Published online Jun 28, 2021. doi: 10.13105/wjma.v9.i3.257
Peer-review started: February 23, 2021
First decision: April 6, 2021
Revised: April 24, 2021
Accepted: June 15, 2021
Article in press: June 15, 2021
Published online: June 28, 2021
Processing time: 139 Days and 7.4 Hours
Gastric adenocarcinoma is a global health concern, and Helicobacter pylori (H. pylori) infection is the main risk factor for its occurrence. Of note, the immune response against the pathogen seems to be a determining factor for gastric oncogenesis, and increasing evidence have emphasized several host and bacterium factors that probably influence in this setting. The development of an inflammatory process against H. pylori involves a wide range of mechanisms such as the activation of pattern recognition receptors and intracellular pathways resulting in the production of proinflammatory cytokines by gastric epithelial cells. This process culminates in the establishment of distinct immune response profiles that result from the cytokine-induced differentiation of T naïve cells into specific T helper cells. Cytokines released from each type of T helper cell orchestrate the immune system and interfere in the development of gastric cancer in idiosyncratic ways. Moreover, variants in genes such as single nucleotide polymorphisms have been associated with variable predispositions for the occurrence of gastric malignancy because they influence both the intensity of gene expression and the affinity of the resultant molecule with its receptor. In addition, various repercussions related to some H. pylori virulence factors seem to substantially influence the host immune response against the infection, and many of them have been associated with gastric tumorigenesis.
Core Tip: Gastric cancer affects more than 1 million people yearly, and Helicobacter pylori (H. pylori) infection is the main risk factor for that malignancy. Moreover, the immune response against the infection seems to play a pivotal role in gastric carcinogenesis. This article provides a broad and updated overview on the main aspects regarding H. pylori infection, immune response, and gastric cancer development.
- Citation: de Brito BB, Lemos FFB, Carneiro CDM, Viana AS, Barreto NMPV, Assis GAS, Braga BDC, Santos MLC, Silva FAFD, Marques HS, Silva NOE, de Melo FF. Immune response to Helicobacter pylori infection and gastric cancer development. World J Meta-Anal 2021; 9(3): 257-276
- URL: https://www.wjgnet.com/2308-3840/full/v9/i3/257.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i3.257
About 1 million people are diagnosed with gastric cancer and more than 700000 individuals die from this neoplasm every year[1]. That incidence makes gastric adenocarcinoma the fifth most common malignancy and the third cause of cancer-related death worldwide[2]. Among the multiple factors that influence the development of this disease, Helicobacter pylori (H. pylori) infection stands out. Gastric colonization by this gram-negative, spiral-shaped microorganism is the main risk factor for the occurrence of gastric adenocarcinoma, and worryingly it infects more than half of the world population[3]. In that context, studies have emphasized the critical role of the interplays between H. pylori and host immune system in carcinogenesis[4].
The immune response activation by H. pylori infection in the gastric mucosa occurs mainly through the triggering of pattern recognition receptors (PRRs), which leads to the activation of intracellular cascades that culminate in the secretion of proinflammatory cytokines[5]. The events taking place in the initial phase of infection lead to the recruitment of T cells, and the establishment of specific immune response profiles by T helper (Th) cells is determinant for the development of H. pylori-related gastric disorders[6]. H. pylori has several virulence factors that favor its perpetuation in the gastric hostile environment. Some mechanisms triggered by these bacterial products play pivotal roles in the regulation of the host immune response and seem to influence the genesis of gastric neoplasms[7]. On the other hand, specific host polymorphisms in genes that encode cytokines also interfere in the risk of developing the disease by altering the expression pattern of these mediators as well as the intensity of the signals that they activate[8].
Given the background, this article aims to provide a broad and updated review on how the immune response against H. pylori infection influences in the development of gastric adenocarcinoma, discussing the main bacterial and host variables that interfere in the pathophysiology of the disease.
Complex host immune responses involving innate and adaptive mechanisms are induced by H. pylori infection[9-11]. Gastric epithelium plays a pivotal role in the innate immune response to the bacterium because its cells make up the only cell phenotype in direct contact with the pathogen in conditions in which tissue damage is absent yet[12,13]. The initial contact of the gastric epithelial cells with the pathogen activates pathogen-associated molecular pattern receptors including NOD1 and toll-like receptors (TLRs). These innate host defense mechanisms trigger cell signaling pathways that induce the activation of nuclear factor kappa B (NF-κB), activating protein-1, and interferon regulatory factors[14]. Concerning the TLRs, it has been reported that gastric epithelial cells express TLR1, TLR2, TLR4, TLR5, TLR9, and TLR10, which interact with various H. pylori antigens such as lipoteichoic acid, lipoproteins, lipopolysaccharide, flagellin, HSP-60, neutrophil-activating protein A, DNA, and RNA[5,15,16]. These receptors are very important to the induction of the expression of proinflammatory and antibacterial factors[5]. For example, the translocation of NF-κB to the nucleus, aiming at activating the expression of genes associated with the inflammatory process, is directly associated with the engagement of TLRs, particularly TLR2, in a myeloid differentiation primary response 88-dependent process[17]. Myeloid differentiation primary response 88 is a key TLR adapter protein used by all TLRs, except TLR3, and transmits signals that result in the induction of inflammatory cytokines[5]. However, although TLRs are the most studied receptors, H. pylori promotes the activation of PRRs other than TLRs. For instance, H. pylori pepti
This inflammatory response is characterized by the chemotaxis of monocytes/ macrophages, dendritic cells (DCs), B and T cells, and in particular, neutrophils, whose main chemoattractant is the interleukin (IL)-8 secreted by gastric epithelial cells as a result of the engagement of NOD1, for instance[25,26]. Neutrophils are recruited to the lamina propria at the beginning of H. pylori infection, and several specific H. pylori factors are known to interact with these cells and modulate their responses[27,28]. One of these factors is a protein produced by H. pylori known as neutrophil-activating protein (HP-NAP or neutrophil-activating protein A). HP-NAP can promote chemotaxis, endothelial adhesion, and production of reactive oxygen intermediates by neutrophils[29-31]. Incubation of these cells with HP-NAP results in significant production of cytokines such as IL-12 and IL-23. The same effects of HP-NAP on cytokine secretion were also observed in macrophages and DCs. Therefore, it is possible to conclude that this protein acts on both neutrophils and monocytes, inducing the production of cytokines[30].
Mononuclear infiltration in the lamina propria is also characteristic of H. pylori-induced chronic infection[13]. Human monocytes and macrophages are important coordinators of the immune response to H. pylori-derived products and signals from epithelial cells in direct contact with the bacterium on the surface of the mucosa[15]. In this infection, both monocytes and macrophages, alongside the DCs, act as activators of adaptive immunity, because they are antigen-presenting cells, capable of expressing class II MHC molecules that activate CD4+ T cells[32]. Furthermore, monocytes and macrophages also produce factors such as IL-12, responsible for inducing a polarized Th1 immune response, IL-1β, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α), which, except for IL-10, induce the amplification of the inflammatory response[16]. Moreover, it is important to note that macrophages are also effector cells that are able to produce nitric oxide derived from the enzyme-inducible nitric oxide synthase (iNOS, NOS2) and reactive oxygen species, both associated with cellular damage[33].
Although in smaller numbers, DCs are also important in the immune response to H. pylori infection, especially because they represent an important bridge between the innate and adaptive immunities[26]. These cells express a broad spectrum of PRRs, which enables them to capture antigens at the periphery and induce T naive cells to direct T cell differentiation[34]. This role is played through three main signals: (1) presentation of foreign antigens in the form of peptides bound to class II MHC molecules to T cells; (2) costimulation of T cell differentiation; and (3) secretion of cytokines, particularly IL-6, IL-8, IL-10, IL-12, IL-1β, and TNF-α[35]. Both aforementioned antigen-presenting cells exhibit remarkable secretion of IL-12, which enables the induction of a Th1-polarized immune response, responsible for the secretion of INF-γ and low amounts of cytokines characteristic of Th2 responses, such as IL-4 and IL-5[36-40].
Finally, mast cells represent an additional innate cell phenotype that is found within the H. pylori-infected gastric mucosa. These cells can be activated by various H. pylori components. For instance, the bacterial virulence factor VacA can induce mast cells to express multiple inflammatory cytokines, including IL-1, TNF, IL-6, IL-23, and IL-10[41,42]. Upon the stimulation of epithelial cells, macrophages, and DCs by H. pylori bacterial factors, CD4+ and CD8+ T cells are recruited to the gastric mucosa, with preferential activation of CD4+ T cells in detriment to CD8+ cells[43-46].
As aforementioned, the triggering of an immune response against H. pylori involves the activation of CD4+ and CD8+ T cells and their migration to the gastric environment[47]. Among the cytokines expressed in that context, those inducing the differentiation of naïve T cells into Th1 (e.g., IL-12), Th17 [e.g., transforming growth factor β (TGF-β), IL-23, and IL-6], and regulatory T cells (Treg) (e.g., IL-2 and TGF-β) cells stand out[48].
The establishment of a proinflammatory Th1 response in the H. pylori infection is associated with the development of corpus gastritis. Depending on further host and environmental variables, the aforementioned condition can result in gastric atrophy and intestinal metaplasia, which are well-known precancerous lesions[48,49]. Of note, our group previously demonstrated that Th1 response varies according to the age among H. pylori-positive individuals. In that study, we observed higher gastric concentrations of Th1-related cytokines IL-2, Il-12p70, and INF-γ in adults than in children. Moreover, the levels of Th1 cytokines were directly correlated with the severity of gastric inflammation[50].
