning generalizability across healthcare settings: insights from multi-site COVID-19 screening. NPJ Digit Med. 2022;5:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 110. | Preti LM, Ardito V, Compagni A, Petracca F, Cappellaro G. Implementation of Machine Learning Applications in Health Care Organizations: Systematic Review of Empirical Studies. J Med Internet Res. 2024;26:e55897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 111. | Deutsch TM, Pfob A, Brusniak K, Riedel F, Bauer A, Dijkstra T, Engler T, Brucker SY, Hartkopf AD, Schneeweiss A, Sidey-Gibbons C, Wallwiener M. Machine learning and patient-reported outcomes for longitudinal monitoring of disease progression in metastatic breast cancer: a multicenter, retrospective analysis. Eur J Cancer. 2023;188:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Matsumura G, Honda S, Kikuchi T, Mizuno Y, Hara H, Kondo Y, Nakamura H, Watanabe S, Hayakawa K, Nakajima K, Takei K. Real-time personal healthcare data analysis using edge computing for multimodal wearable sensors. Device. 2025;3:100597. [DOI] [Full Text] |
| 113. | Kim J, Mun S, Lee S, Jeong K, Baek Y. Prediction of metabolic and pre-metabolic syndromes using machine learning models with anthropometric, lifestyle, and biochemical factors from a middle-aged population in Korea. BMC Public Health. 2022;22:664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Arakaki S, Takenaka S, Sasaki K, Kitaguchi D, Hasegawa H, Takeshita N, Takatsuki M, Ito M. Artificial Intelligence in Minimally Invasive Surgery: Current State and Future Challenges. JMA J. 2025;8:86-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 115. | Mamlook REA, Wells LJ, Sawyer R. Machine-learning models for predicting surgical site infections using patient pre-operative risk and surgical procedure factors. Am J Infect Control. 2023;51:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 116. | Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25:1315-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 117. | Glissen Brown JR, Waljee AK, Mori Y, Sharma P, Berzin TM. Charting a path forward for clinical research in artificial intelligence and gastroenterology. Dig Endosc. 2022;34:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Lekadir K, Frangi AF, Porras AR, Glocker B, Cintas C, Langlotz CP, Weicken E, Asselbergs FW, Prior F, Collins GS, Kaissis G, Tsakou G, Buvat I, Kalpathy-Cramer J, Mongan J, Schnabel JA, Kushibar K, Riklund K, Marias K, Amugongo LM, Fromont LA, Maier-Hein L, Cerdá-Alberich L, Martí-Bonmatí L, Cardoso MJ, Bobowicz M, Shabani M, Tsiknakis M, Zuluaga MA, Fritzsche MC, Camacho M, Linguraru MG, Wenzel M, De Bruijne M, Tolsgaard MG, Goisauf M, Cano Abadía M, Papanikolaou N, Lazrak N, Pujol O, Osuala R, Napel S, Colantonio S, Joshi S, Klein S, Aussó S, Rogers WA, Salahuddin Z, Starmans MPA; FUTURE-AI Consortium. FUTURE-AI: international consensus guideline for trustworthy and deployable artificial intelligence in healthcare. BMJ. 2025;388:e081554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 125] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/