Published online Dec 28, 2021. doi: 10.13105/wjma.v9.i6.557
Peer-review started: June 17, 2021
First decision: July 30, 2021
Revised: August 1, 2021
Accepted: November 5, 2021
Article in press: November 5, 2021
Published online: December 28, 2021
Processing time: 193 Days and 21.1 Hours
Despite the controversies about the effectiveness of the current drug regimens for coronavirus disease 2019 (COVID-19), these drugs are still the only options available. Moreover, the safety of these drugs is yet to be confirmed. A serious concern is the occurrence of various cardiac arrhythmias, particularly QT prolongation.
To summarize the incidence and estimate the risk of QT interval prolongation in patients scheduling for conventional treatment (hydroxychloroquine alone or in combination with azithromycin) for COVID-19.
We comprehensively searched Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane Central Register of Controlled Trials databases until October 31, 2020 for all eligible studies under the considered keywords COVID-19, arrhythmia, QT interval, therapy, azithromycin, and hydroxychloroquine until. The study protocols were established in compliance with PRISMA-P guidelines (Preferred Reporting Items for Systematic Review and Meta-Analysis – Protocols), and a nine-star Newcastle-Ottawa Scale scoring system was used to assess the methodological quality of all eligible studies. Outcome measures were corrected QT (QTc) prolongation, cardiac arrhythmias, or sudden cardiac death.
Fifteen studies enrolling 8298 patients with targeted COVID-19 therapeutic regimes were included. The eligible studies found a significant increase in the mean QTc interval following treatment with the described medications compared to baseline QTc with weighted standard differences in means of 0.766. The pooled prevalence rate of QTc prolongation was estimated to be 9.2% (95% confidence interval: 4.5% to 18.1%).
Hydroxychloroquine ± azithromycin regimen can significantly increase the risk of developing QTc prolongation.
Core Tip: Given the greater importance of coronavirus disease 2019 worldwide, there is an ongoing controversy about the potential harms of anti-viral agents in which caused uncertainties in daily clinical practice. Given the unresolved debate, during this systematic review and meta-analysis, we investigated the association of Hydroxychloroquine (alone or in combination with azithromycin) with the risk of QT interval prolongation, cardiac arrhythmias, and sudden cardiac death. Although there are some studies about the effects of these agents, there are scarce systematic reviews and meta-analyses about both QT prolongation and risk of cardiac arrhythmias which is a distinguishing point for our study.
- Citation: Ashraf H, Ghafouri P, Kazemian S, Soleimani A, Sadat Naseri A, Karbalai S, Kazemi Saeid A. Hydroxychloroquine alone or in combination with azithromycin and corrected QT prolongation in COVID-19 patients: A systematic review. World J Meta-Anal 2021; 9(6): 557-567
- URL: https://www.wjgnet.com/2308-3840/full/v9/i6/557.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i6.557
With the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, we have seen high mortality rates from the disease in almost all countries involved[1]. Besides the pulmonary involvement in form of acute respiratory distress syndrome, one of the main features of this disease is the involvement of other systems, like cardiovascular, gastrointestinal, central nervous system, and even skin and mucosal involvement[2-4]. However, respiratory failure and subsequent cardiovascular compromise were major determinants for patients' survival[5]. So far, there are no successful and safe drug regimens for the treatment and prevention of COVID-19. Current approaches have either failed or have been withheld due to potential side effects. Thus, the side effects have added to the high mortality and morbidity caused by COVID-19[6].
Current evidence suggests that using hydroxychloroquine and azithromycin for COVID-19 increases the risk of cardiac arrhythmias[7,8]. Previous studies reported that these drugs caused corrected QT (QTc) prolongation, leading to life-threatening conditions like torsades de pointes (TdP) and sudden cardiac death[9,10]. Although both in vivo and in vitro studies recommended the combination therapy of azithromycin and hydroxychloroquine, even as the first-line approach in preventing disease, it has also led to QTc prolongation[11]. In addition to cardiac monitoring, identifying patients, who are prone to the side effects, helps to minimize the potential harms. By identifying susceptible individuals, it may be possible to use other drug protocols to maintain patient survival. Herein, we summarize the findings about the prevalence and the risk of QTc prolongation in patients treated with hydroxychloroquine ± azithromycin. Also, we discuss the life-threatening conditions in patients taking these medications.