Regarding Th17 response, although other cytokines such as TGF-β and IL-6 are strongly related to this immune profile, current evidence emphasizes the pivotal role of IL-23 in its induction in the setting of H. pylori infection[51,52]. A study found that chronically infected IL-23(p19)-/- mice had reduced gastric expression of IL-17A as well as milder gastric inflammation and higher levels of H. pylori colonization compared to wild-type H. pylori-positive mice[53]. The IL-17A, in its turn, promotes the migration of polymorphonuclear leukocytes to the infection site and is an important component in the control of H. pylori gastric infection[54]. Previous studies using mice have shown that IL-17A-/- as well as IL-17RA-defficient individuals have a milder gastric neutrophil infiltration against H. pylori infection than wild-type mice. Interestingly, the mice lacking IL-17RA signaling had an enhanced chronic inflammation with intense infiltration of B and CD4+ T cells into the gastric mucosa[55].
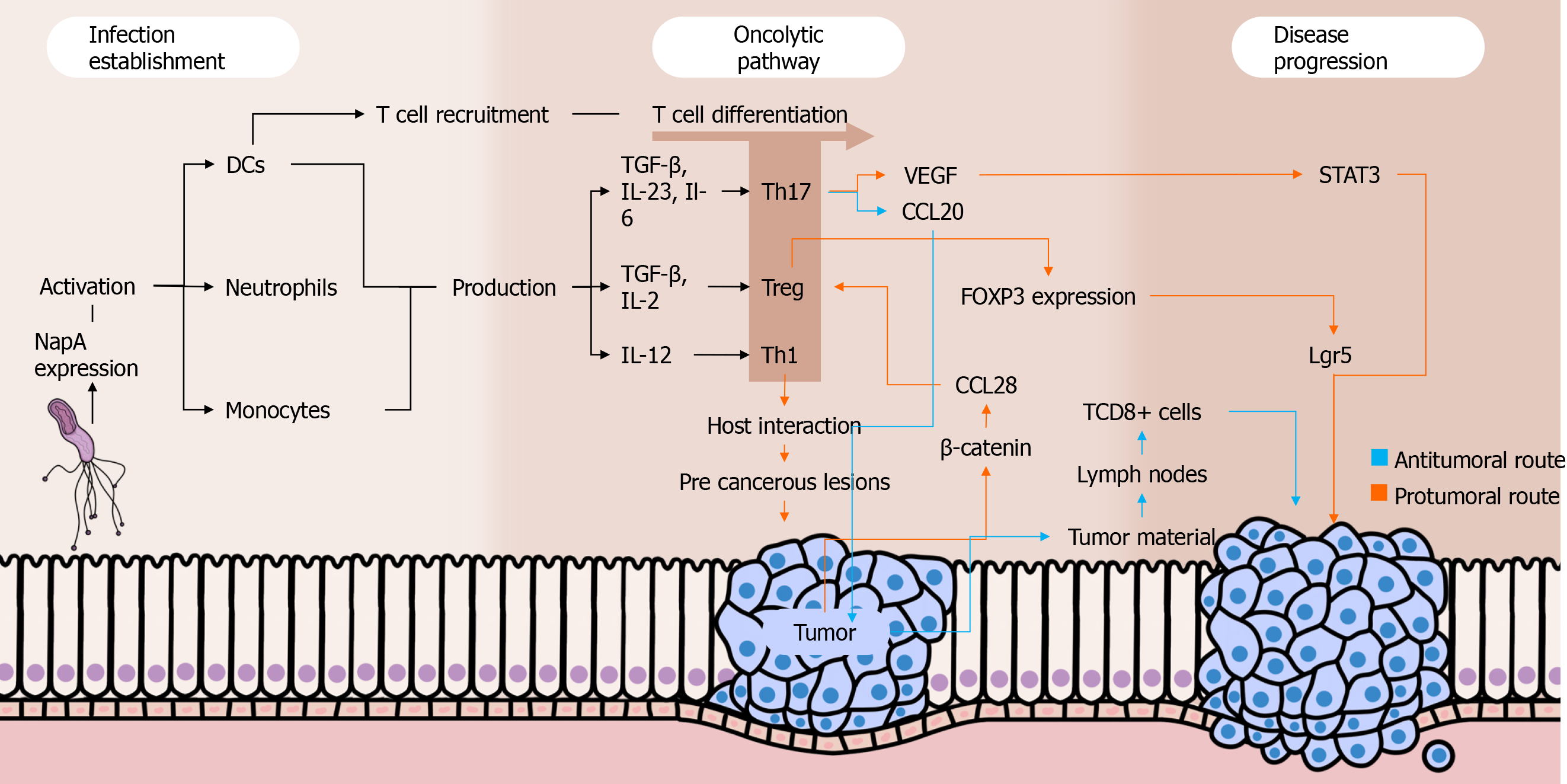
Dual roles have been attributed to Th17 responses in cancer settings. On one hand, this immune profile seems to be important in the immunosurveillance against malignant cells because it stimulates the migration of leukocytes into tumors and promotes the activation of antitumor CD8+ T cells. Intratumoral Th17 cells induce the expression of CCL20, a chemokine that attracts DCs to the tumor environment, as shown in a recently published paper by Chen et al[56]. Subsequently, DCs phagocytose tumor material and migrate to lymph nodes, contributing to the activation of CD8+ T cells that migrate to the tumor environment through their chemotaxis to the Th17-induced CXCL9 and CXCL10[57]. Moreover, studies have shown that Th17 cells can convert into Th1 lymphocytes in vivo, enhancing their antitumor effectiveness[58,59]. When stimulated by IL-23 and IL-12 in an environment with absent or low TGF-β, Th17 cells are able to express IFN-γ and T-bet, important Th1-related molecules. Interestingly, Th17-derived Th1 cells have a more effective antitumor activity compared to other Th1 lymphocytes, and this may be due to the prolonged survival and superior functionality of the former compared to the latter[60].
On the other hand, the Th17 profile is involved in various protumor activities. First, IL-17 seems to promote angiogenesis because elevated intratumoral levels of that cytokine are associated with high expression of vascular endothelial growth factor and increased tumor vascular density[61]. Complementally, the aforementioned cytokine stimulates cancer cells to release IL-6, which besides promoting vascular endothelial growth factor production enhances signal transducer and activator of transcription 3 activation, increasing the survival of malignant cells by suppressing apoptosis[62]. Moreover, studies have described the existence of FOXP3+ CD4+ Th17 cells, which may play regulatory, protumor roles in cancer contexts. This phenomenon seems to occur along with low levels of Il-6 and IL-23 as well as with the presence of TGF-β, which activates FOXP3 expression[63].
A study carried out by Su et al[62] showed that IL-17 and RORγt (the main IL-17A transcription factor) were highly expressed in both tumor microenvironment and peripheral blood mononuclear cells (PBMCs) of gastric cancer patients, mainly among those with metastasis. This data suggests that the presence of Th17 cells is directly associated with the occurrence of gastric cancer and with the severity of the disease. Indeed, a recently published study embracing stage IV gastric cancer patients from four cohorts have reinforced that theory because it found abnormally high levels of Th17 cell differentiation and activation of IL-17 pathways among patients with severe disease[64]. Interestingly, another study evaluating the percentages of Th17 cells in PBMCs among gastric cancer patients before and after tumor resection observed a significant drop in the proportion of Th17 cells after the treatment[65]. Of note, IL-27 has been highlighted as a crucial cytokine that plays dual roles in the regulation of the immune system. As far as this cytokine enhances T-bet expression through IL-27/IL-27Rα signaling and subsequent signal transducer and activator of transcription 1 phosphorylation leading to Th1 cell differentiation, IL-27 impairs Th17 responses by downregulating RORγT[66,67]. In a recently published study, our group showed that H. pylori-infected individuals have higher IL-27 levels in their serum and gastric mucosa than non-infected individuals. In contrast, there was a lack of IL-27 in both serum and gastric environment of gastric cancer patients, who also showed a remarkable Th17-polarized inflammatory pattern[68].
The Treg cells might play pivotal roles in H. pylori-induced gastric adenocarcinoma by favoring infection perpetuation and by repressing immune responses against malignant cells through the secretion of regulatory cytokines. Indeed, studies have observed that Treg cells are positively correlated with increased bacterial colonization[69], being also increased among gastric cancer patients[70,71]. Three types of Treg cells have been described by studies: IL-10-secreting Tr1 cells, TGF-β1-producing Tr3 cells, and FOXP3-expressing CD4+CD25high Treg cells[72]. The latter is a pivotal component in the scenario of H. pylori colonization, favoring the pathogen persistence in the gastric environment by suppressing the immune responses. A study demonstrated that FOXP3, TGF-β1, and IL-10 are highly expressed during H. pylori infection, and the density of FOXP3+ Treg cells was higher in the gastric mucosa of infected individuals than in H. pylori-negative people. These cells have been associated with increased bacterial density among individuals with gastritis[73].
