Published online Jun 28, 2021. doi: 10.13105/wjma.v9.i3.220
Peer-review started: January 25, 2021
First decision: April 19, 2021
Revised: May 7, 2021
Accepted: June 4, 2021
Article in press: June 4, 2021
Published online: June 28, 2021
Processing time: 168 Days and 0.2 Hours
Coronavirus disease 2019 is a pandemic, which has affected millions of people across the globe in the year 2020. This disease is caused by a virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that belongs to the family of coronaviruses and primarily affects the respiratory system. This infection has a wide spectrum of clinical manifestations ranging from asymptomatic form to mild, moderate and severe forms depending upon the age, comorbidity and immunity of an affected individual. Hyper-inflammatory response due to SARS-CoV-2 adversely affect several internal organs. Besides lung injury, which is the main outcome of SARS-CoV-2 infection, it has been reported to adversely impact other organs including the liver and kidneys. SARS-CoV-2 virus can also have a direct adverse impact on liver as well as kidneys due to systemic inflammatory response or drug toxicity, leading to elevated levels of liver injury markers and acute kidney injury. Clinical outcomes of SARS-CoV-2 infection could be worse in patients suffering from pre-existing liver and kidney disease. So far, there have been several reports on the mechanism of liver and kidney injury during SARS-CoV-2 viral attack. However, the long-term impact of this infection on these organs is yet to be understood. This review summarizes the possible causes and effects of SARS-CoV-2 on the liver and kidneys during the infection and post recovery based on available literature.
Core Tip: Coronavirus disease 2019 (COVID-19) infected patients with pre-existing liver and kidney comorbidities are likely to have a poorer clinical prognosis and are at higher risk of severe infection and increased mortality. Data indicates that COVID-19 infection causes acute kidney and liver damage. However, its long-term consequences are yet to be elucidated. Currently in the absence of specific therapy for this viral infection, further clinical studies are needed to understand COVID-19 pathology associated with liver and kidneys. The present review summarizes the effects of COVID-19 on the liver and kidneys during infection and post recovery.
- Citation: Srivastava S, Garg I. Post COVID-19 infection: Long-term effects on liver and kidneys. World J Meta-Anal 2021; 9(3): 220-233
- URL: https://www.wjgnet.com/2308-3840/full/v9/i3/220.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i3.220
Coronaviruses are a large family of viruses causing a variety of diseases. In recent times, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome-CoV) have caused respiratory illness (pneumonia) to thousands of people worldwide, leading to death of many patients. Novel coronavirus-induced pneumonia caused due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a Public Health Emergency of International Concern on January 30, 2020[1] and later named as coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) on the February 11, 2020. SARS-CoV-2 (COVID-19) viral infection was first reported in December 2019 in Wuhan, Hubei Province, China (CDC 2019), and it spread to almost all the parts of the world in a short span of time.
COVID-19 infection causes severe pulmonary disease and has devastating effects. Itinduces a systemic disease attacking multiple organs, potentially causing major damage, including mortality and long-term effects. The mortality rate due to COVID-19 infection is 2%-5% in the general population. However, patients with comorbidities, such as hypertension, diabetes and chronic obstructive pulmonary disease, are considered at higherrisk, with a mortality rate above 50%[2].
There is no definite treatment for infection caused by SARS-CoV-2. Therapeutics currently under consideration by the WHO includeinterleukin6-blockers, colchicines, monoclonal antibodies, anticoagulants and vitamin D along with systemic corticosteroids in patients having severe and critical condition[3]. However, treatment protocols across different countries vary wherein several antiviral candidates are in use for the treatment of infected patients like, the Food and Drug Administration approved remdesivir[4], corticosteroids, oseltamivir (Tamiflu), arbidol hydrochloride, hydroxychloroquine and convalescent plasma therapy[5], although the WHO does not recommend these treatment strategies. Several broad-spectrum antibiotics along with anti-inflammatory drugs are given to prevent acute respiratory distress syndrome. There has been a worldwide endeavour to design a safe and effective vaccine for protection against it. In this regard, several potential vaccines are under various stages of clinical trials.
The infection caused by SARS-CoV-2 virus is predominantly a respiratory disease. However, its adverse effects on other organ system remains unclear. The most common clinical presentation includes respiratory tract involvement with mild to high fever, shortness of breath and cough. However, SARS-CoV-2 infection has a wide spectrum of symptoms ranging from asymptomatic cases and very mild cases with minor symptoms of sore throat and loss of smell or taste[6] to acute respiratory failure and damage to other organs including acute kidney injury (AKI), liver damage, cerebrovascular stroke and gastroenteritis[7,8]. There are several reports on adverse effects of SARS-CoV-2 infection on liver and kidney functions causing liver injury and acute renal injury. The purpose of this review is to ascertain the damaging effects of SARS-CoV-2 infection on the liver and kidneys.
Coronaviruses are enveloped viruses with a positive sense single-stranded RNA genome with sizes between 26-32kb[9]. Four genera of coronavirus viz., α, β, γ, δ have been identified so far, with human coronaviruses detected in α coronavirus and β coronavirus genera[10]. SARS-CoV-2 is a positive-stranded RNA virusbelonging to the genus Beta-coronavirus. It has a crown like structure due to the presence of spike glycoproteins on the envelope[11]. Scientists are trying to find the animal host of this novel coronavirus. However, most groups agree that the intermediate hosts are bats, pangolins or sea animals[12]. Sequencing of the SARS-CoV-2 genome and its phylogenetic analysis revealed that it has 88%-89% similarity with two bat-derived SARS-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21[12].
The virus SARS-CoV-2 mostly enters the body through inhalation, binds to epithe
Although individuals of any age group can succumb to SARS-CoV-2 infection, susceptibility largely varies according to age and comorbidities. Its common clinical features in adults include fever, dry cough, sore throat, headache, fatigue, myalgia and breathlessness[16,17]. The disease manifestations may range from mild pneumonia to moderate pneumonia, which may result in hypoxia requiring hospitalization and critical illness requiring mechanical ventilation, multiorgan dysfunction and possibly death[18]. Individual age, underlying comorbidities and severity of the disease may increase the risk of death due to SARS-CoV-2 up to 49% in critically ill patients[19]. However, the actual mortality rate due to this infection varies in different parts of the world.