We performed this review according to established methods and in compliance with PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis) Protocols. Two investigators searched the manuscript databases including Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane Central Register of Con
Two un-blinded reviewers performed the data abstraction independently in structured collection forms with no divergences in the data collection method. We resolved disagreements by consensus or by involving a third person. One of the authors transferred data into the Review Manager file. We double-checked for correct data entry, comparing the data presented in the systematic review with the data extraction form. The second author spotted-check study characteristics for accuracy against the trial report. The details will be assessed by systematically reviewing the manuscripts are as follows:
The study quality was evaluated based on the following criteria: (1) The systematic review and meta-analysis based on the questions primarily described and formulated; (2) Inclusion and exclusion criteria predefined in the studies as eligibility criteria; (3) Searching the literature performed on a systematic and comprehensive approach; (4) To minimize the bias, two authors reviewed the full texts of the articles; (5) The quality of included studies were rated independently by the reviewers for appraising internal validity; (6) The characteristics and findings of the studies were listed comprehensively; (7) The publication and risk of bias were listed; and (8) Heterogeneity was also assessed. The endpoints were to determine the overall prevalence of QT pro
We assessed the risk of bias for each study with the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreement was resolved by discussion in the whole study team. We assessed the risk of bias according to the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. We judged each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgment in the "Risk of bias" table. We summarized the risk of bias judgments across different studies for each of the domains listed. When considering therapeutical effects, we took the risk of bias into account for studies that contribute to that outcome. We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible, for example, when a study is identified as abstract only. We used the RevMan calculator (version 5.3) to calculate missing standard deviations from other statistics, such as confidence intervals (CI) or P-values. Where it was not possible, we imputed the missing standard deviations and explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. Dichotomous variables were reported as proportions and percentages, and continuous variables as mean values. Binary outcomes from individual studies were combined with both the Mantel-Hansel fixed-effect model. The risk ratios (RRs) and 95%CI for RR were used as summary statistics to compare dichotomous variables and to determine the likelihood of each adverse event after interventions.
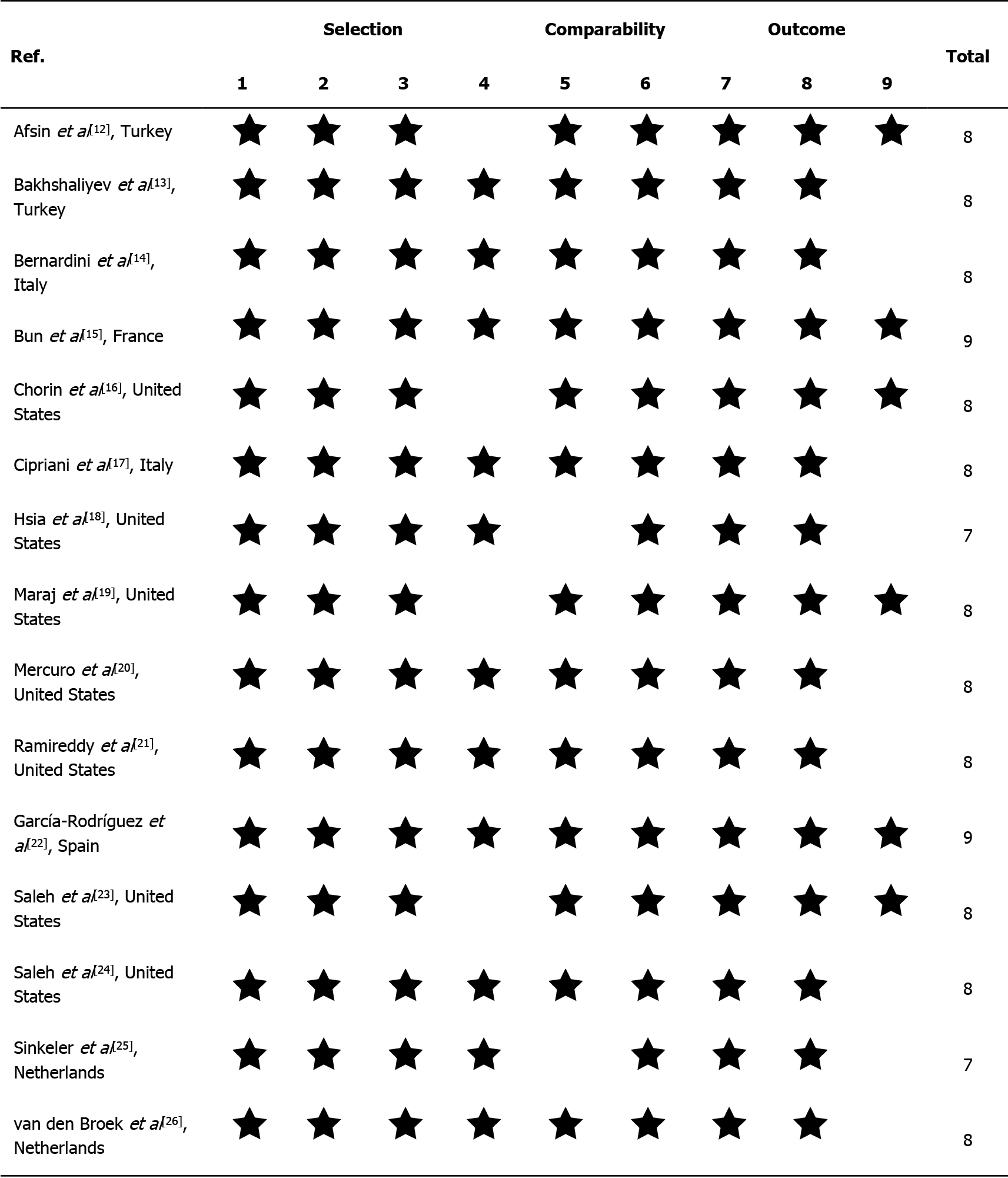
We used Cochran's Q test to estimate the statistical heterogeneity, complemented with the I2 statistic. It quantifies the proportion of total variation across studies that is due to heterogeneity rather than chance. A value of I2 of 0%–25% indicates insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity, and 76%–100% high heterogeneity. Publication bias was assessed by the rank correlation test and also confirmed by the funnel plot analysis. The nine-star Newcastle-Ottawa Scale scoring system was employed to assess the methodological quality of all eligible studies. In this quality assessment technique, each study assessed qualitatively for the three criteria of (1) The selection of the study groups; (2) The comparability of study groups; and (3) The ascertainment of the outcome and is finally scored that the studies awarding 7 stars or over were deemed as high quality. Reported values were two-tailed, and hypothesis testing results were considered statistically significant at P = 0.05. Statistical analysis was performed using the Comprehensive Meta-Analysis Software (CMA, version 3.0).