Advances in the understanding of the interplays between Treg responses and the development of gastric cancer have been achieved. Interestingly, a recent study including gastric cancer patients in various stages of the disease showed that high infiltration of FOXP3+ Treg cells was associated with poor outcomes among individuals with advanced disease, but it was a predictor of better prognosis among patients with early-phase disease[74]. Current evidence emphasizes the role of Wnt/β-catenin signaling in gastric carcinogenesis because 70% of gastric cancer patients have dysregulation in pathways associated with this signaling[75]. β-catenin induces gastric cancer cells to produce CCL28, which strongly attracts Treg cells to the tumor environment. In this sense, a recently published study using Helicobacter felis-colonized mice with gastric cancer found the block of β-catenin-induced CCL28 through anti-CCL28 antibodies leads to the suppression of gastric cancer progression by inhibiting Treg cells infiltration[76].
A surface glycoprotein known as neuropilin-1 seems to be crucial for the immunoregulatory events taking place in the tumor environment. The role of that molecule had already been well described in other malignancies, being related to cell migration, angiogenesis, and invasion[77]. In a new investigation by Kang et al[78], the expression of neuropilin-1 was associated with increased levels of the regulatory cytokines IL-35, IL-10, and TGF-β1 as well as with increased infiltration of Treg cells and M2 macrophages in gastric cancer. Moreover, its expression was positively correlated to poorer outcomes, which indicates that neuropilin-1 has the potential to be used as a prognostic factor in gastric cancer patients.
Besides the aforementioned roles of Treg cells in gastric cancer development, Liu et al[79] found that these cells promote the expression of leucine-rich repeat containing G protein-coupled receptor 5 by tumor cells via TGF-β1 and TGF-β1 signaling pathway, probably involving the aforementioned Wnt/β-catenin signaling. The leucine-rich repeat containing G protein-coupled receptor 5 is a global stem cell marker whose overexpression is observed in gastric cancer, and it has also been positively correlated with tumor invasion, metastasis, and poor prognosis among individuals with that malignancy[80]. Figure 1 summarizes the roles played by Th cells in the setting of gastric carcinogenesis.
The interleukin-1 family has 11 molecules that are able to interact with almost all human cells[81]. Among these, the proinflammatory IL-1β and the antagonist receptor of IL-1 (IL-Ra) have been associated with an increased risk of developing gastric cancer[82-85]. The genes encoding IL-1β and IL-1Ra are called IL1B and IL1RN, respectively[86]. Single nucleotide polymorphisms (SNPs) found in these genes alter the inflammatory response of these cytokines. The SNPs identified in the coding of IL-1B have a C-T transition base at -511, -31, or +3954 positions. For IL-Ra, the allele 2 (IL-RN*2) has been associated with inflammatory responses that increase the risk of disease development[87].
In the presence of any of the three aforementioned SNPs in the sequences that encode IL-1β, the production of this cytokine can be enhanced. This overexpression has been associated with the development of hypochlorhydria and atrophy of the gastric corpus, in addition to an increased risk for gastric cancer, especially on H. pylori positive subjects[88-98]. The production of IL1-Ra is mediated by a diversity of cytokines such as IL-1β, and the antagonistic function of the former controls the inflammatory response of the later. In this sense, the SNP IL-1RN*2 has been linked to an increased secretion of IL-1β[99-102].
IL-8, also known as CXCL8, is a proinflammatory chemokine from the alpha subfamily (CXC)[103]. It can be produced by several cells such as epithelial and endothelial cells, monocytes, macrophages, and tumor cells[104,105]. Increased expression of IL-8 is promoted by various stimuli, including the initiation, modulation, and maintenance of the host inflammatory response against H. pylori infection[106,107]. This molecule induces the migration and proliferation of endothelial cells, contributing to angiogenesis and tumorigenesis, being related to increased cell migration, invasion, and metastasis[108].
The CXCL8 gene is located in chromosome 4q12-21 and possesses three introns, four exons, and a proximal promoter region[109]. The genetic polymorphism IL8-251T> A (rs4073) has been associated with variations in the expression of IL-8 and increased risk of gastric cancer development mainly in Brazilian, Chinese, and Korean populations[110]. Curiously, that polymorphism was not significantly associated with a higher risk of gastric cancer in the Japanese population, which might be related to specific environmental factors and genetic background[103].
As previously discussed in this review, IL-10 is an anti-inflammatory cytokine; therefore, it inhibits the activity of some defense cells and limits the production of proinflammatory cytokines[111]. Polymorphisms -1082, -592 and, less frequently, -819, can modulate IL-10 transcription, decreasing the expression of this cytokine, which can initiate a hyperinflammatory response that increases the risk of gastric lesions and cance[112-116]. Therefore, these polymorphisms have been associated with a possible higher risk of gastric cancer, mainly in Asian populations but also in a study conducted with American subjects[117-120].
IL-2 is a cytokine that plays proinflammatory and anti-inflammatory roles and is encoded by a gene located in chromosome 4q21. Among other repercussions, this molecule contributes to the proliferation of T regulatory cells and regulates the expansion and apoptosis of activated T cells[25,39]. The IL2 GG variant genotype –330T> G in H. pylori-positive Asians and Brazilians as well as the SNP IL-2 + 114T> G and 330g/+ 114T haplotype in H. pylori-positive Brazilians have been associated with an increased risk of developing gastric cancer[121].
Similar to IL-2, IL-4 also plays dual roles in the immune system, being encoded by a gene in chromosome 5q31.1[108]. Its function in tumor progression is mainly related to the inhibition of proinflammatory cytokines in the setting of antitumor immune responses, favoring the perpetuation of malignant cells[107]. Polymorphisms in IL4-590C/T rs2243250 CC, genotype CT + CC, and IL4 haplotypes have been found to be associated with a higher risk of developing gastric cancer in the Chinese population[122].
IL-6 is a cytokine that plays roles as an proinflammatory immune mediator and as an endocrine regulator[79]. This protein is encoded by a gene located in chromosome 7 and has been found to be increased in H. pylori-positive individuals[108]. Polymorphisms in the IL6-174C allele and IL6-174CC genotype are associated with an enhanced prevalence of diffuse-type gastric cancer, whereas the IL6-174CG has been related to intestinal-type gastric cancer[106]. In addition, the IL6 SNP -572 (G> C, rs1800796) has been emphasized as a potential genetic biomarker for increased gastric cancer risk in Asian populations[110].
IL-22 is an anti-inflammatory cytokine that belongs to the IL-10 family. It participates in mucosal repair and epithelial immunity processes[123]. Chinese individuals with the SNP rs1179251 (allele G) encoding IL-22 showed a higher risk of developing gastric cancer associated with H. pylori[124]. Some SNPs of this cytokine have also been found in Chinese patients with increased risk for MALT gastric lymphoma induced by H. pylori (alleles C in rs2227485; A in rs4913428; A in rs1026788 and T in rs7314777)[125].
Infection with cagA-positive H. pylori strains is the main risk factor for the development of gastric cancer[126-129]. CagA is a multifunctional, pore-forming protein that induces vacuolization, cell necrosis, and cell apoptosis in gastric epithelial cells[130-134]. Of note, this virulence factor appears to induce an important modulation of the host immune system[135,136]. A recently published study by He et al[137] using mice revealed that CagA suppresses the expression of proinflammatory cytokines induced by H. pylori infection through the inhibition of the mitogen-activated protein kinase and NF-κB pathways. In addition, the study has shown, for the first time, that this virulence factor downregulates the posttranslational modif
Studies have described that H. pylori has a molecular mechanism of CagA expansion through which its number of copies expands, consequently enhancing its virulence[138,139]. The analysis of the PMSS1 H. pylori strain showed that bacteria that carry more CagA copies also produce more toxin, leading to enhanced cell elongation and IL-8 induction[140,141]. Yamaoka et al[142]. proposes that the levels of IL-8 of the gastric mucosa are related to the presence of CagA and OipA. Both molecules seem to be involved in the induction of interferon regulatory factors and play a role in the complete activation of the IL-8 promoter, using different convergence pathways[143].
Vacuolating cytotoxin A (VacA) is a protein encoded by a monocistronic gene known as vacA. Secreted VacA molecules are 140 kDa initially, but they are rapidly cleaved into a 10 kDa domain (p10) to produce a mature 88 kDa protein[144,145]. Generally, they are secreted as soluble proteins in the extracellular space; however, they are found on the bacterial surface as well[146]. Moreover, this virulence factor is expressed by almost all H. pylori strains[147].
VacA inhibits activation and proliferation of T and B cells, a process that induces the apoptosis of macrophages mainly through the inhibition of INF-β signaling. Moreover, this virulence factor induces an excessive release of IL-8[148]. Specifically in T cells, VacA inhibits the production of IL-2, in addition to regulating the surface expression of the IL2-α receptor. This process is possibly due to the ability of VacA to inhibit the activation of the nuclear factor of activated T-cells, a global transcription factor that regulates immune response genes for T cell activation. The mechanism by which VacA inhibits activation of nuclear factor of activated T-cells is uncertain; however, it is believed that this virulence factor influences the calcium flow in the extracellular medium, which inhibits the calcineurin-dependent Ca2+-calmodulin complex[149]. Other effects on these cells include the activation of intracellular signaling through MAP kinases, such as MKK3/6 and p38 as well as the Rac/Vav-specific nucleotide exchange factor[145]. Studies with primary CD4+ T cells in humans have demonstrated that VacA inhibits the proliferation of activated T cells through a mechanism that is independent of the effect of VacA on nuclear factor of activated T-cells activation and IL-2 expression[150,151]. In antigen presenting cells, VacA seems to interfere with the formation of vesicular compartments in macrophages infected with H. pylori causing homotypic vacuolar fusion and consequent changes in their physiological properties. It has also been reported that VacA can interfere with the antigen presentation of B lymphocytes by interfering in the MHC II of these cells. Finally, blocking the activation and proliferation of this set of cells helps H. pylori to resist the host immune response, establishing a persistent infection and with worse clinical outcomes[146].