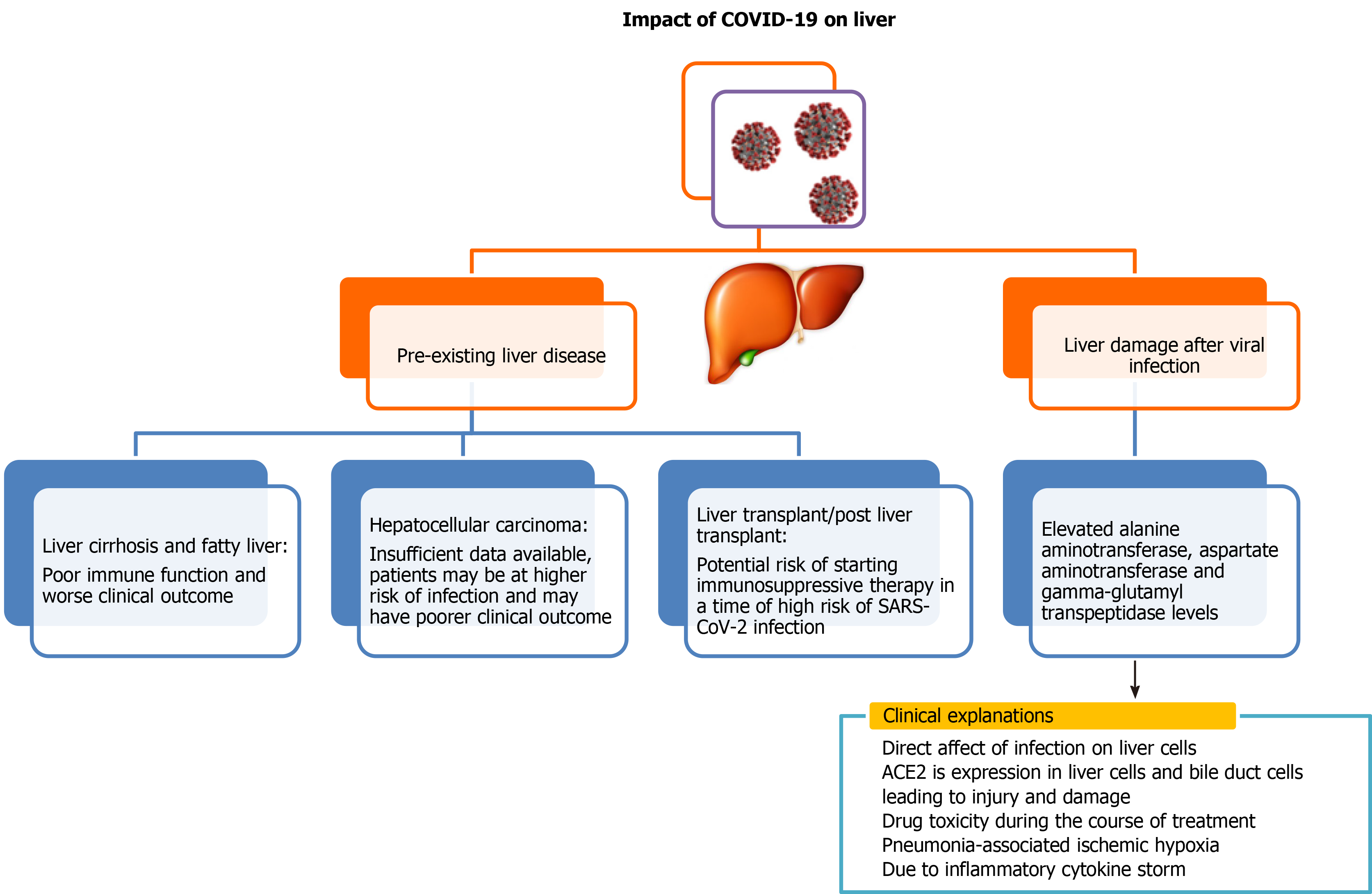
Liver is a vital organ in the human body. COVID-19 infection can either have serious impact on individuals with pre-existing liver disease or can have direct adverse effects on the liver (Figure 1). Although several recently published reports establish that patients with liver diseases are at increased risk and severity of COVID-19 infection, interaction of pre-existing liver disease with SARS-CoV-2 has not been investigated in detail. There have been several studies to demonstrate adverse effects of SARS-CoV-2virus on the liver, and its impairment post SARS-CoV-2 infection is also an emerging concern. Previous studies of SARS coronavirus have shown that up to 60% of patients had a liver impairment showing viral nucleic acid and damage in a liver biopsy[20-22]. Authors in these studies noted that this could be due to treatment of patients with high doses of antibiotics, hepatotoxic antiviral drugs and steroids. Because the process of RNA shedding is well described in the gastrointestinal tract and the production of ACE2 is higher in the colon, biliary system and liver, it is very likely that SARS-CoV-2 may have active replication in these sites, which might in turn result in direct or indirect tissue injury. Because liver is one of the potential entry targets for SARS-CoV-2, the liver damage caused due to infection by SARS-CoV-2 can be attributed to several factors including direct damage by penetrating virus, inflammatory or immune response, increased risk of thrombosis and liver lesions caused by anti-COVID-19 drug therapy[15,23].