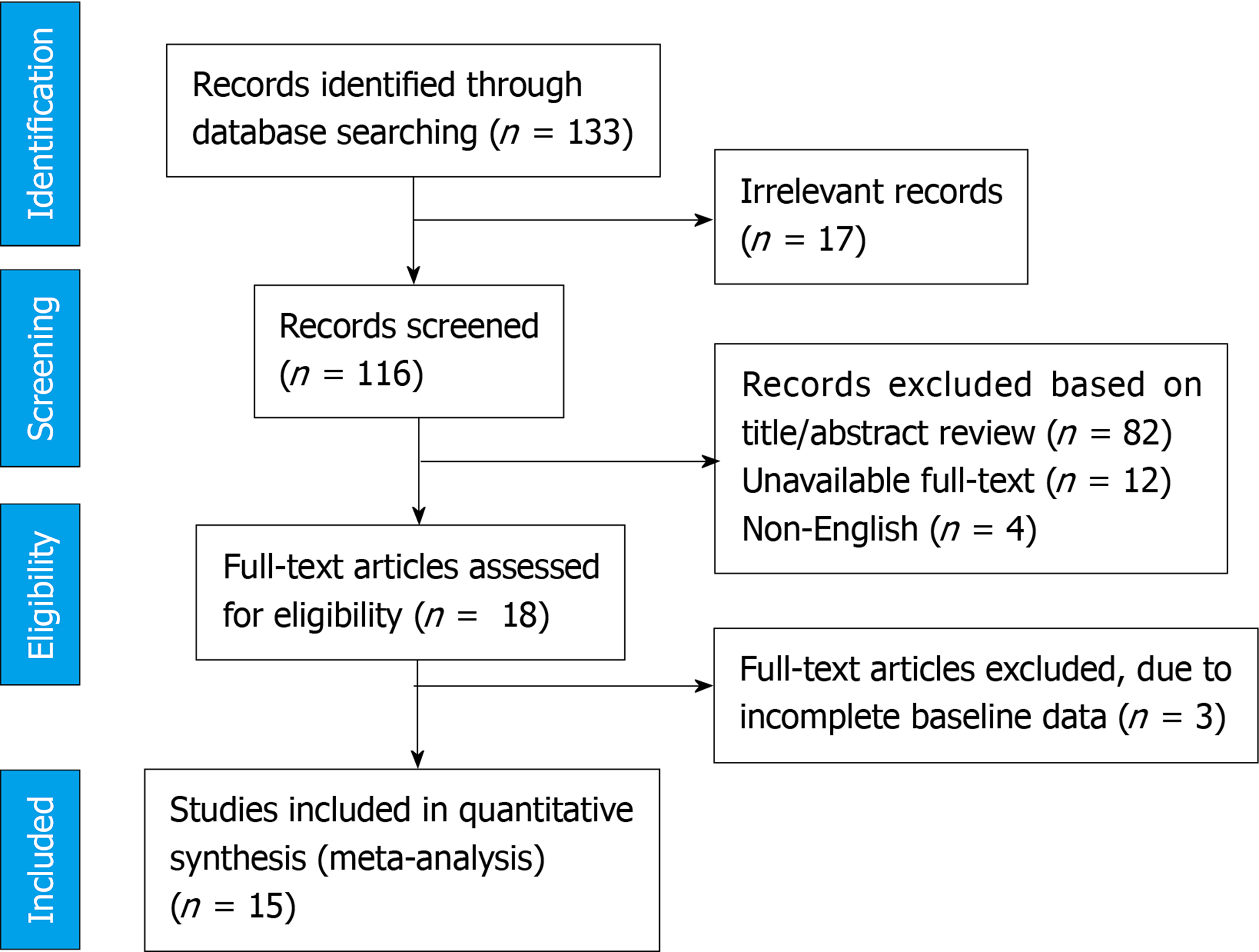
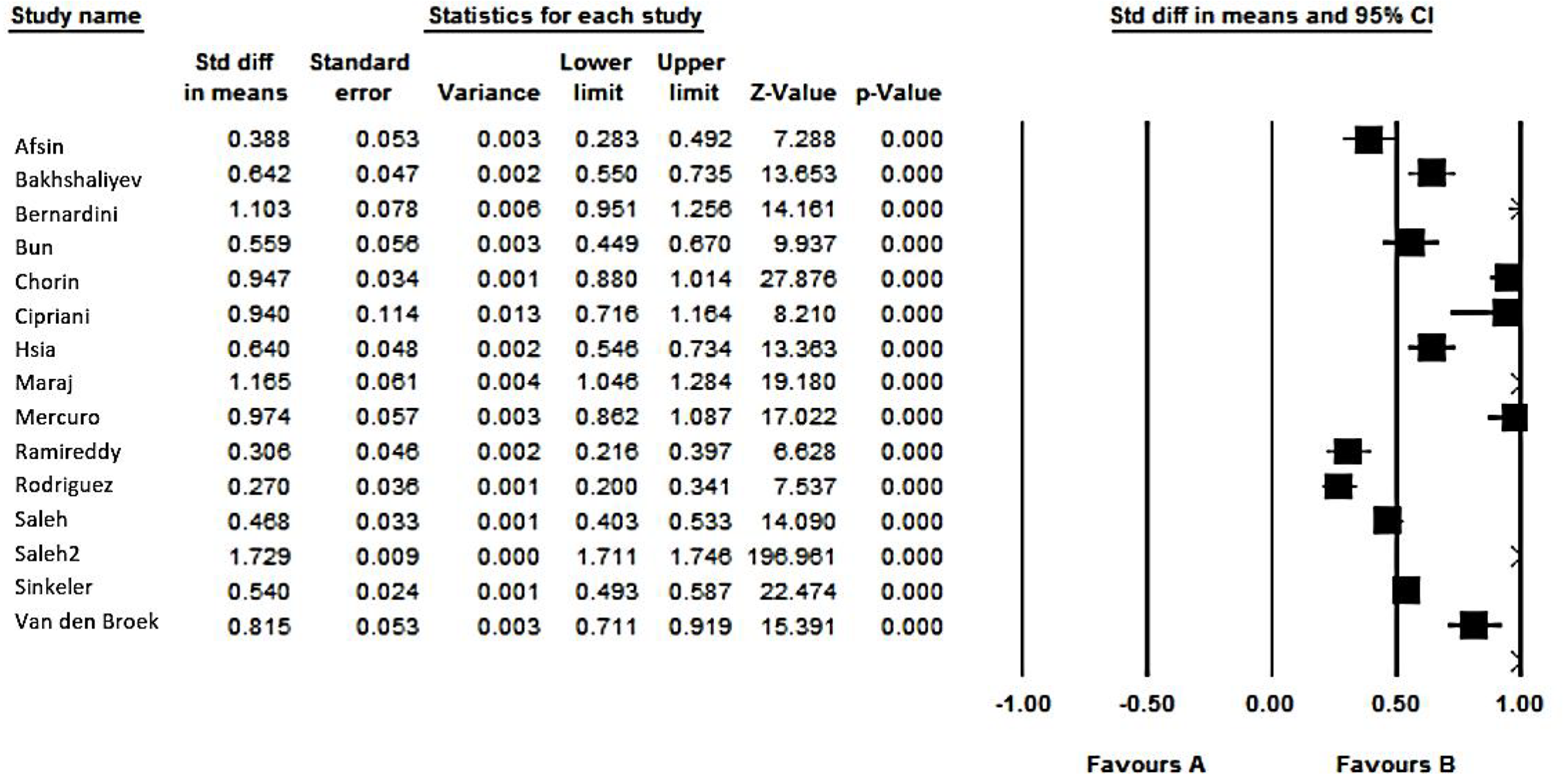
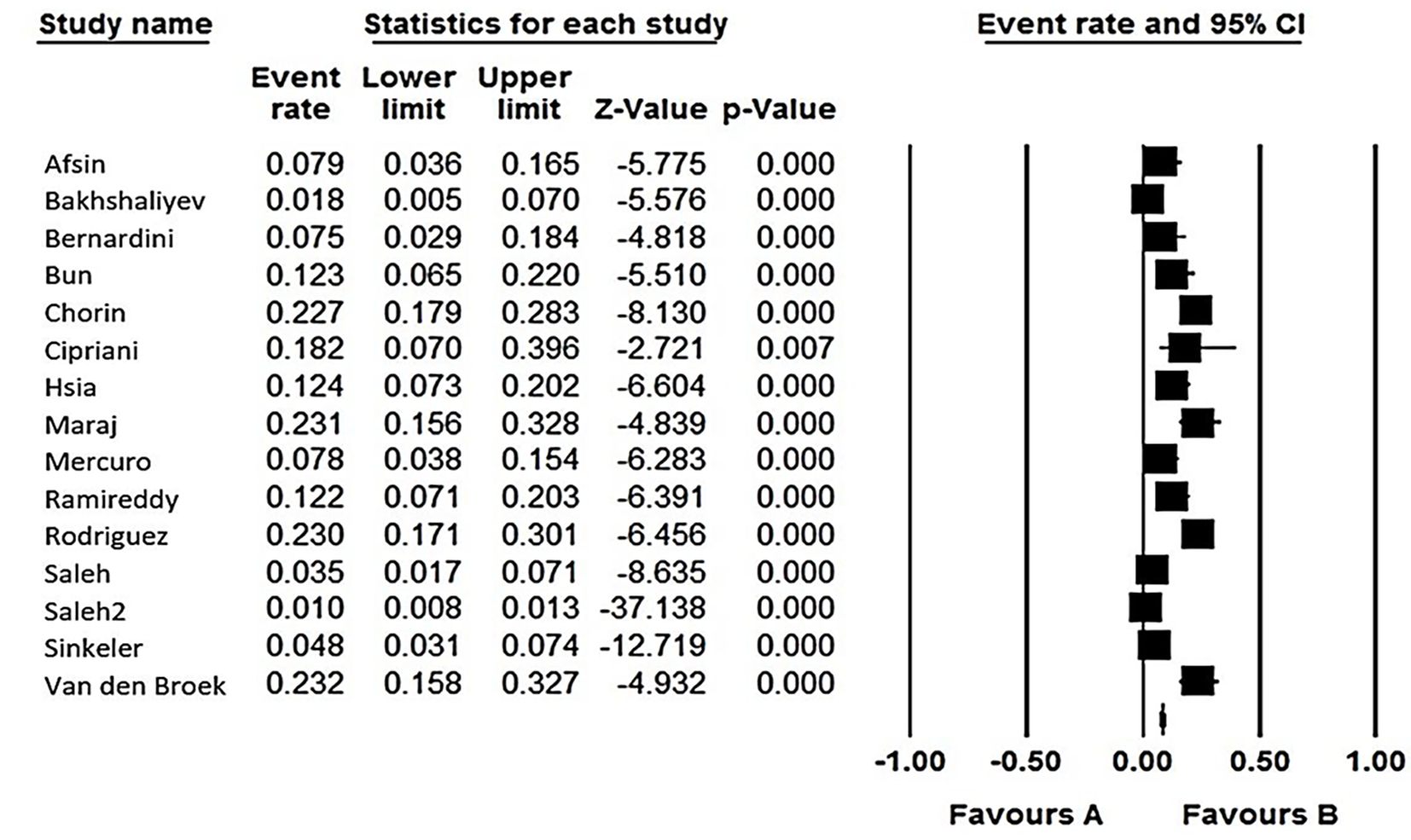
Figure 1 demonstrates the flow diagram of the study selection. Initially, 133 articles were collected by database searching and other sources. After removing duplications, 116 records were primarily under-screened. Based on the titles and abstracts, 98 records were excluded, and the remaining 18 citations were assessed for further eligibility. Of those, 3 were also excluded due to incompleteness of the data and contents. Finally, 15 articles [12-26] were eligible for the final analysis (Figure 1). The studies included were assessed qualitatively by the QUADAS-2 tool. According to our risk of bias assessment, all 15 studies yielded good quality, and none of them had a high risk of