The various positive VacA-linked bacterial genotypes are associated with a higher prevalence of malignant gastric lesions, in addition to a greater severity of inflammation induction by the pathogen. VacA is the most studied toxin in H. pylori due to its versatility in relation to different receptors in different cell types and functions. VacA is directly involved in the formation of intracellular vacuoles, which provide the survival of the bacteria in the gastric environment, even after drug treatment. Therefore, other studies need to be developed with VacA in order to better understand the persistence of the pathogen in the gastric environment[152].
The duodenal ulcer promoter A (DupA) protein is an H. pylori virulence factor whose gene is located in the plasticity zone of the bacterial genome[153]. The DupA gene contains two overlapping open reading frames (jhp0917 and jhp0918) that form a continuous locus[154]. Of note, only strains that harbor both aforementioned segments are able to produce the DupA protein[155].
The initial studies on DupA show that its pathogenicity is closely linked to the development of duodenal ulcer[154]. Based on in vitro and in vivo studies, such an outcome is believed to be due to the role of the DupA gene in the activation of NF-κB and activating protein-1, which enhance the infiltration of neutrophils with consequent expression of IL-8 in the antrum that promotes risk of these injuries[156,157]. These findings have shown that predominant antral gastritis often leads to a reduction in somastatin, greater gastrin secretion, and consequently greater release of gastric acid and formation of duodenal ulcer[158]. In this context, DupA expression is negatively correlated with the risk of gastric atrophy, intestinal metaplasia, and gastric cancer[159,160]. However, it has to be emphasized that DupA has not been associated with the development of duodenal ulcers in western populations[161,162].
The 34 kDa external inflammatory protein A (OipA), encoded by the hopH gene (hp0638), located approximately 100kb from the Cag Pathogenicity Island, belongs to the family of External Membrane Proteins. This protein is associated with gastric inflammation, being one of the main H. pylori virulence factors[163].
The attachment of gastric epithelial cells through OipA occurs with the induction of cellular apoptosis via the Bcl-2 pathway, increased levels of Bax, and cleaved caspase 3[164]. Notably, several studies have shown that positive OipA has been more frequent in individuals with precancerous lesions than those with gastritis alone[149,165-167].
The OipA-positive H. pylori strains are more prone to gastric colonization, being also associated with a higher risk of peptic ulcer disease and gastric cancer. This molecule strongly induces inflammation, and the infiltration of neutrophils as well as the production of IL-8 are significantly higher in oipA-positive strains compared to the negative ones[168]. Some studies indicate that OipA induces the interferon regulatory factors 1, which binds and activates the element similar to the response element stimulated by IFN, to induce the genetic transcription of IL-8 and its production[153]. In addition, NF-κB and activating protein-1 are also involved in the genetic transcription and production of IL-8 by gastric epithelial cells infected with H. pylori[169].
Other proinflammatory cytokines may also be present in H. pylori infection caused by the presence of OipA, such as IL-1, IL-6, IL-8, IL-11 IL-17, matrix metalloproteinase-1, TNF-α, or CC chemokine ligand 5[170]. This is similar to a response linked to the Cag Pathogenicity Island[8]. However, depending on the OipA states in different strains of H. pylori, the secretion of these cytokines may not be observed[171-173].
The genes that express functional OipA are strong factors of bacterial virulence and are linked to the genotypes VacA s1, VacA m1, blood group antigen-binding adhesin 2, and the Cag Pathogenicity Island gene and can act synergistically with each other to induce worse clinical outcomes of diseases caused by H. pylori[155].
The gene induced by contact with epithelium A (iceA) is a virulence marker still poorly described. The functions related to this virulence factor remain unclear and it possesses two variants: IceA1 and IceA2. H. pylori has only one iceA locus from which the protein can be expressed. Therefore, the presence of both aforementioned variations indicates an infection by different strains of the pathogen[149].
The IceA relationship and the clinical outcomes of gastric diseases are still controversial[174]. However, studies have emphasized that strains positive for this gene induce the release of the proinflammatory cytokines IL-6 and IL-8 more intensely than negative strains[175,176]. Feliciano et al[177] demonstrated the possible role of IceA1 in the development of gastric cancer but not in peptic ulcers. In addition, Yakoob et al[178] demonstrated that iceA2-positive H. pylori strains were more often associated with chronic active inflammation, gastric ulcer, and gastric adenocarcinoma. Studies indicate that IceA has its function preserved regardless of the presence of other H. pylori virulence factors[179-181].
To date, there seems to be a consensus that the global prevalence of IceA1 is higher than IceA2[174]. However, although there is a greater expression of IceA1 than IceA2, the latter is associated with greater granulocytic and lymphocytic infiltration as well as atrophic gastritis[182]. Abu-Taleb et al[183] demonstrated in their study that H. pylori-infected individuals who express IceA1 or IceA2 alone do not develop gastric carcinoma. On the other hand, 75% of the patients who had both alleles (IceA1/IceA2) concomitantly developed gastric carcinoma. Strains that have positive IceA2 tend to stimulate IL-1, resulting in an increased risk of precancerous lesions in the gastric mucosa. This process can become worse if associated with the concomitant effects of other virulent factors, worsening inflammatory processes. Taken all together, the iceA gene is an important marker of severe gastric diseases that must be taken into account[149].
The mechanisms related to the BabA pathogenicity are still poorly elucidated. Nonetheless, studies have shown that BabA-dependent H. pylori cell adhesion have great relevance in the initial colonization of the pathogen[184]. Moreover, BabA works by facilitating the entry of CagA and VacA into host cells[185]. BabA-negative H. pylori strains have been associated with the development of mild gastric lesions and are rarely associated with gastric cancer. This means that BabA positivity might increase the risk of serious gastric lesions and carcinomas[186].
A study showed a greater expression of IL-33 mRNA in biopsies from patients infected with H. pylori compared to noninfected individuals. Interestingly, a direct relationship was observed between blood group antigen-binding adhesin 2 and increased gastric levels of that cytokine[187]. IL-33 plays an important role in immune regulation, providing protection after damage to epithelial cells[188]. It also has the potential to reduce colonization in gastrointestinal infections[189]. In addition, recent studies emphasize its likely role in the development of tumorigenesis[190].
Sialic acid A adhesin (SabA) is an H. pylori membrane protein whose expression has been explored as a biomarker for increased risk of developing gastric cancer[191,192]. Yamaoka et al[193] demonstrated that SabA is positively associated with gastric cancer, intestinal metaplasia, and body atrophy and is negatively associated with duodenal ulcer. H. pylori uses SabA to recognize the Lewis X antigen from gastric epithelial cells, and this virulence factor has been associated with non-opsonic activation of human neutrophils[194,195]. SabA mediates antigen binding to sialyl-Lewis, which is an established tumor and gastric dysplasia marker[196]. The available data on this issue highlights how harmful such adhesion can be to the gastric epithelium; however, further studies are needed to better understand the underlying immune system responses related to this molecule[197]. Table 1 shows how the H. pylori virulence factors interact with the immune system.
| Virulence factor | Effects over immune response | Ref. |
| CagA | IL-8 stimulation; Suppression of proinflammatory cytokines through the inhibition of MAPK and NF-κB | Schumacher and Schwarz[81] and Garlanda et al[83] |
| VacA | Inhibition of activation and proliferation of T and B cells; Induction of the apoptosis of macrophages (inhibition of INF-β signaling); IL-8 stimulation; Inhibition of IL-2 production by inhibiting NFAT; Impairment of antigen presentation by B lymphocytes | Drici et al[86] Dinarello[89], Hollegaard and Bidwell[90], Sicinschi et al[91] and Garza-González et al[92] |
| DupA | Promotion of neutrophilic infiltration | Zeng et al[94] |
| OipA | Promotion of neutrophilic infiltration; Stimulation of IL-1, IL-6, IL-8, IL-11, IL-17, MMP-1, TNF-α, and IRF-1 | Gehmert et al[97] |
| IceA | Stimulation of IL-1, IL-6, and IL-8 | Abbasian et al[101] |
| BabA | Stimulation of IL-33 | Xue et al[108] |
Heat-shock protein 60 (hsp60) is known to have substantial immunogenic properties. Studies have demonstrated that hsp60 promotes cell signaling upon myeloid and vascular endothelial cells[198]. H. pylori-expressed hsp60 seems to play a role in bacterial adhesion to gastric epithelial cells and mucin[199]. In addition, that virulence factor has been shown to effectively inhibit human PBMCs. A study by Maguire et al[200] demonstrated that the inhibitor effect over human PBMCs was more potent with hsp60 from H. pylori than hsp60 from Chlamydia pneumoniae or human mitochondria. Evidence has shown that hsp60 also promotes immune system responses through the activation of TLRs in human gastric epithelial cells and induces IL-8 expression through TLR-2 and mitogen-activated protein kinase pathways in human monocytes[201,202]. Another study evaluating the effects of hsp60 over human monocytes demonstrated that it seems to promote an upregulation of cytokines such as IL-1a, IL-8, IL-10, IFN-γ, TNF-α, and TGF-β[203]. Regarding the oncogenic roles related to this molecule, an enhanced gastric cancer cell and promotion of tube formation by umbilical vein endothelial cells have been positively associated with hsp60, but effects on cell proliferation and cell death prevention have not been attributed to the protein[204].
The knowledge on the relationship between H. pylori infection, the immune system, and oncogenesis is crucial for the understanding of the mechanisms involved in the onset and progression of gastric cancer. Although considerable advances have been achieved in this research field, much has to be done in order to describe underlying mechanisms related to the H. pylori-related carcinogenesis. A better comprehension on this issue could be useful for the development of tools that may aid in the prevention as well as in the prognostic prediction and treatment of such an important disease. Here, we gathered data showing that H. pylori infection promotes multiple immune response activities, such as Th cell polarizations that are closely related to mechanisms associated with gastric carcinogenesis.
| 1. | Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 2. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 773] [Article Influence: 96.6] [Reference Citation Analysis (1)] |
| 3. | Wroblewski LE, Peek RM Jr. Helicobacter pylori, Cancer, and the Gastric Microbiota. Adv Exp Med Biol. 2016;908:393-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Camilo V, Sugiyama T, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22 Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (3)] |
| 5. | Smith SM. Role of Toll-like receptors in Helicobacter pylori infection and immunity. World J Gastrointest Pathophysiol. 2014;5:133-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 605] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 7. | Chang WL, Yeh YC, Sheu BS. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci. 2018;25:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | de Brito BB, da Silva FAF, de Melo FF. Role of polymorphisms in genes that encode cytokines and Helicobacter pylori virulence factors in gastric carcinogenesis. World J Clin Oncol. 2018;9:83-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Yoshikawa T, Naito Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic Res. 2000;33:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Crabtree JE, Mahony MJ, Taylor JD, Heatley RV, Littlewood JM, Tompkins DS. Immune responses to Helicobacter pylori in children with recurrent abdominal pain. J Clin Pathol. 1991;44:768-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1205] [Article Influence: 70.9] [Reference Citation Analysis (1)] |
| 12. | Suarez G, Reyes VE, Beswick EJ. Immune response to H. pylori. World J Gastroenterol. 2006;12:5593-5598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Nagashima H, Iwatani S, Cruz M, Jiménez Abreu JA, Uchida T, Mahachai V, Vilaichone RK, Graham DY, Yamaoka Y. Toll-like Receptor 10 in Helicobacter pylori Infection. J Infect Dis. 2015;212:1666-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Meliț LE, Mărginean CO, Mărginean CD, Mărginean MO. The Relationship between Toll-like Receptors and Helicobacter pylori-Related Gastropathies: Still a Controversial Topic. J Immunol Res. 2019;2019:8197048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Cadamuro AC, Rossi AF, Maniezzo NM, Silva AE. Helicobacter pylori infection: host immune response, implications on gene expression and microRNAs. World J Gastroenterol. 2014;20:1424-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (3)] |
| 17. | Ding SZ, Torok AM, Smith MF Jr, Goldberg JB. Toll-like receptor 2-mediated gene expression in epithelial cells during Helicobacter pylori infection. Helicobacter. 2005;10:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2423] [Cited by in RCA: 2825] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 19. | Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, Mak TW, Núñez G, Chinnaiyan AM, Fukase K, Inohara N. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Kumar Pachathundikandi S, Brandt S, Madassery J, Backert S. Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS One. 2011;6:e19614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997;12:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | George JT, Boughan PK, Karageorgiou H, Bajaj-Elliott M. Host anti-microbial response to Helicobacter pylori infection. Mol Immunol. 2003;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 890] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 25. | Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori. Aliment Pharmacol Ther. 1996;10 Suppl 1:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Andres S, Schmidt HM, Mitchell H, Rhen M, Maeurer M, Engstrand L. Helicobacter pylori defines local immune response through interaction with dendritic cells. FEMS Immunol Med Microbiol. 2011;61:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19:597-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Evans DJ Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Montecucco C, de Bernard M. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 2003;5:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Gobert AP, Mersey BD, Cheng Y, Blumberg DR, Newton JC, Wilson KT. Cutting edge: urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J Immunol. 2002;168:6002-6006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998-6004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 760] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 35. | Rad R, Brenner L, Krug A, Voland P, Mages J, Lang R, Schwendy S, Reindl W, Dossumbekova A, Ballhorn W, Wagner H, Schmid RM, Bauer S, Prinz C. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology 2007; 133: 150-163. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Haeberle HA, Kubin M, Bamford KB, Garofalo R, Graham DY, El-Zaatari F, Karttunen R, Crowe SE, Reyes VE, Ernst PB. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229-4235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Meyer F, Wilson KT, James SP. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect Immun. 2000;68:6265-6272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Guiney DG, Hasegawa P, Cole SP. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect Immun. 2003;71:4163-4166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Kranzer K, Eckhardt A, Aigner M, Knoll G, Deml L, Speth C, Lehn N, Rehli M, Schneider-Brachert W. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect Immun. 2004;72:4416-4423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Nakajima S, Krishnan B, Ota H, Segura AM, Hattori T, Graham DY, Genta RM. Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology. 1997;113:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | de Bernard M, Cappon A, Pancotto L, Ruggiero P, Rivera J, Del Giudice G, Montecucco C. The Helicobacter pylori VacA cytotoxin activates RBL-2H3 cells by inducing cytosolic calcium oscillations. Cell Microbiol. 2005;7:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 399] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 798] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 45. | Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, Fonseca de Castro LP, Carvalho SD, Bittencourt PF, de Oliveira CA, Corrêa-Oliveira R, Magalhães Queiroz DM. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect. 2012;14:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 47. | Niu Q, Zhu J, Yu X, Feng T, Ji H, Li Y, Zhang W, Hu B. Immune Response in H. pylori-Associated Gastritis and Gastric Cancer. Gastroenterol Res Pract. 2020;2020:9342563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Su Z, Sun Y, Zhu H, Liu Y, Lin X, Shen H, Chen J, Xu W, Xu H. Th17 cell expansion in gastric cancer may contribute to cancer development and metastasis. Immunol Res. 2014;58:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Freire de Melo F, Rocha GA, Rocha AM, Teixeira KN, Pedroso SH, Pereira Junior JB, Fonseca de Castro LP, Cabral MM, Carvalho SD, Bittencourt PF, de Oliveira CA, Queiroz DM. Th1 immune response to H. pylori infection varies according to the age of the patients and influences the gastric inflammatory patterns. Int J Med Microbiol. 2014;304:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Zhang S. The role of transforming growth factor β in T helper 17 differentiation. Immunology. 2018;155:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 52. | Harbour SN, DiToro DF, Witte SJ, Zindl CL, Gao M, Schoeb TR, Jones GW, Jones SA, Hatton RD, Weaver CT. TH17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 53. | Horvath DJ Jr, Washington MK, Cope VA, Algood HM. IL-23 Contributes to Control of Chronic Helicobacter Pylori Infection and the Development of T Helper Responses in a Mouse Model. Front Immunol. 2012;3:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Caruso R, Pallone F, Monteleone G. Emerging role of IL-23/IL-17 axis in H pylori-associated pathology. World J Gastroenterol. 2007;13:5547-5551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Algood HM, Allen SS, Washington MK, Peek RM Jr, Miller GG, Cover TL. Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. J Immunol. 2009;183:5837-5846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Chen W, Qin Y, Liu S. CCL20 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1231:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Bronte V. Th17 and cancer: friends or foes? Blood. 2008;112:214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Bending D, De la Peña H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 442] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 59. | Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 Lineage. Immunity. 2009;30:92-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 849] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 60. | Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 61. | Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 62. | Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 63. | Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 1072] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 64. | Zhang X, Yang F, Wang Z. Tumor microenvironment characterization in stage IV gastric cancer. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Zheng X, Dong L, Wang K, Zou H, Zhao S, Wang Y, Wang G. MiR-21 Participates in the PD-1/PD-L1 Pathway-Mediated Imbalance of Th17/Treg Cells in Patients After Gastric Cancer Resection. Ann Surg Oncol. 2019;26:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886-4890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 438] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 67. | Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748-5756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 68. | Rocha GA, de Melo FF, Cabral MMDA, de Brito BB, da Silva FAF, Queiroz DMM. Interleukin-27 is abrogated in gastric cancer, but highly expressed in other Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2020;25:e12667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Kandulski A, Malfertheiner P, Wex T. Role of regulatory T-cells in H. pylori-induced gastritis and gastric cancer. Anticancer Res. 2010;30:1093-1103. [PubMed] |