Several independent reports are available to demonstrate liver function abnorma
| No. | Study | Finding | Ref. |
| 1 | Large scale study: n = 1099 | AST/ALT were elevated in 18.2%/19.8% of mild COVID-19 patientsand 39.4%/28.1% in severe COVID-19 patients | Guan et al[7] |
| 2 | Small scale study: n = 41 | AST was elevated in 62% of patients in the ICU compared with 25% in those who did not require care in the ICU | Huang et al[8] |
| 3 | n = 99 | AST (U/L): All patients: 34 (26-48); ALT (U/L): All patients: 39 (21-55) | Chen et al[17] |
| 4 | n = 113 | AST (U/L): All patients: 16 (22-46); ALT (U/L): All patients: 22%; Deaths: 27% | Chen et al[25] |
| 5 | n = 115 | AST (U/L): All patients: 28.3 ± 15.6; ULN ≤ 50 U/L: 85%; 50-150 U/L: 15%; > 150: None; ALT (U/L): All patients: 34 (18-67); Moderate: 28 (21-43.5); Severe: 36.5 (17.5-71.5) | Zhang et al[30] |
| 6 | French Cohort Study, n = 281 patients | 102 (36.3%) patients had liver dysfunctions. High level of GGT was the most common perturbation (25.3%). Elevated levels of AST (24.3%) and ALT (12.8%) | Chaibi et al[81] |
| 7 | Large scale study: China, n = 350 | ALT (U/L) and AST (U/L) were profoundly elevated in critically ill patients (33; 49) in comparison to severe patients (23; 29) and mild patients (22; 26) | Fu et al[82] |
| 8 | n = 79 | AST (U/L): All patients: 30 (20-50); Moderate: 28 (22-48); Severe: 35 (22-55). ALT (U/L): All patients: 34 (18-67); Moderate: 28 (21-43.5); Severe: 36.5 (17.5-71.5) | Xie et al[83] |
| 9 | n = 104 | ALT (U/L) and AST (U/L) were profoundly elevated in patients (36.5; 33) | Li et al[84] |
| 10 | Large scale study: China, n = 657 | ALT (U/L) was highly elevated in critically ill patients (41) in comparison to moderate patients (25) | Wang et al[85] |
| 11 | Large scale study: China, n = 1827 | AST (U/L): Abnormal in pre-hospitalization patients (20.3%); Admission (66.9%) and peak hospitalization (83.4%) patients with COVID-19; ALT (U/L): Abnormal in pre-hospitalization patients (19.1%); Admission (41.6%) and peak hospitalization (61.6%) patients with COVID-19 infection | Hundt et al[86] |
| 12 | Large scale study: New York, n = 5700 | 58.4% developed AST values > 40U/L and 39% ALT > 60U/L. 56 patients (2.1%) developed acute hepatic injury defined as an elevation in AST or ALT of >15 times the upper limit of normal | Richardson et al[87] |
Apart from cohort studies, numerous case reports and meta-analyses are also available, which depict severe clinical outcome of viral infection to those patients who were having pre- and post-liver abnormalities[31,32]. Liver injury in SARS-CoV-2 infection is one side of the coin, but little is known about SARS-CoV-2 clinical presentation in the context of liver transplant. It is a well-known fact that these patients are immunosuppressed and are more susceptible to various opportunistic infections. Few case reports are available, which mainly focused on patients who underwent liver transplant a few months or year before SARS-CoV-2 infection but after getting SARS-CoV-2 infection, disease progressed rapidly from mild to critical stage and ultimately led to death despite giving various therapies and treatment[33-35].Few case reports showed promising recovery of these kinds of patients after dynamic surveillance and treatment[35,36]. There is a prerequisite to plan strategies to manage SARS-CoV-2 infection in the post-transplant situation to reduce mortality.
Youssef et al[37] reported a meta-analysis of 3428 patients having SARS-CoV-2 infection from 20 retrospective studies and found that these patients exhibited elevated levels of ALT and AST. They concluded that dysfunctionality of the liver is associated with a critical outcome of SARS-CoV-2 infection, and precise monitoring is very much required in these patients to avoid serious clinical outcomes[37]. Recent meta-analysis done by Del Zompo et al[38] included 20724 COVID-19 patients out of 36 studies and showed that there is an elevation of AST 26.5% and ALT 22.8% at the time of admission. In another meta-analysis, it has been also demonstrated that liver enzymes are firmly linked with 12882 confirmed COVID-19 patients as they showed that COVID 19 infected patients has elevated levels of AST (41.1%) and ALT (29.1%)[39]. It has been reported that COVID-19 associated liver injury is more common in severe COVID-19 than non-severe COVID-19.Wong et al[40] reported the pooled odds ratio for elevated ALT (odds ratio = 2.5, 95% confidence interval: 1.6-3.7, I2 = 57%) and AST (odds ratio = 3.4, 95% confidence interval: 2.3-5.0, I2 = 56) were higher subjects in critical 5961 COVID-19 patients.
Díazet al[41] performed a meta-analysis to characterize hepatic pathological findings in COVID-19 patients. They included 18 studies, which were all case reports and case series from autopsies and reported that 55.1% of patients had hepatic steatosis, 34.7% had congestion of hepatic sinuses, 29.4% had venous thrombosis, 20.5% patients were with fibrosis, 13.5% patients represented Kupffer cell hyperplasia, 13.2% had portal inflammation, and 11.6% had lobular inflammation. Thus, there was a high prevalence of hepatic steatosis and vascular thrombosis as major histological liver features[41].
Finally, there is an utmost need to pay more attention towards the occurrence of liver damage in the diagnosis and treatment of SARS-CoV-2 infection. It is also advisable to clinicians to closely monitor the progression of liver dysfunction in mild to moderate as well as severe COVID-19 patients so that appropriate medical treatment could be given based on severity. It has become of utmost importance to get deep insight into the COVID-19 pathogenesis of liver in patients pre- and post-infection to develop an effective treatment regime for patients.
Scanty information is available regarding liver impairment in alcoholic COVID-19 patients in comparison to nonalcoholic COVID-19 patients. Although, it is well known that alcohol is a major hepatotoxin, and its consumption is responsible for more than 40% of all deaths from liver disease as it interferes with the immune system and compromises its function[42]. A wide spectrum of liver disease ranging from mild to severe comes under the umbrella of alcoholic hepatitis, which increases the risk of severe COVID-19 infection. It has been observed that the patients with alcoholic liver disease infected with SARS-CoV-2 have a worse prognosis. As we are evolving everyday for current pandemics, there are not straight and clear recommendations for the management of alcoholic liver disease in the COVID-19 pandemic[43]. There is little information on the interaction of alcoholic hepatitis and COVID-19[44]; however, it is well-known that patients with advanced alcoholic liver disease are more prone to develop numerous kinds of respiratory distress[45].