bias. Therefore, the pooled results should be persuasive (Figure 2). In total, 15 studies (twelve retrospective and three prospective) were included in our final analysis. The anti-COVID-19 medications focused in the studies were mostly a combination of hydroxychloroquine plus azithromycin[13-24], hydroxychloroquine plus moxifloxacin[12], and hydroxychloroquine alone[25,26]. Overall, 8298 patients suffering from COVID-19 were treated with these regimens. The details of the participants are summarized in Table 1. As shown in Table 2, all studies found a significant increase in the mean QTc compared to the baseline. The weighted standard differences in means are 0.766 (95%CI: 0.394 to 1.137, P < 0.001), with significant heterogeneity across the studies relevant to the I2 value of 99.33% (P < 0.001) (Figures 3 and 4). In this regard, the pooled prevalence rate of QT prolongation was estimated to be 9.2% (95%CI: 4.5% to 18.1%) with a significant level of heterogeneity across the studies (I2 = 98.10%, P < 0.001). The Egger test also detected significant publication bias for all assessments.
Across all studies, 132 patients stopped taking the medications due to QTc ≥ 500 ms or an increase of more than 60 ms in QTc. The pooled prevalence was 0.9% (95%CI: 0.6% to 1.1%) with significant level of heterogeneity across the studies (I2= 81.50%, P < 0.001).
Studies did not report any mortality caused by sudden cardiac death or arrhythmogenic death. However, 4 cases of TdP and 34 cases of ventricular tachycardia/fibrillation were reported with a pooled prevalence of 0.1% < and 0.4% (95%CI: 0.2% to 0.5%), respectively.