| 70. | Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 71. | Enarsson K, Lundgren A, Kindlund B, Hermansson M, Roncador G, Banham AH, Lundin BS, Quiding-Järbrink M. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin Immunol. 2006;121:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Heiber JF, Geiger TL. Context and location dependence of adaptive Foxp3(+) regulatory T cell formation during immunopathological conditions. Cell Immunol. 2012;279:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Gil JH, Seo JW, Cho MS, Ahn JH, Sung HY. Role of Treg and TH17 cells of the gastric mucosa in children with Helicobacter pylori gastritis. J Pediatr Gastroenterol Nutr. 2014;58:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Liu X, Xu D, Huang C, Guo Y, Wang S, Zhu C, Xu J, Zhang Z, Shen Y, Zhao W, Zhao G. Regulatory T cells and M2 macrophages present diverse prognostic value in gastric cancer patients with different clinicopathologic characteristics and chemotherapy strategies. J Transl Med. 2019;17:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, Cheng LL, Lee J, Rha SY, Chung HC, Ganesan K, So J, Soo KC, Lim D, Chan WH, Wong WK, Bowtell D, Yeoh KG, Grabsch H, Boussioutas A, Tan P. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 76. | Ji L, Qian W, Gui L, Ji Z, Yin P, Lin GN, Wang Y, Ma B, Gao WQ. Blockade of β-Catenin-Induced CCL28 Suppresses Gastric Cancer Progression via Inhibition of Treg Cell Infiltration. Cancer Res. 2020;80:2004-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 77. | Tse BWC, Volpert M, Ratther E, Stylianou N, Nouri M, McGowan K, Lehman ML, McPherson SJ, Roshan-Moniri M, Butler MS, Caradec J, Gregory-Evans CY, McGovern J, Das R, Takhar M, Erho N, Alshalafa M, Davicioni E, Schaeffer EM, Jenkins RB, Ross AE, Karnes RJ, Den RB, Fazli L, Gregory PA, Gleave ME, Williams ED, Rennie PS, Buttyan R, Gunter JH, Selth LA, Russell PJ, Nelson CC, Hollier BG. Neuropilin-1 is upregulated in the adaptive response of prostate tumors to androgen-targeted therapies and is prognostic of metastatic progression and patient mortality. Oncogene. 2017;36:3417-3427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Kang JY, Gil M, Kim KE. Neuropilin1 Expression Acts as a Prognostic Marker in Stomach Adenocarcinoma by Predicting the Infiltration of Treg Cells and M2 Macrophages. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Liu XS, Lin XK, Mei Y, Ahmad S, Yan CX, Jin HL, Yu H, Chen C, Lin CZ, Yu JR. Regulatory T Cells Promote Overexpression of Lgr5 on Gastric Cancer Cells via TGF-beta1 and Confer Poor Prognosis in Gastric Cancer. Front Immunol. 2019;10:1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 80. | Simon E, Petke D, Böger C, Behrens HM, Warneke V, Ebert M, Röcken C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One. 2012;7:e35486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Schumacher A, Schwarz R. [Histomorphologic and catamnestic studies of 226 patients with cervical carcinoma in stage Ia in the years 1966 to 1986]. Zentralbl Allg Pathol. 1989;135:639-648. [PubMed] |
| 82. | Chang YW, Oh CH, Kim JW, Lee JW, Park MJ, Shim JJ, Lee CK, Jang JY, Dong SH, Kim HJ, Kim SS, Kim BH. Combination of Helicobacter pylori infection and the interleukin 8 -251 T > A polymorphism, but not the mannose-binding lectin 2 codon 54 G > A polymorphism, might be a risk factor of gastric cancer. BMC Cancer. 2017;17:388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1506] [Article Influence: 125.5] [Reference Citation Analysis (1)] |
| 84. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1687] [Article Influence: 64.9] [Reference Citation Analysis (3)] |
| 85. | Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, Sobrinho-Simões M. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 309] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 86. | Drici Ael-M, Moulessehoul S, Tifrit A, Diaf M, Turki DK, Bachir M, Tou A. Effect of IL-1β and IL-1RN polymorphisms in carcinogenesis of the gastric mucosa in patients infected with Helicobacter pylori in Algeria. Libyan J Med. 2016;11:31576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Irtiza S, Samie AU, Ali S, Siddiqi MA, Naqash SH, Sameer AS. IL-1β polymorphism and expression associated with decreased risk of gastric carcinoma: a case control study in the ethnic Kashmiri population, India. Asian Pac J Cancer Prev. 2015;16:1987-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Ruzzo A, Graziano F, Pizzagalli F, Santini D, Battistelli V, Panunzi S, Canestrari E, Catalano V, Humar B, Ficarelli R, Bearzi I, Cascinu S, Naldi N, Testa E, Magnani M. Interleukin 1B gene (IL-1B) and interleukin 1 receptor antagonist gene (IL-1RN) polymorphisms in Helicobacter pylori-negative gastric cancer of intestinal and diffuse histotype. Ann Oncol. 2005;16:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human disease: on-line databases, Supplement 3. Genes Immun. 2006;7:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Sicinschi LA, Lopez-Carrillo L, Camargo MC, Correa P, Sierra RA, Henry RR, Chen J, Zabaleta J, Piazuelo MB, Schneider BG. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer. 2006;118:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Pérez-Pérez GI. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Chang YW, Jang JY, Kim NH, Lee JW, Lee HJ, Jung WW, Dong SH, Kim HJ, Kim BH, Lee JI, Chang R. Interleukin-1B (IL-1B) polymorphisms and gastric mucosal levels of IL-1beta cytokine in Korean patients with gastric cancer. Int J Cancer. 2005;114:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Zeng ZR, Hu PJ, Hu S, Pang RP, Chen MH, Ng M, Sung JJ. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Ismaili A, Yari K, Moradi MT, Sohrabi M, Kahrizi D, Kazemi E, Souri Z. IL-1B (C+3954T) gene polymorphism and susceptibility to gastric cancer in the Iranian population. Asian Pac J Cancer Prev. 2015;16:841-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Holds JB, Fogg SG, Anderson RL. Botulinum A toxin injection. Failures in clinical practice and a biomechanical system for the study of toxin-induced paralysis. Ophthalmic Plast Reconstr Surg. 1990;6:252-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Gehmert S, Velapatiño B, Herrera P, Balqui J, Santivañez L, Cok J, Vargas G, Combe J, Passaro DJ, Wen S, Meyer F, Berg DE, Gilman RH. Interleukin-1 beta single-nucleotide polymorphism's C allele is associated with elevated risk of gastric cancer in Helicobacter pylori-infected Peruvians. Am J Trop Med Hyg. 2009;81:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28:2598-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 99. | Siech M, Letko G. Influence of ethanol on survival of acinar cells isolated from rat pancreas. Res Exp Med (Berl). 1992;192:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 100. | Ingerslev J, Wallevik K. Clinical experience with Hemofil M in a hemophilia patient exhibiting inhibitors. Ann Hematol. 1991;63:152-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 101. | Abbasian MH, Abbasi B, Ansarinejad N, Motevalizadeh Ardekani A, Samizadeh E, Gohari Moghaddam K, Hashemi MR. Association of interleukin-1 gene polymorphism with risk of gastric and colorectal cancers in an Iranian population. Iran J Immunol. 2018;15:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 102. | Moghimi M, Dastgheib SA, Heiranizadeh N, Zare M, Sheikhpour E, Neamatzadeh H. ASSOCIATION OF IL-8 -251T>A (RS4073) POLYMORPHISM WITH SUSCEPTIBILITY TO GASTRIC CANCER: A SYSTEMATIC REVIEW AND META-ANALYSIS BASED ON 33 CASE-CONTROL STUDIES. Arq Gastroenterol. 2020;57:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Felipe AV, Silva TD, Pimenta CA, Kassab P, Forones NM. lnterleukin-8 gene polymorphism and susceptibility to gastric cancer in a Brazilian population. Biol Res. 2012;45:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Zhang Y, Zeng X, Lu H, Li Y, Ji H. Association between Interleukin-8-251A/T polymorphism and gastric cancer susceptibility: a meta-analysis based on 5286 cases and 8000 controls. Int J Clin Exp Med. 2015;8:22393-22402. [PubMed] |
| 105. | Ma J, Wu D, Hu X, Li J, Cao M, Dong W. Associations between cytokine gene polymorphisms and susceptibility to Helicobacter pylori infection and Helicobacter pylori related gastric cancer, peptic ulcer disease: A meta-analysis. PLoS One. 2017;12:e0176463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 106. | Negovan A, Iancu M, Fülöp E, Bănescu C. Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions. World J Gastroenterol. 2019;25:4105-4124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Yuzhalin A. The role of interleukin DNA polymorphisms in gastric cancer. Hum Immunol. 2011;72:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Xue H, Liu J, Lin B, Wang Z, Sun J, Huang G. A meta-analysis of interleukin-8 -251 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7:e28083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Cheng D, Hao Y, Zhou W, Ma Y. Positive association between Interleukin-8 -251A > T polymorphism and susceptibility to gastric carcinogenesis: a meta-analysis. Cancer Cell Int. 2013;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Wang X, Yang F, Xu G, Zhong S. The roles of IL-6, IL-8 and IL-10 gene polymorphisms in gastric cancer: A meta-analysis. Cytokine. 2018;111:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771-5777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1489] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 112. | Chand-Bhayal A, Krishnaveni D, Pandu-Ranga-Rao K, Prabhakar B, Vidyasagar A, Murali-Krishna B, Anita P, Jyothy A, Nallari P, Venkateshwari A. Association of interleukin-10 promoter polymorphism (-1082 g/a) and gastric cancer in andhra pradesh population of South India. Iran J Cancer Prev. 2012;5:117-123. [PubMed] |
| 113. | Liu S, Liu JW, Sun LP, Gong YH, Xu Q, Jing JJ, Yuan Y. Association of IL10 gene promoter polymorphisms with risks of gastric cancer and atrophic gastritis. J Int Med Res. 2018;46:5155-5166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Namazi A, Forat-Yazdi M, Jafari M, Farahnak S, Nasiri R, Foroughi E, Abolbaghaei SM, Neamatzadeh H. Association of interleukin-10-1082 A/G (RS1800896) polymorphism with susceptibility to gastric cancer: Meta-analysis of 6,101 cases and 8,557 controls. Arq Gastroenterol. 2018;55:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Yu T, Lu Q, Ou XL, Cao DZ, Yu Q. Clinical study on gastric cancer susceptibility genes IL-10-1082 and TNF-α. Genet Mol Res. 2014;13:10909-10912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 116. | Qi M, Liu DM, Pan LL, Lin YX. Interleukin-10 gene -592C>A polymorphism and susceptibility to gastric cancer. Genet Mol Res. 2014;13:8954-8961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Li C, Tong W, Liu B, Zhang A, Li F. The -1082A>G polymorphism in promoter region of interleukin-10 and risk of digestive cancer: a meta-analysis. Sci Rep. 2014;4:5335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 118. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 119. | Nishikawa RM, Yaffe MJ. Effect of various noise sources on the detective quantum efficiency of phosphor screens. Med Phys. 1990;17:887-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni JF Jr, Chow WH. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 680] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 121. | Melchiades JL, Zabaglia LM, Sallas ML, Orcini WA, Chen E, Smith MAC, Payão SLM, Rasmussen LT. Polymorphisms and haplotypes of the interleukin 2 gene are associated with an increased risk of gastric cancer. The possible involvement of Helicobacter pylori. Cytokine. 2017;96:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Yun Y, Dong W, Chen C, Zhang H, Shi N, He M, Chen X. Roles of IL-4 genetic polymorphisms and haplotypes in the risk of gastric cancer and their interaction with environmental factors. Int J Clin Exp Pathol. 2017;10:8936-8943. [PubMed] |
| 123. | Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 124. | Wang YM, Li ZX, Tang FB, Zhang Y, Zhou T, Zhang L, Ma JL, You WC, Pan KF. Association of genetic polymorphisms of interleukins with gastric cancer and precancerous gastric lesions in a high-risk Chinese population. Tumour Biol. 2016;37:2233-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Okabe H, Kitamura R, Shibata H, Hase T, Nakanishi Y, Masamune Y. [In vitro replication of plasmid pKYM]. Yakugaku Zasshi. 1989;109:582-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 126. | Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 127. | Alipour M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer. 2021;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 128. | Sukri A, Hanafiah A, Mohamad Zin N, Kosai NR. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS. 2020;128:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 129. | Hatakeyama M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:196-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 130. | Lin L, Wei H, Yi J, Xie B, Chen J, Zhou C, Wang L, Yang Y. Chronic CagA-positive Helicobacter pylori infection with MNNG stimulation synergistically induces mesenchymal and cancer stem cell-like properties in gastric mucosal epithelial cells. J Cell Biochem. 2019;120:17635-17649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Ansari S, Yamaoka Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 132. | McClain MS, Beckett AC, Cover TL. Helicobacter pylori Vacuolating Toxin and Gastric Cancer. Toxins (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 133. | El Khadir M, Alaoui Boukhris S, Benajah DA, El Rhazi K, Ibrahimi SA, El Abkari M, Harmouch T, Nejjari C, Mahmoud M, Benlemlih M, Bennani B. VacA and CagA Status as Biomarker of Two Opposite End Outcomes of Helicobacter pylori Infection (Gastric Cancer and Duodenal Ulcer) in a Moroccan Population. PLoS One. 2017;12:e0170616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 134. | Chmiela M, Karwowska Z, Gonciarz W, Allushi B, Stączek P. Host pathogen interactions in Helicobacter pylori related gastric cancer. World J Gastroenterol. 2017;23:1521-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 135. | Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1578] [Article Influence: 78.9] [Reference Citation Analysis (1)] |
| 136. | Sayehmiri F, Kiani F, Sayehmiri K, Soroush S, Asadollahi K, Alikhani MY, Delpisheh A, Emaneini M, Bogdanović L, Varzi AM, Zarrilli R, Taherikalani M. Prevalence of cagA and vacA among Helicobacter pylori-infected patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries. 2015;9:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 137. | He H, Liu J, Li L, Qian G, Hao D, Li M, Zhang Y, Hong X, Xu J, Yan D. Helicobacter pylori CagA Interacts with SHP-1 to Suppress the Immune Response by Targeting TRAF6 for K63-Linked Ubiquitination. J Immunol. 2021;206:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 139. | Ferreira RM, Machado JC, Figueiredo C. Clinical relevance of Helicobacter pylori vacA and cagA genotypes in gastric carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 140. | Dela Pena-Ponce MG, Jimenez MT, Hansen LM, Solnick JV, Miller LA. The Helicobacter pylori type IV secretion system promotes IL-8 synthesis in a model of pediatric airway epithelium via p38 MAP kinase. PLoS One. 2017;12:e0183324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 141. | Jang S, Su H, Blum FC, Bae S, Choi YH, Kim A, Hong YA, Kim J, Kim JH, Gunawardhana N, Jeon YE, Yoo YJ, Merrell DS, Ge L, Cha JH. Dynamic Expansion and Contraction of cagA Copy Number in Helicobacter pylori Impact Development of Gastric Disease. mBio. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 142. | Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126:1030-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 143. | Horridge DN, Begley AA, Kim J, Aravindan N, Fan K, Forsyth MH. Outer inflammatory protein a (OipA) of Helicobacter pylori is regulated by host cell contact and mediates CagA translocation and interleukin-8 response only in the presence of a functional cag pathogenicity island type IV secretion system. Pathog Dis. 2017;75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 144. | Nejati S, Karkhah A, Darvish H, Validi M, Ebrahimpour S, Nouri HR. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb Pathog. 2018;117:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 145. | Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 146. | Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 147. | Chauhan N, Tay ACY, Marshall BJ, Jain U. Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: An overview. Helicobacter. 2019;24:e12544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 148. | Sukhan DS, Vernygorodskyi S V. , Haidukov N V., Ludkevich HP. Molecular and Genetic Aspects of Helicobacter pylori Interaction with Cells of Gastric Mucosa. Cytol Genet. 2020;54:147-153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 149. | Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 150. | Utsch C, Haas R. VacA's Induction of VacA-Containing Vacuoles (VCVs) and Their Immunomodulatory Activities on Human T Cells. Toxins (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci USA. 2004;101:7727-7732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 152. | Abdullah M, Greenfield LK, Bronte-Tinkew D, Capurro MI, Rizzuti D, Jones NL. VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori infection. Sci Rep. 2019;9:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 153. | Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1387] [Cited by in RCA: 1362] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 154. | Talebi Bezmin Abadi A, Perez-Perez G. Role of dupA in virulence of Helicobacter pylori. World J Gastroenterol. 2016;22:10118-10123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 155. | Souod N, Sarshar M, Dabiri H, Momtaz H, Kargar M, Mohammadzadeh A, Abdi S. The study of the oipA and dupA genes in Helicobacter pylori strains and their relationship with different gastroduodenal diseases. Gastroenterol Hepatol Bed Bench. 2015;8:S47-S53. [PubMed] |
| 156. | Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 157. | Youssefi M, Ghazvini K, Farsiani H, Tafaghodi M, Keikha M. A systematic review and meta-analysis of outcomes of infection with Helicobacter pylori dupA+ strains in Iranian patients. Gene Rep. 2020;19:100650. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 158. | Miftahussurur M, Yamaoka Y. Helicobacter pylori virulence genes and host genetic polymorphisms as risk factors for peptic ulcer disease. Expert Rev Gastroenterol Hepatol. 2015;9:1535-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 159. | Fatahi G, Talebi Bezmin Abadi A, Peerayeh SN, Forootan M. Carrying a 112 bp-segment in Helicobacter pylori dupA may associate with increased risk of duodenal ulcer. Infect Genet Evol. 2019;73:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 160. | Shiota S, Nguyen LT, Murakami K, Kuroda A, Mizukami K, Okimoto T, Kodama M, Fujioka T, Yamaoka Y. Association of helicobacter pylori dupA with the failure of primary eradication. J Clin Gastroenterol. 2012;46:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 161. | Argent RH, Burette A, Miendje Deyi VY, Atherton JC. The presence of dupA in Helicobacter pylori is not significantly associated with duodenal ulceration in Belgium, South Africa, China, or North America. Clin Infect Dis. 2007;45:1204-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 162. | Paredes-Osses E, Sáez K, Sanhueza E, Hebel S, González C, Briceño C, García Cancino A. Association between cagA, vacAi, and dupA genes of Helicobacter pylori and gastroduodenal pathologies in Chilean patients. Folia Microbiol (Praha). 