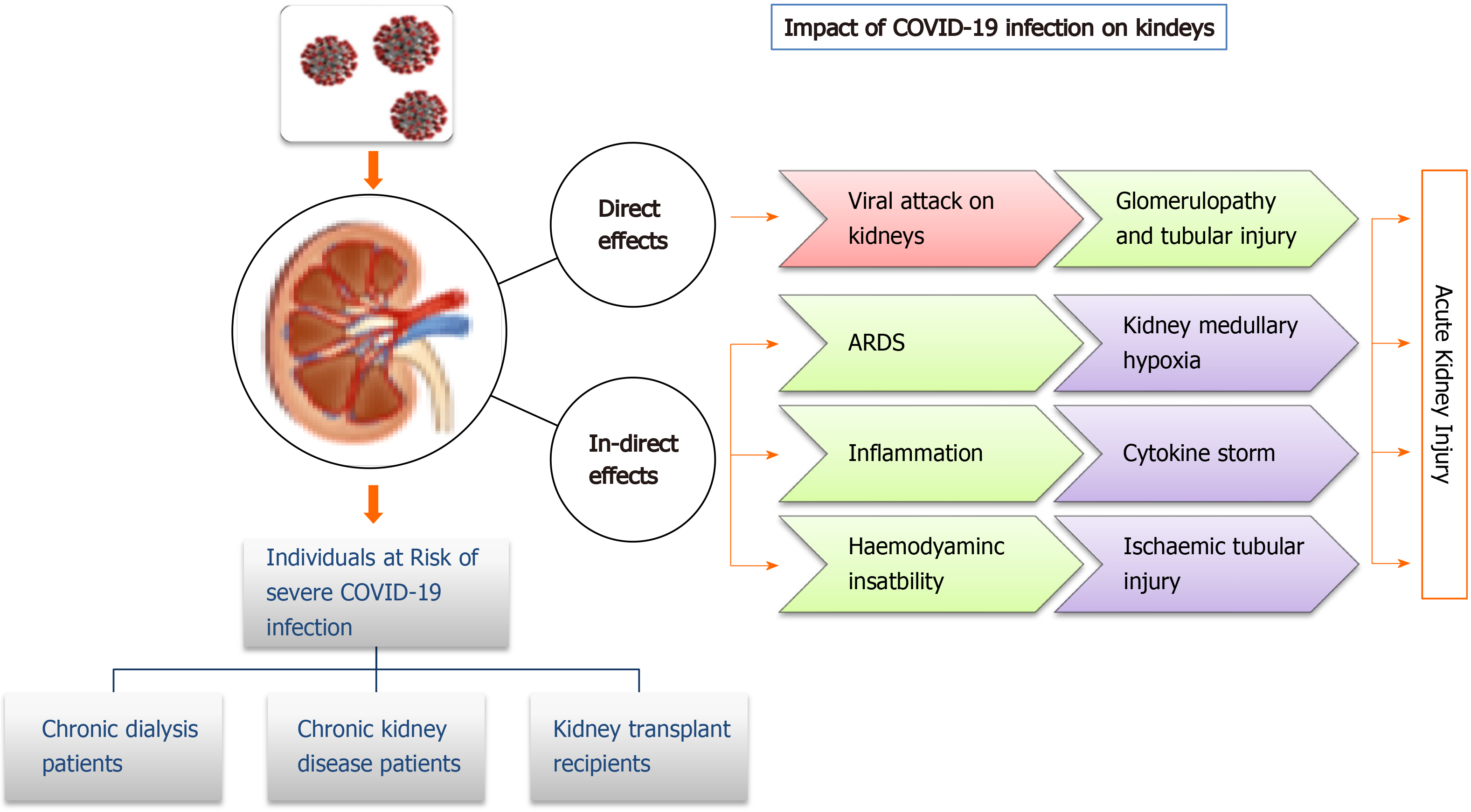
Coronavirus infections have been associated with multiple organ dysfunction, including AKI (Figure 2). Several reports have shown higher frequencies of renal abnormalities in COVID-19 patients. Some studies have classified kidney impairment as an independent risk factor for mortality in COVID-19 patients admitted to hospitals[46,47]. It is important for clinicians to understand whether accompanying AKI phenomenon occurs due to infection or is an important marker of disease severity[48]. AKI occurrence at an early stage is considered a negative prognostic factor for survival and is more common in severely infected patients, especially those admitted to an intensive care unit (ICU)[49,50]. Individuals with no underlying kidney problems also show signs of kidney damage after getting infected with SARS-CoV-2. Renal injury by SARS-CoV-2 can also be attributed to multiple factors such as direct injury due to virus infection or due to systemic effects including host immune clearance and immune response, endothelium-mediated vasculitis, thrombus formation, glucose and lipid metabolism disorder and hypoxia[51]. In the early stages of pandemic spread, incidence of AKI was reported to be in 3% to 9% of patients. However, later studies reported an incidence rate of 15%[52,53]. The COVID-19 associated AKI is managed by avoiding nephrotoxic drugs and by including supportive treatment like renal replacement therapy[47]. Continuous renal replacement therapy by hemofiltration and hemodiafiltration has been used in the past to treat SARS, Middle East respiratory syndrome and sepsis. Thus, it is speculated that continuous renal replacement therapy may be beneficial in patients with COVID-19[54]; however, it needs to be critically evaluated.