| Ref. | Study design | Population | Mean age | Male/female | HTN | DM | Medication |
| Afsin et al[12], Turkey | Retrospective | 76 | 61 ± 14 | 32/44 | 41 | 26 | Hydroxychloroquine plus Moxifloxacin |
| Bakhshaliyev et al[13], Turkey | Retrospective | 109 | 57 ± 14 | 48/61 | 49 | 32 | Hydroxychloroquine plus azithromycin |
| Bernardini et al[14], Italy | Retrospective | 53 | 67 ± 12 | 37/16 | 21 | 6 | Hydroxychloroquine plus azithromycin |
| Bun et al[15], France | Prospective | 73 | 62 ± 14 | 49/24 | 33 | 19 | Hydroxychloroquine plus azithromycin |
| Chorin et al[16], United States | Retrospective | 251 | 64 ± 13 | 75/176 | 54 | 27 | Hydroxychloroquine plus azithromycin |
| Cipriani et al[17], Italy | Retrospective | 22 | 64 ± 11 | 18/4 | 12 | 6 | Hydroxychloroquine plus azithromycin |
| Hsia et al[18], United States | Retrospective | 105 | 67 ± 15 | 58/47 | 51 | 41 | Hydroxychloroquine plus azithromycin |
| Maraj et al[19], United States | Retrospective | 91 | 62 ± 15 | 51/40 | 42 | 26 | Hydroxychloroquine plus azithromycin |
| Mercuro et al[20], United States | Prospective | 90 | 60 ± 16 | 46/44 | 48 | 26 | Hydroxychloroquine plus azithromycin |
| Ramireddy et al[21], United States | Retrospective | 98 | 62 ± 17 | 60/38 | 59 | 22 | Hydroxychloroquine plus azithromycin |
| Rodrıguez et al[22], Spain | Retrospective | 161 | 63 ± 14 | 103/42 | 71 | 25 | Hydroxychloroquine plus azithromycin |
| Saleh et al[23], United States | Prospective | 201 | 58 ± 9 | 115/86 | 84 | 65 | Hydroxychloroquine plus azithromycin |
| Saleh et al[24], United States | Retrospective | 6476 | 64 ± 15 | 3980/2496 | 3184 | 2161 | Hydroxychloroquine plus azithromycin |
| Sinkeler et al[25], Netherlands | Retrospective | 397 | 67 ± 12 | 262/135 | --- | --- | Hydroxychloroquine |
| van den Broek et al[26], Netherlands | Retrospective | 95 | 65 ± 12 | 63/32 | --- | --- | Hydroxychloroquine |
| Ref. | Mean QTc (base) | Mean QTc (treatment) | QT prolongation | Overall mortality | Torsades de pointes | Other ventricular arrhythmias | Sudden cardiac death | Stopped medication | Arrhythmo | Special considerations |
| Afsin et al[12], Turkey | 424 ± 28 | 442 ± 42 | 6 | 5 | 0 | 0 | 0 | 0 | 0 | N/A |
| Bakhshaliyev et al[13], Turkey | 435 ± 32 | 459 ± 38 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| Bernardini et al[14], Italy | 424 ± 24 | 452 ± 26 | 4 | 20 | 0 | 0 | 0 | 0 | 0 | Atrial tachyarrhythmia: n = 9 (8%); Premature atrial or ventricular ectopies: n = 17 (15.2%); First-degree AV block: n = 3 (2.6%) |
| Bun et al[15], France | 436 ± 44 | 460 ± 39 | 9 | 0 | 0 | 0 | 0 | 1 | 0 | Counterclockwise atrial flutter: n = 1 (1.3%) |
| Chorin et al[16], United States | 439 ± 29 | 473 ± 36 | 57 | 20 | 1 | 0 | 0 | 8 | 0 | N/A |
| Cipriani et al[17], Italy | 426 ± 26 | 450 ± 22 | 4 | 0 | 0 | Non-sustained VT, n = 1 | 0 | 0 | 0 | N/A |
| Hsia et al[18], United States | 439 ± 28 | 459 ± 32 | 13 | 29 | 0 | VT, n = 1 | 0 | 21 | 0 | N/A |
| Maraj et al[19], United States | 437 ± 25 | 473 ± 31 | 21 | 8 | 1 | VF, n = 1 | 0 | 0 | 6 | Bradyarrhythmia, n = 9 (10%) |
| Mercuro et al[20], United States | 442 ± 26 | 473 ± 32 | 7 | 4 | 1 | 0 | 0 | 10 | 0 | N/A |
| Ramireddy et al[21], United States | 448 ± 29 | 459 ± 36 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| Rodrıguez et al[22], Spain | 435 ± 25 | 443 ± 30 | 37 | 7 | 0 | 0 | 0 | 0 | 0 | N/A |
| Saleh et al[23], United States | 439 ± 24 | 463 ± 42 | 7 | 0 | 0 | Non-sustained monomorphic VT, n = 1; Sustained monomorphic VT, n = 1 | 0 | 7 | 0 | Atrial fibrillation: n = 17 (8.4%) |
| Saleh et al[24], United States | 473 ± 35 | 532 ± 31 | 67 | 0 | 1 | Non-sustained monomorphic VT, n = 18; Sustained monomorphic VT, n = 5; VF, n = 4 Sustained polymorphic VT, n = 1 | 0 | 58 | 0 | N/A |
| Sinkeler et al[25], Netherlands | 448 ± 34 | 468 ± 38 | 19 | 0 | 0 | Non-sustained monomorphic VT, n = 1 | 0 | 27 | 0 | N/A |
| van den Broek et al[26], Netherlands | 444 ± 32 | 479 ± 42 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
There are controversies about the effectiveness and safety of medications used to treat COVID-19. In some cases, serious side effects following the use of these drugs may contribute to the morbidity caused by the disease and even may lead to its progression. QTc prolongation is a potential side effect of hydroxychloroquine and, also, one of the most critical complications, leading to some fatal arrhythmias like TdP. Interestingly, mechanisms other than drug-related toxicity have been suggested to cause QTc prolongation. In this regard, we can mention cardiac ischemia due to direct viral invasion, electrolyte imbalances, activation of inflammatory cascades, and oxidative stress destroying myocardial tissue[27]. Studies have shown that myocardial ischemia may result in repolarization abnormality leading to cardiac arrhythmias[28]. Also, inflammatory processes, per se, may lead to disruption of cardiomyocyte ion channels by enhancing inward calcium currents and delaying outward potassium currents. It causes prolonged action potential duration and stimulation of car
Besides, there is strong evidence that using azithromycin can induce QTc prolongation. According to case-control studies, azithromycin has increased the risk of QTc prolongation up to 1.5 times[30]. Of course, it seems that pre-existing cardi
Overall, a significant proportion of patients with COVID-19 have experienced QTc prolongation. According to our meta-analysis, 4.5% to 18.1% of COVID-19 patients have episodes of QTc prolongation, regardless of the drugs they are taking. Despite this, based on the studies, mortality from the disease does not appear to be due to arrhythmogenic events. However, providing reliable guidelines is essential for managing patients who develop QTc prolongation during treatment. A conventional cut-off for discontinuing treatment with QT-prolonging drugs is a QTc ≥ 500 ms or a rise in QTc more than 60 ms. This strategy is widely popular among the studies that we reviewed, and it led to 4 episodes of TdP and 34 episodes of ventricular tac
In conclusion, according to our systematic review and meta-analysis, a significant change in QTc interval following the use of hydroxychloroquine alone or in combination with azithromycin is highly expected that may be life-threatening. However, it should be noted that these changes may not be solely due to the toxicity of drugs. Interventional studies are required to confirm this hypothesis.
Current evidence suggests that using hydroxychloroquine and azithromycin for coronavirus disease 2019 (COVID-19) increases the risk of cardiac arrhythmias. Previous studies reported that these drugs caused corrected QT (QTc) prolongation, leading to life-threatening conditions like torsades de pointes and sudden cardiac death. Although both in vivo and in vitro studies recommended the combination therapy of azithromycin and hydroxychloroquine, even as the first-line approach in preventing disease, it has also led to QTc prolongation.
In addition to cardiac monitoring, identifying patients, who are prone to side effects, helps to minimize the potential harms. By identifying susceptible individuals, it may be possible to use other drug protocols to maintain patient survival.
We summarize the findings about the prevalence and the risk of QTc prolongation in patients treated with hydroxychloroquine ± azithromycin. Also, we discuss the life-threatening conditions in patients taking these medications.
We comprehensively searched Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane Central Register of Controlled Trials databases until October 31, 2020 for all eligible studies under the considered keywords COVID-19, arrhythmia, QT interval, therapy, azithromycin, and hydroxychloroquine until. The study protocols were established in compliance with PRISMA-P guidelines. Outcome measures were QTc prolongation, cardiac arrhythmias, or sudden cardiac death.
Fifteen studies enrolling 8298 patients with targeted COVID-19 therapeutic regimes were included. The eligible studies found a significant increase in the mean QTc interval following treatment with the described medications compared to baseline QTc with weighted standard differences in means of 0.766. The pooled prevalence rate of QTc prolongation was estimated to be 9.2% (95%CI: 4.5% to 18.1%).
Hydroxychloroquine ± azithromycin regimen can significantly increase the risk of developing QTc prolongation.
According to our systematic review and meta-analysis, a significant change in QTc interval following the use of hydroxychloroquine alone or in combination with azithromycin is highly expected that may be life-threatening. However, it should be noted that these changes may not be solely due to the toxicity of drugs. Interventional studies are required to confirm this hypothesis.
We are indebted to Research Development Center of Sina Hospital for their technical help. We also thank Sherry Hughes Garne for editing this paper for proper English language, grammar, punctuation, spelling, and overall style. The authors are grateful to Mrs. Mahin Ahmadi Pishkuhi for statistical consultation and for evaluating the statistical methods and tests mentioned.
| 1. | Kakodkar P, Kaka N, Baig MN. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19). Cureus. 2020;12:e7560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 2. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1268] [Article Influence: 211.3] [Reference Citation Analysis (2)] |
| 3. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 761] [Article Influence: 126.8] [Reference Citation Analysis (2)] |
| 4. | Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 5. | Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 6. | Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. 2020;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Das RR, Jaiswal N, Dev N, Naik SS, Sankar J. Efficacy and Safety of Anti-malarial Drugs (Chloroquine and Hydroxy-Chloroquine) in Treatment of COVID-19 Infection: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2020;7:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 9. | Offerhaus JA, Wilde AAM, Remme CA. Prophylactic (hydroxy)chloroquine in COVID-19: Potential relevance for cardiac arrhythmia risk. Heart Rhythm. 2020;17:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 10. | Stevenson A, Kirresh A, Conway S, White L, Ahmad M, Little C. Hydroxychloroquine use in COVID-19: is the risk of cardiovascular toxicity justified? Open Heart. 2020;7:e001362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Sciaccaluga C, Cameli M, Menci D, Mandoli GE, Sisti N, Cameli P, Franchi F, Mondillo S, Valente S. COVID-19 and the burning issue of drug interaction: never forget the ECG. Postgrad Med J. 2021;97:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Afsin A, Ecemis K, Asoglu R. Effects of Short-Term Hydroxychloroquine Plus Moxifloxacin Therapy on Corrected QT Interval and Tp-e Interval in Patients With COVID-19. J Clin Med Res. 2020;12:604-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Bakhshaliyev N, Uluganyan M, Enhos A, Karacop E, Ozdemir R. The effect of 5-day course of hydroxychloroquine and azithromycin combination on QT interval in non-ICU COVID19(+) patients. J Electrocardiol. 2020;62:59-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Bernardini A, Ciconte G, Negro G, Rondine R, Mecarocci V, Viva T, Santini F, de Innocentiis C, Giannelli L, Witkowska E, Locati ET, Castelvecchio S, Marrocco-Trischitta MM, Vicedomini G, Menicanti L, Pappone C. Assessing QT interval in COVID-19 patients:safety of hydroxychloroquine-azithromycin combination regimen. Int J Cardiol. 2021;324:242-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Bun SS, Taghji P, Courjon J, Squara F, Scarlatti D, Theodore G, Baudouy D, Sartre B, Labbaoui M, Dellamonica J, Doyen D, Marquette CH, Levraut J, Esnault V, Bun SS, Ferrari E. QT Interval Prolongation Under Hydroxychloroquine/Azithromycin Association for Inpatients With SARS-CoV-2 Lower Respiratory Tract Infection. Clin Pharmacol Ther. 2020;108:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 16. | Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Kogan E, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, Park DS, Stefano C, Chinitz LA, Jankelson L. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 17. | Cipriani A, Zorzi A, Ceccato D, Capone F, Parolin M, Donato F, Fioretto P, Pesavento R, Previato L, Maffei P, Saller A, Avogaro A, Sarais C, Gregori D, Iliceto S, Vettor R. Arrhythmic profile and 24-hour QT interval variability in COVID-19 patients treated with hydroxychloroquine and azithromycin. Int J Cardiol. 2020;316:280-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Hsia BC, Greige N, Quiroz JA, Khokhar AS, Daily J, Di Biase L, Ferrick KJ, Fisher JD, Krumerman A. QT prolongation in a diverse, urban population of COVID-19 patients treated with hydroxychloroquine, chloroquine, or azithromycin. J Interv Card Electrophysiol. 2020;59:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Maraj I, Hummel JP, Taoutel R, Chamoun R, Workman V, Li C, Tran L, DelVecchio A, Howes C, Akar JG. Incidence and determinants of QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin. J Cardiovasc Electrophysiol. 2020;31:1904-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 489] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 21. | Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, Cingolani E, Cheng S, Marban E, Albert CM, Chugh SS. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020;9:e017144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | García-Rodríguez D, Remior P, García-Izquierdo E, Toquero J, Castro V, Fernández Lozano I. Drug-induced QT prolongation in COVID-19 pneumonia: influence on in-hospital survival. Rev Esp Cardiol (Engl Ed). 2021;74:111-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Saleh M, Gabriels J, Chang D, Soo Kim B, Mansoor A, Mahmood E, Makker P, Ismail H, Goldner B, Willner J, Beldner S, Mitra R, John R, Chinitz J, Skipitaris N, Mountantonakis S, Epstein LM. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol. 2020;13:e008662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (1)] |
| 24. | Saleh M, Gabriels J, Chang D, Fishbein J, Qiu M, Mountantonakis SE, Epstein LM; Northwell COVID-19 Research Consortium. Safely Administering Potential QTc Prolonging Therapy Across a Large Health Care System in the COVID-19 Era. Circ Arrhythm Electrophysiol. 2020;13:e008937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Sinkeler FS, Berger FA, Muntinga HJ, Jansen MMPM. The risk of QTc-interval prolongation in COVID-19 patients treated with chloroquine. Neth Heart J. 2020;28:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | van den Broek MPH, Möhlmann JE, Abeln BGS, Liebregts M, van Dijk VF, van de Garde EMW. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J. 2020;28:406-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 27. | Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, Cheung JW, Patel P, Sotomonte J, Lampert R, Han JK, Rajagopalan B, Eckhardt L, Joglar J, Sandau KE, Olshansky B, Wan E, Noseworthy PA, Leal M, Kaufman E, Gutierrez A, Marine JE, Wang PJ, Russo AM. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17:e233-e241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 28. | Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, Hochman JS. ST-Segment Elevation in Patients with Covid-19 - A Case Series. N Engl J Med. 2020;382:2478-2480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 609] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 29. | Lazzerini PE, Laghi-Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, Selvi E, Bacarelli MR, Finizola F, Vanni F, Lazaro D, Aromolaran A, El Sherif N, Boutjdir M, Capecchi PL. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart. 2017;103:1821-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Maisch NM, Kochupurackal JG, Sin J. Azithromycin and the risk of cardiovascular complications. J Pharm Pract. 2014;27:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan MKA S-Editor: Liu M L-Editor: A P-Editor: Liu M