2017;62:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 163. | Dossumbekova A, Prinz C, Mages J, Lang R, Kusters JG, Van Vliet AH, Reindl W, Backert S, Saur D, Schmid RM, Rad R. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J Infect Dis. 2006;194:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 164. | Teymournejad O, Mobarez AM, Hassan ZM, Talebi Bezmin Abadi A. Binding of the Helicobacter pylori OipA causes apoptosis of host cells via modulation of Bax/Bcl-2 Levels. Sci Rep. 2017;7:8036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 165. | Braga LLBC, Batista MHR, de Azevedo OGR, da Silva Costa KC, Gomes AD, Rocha GA, Queiroz DMM. oipA "on" status of Helicobacter pylori is associated with gastric cancer in North-Eastern Brazil. BMC Cancer. 2019;19:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 166. | Bartpho TS, Wattanawongdon W, Tongtawee T, Paoin C, Kangwantas K, Dechsukhum C. Precancerous Gastric Lesions with Helicobacter pylori vacA +/babA2+/oipA + Genotype Increase the Risk of Gastric Cancer. Biomed Res Int. 2020;2020:7243029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 167. | Sallas ML, Dos Santos MP, Orcini WA, David ÉB, Peruquetti RL, Payão SLM, Rasmussen LT. Status (on/off) of oipA gene: their associations with gastritis and gastric cancer and geographic origins. Arch Microbiol. 2019;201:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 168. | Yamaoka Y. Pathogenesis of Helicobacter pylori-Related Gastroduodenal Diseases from Molecular Epidemiological Studies. Gastroenterol Res Pract. 2012;2012:371503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 169. | Zhang J, Qian J, Zhang X, Zou Q. Outer membrane inflammatory protein A, a new virulence factor involved in the pathogenesis of Helicobacter pylori. Mol Biol Rep. 2014;41:7807-7814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 170. | Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1058] [Article Influence: 66.1] [Reference Citation Analysis (1)] |
| 171. | Odenbreit S, Kavermann H, Püls J, Haas R. CagA tyrosine phosphorylation and interleukin-8 induction by Helicobacter pylori are independent from alpAB, HopZ and bab group outer membrane proteins. Int J Med Microbiol. 2002;292:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 172. | Ando T, Peek RM Jr, Lee YC, Krishna U, Kusugami K, Blaser MJ. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin Diagn Lab Immunol. 2002;9:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 173. | Teymournejad O, Mobarez AM, Hassan ZM, Moazzeni SM, Ahmadabad HN. In vitro suppression of dendritic cells by Helicobacter pylori OipA. Helicobacter. 2014;19:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 174. | Huang X, Deng Z, Zhang Q, Li W, Wang B, Li M. Relationship between the iceA gene of Helicobacter pylori and clinical outcomes. Ther Clin Risk Manag. 2016;12:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 175. | Xu Q, Blaser MJ. Promoters of the CATG-specific methyltransferase gene hpyIM differ between iceA1 and iceA2 Helicobacter pylori strains. J Bacteriol. 2001;183:3875-3884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 176. | Dabiri H, Jafari F, Baghaei K, Shokrzadeh L, Abdi S, Pourhoseingholi MA, Mohammadzadeh A. Prevalence of Helicobacter pylori vacA, cagA, cagE, oipA, iceA, babA2 and babB genotypes in Iranian dyspeptic patients. Microb Pathog. 2017;105:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 177. | Feliciano O, Gutierrez O, Valdés L, Fragoso T, Calderin AM, Valdes AE, Llanes R. Prevalence of Helicobacter pylori vacA, cagA, and iceA Genotypes in Cuban Patients with Upper Gastrointestinal Diseases. Biomed Res Int. 2015;2015:753710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 178. | Yakoob J, Abbas Z, Khan R, Salim SA, Abrar A, Awan S, Ahmad Z. Helicobacter pylori: correlation of the virulence marker iceA allele with clinical outcome in a high prevalence area. Br J Biomed Sci. 2015;72:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 179. | Shiota S, Watada M, Matsunari O, Iwatani S, Suzuki R, Yamaoka Y. Helicobacter pylori iceA, clinical outcomes, and correlation with cagA: a meta-analysis. PLoS One. 2012;7:e30354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 180. | Boyanova L, Yordanov D, Gergova G, Markovska R, Mitov I. Association of iceA and babA genotypes in Helicobacter pylori strains with patient and strain characteristics. Antonie Van Leeuwenhoek. 2010;98:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 181. | Ladeira MS, Rodrigues MA, Salvadori DM, Neto PP, Achilles P, Lerco MM, Rodrigues PA, Gonçalves I Jr, Queiroz DM, Freire-Maia DV. Relationships between cagA, vacA, and iceA genotypes of Helicobacter pylori and DNA damage in the gastric mucosa. Environ Mol Mutagen. 2004;44:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 182. | Chiurillo MA, Moran Y, Cañas M, Valderrama E, Alvarez A, Armanie E. Combination of Helicobacter pylori-iceA2 and proinflammatory interleukin-1 polymorphisms is associated with the severity of histological changes in Venezuelan chronic gastritis patients. FEMS Immunol Med Microbiol. 2010;59:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 183. | Abu-Taleb AMF, Abdelattef RS, Abdel-Hady AA, Omran FH, El-Korashi LA, Abdel-Aziz El-Hady H, El-Gebaly AM. Prevalence of Helicobacter pylori cagA and iceA Genes and Their Association with Gastrointestinal Diseases. Int J Microbiol. 2018;2018:4809093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 184. | Yılmaz N, Koruk Özer M. The Prevalence of Helicobacter Pylori babA, homB, aspA, and sabA Genes and Its Relationship with Clinical Outcomes in Turkey. Can J Gastroenterol Hepatol. 2019;2019:1271872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 185. | Yamaoka Y. Roles of Helicobacter pylori BabA in gastroduodenal pathogenesis. World J Gastroenterol. 2008;14:4265-4272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 186. | Fujimoto S, Olaniyi Ojo O, Arnqvist A, Wu JY, Odenbreit S, Haas R, Graham DY, Yamaoka Y. Helicobacter pylori BabA expression, gastric mucosal injury, and clinical outcome. Clin Gastroenterol Hepatol. 2007;5:49-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 187. | Shahi H, Reiisi S, Bahreini R, Bagheri N, Salimzadeh L, Shirzad H. Association Between Helicobacter pylori cagA, babA2 Virulence Factors and Gastric Mucosal Interleukin-33 mRNA Expression and Clinical Outcomes in Dyspeptic Patients. Int J Mol Cell Med. 2015;4:227-234. [PubMed] |
| 188. | Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F, Fang M. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3⁺ regulatory T-cell responses in mice. Mol Med. 2012;18:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 189. | Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, Kurt-Jones EA, Wang TC, Sivaprasad U, Hershey GK, Herbert DR. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209:607-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 190. | Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, Kim J, Kim YM, Kwon YG. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 191. | Pakbaz Z, Shirazi MH, Ranjbar R, Pourmand MR, Khalifeh Gholi M, Aliramezani A, Vaise Malekshahi Z. Frequency of sabA Gene in Helicobacter pylori Strains Isolated From Patients in Tehran, Iran. Iran Red Crescent Med J. 2013;15:767-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 192. | Su YL, Huang HL, Huang BS, Chen PC, Chen CS, Wang HL, Lin PH, Chieh MS, Wu JJ, Yang JC, Chow LP. Combination of OipA, BabA, and SabA as candidate biomarkers for predicting Helicobacter pylori-related gastric cancer. Sci Rep. 2016;6:36442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 193. | Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, El-Zimaity HM, Reddy R, Arnqvist A, Graham DY. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55:775-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 194. | Nakasato F, Shimoyama T, Yoshimura T, Mikami T, Munakata A, Fukuda S. Infection of sabA-positive H. pylori does not induce anti-Lewis X antibody in host. Hepatogastroenterology. 2008;55:1122-1125. [PubMed] |
| 195. | Sheu BS, Odenbreit S, Hung KH, Liu CP, Sheu SM, Yang HB, Wu JJ. Interaction between host gastric Sialyl-Lewis X and H. pylori SabA enhances H. pylori density in patients lacking gastric Lewis B antigen. Am J Gastroenterol. 2006;101:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 196. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Borén T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 674] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 197. | de Jonge R, Pot RG, Loffeld RJ, van Vliet AH, Kuipers EJ, Kusters JG. The functional status of the Helicobacter pylori sabB adhesin gene as a putative marker for disease outcome. Helicobacter. 2004;9:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 198. | Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 199. | Huesca M, Borgia S, Hoffman P, Lingwood CA. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 200. | Maguire M, Poole S, Coates AR, Tormay P, Wheeler-Jones C, Henderson B. Comparative cell signalling activity of ultrapure recombinant chaperonin 60 proteins from prokaryotes and eukaryotes. Immunology. 2005;115:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 201. | Zhao Y, Yokota K, Ayada K, Yamamoto Y, Okada T, Shen L, Oguma K. Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes. J Med Microbiol. 2007;56:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 202. | Takenaka R, Yokota K, Ayada K, Mizuno M, Zhao Y, Fujinami Y, Lin SN, Toyokawa T, Okada H, Shiratori Y, Oguma K. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology (Reading). 2004;150:3913-3922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 203. | Lin CY, Huang YS, Li CH, Hsieh YT, Tsai NM, He PJ, Hsu WT, Yeh YC, Chiang FH, Wu MS, Chang CC, Liao KW. Characterizing the polymeric status of Helicobacter pylori heat shock protein 60. Biochem Biophys Res Commun. 2009;388:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 204. | Lin CS, He PJ, Tsai NM, Li CH, Yang SC, Hsu WT, Wu MS, Wu CJ, Cheng TL, Liao KW. A potential role for Helicobacter pylori heat shock protein 60 in gastric tumorigenesis. Biochem Biophys Res Commun. 2010;392:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Katayama Y, Mi Y S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL