INTRODUCTION
Surgery was the only curative option for the treatment of liver cancer for a long time. However, in the early 1980s percutaneous ethanol injection (PEI) under real-time, grayscale ultrasonography (US) guidance suggested that small hepatocellular carcinomas (HCCs) could effectively be treated with minimally invasive techniques[1,2].
Ethanol injected into the tumor through very fine needles (from 19 to 21 gauge) induces rapid dehydration of cytoplasmic proteins with subsequent coagulation necrosis of the tumoral tissue, necrosis of endothelial cells with platelet aggregation and thrombosis of small vessels, and tissue ischemia. Moreover, the presence of peripheral capsula in most HCCs, their hypervascularity, and the high density of the cirrhotic tissue surrounding the tumor allow ethanol to achieve high intratumoral concentrations, making PEI an effective, safe, and cheap treatment for HCC lesions under 2 cm in diameter[3-5].
However, PEI has shown some major limitations. First, the volume of ethanol that could be injected in a single session was low, leading to multiple treatment sessions even for small lesions. The attempts of injecting large volumes of ethanol in a single session under general anesthesia yielded questionable results and some severe complications, with mortality rates up to 4.5%-5.0%[6,7]. Second, PEI was not effective to treat HCC satellites. Therefore, local tumor progression (LTP) was not infrequent. Finally, PEI was proven to be unsuccessful in the treatment of liver metastases due to their poor vascularity and absence of a peripheral capsule[8].
Some preliminary reports on other attempts of chemical ablation with acetic acid[9] or hot saline[10] were not followed by larger trials with longer follow-up, and these methods were abandoned. As a result, PEI remained the only percutaneous technique that was used worldwide until the early 1990s. However, PEI was limited to the treatment of small HCC lesions not suitable for surgery due to patient comorbidity or poor liver function.
THERMAL ABLATION
The shift from chemical to thermal ablation represented a milestone in the percutaneous treatment of liver tumors, in particular with the “hot” approach. Despite the interesting physical characteristics and good cytotoxic effect of the “cold” approach of thermal ablation, cryoablation was only sporadically used to treat liver tumors due to its high rates of severe complications and high costs[11,12].
The first “hot” technique introduced in clinical practice was interstitial laser photocoagulation. This technique converts light absorption into heat, provoking tissue coagulation[13]. However, interstitial laser photocoagulation produced small thermal lesions (20 mm or less) and was technically challenging because multiple, regularly-spaced laser fibers had to be inserted into the tumor. Therefore, it had limited clinical diffusion, mainly in the treatment of small multiple liver metastases[14,15].
The first experiences with radiofrequency ablation (RFA) in the 1990s on both animals and humans changed the history of thermal ablation of liver tumors[16,17]. Radiofrequency waves induce ionic agitation resulting in production of heat by friction because the surrounding tissue opposes resistance to the current of the flow. Heat is generated within the tissue rather than at the tip of the probe. The heat produced inside the tumor results from the difference between the heat generated by radiofrequency waves and the entity of heat dispersion, depending on tissue conductivity and proximity of blood vessels (the so-called heat-sink effect).
The size and shape of the coagulation area depend on the diameter of the electrode, length of the exposed needle tip, temperature around the exposed tip, and time of application[18]. However, charring of the tissue surrounding the needle tip hampers heat propagation within the tumor, remarkably reducing the size of the necrotic area. To solve this problem, probes with an internal cooling system were introduced in 1995[19], and further technical improvements aimed at increasing the size of the thermal lesion (multiprobe cooled tip arrays, perfusion needles, probes with side-deployed arrays, and so on) were launched into clinical practice[20].
Several randomized trials and meta-analyses showed that RFA was superior to PEI in the treatment of small HCC in terms of primary technique efficacy rate, LTP, and overall survival (OS)[21-24]. Indeed, in tumors under 3 cm in size, RFA frequently obtained a 0.5-1.0 cm safety margin, significantly reducing LTP rates and achieving OS rates comparable to surgical resection[25]. Based on these results, it was included with the curative treatment options for early-stage HCC[26]. Unlike PEI, RFA is also effective in the treatment of small liver metastases[19,27,28]. A phase II randomized trial reported that systemic chemotherapy combined with RFA significantly prolonged OS compared to chemotherapy alone in patients with colorectal liver metastases[29]. However, despite the ongoing technical improvements, RFA remains poorly effective in the treatment of nodules ≥ 3 cm in size. Moreover, the heat sink effect limits its efficacy when the tumor is close to large blood vessels.
In the late 1990s, microwave ablation (MWA) was proposed as an alternative to RFA[30]. Microwave energy applied to tumor tissue creates a rapidly alternating electromagnetic field, and polar molecules (primarily H2O) are forced to continuously realign with the oscillating electric field. Their kinetic energy increases, which increases the tissue temperature and causes coagulation necrosis. Microwaves are produced by a generator that works at a wavelength of 915 or 2450 MHz and are delivered to tissues through needle-like antennas. Due to the radiating nature of the antenna, direct heating occurs in a volume of tissue around the antenna.
This mechanism of heating differs substantially from RFA and offers some advantages. RFA heating requires an electrically conductive path, is limited in areas of low electrical conductivity, and heats only tissues immediately adjacent to the electrode. Conversely, microwaves are capable of propagating through tissues with low electrical conductivity, high impedance, or low thermal conductivity and are not influenced by the heat sink effect. Therefore, larger ablation volumes and a more predictable shape of the ablation volume should be expected[31].
Despite these theoretical advantages, the first experiences with MWA did not yield better results than RFA[32,33]. The main limit of the first-generation MWA systems was the back heating effect due to reflected waves along the antenna. The effects of this were the need to deliver energy at low power for a short time, small coagulation areas, and the requirement of multiple antennas to treat HCC lesions up to 2 cm[32,33].
Technical improvements, such as a cooling jacket around the antenna to decrease cable heating, and the introduction in the distal portion of the antenna of a miniaturized device for microwave confinement to minimize the back heating effect enabled the delivery of high-power energy for a longer time, achieving large ablation areas. The first in vivo randomized comparison between the most recent generation of the MWA system and an internally cooled RFA system found significantly larger ablation areas for MWA than for RFA[34]. Subsequent studies confirmed the efficacy of MWA in the treatment of both HCC and liver metastases from 3-5 cm in size, suggesting that the so-called 3-cm barrier was finally going to be broken[35-38].
US-GUIDANCE: FROM GRAYSCALE TO FUSION IMAGING
Grayscale US was the first imaging technique used to guide needle placement in the percutaneous ablation of liver tumors[1,2,17,19], and US still remains the most used guidance tool worldwide. However, confident visualization of the lesions can sometimes be poor because of small size, deep location, obscuration by overlying structures, or patient characteristics such as obesity or meteorism[39].
Computed tomography (CT) provides a larger field of view and better visualization of the target lesion and is less limited by patient characteristics. However, it requires radiation exposure, does not allow real-time visualization of intrahepatic vessels, and offers a limited angle for needle insertion[39]. Magnetic resonance imaging (MRI) is the most sensitive imaging technique to detect small liver lesions, does not require radiation exposure, and allows near real-time thermal monitoring to adjust power settings and optimize treatment duration. However, it is expensive, its availability is limited, and the ablation procedure is complex and time-consuming[39].
Therefore, despite the limits of US guidance, the vast majority of interventional oncology centers still consider US the first-choice to guide percutaneous ablation. Indeed, US guidance is cheap, readily available, does not require radiation exposure, enables real-time monitoring of each step of the ablation procedure, and allows the placement of the patient in the most suitable position to reach the target area.
In the late 1990s, the implementation of harmonic imaging, which exploits non-linear propagation of US waves through the tissues, decreased reverberation artifacts and improved the signal-to-noise ratio, providing better quality images compared to conventional US[40]. However, a major advancement in US imaging was the introduction of second-generation US contrast media that consisted of small (3-7 µm) gas-containing microbubbles with an external lipidic, proteic, or polymeric shell. Intravenous microbubbles are confined to the blood pool and when they are exposed to the ultrasound beam experience a resonance phenomenon with a frequency from 2-15 MHz[41].
Exploiting this non-linear interaction of the US wave with microbubbles and using low mechanical index, contrast-specific techniques to minimize microbubble destruction, it is possible to achieve continuous, real-time imaging of macrovessels and microvessels in normal and pathologic tissues. Contrast-enhanced US (CEUS) dramatically improved both characterization and detection of liver lesions not visible upon conventional US to such an extent that it is similar to CT or MRI[42]. Even more importantly in interventional oncology, CEUS can differentiate perfuse, viable tissues from necrotic tissues enabling the assessment of the completion of the ablation after the end the procedure and guiding the immediate retreatment of residual viable foci in the tumor tissue, which significantly reduces LTP rates[43,44].
Unfortunately, CEUS unavoidably shares some limitations similar to conventional US. First, US wave penetration is decreased in obese or meteoric patients or in presence of fatty liver. Second, small and deep lesions or lesions at the hepatic dome are extremely difficult to visualize. Third (and most important) in the assessment of ablation completion, CEUS images are two-dimensional, but the tumor and thermal lesion are three-dimensional, leading to the possibility of missing residual viable foci[45].
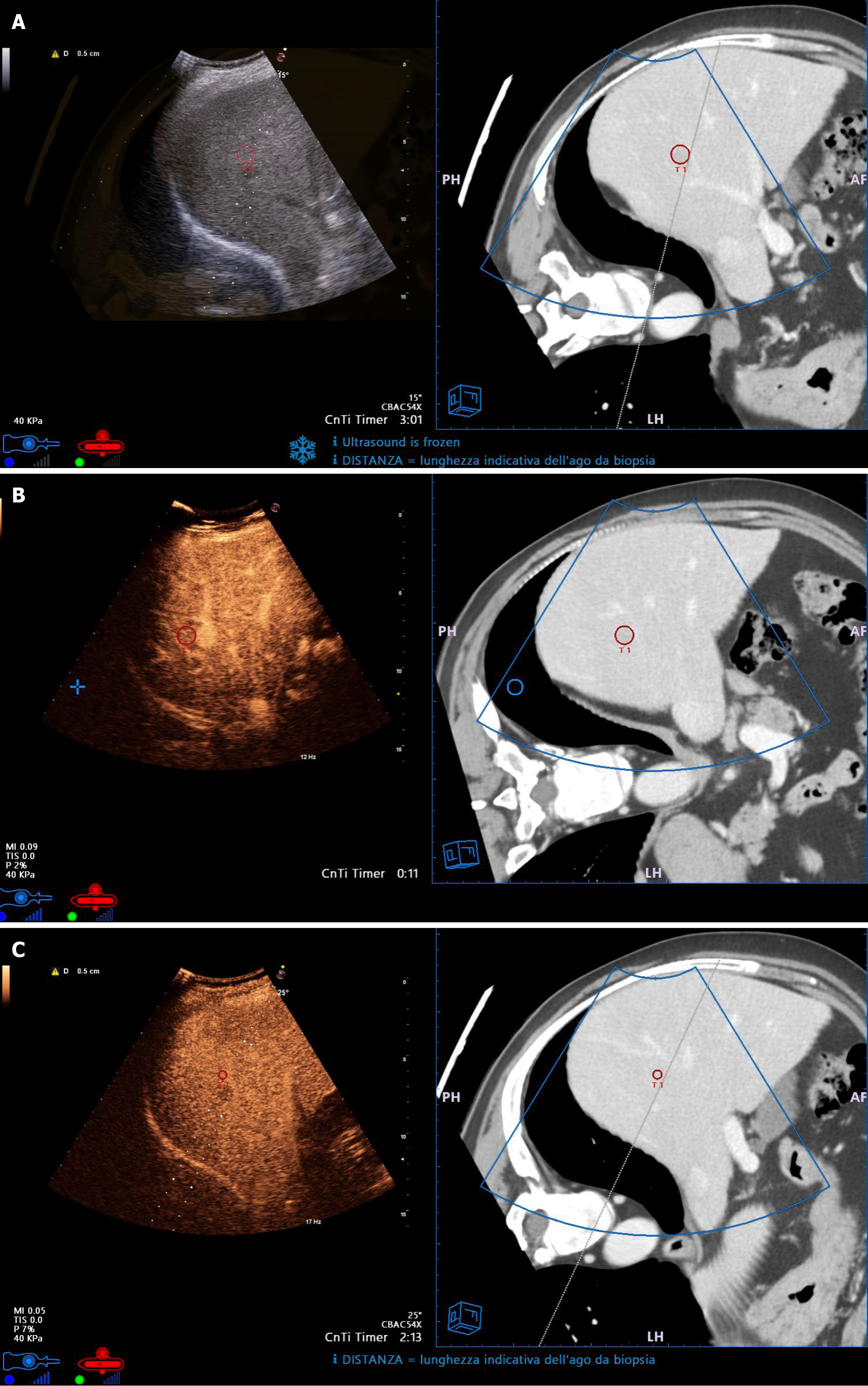
Therefore, further advancements are still needed for US and CEUS to remain the first-choice to guide percutaneous ablation. Recent technical advancements such as navigation systems and fusion imaging (FI) (i.e. the fusion of real-time US and CEUS with previously acquired CT or MRI scans) are moving in this direction. They enable precise localization (Figure 1A), allow characterization and correct targeting (Figure 1B and C) of lesions that are difficult to visualize (thus expanding the indications for US-guided or CEUS-guided ablation of tumors missed at US or CEUS), and lead to visualization of tumors in high-risk locations (Figure 2A). Moreover, FI provides a better assessment of the ablation area (Figure 2B), which improves the evaluation of ablation completion and permits the treatment of large tumors with multiple needle insertions[46-48].
Figure 1 Small hepatocellular carcinoma with low visibility on ultrasound.
A: Fusion Imaging (FI) allows to precisely identify and target the lesion (red circlet in both left and right sides of the split screen); B: Contrast-enhanced ultrasound (CEUS) shows hyperhancement in the arterial phase (left side of the split screen); C: CEUS shows wash-out in the portal-venous phase (left side of the split screen). FI allows correct guidance of the needle electrode insertion for thermal ablation (dotted line in CEUS images, continuous line in Computed Tomography images).
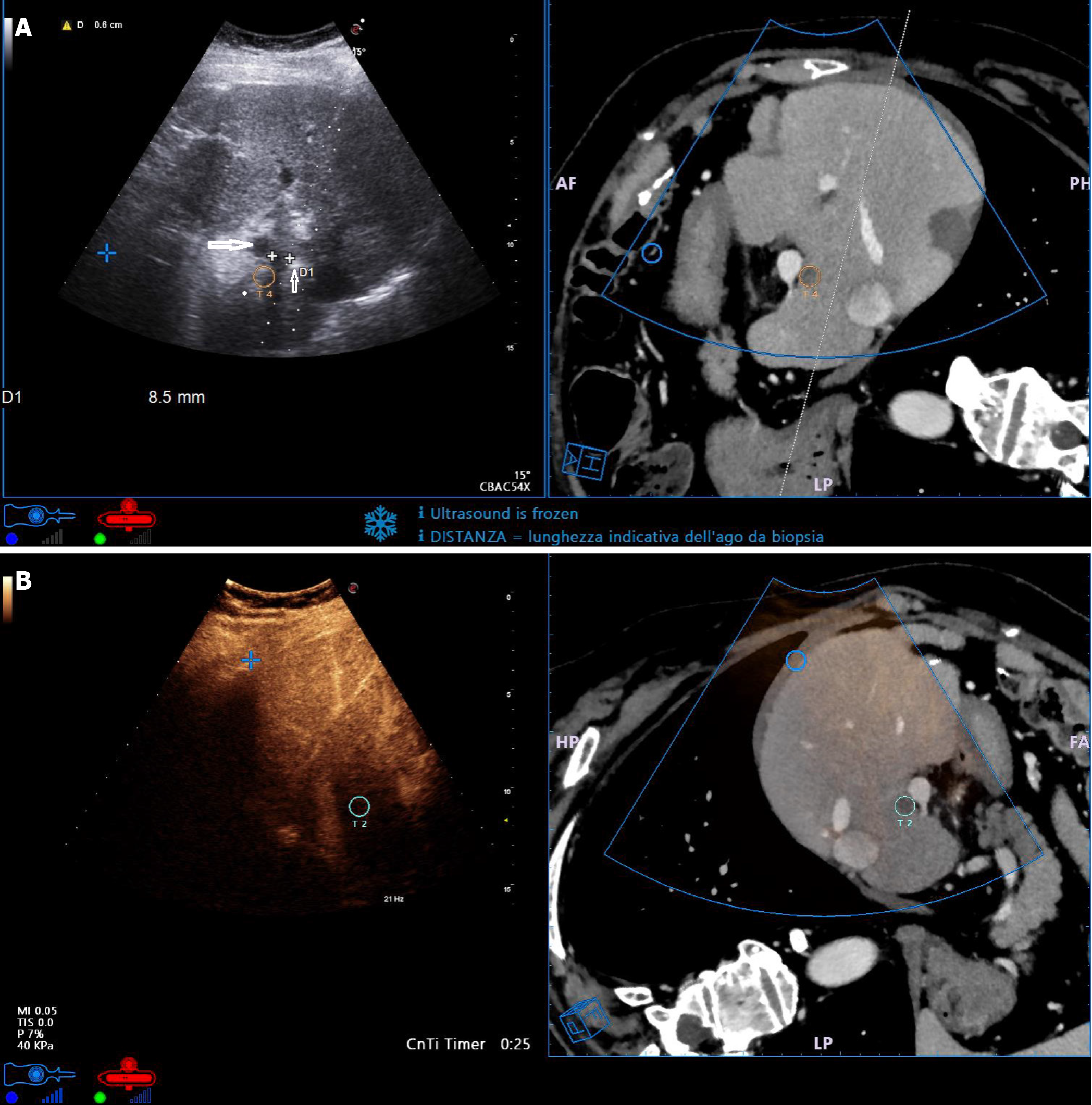
Figure 2 Fusion imaging of small metastasis from colorectal cancer in difficult and high-risk location.
A: The lesion was close to the portal vein (white cross-shaped markers in ultrasound image, yellow circle in computed tomography image). The needle tip was correctly placed lateral of the lesion (thin arrow) to maintain a safe distance from the portal vein (large arrow); B: CEUS scan performed 10 min after thermal ablation (with ultrasound transducer orientation inverted compared to Figure 3A) showed complete necrosis. Fusion imaging confirmed the presence of safety ablation margins around the targeted lesion.
The complete workflow of FI consists of acquiring baseline images, registering and creating a fusion image, validating the fused image, employing the fused image for real-time guidance during the procedure, and evaluating the post-procedural result of the treatment. FI originates from both different (i.e. CT-US or MRI-US) and identical imaging modalities (i.e. US-US) acquired before, during, and after thermal ablation[46]. FI is mostly based on the fusion of real-time US images with pre-ablation CT or MRI, but interesting results have also been reported for three-dimensional US-US FI. In this regard, US-US FI has been recently reported to achieve a higher successful registration rate than multimodality FI due to the higher anatomical consistency in monomodality imaging[49].
Regardless of imaging modalities used, intraprocedural real-time FI improves safety and efficacy of percutaneous ablation. A systematic review that analyzed 22 studies confirmed the usefulness of FI in planning and guiding the treatment as well as in monitoring treatment efficacy[47]. In particular, some studies reported immediate technical success rates ranging from 80.7% to 100% and LTP rates ranging from 0% to 14.8%[50,51]. Moreover, other authors reported an improved one-time ablation rate and OS rate as well as a decreased major complication rate[48]. Furthermore, FI allows the identification of incomplete ablation. This leads to accurate and safe guidance of either retreatment or additional needle insertions when large tumors are treated.
It is known that the LTP rate strongly depends on tumor size: As it increases, its spatial shape becomes more complex, making it more difficult to obtain adequate ablation margins and increasing the risk of thermal damage to adjacent structures[48,52,53]. Moreover, ablation planning is more difficult, and multiple needle insertions are needed to cover the entire volume of the tumor. Using FI guidance, Yang et al[48] achieved a technical success rate of 97.1% in the treatment of primary and secondary liver tumors 3-7 cm in size. They were able to use FI to stereoscopically define the profile of both the lesions and surrounding structures and to guide the optimal needle insertion, correctly stating the ablation volume and avoiding thermal damage. Interestingly, no difference in technique efficacy rate was found between 3.0-5.0 cm and 5.1-7.0 cm nodules[48], suggesting that US FI might play a key role in the treatment of medium to large-sized liver tumors, particularly using MWA systems.
ARTIFICIAL INTELLIGENCE APPLIED TO US GUIDANCE SYSTEMS: WHERE COULD IT LEAD PERCUTANEOUS ABLATION OF LIVER TUMORS?
Artificial intelligence (AI) is revolutionizing the landscape of modern medicine, assisting physicians in complex interventional procedures that rely on advanced imaging modalities such as MRI, CT, and US.
AI for automatic registration in abdominal FI
It is well recognized that real-time FI can increase safety and efficacy of percutaneous ablation, but achieving perfect synchronization between the two selected imaging modalities is crucial to improve treatment outcomes. Synchronization requires very skilled operators and is complex and time-consuming. AI can aid clinicians in this crucial phase. Machine learning algorithms can work like a very expert clinician who has performed many evaluations. These algorithms are trained to understand and optimize the integration of data from various imaging modalities in real-time. By automating the synchronization process, AI ensures precise alignment and registration of imaging data, enabling the lesion to be targeted more accurately and permitting the precise placement of the needle, ultimately improving the overall accuracy of the ablative procedure[54].
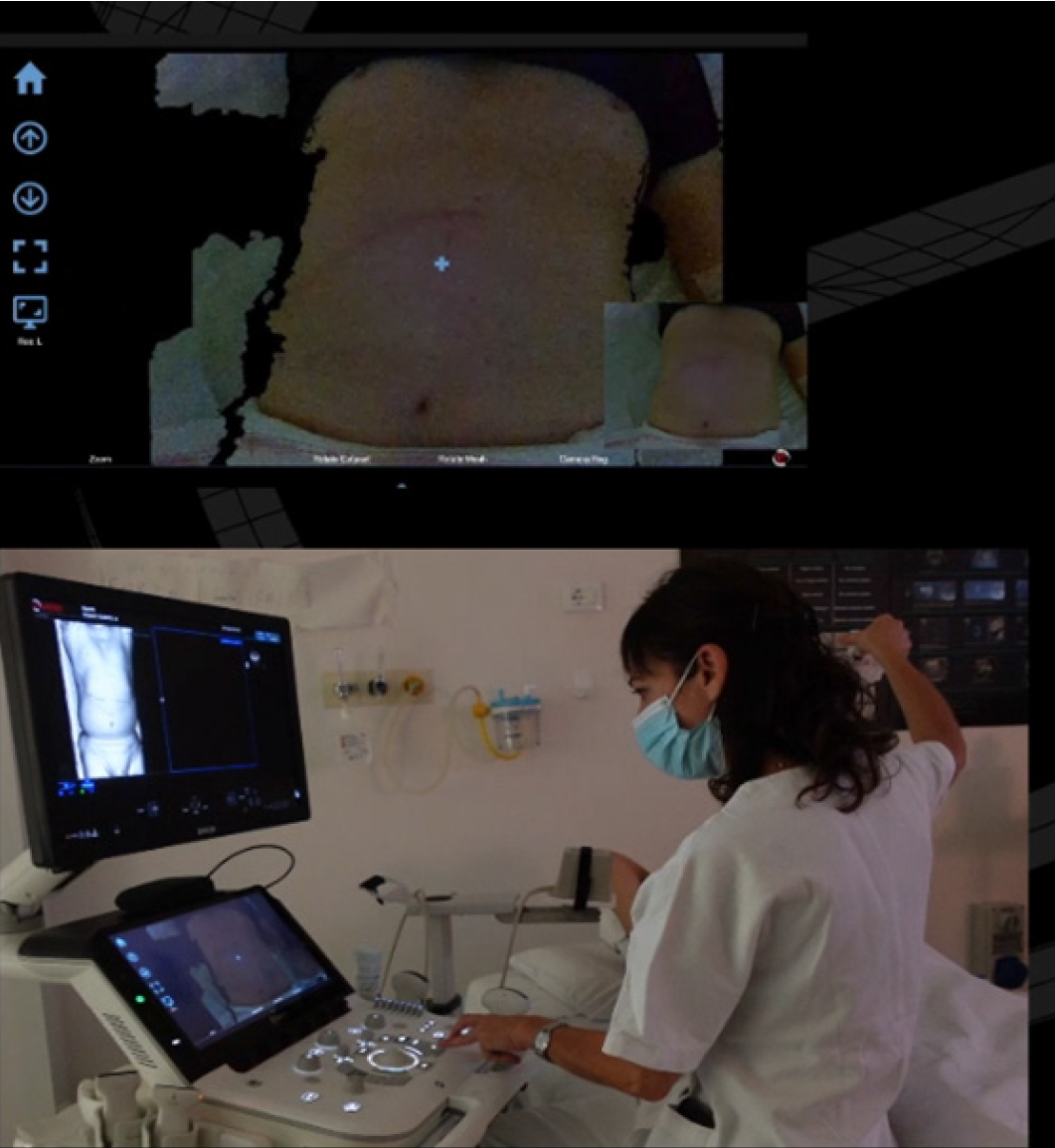
In this regard, an innovative tool introduces a sophisticated approach to FI (AutoSync®; Esaote S.p.A., Genova, Italy). This system utilizes a three-dimensional camera positioned over the patient’s abdomen to capture detailed photographs of the skin surface (Figure 3). Subsequently, an advanced algorithm conducts a pixel-by-pixel comparison between these images and the three-dimensional volume derived from CT or MRI datasets, achieving precise alignment and registration. AutoSync® can enable clinicians to promptly verify registration accuracy and to proceed with fine-tuning adjustments using conventional tools, particularly focusing on areas of interest. This system significantly expedites the initial phase of imaging synchronization and has the advantage of being independent of patient positioning. The algorithm comprehensively recognizes the entire surface of datasets, allowing synchronization even with patients positioned in lateral decubitus and ensuring seamless integration and alignment.
Figure 3
Three-dimensional camera positioned over the patient’s abdomen captured detailed images of the skin surface.
AI for post-procedure control of ablation volume
A concentric ablative margin of at least 5 mm significantly decreases the LTP rate[27,55]. Post-procedure CEUS and FI between pre-ablation CT and post-ablation CEUS can help assess the completeness of the ablation and the size of the safety margin to guide further needle insertions[43,44,46-48]. Nevertheless, the side-by-side comparison between pre-procedure and post-procedure contrast-enhanced cross-sectional images is undoubtedly more reliable[20]. However, it too relies on visual evaluation by clinicians and has a non-negligible margin of error because of the misalignment of the liver due to the patient’s position and breath movements as well as the tissue biomechanical changes after ablation[56].
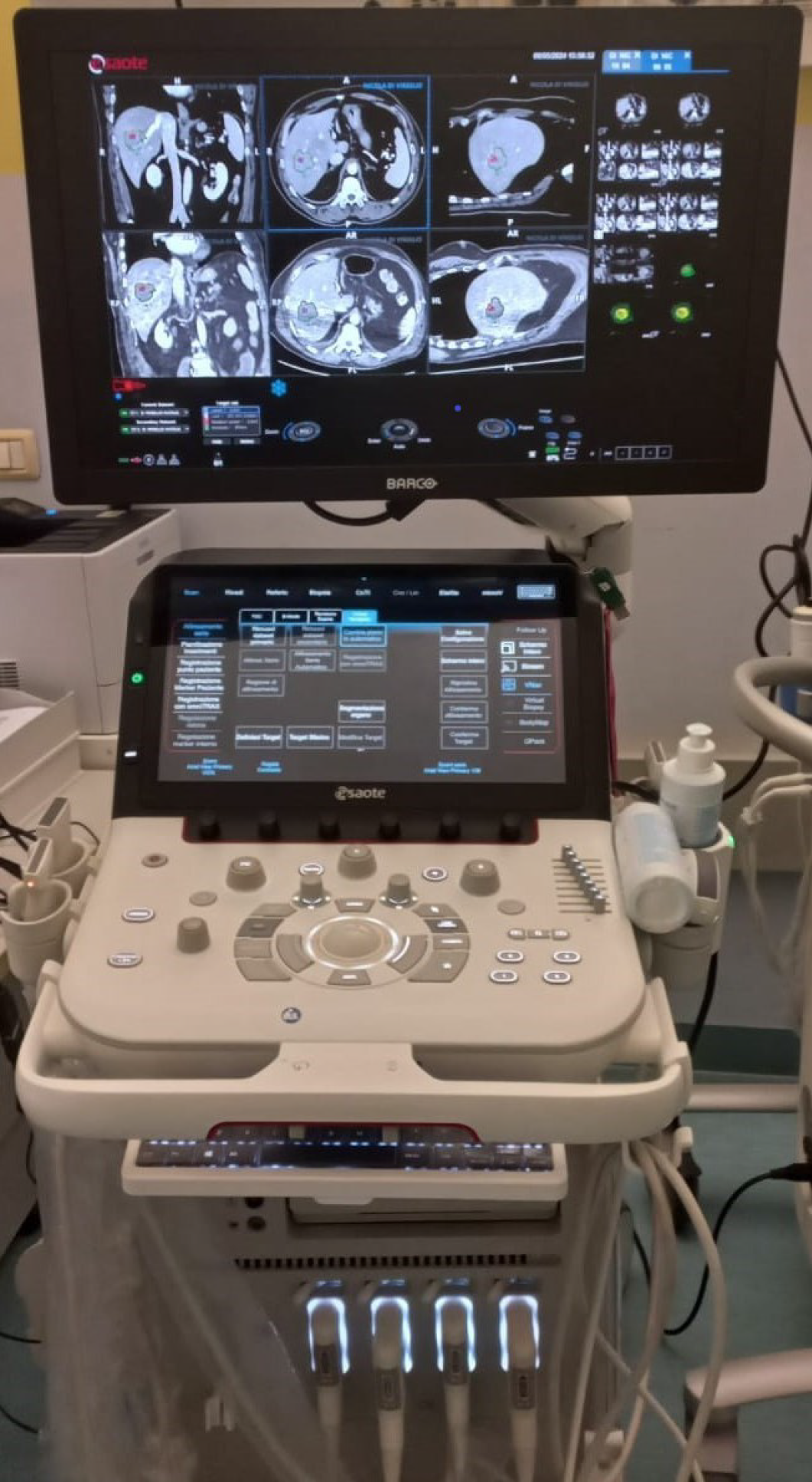
Recently, various software have been developed to provide objective and precise assessment of ablation margins using the non-rigid registration of pre-procedure and post-procedure CT or MRI[57,58]. These tools are becoming mandatory for the precise assessment of the technical success of percutaneous ablation. However, they are external to the US machine, are not user-friendly, and are time-consuming. These limits have led to the development of a new technology. The Augmented Ablation Suite (AuAS®; Esaote, Genova, Italy) integrates a pre-ablation and post-ablation control software directly into the most advanced Esaote US machines (Figure 4). Powered by AI, AuAS® combines pre-ablation and post-ablation CT datasets through an elastic registration, which follows a preliminary segmentation of the abdominal organs. Afterwards, it compares the pre-procedure planned margins (Figure 5) with the necrotic area obtained by ablation (Figure 6), identifying and quantifying any residual viable tumor tissue or insufficient safety margin (Figures 7 and 8) and displaying them as a new target. The development and introduction of this technology will very likely allow significant increases in precision targeting of percutaneous ablation, optimizing technical success and patient outcomes.
Figure 4 Augmented Ablation Suite.
Pre-ablation and post-ablation computed tomography datasets are displayed on the monitor of the ultrasound machine.
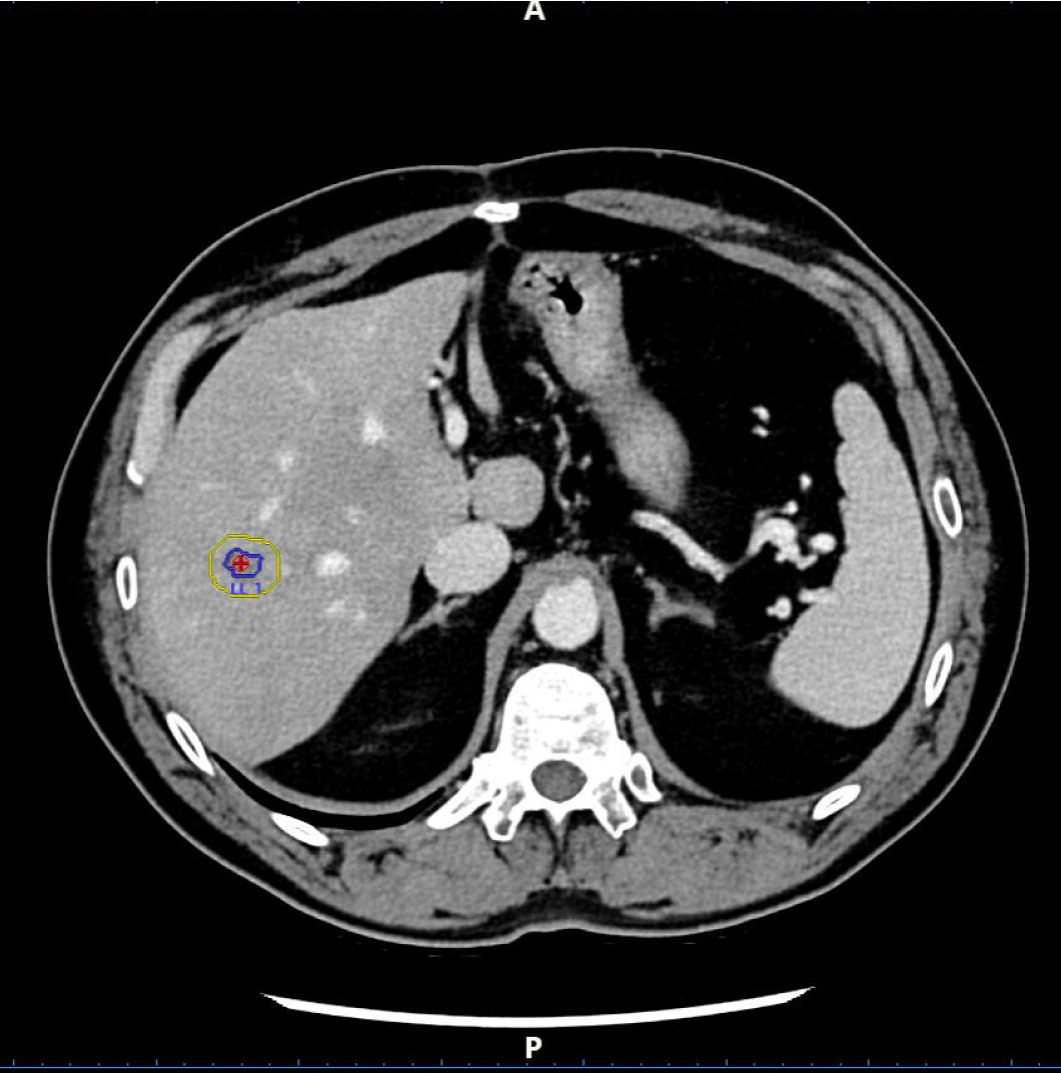
Figure 5 Pre-ablation two-dimensional computed tomography.
The target lesion (red circle) was circumscribed scan by scan by the operator (blue line), and the software calculated the safety margin (yellow line).
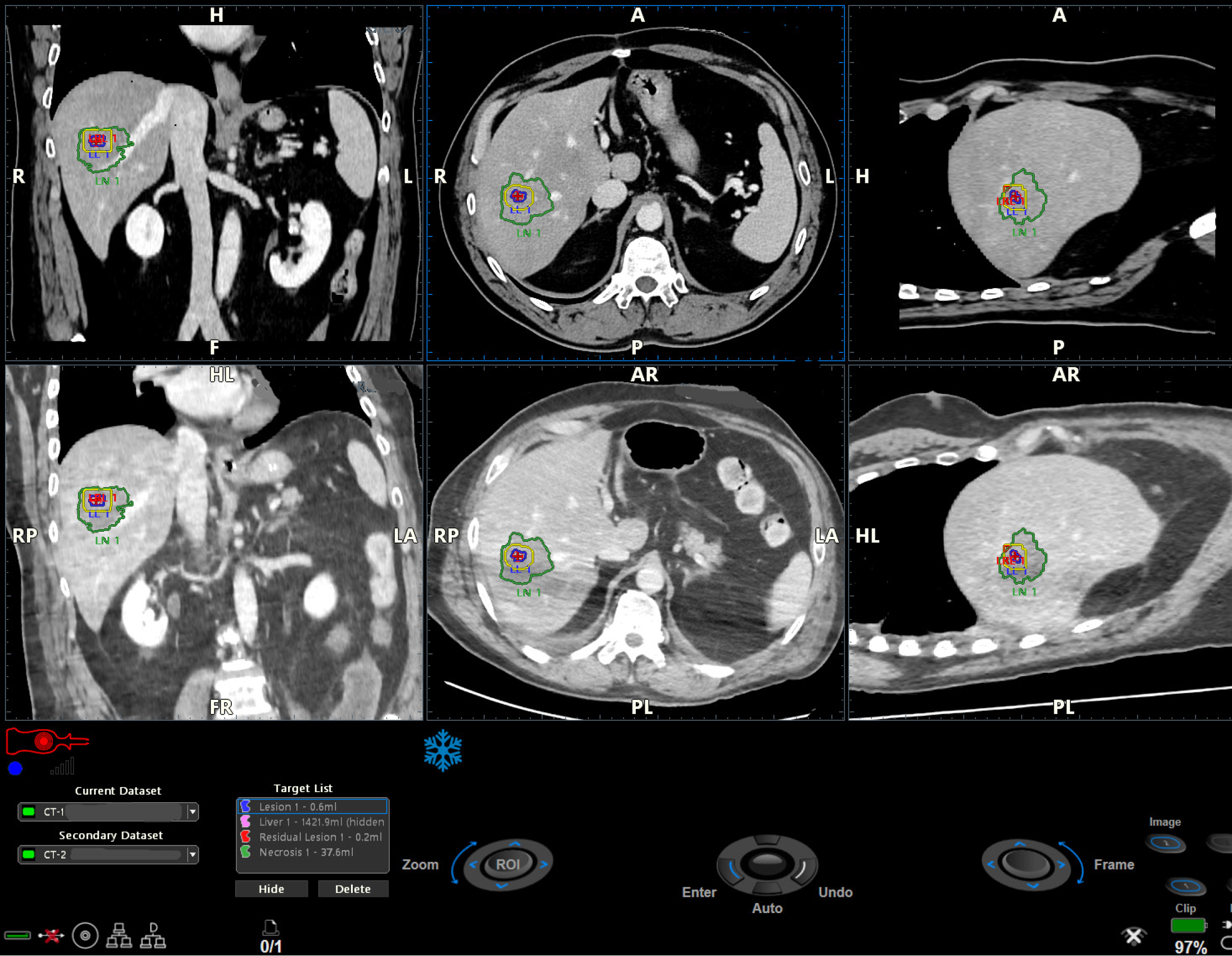
Figure 6
Detail of Figure 4: The pre-ablation dataset (upper series), the post-ablation dataset (lower series), the lesion (red circle and blue line), the safety margin calculated by the software (yellow perimeter), and the necrosis area (green perimeter) are displayed in the three plans.
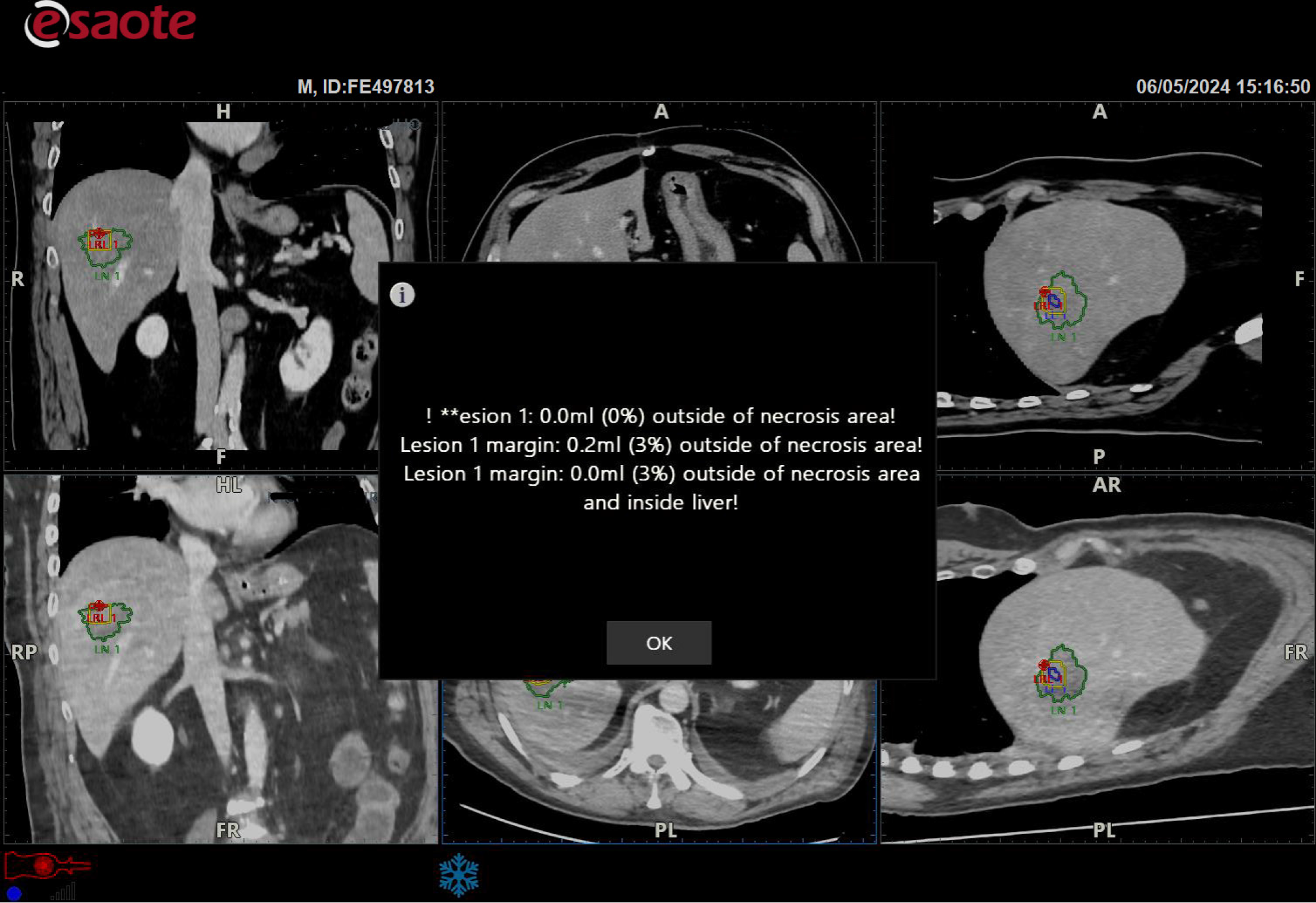
Figure 7
The target lesion was within the necrotic area, but the software warned that the safety margin was insufficient (red spot).
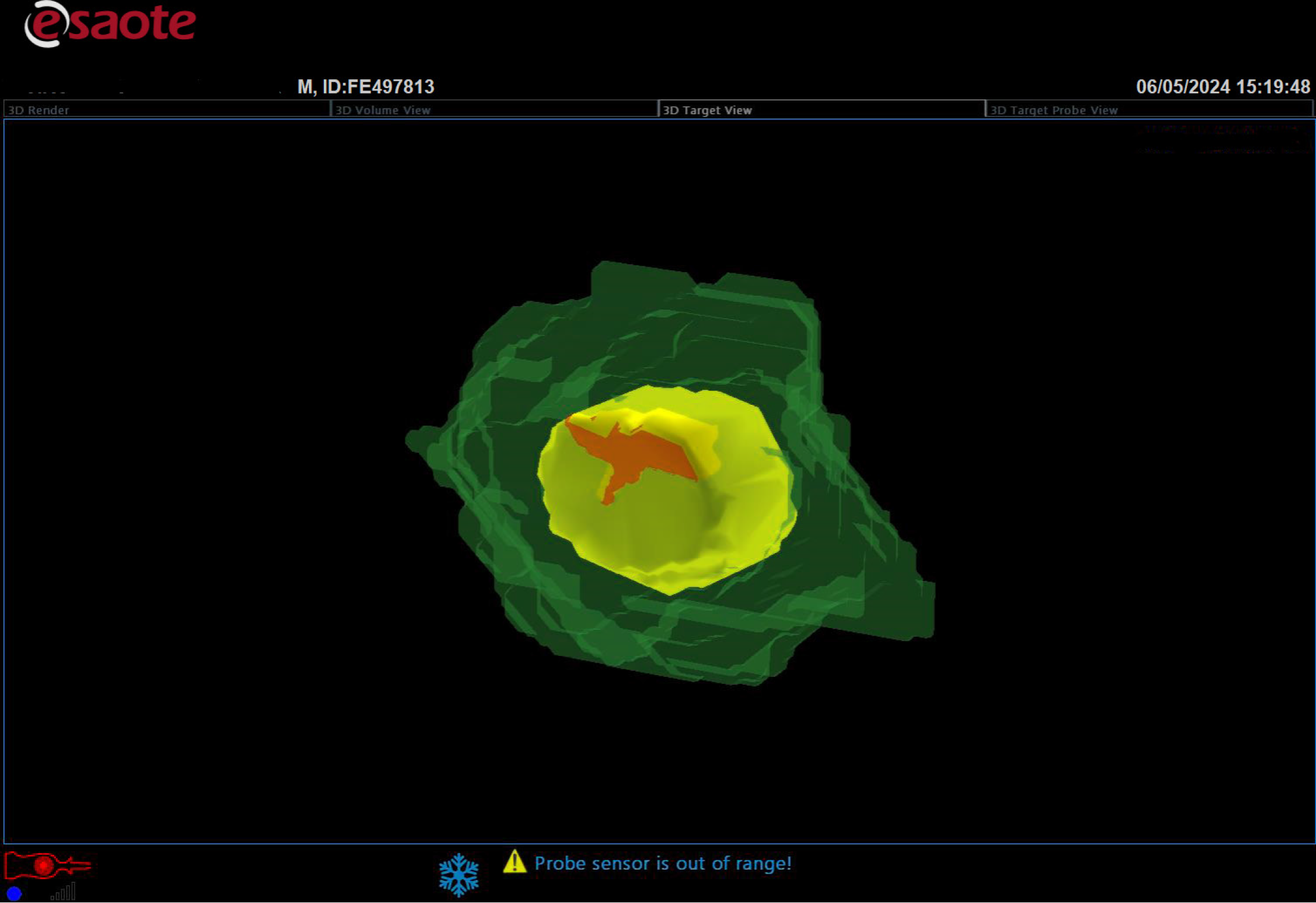
Figure 8
Three-dimensional reconstruction of the ablation result showed the necrotic volume (deep green), planned safety margin (yellow), completely necrotic lesion (light green), and planned safety margin outside of necrosis volume (red).
CONCLUSION
Starting from grayscale US-guided PEI of small HCC lesions, constant technical advancements in both thermal ablation and US imaging have led interventional oncology clinicians to break the so-called 3-cm barrier in the percutaneous treatment of primary and secondary liver tumors[35-38]. The latest generation of MWA systems and advanced software powered by AI can precisely target tumor margins and/or residual viable tumor tissue to enable us to attack the 5-cm (and hopefully the 7-cm) barrier.
Interventional oncologists are ready to fight!
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Emanuela Mo for her outstanding contribution to the assembly work of this manuscript.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Chowdhury D S-Editor: Liu JH L-Editor: A P-Editor: Wang WB