Studies report that normal kidneys and intestinal tract have higher ACE2 expression compared to the lungs[28]. ACE2 receptors on the cell walls of the kidney allow the entry of virus into the cells. Thus, kidneys can be directly impacted by the SARS-CoV-2 virus. Diao et al[55] examined post-mortem kidney tissue from 6 infected patients and observed severe acute tubular necrosis and lymphocyte infiltration[56]. Additionally, SARS-CoV-2 nucleocapsid protein and clusters has been detected in kidney tubules by immunohistochemistry[55] and in the tubular epithelium and podocytes using electron microscopy[56]. Thus, this pathogenetic nature of a virus causing injury in tubular, glomerular and vascular parts of the kidney leads to AKI. In a recent observational study of 85 deceased patients, it was found that 54% of the patients had severe AKI, and non-recovery from severe AKI was associated with the presence of pigmented casts, as found on kidney biopsies. Researchers further reported that inflammatory markers and medications were associated with specific histopathologic findings in patients dying due to SARS-CoV-2 virus infection[57]. Some selected studies showing kidney abnormalities in COVID-19 patients have been listed in Table 2.
| No. | Study | Finding | Ref. |
| 1 | n = 191 patients; 137 were discharged and 54 died in hospital | The incidence of AKI was in 15% in the COVID-19 infected patients, out of which 50% were non-survivors and 1% were survivors | Zhou et al[2] |
| 2 | n = 41 patients | 10% patients had elevated creatinine level (> 133 μmol/L) on admission and 7% had AKI. Incidence rate was further increased to 23% in critically ill patients in ICU | Huang et al[8] |
| 3 | n = 193 patients | After hospital admission, 59% of patients developed proteinuria, 44% with haematuria, 14% with increased levels of blood urea nitrogen, and 10% with increased levels of serum creatinine | Li et al[46] |
| 4 | n = 701 consecutive hospitalized patients | 11.9% of those with elevated baseline creatinine developed AKI compared to 4.0% in patients with normal baseline creatinine. 44% of patients had proteinuria and haematuria. Mortality rate was higher in patients with proteinuria, haematuria, elevated baseline creatinine and urea, and AKI stage 2-3 | Cheng et al[47] |
| 5 | Observational study of n = 287 patients | Most patients recover from AKI stage 1. Patients who progress to AKI stage 2 or 3 have a very high mortality rate | Xiao et al[53] |
| 6 | Retrospective study of n = 333 patients | About 75% experienced urine dipstick abnormalities or AKI. Patients who presented kidney dysfunction had higher mortality rates (11.2%) than patients without kidney involvement (1.2%) | Pei et al[88] |
| 7 | Observational study of n = 5449 hospitalized patients | The incidence of AKI was 36.6% with 14.3% of patients requiring dialysis. It was higher in patients admitted to the ICU. Patients with AKI had higher mortality compared to those without AKI (35% and 16.3%, respectively) | Hirsch et al[89] |
| 8 | n = 116 patients | Most of the patients with AKI were critical (52.4%) and incidence of AKI was 18.1% among patients admitted with COVID-19 | Cui et al[90] |
Patients with chronic kidney disease have functional defects in innate and adaptive immunity, thus having a persistent proinflammatory state. These patients are at higher risk of infections in the upper respiratory tract; hence they could be prone to severe SARS-CoV-2 infection[47]. Although, no systematic study has been done on this so far, Henry and Lippi[58] found a significant association of chronic kidney disease with severe COVID-19while analysing different available studies.
Comorbidity such as nephropathy could lead to poor prognosis of COVID-19. Wang[59] studied 230 haemodialysis (HD) COVID-19 patients at Renmin Hospital, Wuhan University. They found 37 individuals amongst them were infected with SARS-CoV-2. Most of these patients presented mild symptoms and did not required admission to an intensive care unit. During this observational study, 7HD patients died, out of which 6 died due to SARS-CoV-2 infection. Another study on HD patients revealed that the most common symptoms of COVID-19, such as fever, cough, and dyspnoea, were not present in these patients. Infact, diarrhoea was the most common symptom, which made the diagnosis even more difficult[60]. In a large-scale retrospective study of 7154 HD patients, SARS-CoV-2 infection was found in 2% of patients, and only about 50% of them had fever while about 20% were asymptomatic. Also, the mortality rate was as high as 31%, much higher compared to the general population[61]. Studies report that HD patients with SARS-CoV-2 infection have reduced inflammatory response, such as levels of circulating CD4 and CD8 T cells, natural killer cells and proinflammatory cytokines and mild symptoms with a lower risk of acute respiratory distress syndrome compared to other general SARS-CoV-2 infected patients[62]. However, this reduced or impaired inflammatory response may lead to a severe outcome of the disease. Thus, HD patients might need to be extra cautious and take all preventive measures to avoid contact with an infected person.
To prevent graft rejection and to prevent inflammatory response, kidney transplant recipients are given immunosuppressants. Many countries with community transfer of COVID-19 have withheld transplantation procedures unless required in highly selected cases when required as a life-saving procedure. Common symptoms of COVID-19 in transplant recipients include fever, cough, asthenia, myalgias and diarrhoea[63]. In different studies on COVID-19 infection in transplant patients showed numerous radiopacity and patchy shadows on chest radiographs[64-66], while in another study on 15 kidney recipients, 33% had no acute radiographic findings (Columbia University Kidney Transplant Program)[67].
Complete withdrawal of immunosuppressants such as calcineurin inhibitors is not recommended in COVID-19 patients in the absence of pneumonia, according to the European Renal Association-European Dialysis and Transplant Association guidelines[68]. However, reduction in its dose and withdrawal of mycophenolate, azathioprine or mTOR-inhibitors can be considered based on the severity of infection. Also, in such cases the prescription of antiviral and anti-inflammatory drugs should be done with caution, considering their drug-drug interactions with immunosuppressants[68]. Sudden withdrawal of immunosuppressants to clear viral load may lead to immune reconstitution and kidney rejection.
Most people who get infected by SARS-CoV-2have mild or moderate symptoms and can recover completely after supportive medical care. Whereas some people, especially the elderly population with weaker immune systems and comorbidities, might suffer from severe symptoms and do not survive[69]. Currently there are no drugs licensed for the treatment or prevention of COVID-19. As treatment with several different drugs is under trial, the WHO is keeping a close watch on the efforts to develop medicines to treat COVID-19. In such cases, misuse of several drugs can lead to serious side effects and complications[70]. Meanwhile, non-pharmaceutical measures such as personal hygiene like hand washing and face masks, environmental sanitizing, social distancing and community-based decisions such as closure of schools, pubs, workplace restrictions etc. are followed at various levels to contain the infection spread.
The majority of people infected with SARS-CoV-2 make a complete recovery in 2-3 wk. Inconclusive studies are available on how long a person remains infectious after the symptoms of infection vanish. Doctors and medical experts across the globe are putting their best efforts to fight this novel coronavirus pandemic.With every passing day new information about its early symptoms, spread, variants, possible treatment strategies and post recovery complications is being added. Still there is no definite answer to the long-term implications of COVID-19. However, some emerging data point out that people suffer from shortness of breath, fatigue, joint pain and headache post recovery from infection[71]. Although, a patient’s recovery may happen in about 2 wk of medical care, people may suffer from other ailments of the kidney, lungs and heart post-recovery. This condition is referred to as ‘post COVID-19 syndrome’ or ‘long COVID19’, which occurs due to persistent symptoms or delayed long-term complications beyond 4 wk. Older aged people, especially those with comorbidities, are more likely to experience lingering COVID-19 symptoms. Nalbandian et al[72] recently summarized the epidemiology and organ-specific sequelae of post-acute COVID-19 and the management considerations needed for interdisciplinary comprehensive care of such patients.
Most of the human viral diseases such as influenza virus do not produce a stable immune response. Understanding the immunity development and its long-lasting effects post SARS-CoV-2 infection needs systematic large-scale studies. Conclusive data on post infection immunity against COVID-19 is lacking. However, antibodies present post recovery would confer to immunity against re-infection, atleast temporarily. During recovery from COVID-19, immunoglobulin (Ig) M and IgG antibodies develop within days to weeks from the onset of symptoms[73,74]. However, there is not a clear and direct relationship between detectable antibody response and clinical improvement in a patient’s condition[73,74]. Viral load peaks during the early stage of infection and then gradually declines as antibodies develop during the subsequent 2-3 wk[74,75]. The stability of neutralizing antibodies, primarily IgG, against COVID-19 is yet to be confirmed. During infection with SARS-CoV, IgG remained high for over 4-5 mo and subsequently declined during the next 2 years to 3 years[76]. It persisted upto 34 mo in patients recovered from Middle East respiratory syndrome-CoV[77].
There have been reports from different parts of the world about re-infection, possible by a distinct molecular form of the same virus[78]. A few studies have shown that patients who had mild symptoms of COVID-19 developed a weaker and shorter immune response and show a decrease in antibody levels after 2-3 mo of infection[79,80]. After almost a year of the COVID-19 pandemic, there is no consensus regarding persistence of neutralizing antibodies and re-infection by SARS-CoV-2.
COVID-19 or SARS-CoV-2 infected patients with pre-existing liver and kidney comorbidities are likely to have a poorer clinical prognosis. Such patients are at high risk of severe infection, and the mortality rate is higher compared to that of the general population. They should take utmost precautions to avoid contact with infected persons. SARS-CoV-2 infection can adversely affect liver and kidney functions in healthy individuals. Data available so far indicates that SARS-CoV-2 causes acute kidney and liver damage. However, the extent of damage and long-term consequences are yet to be elucidated. Currently in the absence of specific therapy for this viral infection and newly developed vaccines being unavailable to the majority of populations worldwide, further clinical studies are needed to understand COVID-19 pathology in relation to liver and kidney damage. This could further help clinicians to modify therapeutic approaches for better management of SARS-CoV-2 infection.
We acknowledge and thank all clinicians, medical staff and researchers who collected the clinical data of COVID-19 patients, which is very helpful in further understanding and formulating strategies to deal with this pandemic.
| 1. | Ramzy A, Mc Neil DG. World Health Organization declares global emergency as Wuhan Coronavirus spreads. The New York Times 2020; Jan 30. [cited 30 March 2020]. In: Myti.ms [Internet]. Available from: http://myti.ms/2RER70M. |
| 2. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18390] [Article Influence: 3065.0] [Reference Citation Analysis (13)] |
| 3. | World Health Organization. WHO 2019-nCoV therapeutics. [cited 5 May 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.14. |
| 4. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5220] [Article Influence: 870.0] [Reference Citation Analysis (0)] |
| 5. | Tobaiqy M, Qashqary M, Al-Dahery S, Mujallad A, Hershan AA, Kamal MA, Helmi N. Therapeutic management of patients with COVID-19: a systematic review. Inf PreventPract. 2020;2:100061. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Patel A, Charani E, Ariyanayagam D, Abdulaal A, Denny SJ, Mughal N, Moore LSP. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19019] [Article Influence: 3169.8] [Reference Citation Analysis (9)] |
| 8. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30466] [Article Influence: 5077.7] [Reference Citation Analysis (12)] |
| 9. | Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1725] [Cited by in RCA: 1911] [Article Influence: 191.1] [Reference Citation Analysis (1)] |
| 10. | Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1309] [Cited by in RCA: 1175] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 11. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3736] [Cited by in RCA: 3230] [Article Influence: 538.3] [Reference Citation Analysis (1)] |
| 12. | Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1670] [Cited by in RCA: 1780] [Article Influence: 296.7] [Reference Citation Analysis (1)] |
| 13. | Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 518] [Article Influence: 86.3] [Reference Citation Analysis (1)] |
| 14. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2935] [Article Influence: 489.2] [Reference Citation Analysis (0)] |
| 15. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14571] [Article Influence: 2428.5] [Reference Citation Analysis (3)] |
| 16. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14867] [Article Influence: 2477.8] [Reference Citation Analysis (1)] |
| 17. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13058] [Article Influence: 2176.3] [Reference Citation Analysis (4)] |
| 18. | Kordzadeh-Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID-19). Future Microbiol. 2020;15:1287-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11609] [Article Influence: 1934.8] [Reference Citation Analysis (2)] |
| 20. | Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1609] [Article Influence: 70.0] [Reference Citation Analysis (1)] |
| 21. | Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY; SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2097] [Cited by in RCA: 2170] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 22. | Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 731] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 23. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv: 2020.02.03.931766. [DOI] [Full Text] |
| 24. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 667] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 25. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2565] [Article Influence: 427.5] [Reference Citation Analysis (2)] |
| 26. | Altaf A, Abbas Z, Mandviwalla HA, Qadeer MA, Siyal M, Tariq M, Ghafoor A, Karamat M, Shahid B, Ali M. Severe COVID-19 Associated With Liver Injury in Patients Without Preexisting Liver Disease. Cureus. 2021;13:e14705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Zhao XY, Xu XX, Yin HS, Hu QM, Xiong T, Tang YY, Yang AY, Yu BP, Huang ZP. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 28. | Du M, Cai G, Chen F, Christiani DC, Zhang Z, Wang M. Multiomics Evaluation of Gastrointestinal and Other Clinical Characteristics of COVID-19. Gastroenterology 2020; 158: 2298-2301. e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 29. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1214] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 30. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 31. | Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, Wang L, Chen Y, Liu W, Zhang K, Wu Y, Yang Z, Tao J, Feng J, Liu K, Ye X, Wang R, Zhang X, Zha Y. A 55-Day-Old Female Infant Infected With 2019 Novel Coronavirus Disease: Presenting With Pneumonia, Liver Injury, and Heart Damage. J Infect Dis. 2020;221:1775-1781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 32. | Makarem J, Naghibi N, Beigmohammadi MT, Foroumandi M, Mehrpooya M. A Case Report of Progressive Liver Failure Inappropriate to Decompensated Heart Failure Following Infection With COVID-19. Cureus. 2020;12:e10142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Huang JF, Zheng KI, George J, Gao HN, Wei RN, Yan HD, Zheng MH. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20:1907-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Nikoupour H, Kazemi K, Arasteh P, Ghazimoghadam S, Eghlimi H, Dara N, Gholami S, Nikeghbalian S. Pediatric liver transplantation and COVID-19: a case report. BMC Surg. 2020;20:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Wei L, Liu B, Zhao Y, Chen Z. Prolonged shedding of SARS-CoV-2 in an elderly liver transplant patient infected by COVID-19: a case report. Ann Palliat Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Nikoupour H, Arasteh P, Gholami S, Nikeghbalian S. Liver transplantation and COVID-19: a case report and cross comparison between two identical twins with COVID-19. BMC Surg. 2020;20:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, S Farhoud A, Elnahla A, Shihabi A, Toraih EA, S Fawzy M, Kandil E. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072-13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 39. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 40. | Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693-7706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 42. | Da BL, Im GY, Schiano TD. Coronavirus Disease 2019 Hangover: A Rising Tide of Alcohol Use Disorder and Alcohol-Associated Liver Disease. Hepatology. 2020;72:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 43. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 44. | Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020;19:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Simet SM, Sisson JH. Alcohol's Effects on Lung Health and Immunity. Alcohol Res. 2015;37:199-208. [PubMed] |
| 46. | Li Z, Wu M, Yao J, Guo J, Liao X, Song S, Li J, Duan G, Zhou Y, Wu X, Zhou Z, Wang T, Hu M, Chen X, Fu Y, Lei C, Dong H, Xu C, Hu Y, Han M, Jia H, Yan J. Caution on kidney dysfunctions of COVID-19 patients. 2020 Preprint. Available from: medRxiv:2020.02.08.20021212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 47. | Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1757] [Cited by in RCA: 1835] [Article Influence: 305.8] [Reference Citation Analysis (0)] |
| 48. | Li Q, Zhang T, Li F, Mao Z, Kang H, Tao L, Zhou F, Cai Y. Acute Kidney Injury Can Predict In-Hospital Mortality in Elderly Patients with COVID-19 in the ICU: A Single-Center Study. Clin Interv Aging. 2020;15:2095-2107. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Joseph A, Zafrani L, Mabrouki A, Azoulay E, Darmon M. Acute kidney injury in patients with SARS-CoV-2 infection. Ann Intensive Care. 2020;10:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Xu S, Fu L, Fei J, Xiang H-X, Xiang Y, Tan Z-X, et al Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: a hospital-based retrospective analysis. 2020 Preprint. Available from: medRxiv:2020.03.24.20042408. [DOI] [Full Text] |
| 51. | Wang M, Xiong H, Chen H, Li Q, Ruan XZ. Renal Injury by SARS-CoV-2 Infection: A Systematic Review. Kidney Dis (Basel). 2021;7:100-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, Qiang M, Xiang J, Zhang B, Chen Y, Gao C. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020;189:428-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 53. | Xiao G, Hu H, Wu F, Sha T, Huang Q, Li H, Han J, Song W, Chen Z, Zeng Z. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. 2020 Preprint. Available from: medRxiv:2020.04.06.20055194. [DOI] [Full Text] |
| 54. | Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 465] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 55. | Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, Tan Y, Wang H, Liu L, Liu Y, Wang G, Yuan Z, Hou X, Ren L, Wu Y, Chen Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12:2506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 56. | Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1278] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 57. | Rivero J, Merino-López M, Olmedo R, Garrido-Roldan R, Moguel B, Rojas G, Chavez-Morales A, Alvarez-Maldonado P, Duarte-Molina P, Castaño-Guerra R, Ruiz-Lopez IK, Soria-Castro E, Luna C, Bonilla-Méndez A, Baranda F, Zabal C, Madero M, Valdez-Ortiz R, Soto-Abraham MV, Vazquez-Rangel A. Association between Postmortem Kidney Biopsy Findings and Acute Kidney Injury from Patients with SARS-CoV-2 (COVID-19). Clin J Am Soc Nephrol. 2021;16:685-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 58. | Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 59. | Wang H. Maintenance Hemodialysis and COVID-19: Saving Lives With Caution, Care, and Courage. Kidney Med. 2020;2:365-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Wang R, Liao C, He H, Hu C, Wei Z, Hong Z, Zhang C, Liao M, Shui H. COVID-19 in Hemodialysis Patients: A Report of 5 Cases. Am J Kidney Dis. 2020;76:141-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 61. | Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, Liu J, Dong JW, Chen WL, Wang XH, Luo D, Shi M, Miao XP, Zhang C. Clinical Characteristics of and Medical Interventions for COVID-19 in Hemodialysis Patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 62. | Ma Y, Diao B, Lv X, Zhu J, Liang W, Liu L, Zhang S, Shen B, Wang H. COVID-19 in hemodialysis (HD) patients: report from one HD center in Wuhan, China. 2020 Preprint. Available from: medRxiv:2020.02.24.20027201. [DOI] [Full Text] |
| 63. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 64. | Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 65. | Guillen E, Pineiro GJ, Revuelta I, Rodriguez D, Bodro M, Moreno A, Campistol JM, Diekmann F, Ventura-Aguiar P. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 66. | Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Zambetti N, Moscato M, Venturini M, Affatato S, Gaggiotti M, Bossini N, Scolari F. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 67. | Columbia University Kidney Transplant Program. Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York. J Am Soc Nephrol. 2020;31:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 68. | Maggiore U, Abramowicz D, Crespo M, Mariat C, Mjoen G, Peruzzi L, Sever MS, Oniscu GC, Hilbrands L, Watschinger B. How should I manage immunosuppression in a kidney transplant patient with COVID-19? Nephrol Dial Transplant. 2020;35:899-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 69. | Santesmasses D, Castro JP, Zenin AA, Shindyapina AV, Gerashchenko MV, Zhang B, Kerepesi C, Yim SH, Fedichev PO, Gladyshev VN. COVID-19 is an emergent disease of aging. 2020 Preprint. Available from: medRxiv:2020.04.15.20060095. [DOI] [Full Text] |
| 70. | Alessi J, de Oliveira GB, Schaan BD, Telo GH. Dexamethasone in the era of COVID-19: friend or foe? Diabetol Metab Syndr. 2020;12:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 71. | McCallum K. Post-COVID Syndrome: What Should You Do If You Have Lingering COVID-19 Symptoms? [cited 19 Nov 2020]. In: Houston Methodist [Internet]. Available from: https://www.houstonmethodist.org/blog/articles/2020/nov/post-covid-syndrome-what-should-you-do-if-you-have-lingering-covid-19-symptoms/. |
| 72. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 3147] [Article Influence: 629.4] [Reference Citation Analysis (0)] |
| 73. | Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 1855] [Article Influence: 309.2] [Reference Citation Analysis (0)] |
| 74. | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4682] [Cited by in RCA: 4849] [Article Influence: 808.2] [Reference Citation Analysis (0)] |
| 75. | To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, Lau DP, Choi CY, Chen LL, Chan WM, Chan KH, Ip JD, Ng AC, Poon RW, Luo CT, Cheng VC, Chan JF, Hung IF, Chen Z, Chen H, Yuen KY. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2296] [Cited by in RCA: 2273] [Article Influence: 378.8] [Reference Citation Analysis (4)] |
| 76. | Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, Zheng L, Lan T, Wang LF, Liang GD. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562-1564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 77. | Payne DC, Iblan I, Rha B, Alqasrawi S, Haddadin A, Al Nsour M, Alsanouri T, Ali SS, Harcourt J, Miao C, Tamin A, Gerber SI, Haynes LM, Al Abdallat MM. Persistence of Antibodies against Middle East Respiratory Syndrome Coronavirus. Emerg Infect Dis. 2016;22:1824-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 78. | To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, Fong CH, Yuan S, Tsoi HW, Ng AC, Lee LL, Wan P, Tso E, To WK, Tsang D, Chan KH, Huang JD, Kok KH, Cheng VC, Yuen KY. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 487] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 79. | Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, Wei P, Ge J, Gou M, Li X, Sun L, Cao T, Wang P, Zhou C, Zhang R, Liang P, Guo H, Wang X, Qin CF, Chen F, Dong C. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020; 52: 971-977. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 823] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 80. | Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, Hemmings O, O'Byrne A, Kouphou N, Galao RP, Betancor G, Wilson HD, Signell AW, Winstone H, Kerridge C, Huettner I, Jimenez-Guardeño JM, Lista MJ, Temperton N, Snell LB, Bisnauthsing K, Moore A, Green A, Martinez L, Stokes B, Honey J, Izquierdo-Barras A, Arbane G, Patel A, Tan MKI, O'Connell L, O'Hara G, MacMahon E, Douthwaite S, Nebbia G, Batra R, Martinez-Nunez R, Shankar-Hari M, Edgeworth JD, Neil SJD, Malim MH, Doores KJ. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 954] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 81. | Chaibi S, Boussier J, Hajj WE, Abitbol Y, Taieb S, Horaist C, Jouannaud V, Wang P, Piquet J, Maurer C, Lahmek P, Nahon S. Liver function test abnormalities are associated with a poorer prognosis in Covid-19 patients: Results of a French cohort. Clin Res Hepatol Gastroenterol. 2020;101556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Fu L, Fei J, Xu S, Xiang HX, Xiang Y, Hu B, Li MD, Liu FF, Li Y, Li XY, Zhao H, Xu DX. Liver Dysfunction and Its Association with the Risk of Death in COVID-19 Patients: A Prospective Cohort Study. J Clin Transl Hepatol. 2020;8:246-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 84. | Li C, Chen Q, Wang J, Lin H, Lin Y, Lin J, Peng F, Chen J, Yang Z. Clinical characteristics of chronic liver disease with coronavirus disease 2019 (COVID-19): a cohort study in Wuhan, China. Aging (Albany NY). 2020;12:15938-15945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Wang M, Yan W, Qi W, Wu D, Zhu L, Li W, Wang X, Ma K, Ni M, Xu D, Wang H, Chen G, Yu H, Ding H, Xing M, Han M, Luo X, Chen T, Guo W, Xi D, Ning Q. Clinical characteristics and risk factors of liver injury in COVID-19: a retrospective cohort study from Wuhan, China. Hepatol Int. 2020;14:723-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 87. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6573] [Article Influence: 1095.5] [Reference Citation Analysis (0)] |
| 88. | Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol. 2020;31:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 559] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 89. | Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 939] [Cited by in RCA: 1037] [Article Influence: 172.8] [Reference Citation Analysis (1)] |
| 90. | Cui X, Yu X, Wu X, Huang L, Tian Y, Huang X, Zhang Z, Cheng Z, Guo Q, Zhang Y, Cai Y, Zhan Q. Acute Kidney Injury in Patients with the Coronavirus Disease 2019: A Multicenter Study. Kidney Blood Press Res. 2020;45:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Gracia-Ramos AE, Khader MA S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH