Published online Mar 31, 2019. doi: 10.13105/wjma.v7.i3.80
Peer-review started: February 20, 2019
First decision: March 5, 2019
Revised: March 12, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: March 31, 2019
Processing time: 39 Days and 0.6 Hours
Hepatocellular carcinoma (HCC) is a highly aggressive malignant disease, with a poor clinical prognosis. Many standard therapies are often considered for HCC treatment today; however, these conventional therapies often fail to achieve sufficiently effective clinical results. Today, HCC therapy is set to undergo a major revolution, owing to rapid developments in cancer immunotherapy, particularly immune checkpoint inhibitor therapy. Cancer immunotherapy is a novel and promising treatment strategy that differs significantly from conventional therapies in its approach to achieve antitumor effects. In fact, many cancer immunotherapies have been tested worldwide and shown to be effective against various types of cancer; HCC is no exception to this trend. For example, we identified a specific cancer antigen called glypican-3 (GPC3) and performed clinical trials of GPC3-targeted peptide vaccine immunotherapy in patients with HCC. Here, we present an overview of the immune mechanisms for development and progression of HCC, our GPC3-based immunotherapy, and immune checkpoint inhibitor therapy against HCC. Finally, we discuss the future prospects of cancer immunotherapy against HCC. We believe that this review and discussion of cancer immunotherapy against HCC could stimulate more interest in this promising strategy for cancer therapy and help in its further development.
Core tip: Hepatocellular carcinoma (HCC) is a highly aggressive malignant disease, with a poor prognosis. Recent developments and advances in cancer immunotherapy, particularly immune checkpoint inhibitor therapy, could lead to a major paradigm shift in standard HCC therapy. This review aims to provide an overview of novel immunotherapies, including antigen-based immunotherapies such as glypican-3-targeted immunotherapy, and immune checkpoint inhibitor therapy against HCC. It also discusses the future prospects of cancer immunotherapy against HCC.
- Citation: Akazawa Y, Suzuki T, Yoshikawa T, Mizuno S, Nakamoto Y, Nakatsura T. Prospects for immunotherapy as a novel therapeutic strategy against hepatocellular carcinoma. World J Meta-Anal 2019; 7(3): 80-95
- URL: https://www.wjgnet.com/2308-3840/full/v7/i3/80.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i3.80
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related death[1]. Approximately 750000 people are affected and about 700000 result in death in worldwide every year; the incidence is particularly high in Asia and Africa[2,3]. Infections with hepatitis B virus (HBV) and hepatitis C virus (HCV), which induce the formation of chronic inflammatory microenvironments in the liver, are considered major risk factors for HCC[4]. Other factors, including alcohol intake, obesity, diabetes, and exposure to aflatoxin, have also been implicated in the cause and progression of HCC[5]. Early detection can ensure better clinical results for patients with HCC, as evidenced by the high 5-year survival rate (more than 70%) for early-stage HCC patients[6]. In contrast, most late-stage HCC patients show poor prognosis, with a 5-year survival rate of less than 16%[6,7]. One of the causes of making early diagnosis of HCC difficult is the existence of background liver of patients including chronic liver inflammation and cirrhosis, which makes it difficult to obtain clear images from ultrasonography, computerized tomography (CT), and magnetic resonance imaging (MRI). Furthermore, highly accurate biomarkers for early-stage HCC detection have not yet been established[8]. Also, patients with HCC have higher recurrence rates than those with other solid cancers; as HCC is initiated from injured hepatocytes, even after tumor removal, the patient has a high risk of recurrence as long as the background liver disease persists. Therefore, these above factors make HCC one of the most aggressive diseases, with poor survival prognosis.
Currently, there are various options for HCC therapy, depending on the clinical stage. Surgical hepatic resection and radiofrequency ablation therapy (RFA) is considered as ideal for early-stage HCC patients, who have adequate liver function and no evidence of portal hypertension or vascular invasion[9-13]. For early-stage HCC patients with relatively poor liver function, liver transplantation is an effective therapeutic procedure[14]. Also, many reports have demonstrated the effectiveness of RFA and transarterial embolization (TAE) for HCC patients. However, these conventional strategies are limited by considerations of tumor size, number of intrahepatic metastases, and adequate hepatic reserve capacity; they are therefore unsuitable for many patients[15,16]. Meanwhile, for patients with advanced HCC, transcatheter arterial chemoembolization and molecular targeted drugs have been conducted. Systemic chemotherapy, which is other treatment method, and has been reported to show a high frequency of adverse events and strong tolerance, with poor clinical effectivity[17]. Molecular targeted therapy, one of the more modern strategies, is based on specific molecular attributes of cancer types. Sorafenib, an inhibitor of tyrosine kinase, is the first molecular targeted drug against HCC that is approved by the Food and Drug Administration (FDA). Indeed, in a phase-III clinical trial for advanced HCC, patients who received sorafenib had better overall survival (OS) than placebo-treated patients (10.7 mo vs 7.9 mo)[18-21]. However, because of its low response rate for HCC patients and the relatively high incidence of adverse events, sorafenib may not be the most suitable strategy for HCC therapy[18-20,22,23]. Today, the development of other molecular targeted drugs is under way[24,25]. Indeed, as novel molecular targeted drug against HCC, Regorafenib and Lenvatinib were approved by the FDA in April 2017 and August 2018, respectively. In any case, the development of a new therapeutic strategy of HCC with adequate antitumor effect and few adverse events would be urgent[26,27].
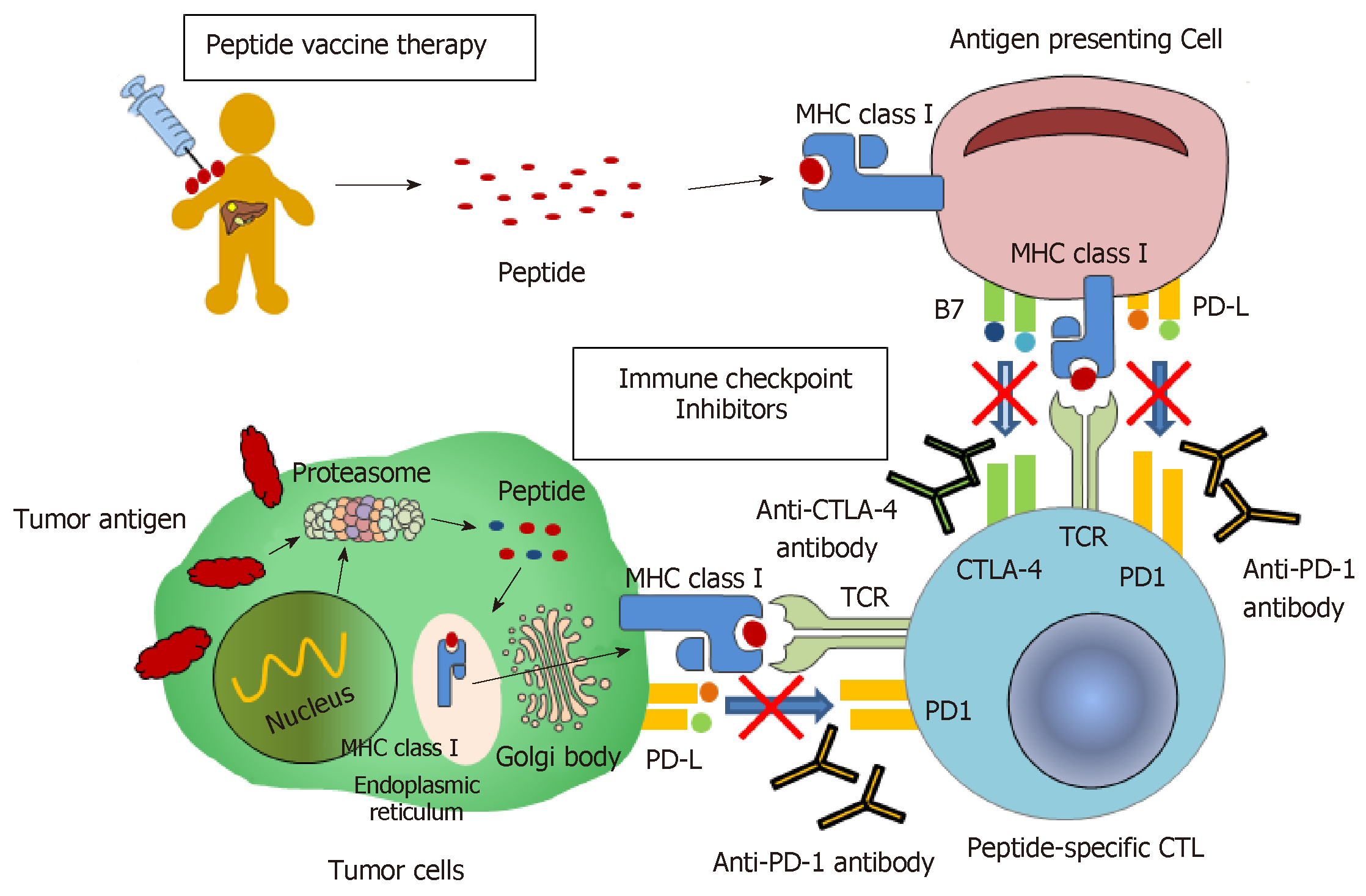
In recent years, immunotherapy has attracted a lot of attention from both basic scientists and clinicians as a promising new method of cancer therapy. It has been demonstrated that several immunotherapies have high antitumor effects against various cancer types, including malignant melanomas and hematological malignancies[28,29]. Also, immunotherapy could theoretically be ideal for HCC therapy, as: (1) it exerts antitumor effects through mechanisms different from those of existing therapies; and (2) it produces recurrence prevention effects along with curative effects[30-32]. Indeed, immunotherapy against HCC has been studied for decades[33,34], and many clinical trials have been performed on HCC patients[35,36]. Several randomized controlled trials have established the use of immunotherapy as an adjuvant therapy to reduce the risk of cancer recurrence[37-39]. Therefore, it is almost certain that immunotherapy will be one of the major options for HCC therapy in the near future. Here, we introduce novel immunotherapeutic strategies for HCC therapy, including immune checkpoint inhibitor therapy; we also elaborate on immunotherapy using GPC3, which is a cancer-specific antigen we identified (Figure 1). We believe this review could awaken more interest in immunotherapy for HCC, which would help improve the survival prognosis of HCC patients.
The liver has a role as a lymphatic system of the whole body, and HCC occurring in these tissues often has properties different from other cancers. Commonly, most HCC patients have a background of chronic hepatitis or liver cirrhosis. Liver cirrhosis is often a highly genotoxic environment with persistent inflammation and fibrosis, which could promote the onset of HCC. Generally, HCC is a malignant disease induced by inflammation; the carcinogenesis of HCC usually involves DNA oxidative injury accumulated under a sustained inflammatory environment caused by HBV, HCV, or other factors.
During the development and progression of HCC, patients show a unique anti- or pro-tumor response[29]. Previously, it had been reported that patients with HCC showed spontaneous T-cell response to many tumor antigens, including alpha fetoprotein (AFP), glypican-3 (GPC3), NY-ESO-1, SSX-2, MAGE-A-10, and p53[40-46]. However, the anti-tumor effects of these immune responses are not sufficient to cause tumor regression or inhibit disease progression. Several mechanisms have been proposed to explain this phenomenon. First, there would be a change in the expression levels of major histocompatibility complex (MHC) class I, which plays a role in antigen presentation to cytotoxic T-lymphocytes (CTLs). Indeed, the downregulation of MHC class I has been reported in many advanced cancers, including HCC[47-49]. However, changes in the expression levels of HLA class I have not been consistent in previous studies, and remain unclear. In addition, a decrease in the expression of co-stimulatory molecules B7-1 and B7-2 has been reported in HCC[50]. Second, there would be excessive activation of immunosuppressive cells, including regulatory T-cells (Tregs) and bone marrow-derived suppressor cells (MDSCs)[51]. Tregs are the most well-known players in cancer evasion from immunosurveillance. In fact, Tregs have been shown to suppress anti-tumor immunity[52,53]. Patients with HCC have increased number of Treg in peripheral blood mononuclear cells (PBMCs) and tumor-infiltrating lymphocytes (TILs), which is correlated with disease progression and poor prognosis[54-56]. MDSCs are the heterogeneous cell population of early bone marrow progenitor cells; they suppress the cytotoxic activity of NK cells and adaptive immune responses mediated by CD4+ and CD8+ T-cells. Specifically, MDSCs have been reported to induce the production of Foxp3 and interleukin-10 in CD4+ T-cells through arginase activity; they also suppress T-cell function through the induction of Tregs. In addition, the frequency of MDSCs in the peripheral blood is positively correlated with the risk of recurrence of HCC in patients who received RFA therapy[57]. Third, there is the presence of immune checkpoint factors. It has been shown that, due to escape from immune monitoring, especially from specific T-cell responses for tumor antigens, several immune checkpoint pathways could be actively exploited by tumors. Indeed, some immune checkpoint molecules such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) have been detected in the tumor microenvironment and are often overexpressed[58-61]. Recent years have seen a rapid advance in research on these immune checkpoint molecules. In some cancers, particularly malignant melanomas, inhibitors of immune checkpoint molecules have been shown to cause a major antitumor effect. Today, inhibitors targeting immune checkpoint molecules are being extensively studied worldwide. Finally, persistent infection of the liver could lead to immune escape mechanisms. It is well known that NK cells, which kill cancer cells by recognizing them as “non-self,” can inhibit the progression of cancer. A recent report showed increased blood levels of MHC class I-related chain A, which is involved in the recognition of cancer cells by NK cells, during the progression of chronic liver disease; this attenuates the NKG2D-mediated cytotoxic activity of NK cells, which could contribute to the development of liver cancer[62,63]. In addition, the presence of the unique liver cells such as sinusoidal endothelial cells and Kupffer cells (macrophages that act as antigen-presenting cells) is known to induce immune tolerance. Thus, hindering these immune escape mechanisms and promoting immune response with stronger antitumor effects could improve the clinical efficiency of cancer therapies.
Recent studies have focused on immune responses in patients who received existing conventional therapies for HCC. Briefly, anti-cancer immune response induced by tumor-specific T-cells have been confirmed in HCC patients treated with RFA and TAE[64-67]. In addition, the frequency of natural killer T-cells (NKT cells), which have anti-tumor effect, has been reported to increase in the peripheral blood after RFA treatment[68]. These results suggest that traditional therapies for HCC are, at least in some cases, involved in immune responses; this opens up the possibility of combining with novel immunotherapy.
In 2001, we identified GPC3, a cancer-specific antigen expressed in some cancer cells, from tens of thousands of genes collected using cDNA microarray against several cancer tissues, several surrounding non-cancer tissues, and various normal tissues[69]. GPC3, a 65-kDa protein comprising 580 amino acids, is a heparan sulfate proteoglycan bound to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor. It is hardly expressed in any normal tissue, except in the embryonic liver, embryonic kidney, placenta, and parts of the kidney tubules. However, it is expressed in tissues of HCC, ovarian clear cell carcinoma, malignant melanoma, lung squamous cell carcinoma, hepatoblastoma, Wilms tumor, and AFP-producing gastric cancer, but not in other cancer tissues. There are two types of GPC3, a membrane-bound type and a secreted type, but it is not clear how these types influence the development and progression of cancer. In HCC, they are known to be involved in neoplastic transformation[70]. The expression of GPC3 in HCC has also been reported to be associated with clinical prognosis[71]. We had evaluated the relationship between GPC3 expression in HCC and long-term prognosis in 33 HCC patients who underwent radical surgery (in preparation). The 5-year survival rates were 42.9% and 83.3% in GPC3-positive (21 cases) and -negative (12 cases) patients, respectively. Thus, GPC3-negative patients had significantly better OS than GPC3-positive patients (log-rank test, P = 0.02). This result was also consistent with that of a previous report[71]. With such high cancer-specificity, GPC3 could be an ideal target for immunotherapy against cancer. Nakano et al[72] also independently identified GPC3 as a cancer-specific antigen almost at the same time as our group. They also developed an antibody therapy targeting GPC3, and are being progressed research using its antibody[72]. In addition, phase I/II clinical trials of GC33, a novel GPC-3 antibody, are ongoing under world scale in patients with advanced HCC (Table 1)[73,74]. We also showed that GPC3-derived peptides could restrict HLA-A24 and HLA-A2, thus inducing peptide-specific CTLs[43,75]. HLA-A24 is present in approximately 60% of the Japanese population, while HLA-A2 is present in 40% of the Japanese population and is also a major haplotype in the Caucasian population[43,75]. Further, we performed clinical trials for a peptide vaccine therapy using HLA-A24- and HLA-A2-restricted GPC3-derived peptides (Table 1)[76]. Here, we present the results of these trials and our attempts to develop novel immunotherapies using GPC-3.
| Immunotherapy | Title | Trial no. | Phase | n | Primary endpoint | Result | Status | Ref. | ||
| GPC3-based immunotherapy | ||||||||||
| GPC3 peptide vaccination | ||||||||||
| HLA-A 24:02–restricted GPC3298–306 peptide vaccine, and HLA-A 02:01–restricted GPC3144-152 peptide vaccine | Phase I trial of a glypican-3-derived peptide vaccine for advanced HCC | UMIN000001395 | I | 33 | The safety and immune ; response to GPC3 vaccination | Well-tolerated. The GPC3 vaccine induced a GPC3-specific CTL response in 90.1% patients (30/33) | Completed | [29] | ||
| HLA-A 24:02–restricted GPC3298–306 peptide vaccine, and HLA-A 02:01–restricted GPC3144-152 peptide vaccine | Immunological efficacy of glypican-3 peptide vaccine in patients with advanced HCC | UMIN000005093 | I | 11 | The frequency of peptide-specific ; CD8+ T-cells in PBMCs and infiltration into the tumor after vaccination | The number of peptide-specific CD8+ T-cells in PBMCs increased in 9 out of 11 cases. In 3 cases, they infiltrated into the tumor after the vaccination | Completed | [78] | ||
| HLA-A 24:02–restricted GPC3298–306 peptide vaccine, and HLA-A 02:01–restricted GPC3144-152 peptide vaccine | Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for HCC patients | UMIN000002614 | II | 35 | The 1- and 2-y recurrence rate | The 1- and 2-yr recurrence rates were 24.4% and 53.7%, respectively | Completed | [80] | ||
| Anti GPC3 antibody | ||||||||||
| GC33 | First-in-man Phase I study of GC33, a novel recombinant; humanized antibody against GPC3, in patients with advanced HCC | NCT00746317 | I | 20 | Tolerability and tumor response | Well-tolerated. The median TTP was 26.0 wk in patients with GPC3-high HCC | Completed | [73] | ||
| GC33 | Japanese phase I study of GC33, a humanized antibody against GPC3 for advanced HCC | Japic CTI-101255 | I | 13 | Determined maximum tolerated dose of GC13 | Well-tolerated for GC33 dose of 20 mg/kg in Japanese patients with HCC | Completed | [74] | ||
| GC33 | - | NCT01507168 | II | 185 | Ongoing | - | ||||
| Anti-GPC3 CAR-T based GC33 | - | NCT02395250 | I | 13 | Ongoing | - | ||||
| Immune checkpoint inhibitor therapy | ||||||||||
| Anti-PD-1 antibody | ||||||||||
| Nivolumab | Nivolumab in patients with advanced HCC: an open-label, noncomparative, phase 1/2 dose escalation and expansion trial | NCT01658878 | I /II | 262 | Safety, tolerability, and clinical efficacy, including ORR, DCR, DOR, and PFS | ORR was 20%. DCR was 64% (CR and PR; 3 and 39 cases). The median DOR and PFS was 9.9 and 4.0 mo, respectively | Completed | [94] | ||
| Nivolumab | - | NCT02576509 | III | 726 | Ongoing | - | ||||
| Pembrolizumab | Pembrolizumab in patients with advanced HCC previously treated with sorafenib: non-randomised, open-label phase 2 trial | NCT02702414 | II | 104 | Clinical efficacy, including ORR, DCR, DOR, and PFS | ORR was 16.3%. DCR was 61.5% (CR and PR; 1 and 16 cases). The median DOR and PFS were 2.1 and 4.8 mo, respectively | Completed | [96] | ||
| Pembrolizumab | - | NCT02702401 | III | 408 | Ongoing | - | ||||
| Pembrolizumab (with Lenvatinib) | - | NCT03006926 | I | 104 | Ongoing | - | ||||
| Anti-CTLA-4 antibody | ||||||||||
| Tremelimumab | A clinical trial of CTLA-4 blockade with tremelimumab in patients with HCC and chronic hepatitis C | NCT01008358 | II | 21 | Clinical efficacy, including ORR, TTP, and OS | PR (n = 3) and SD (n = 10) rate were 17.6% and 58.8, respectively. The median TTP and OS were 6.48 and 8.2 mo, respectively | Completed | [97] | ||
| Tremelimumab (with RFA or TAE) | Tremelimumab in combination with ablation in patients with advanced HCC | NCT01853618 | II | 32 | Clinical efficacy as adjuvant therapy after RFA or TAE | PR rate was 26%. The median TTP and OS were 7.4 and 12.3 mo, respectively | Completed | [101] | ||
| Tremelimumab (with Durvalumab) | - | NCT02519348 | II | 144 | Ongoing | - | ||||
Phase I clinical trial of GPC3 peptide vaccine for advanced HCC: A Phase I clinical trial for a GPC3 peptide vaccine was performed between February 2007 and November 2009 in 33 patients with advanced HCC at the National Cancer Research Center East Hospital (Kashiwa, Japan) (UMIN Clinical Trials Registry: 000001395)[29,77]. The primary endpoint was the safety of the GPC3 vaccine and the immune response against it. No dose-limiting toxicity was observed in any of the enrolled patients, and the GPC3 vaccine showed high tolerability. In addition, IFN-r ELSPOT assay revealed that the GPC3 vaccine induced adequate number of GPC3 peptide-specific CTLs in 30 (90.1%) out of 33 patients. Also, the disease control rate (DCR) was 60.6% after 2 months after administration of vaccine, and the median time to progression (TTP) and OS was 3.4 and 9.0 mo, respectively. We also demonstrated that GPC3 vaccination could induce immunological responses, including a decrease in tumor markers and an increase in GPC3 peptide-specific CTLs in peripheral blood. In addition, we evaluated the immunological changes before and after vaccination using biopsy specimens of the tumor. We observed that more CTLs had infiltrated into tumor in the post-vaccination than the pre-vaccination, which proved that the vaccination caused immunological effects[29,77].
In a subsequent phase I trial, we investigated the extent of CTL infiltration into the PBMCs and tumors in 11 patients with advanced HCC who had undergone GPC3 vaccination and were resistant to sorafenib treatment (UMIN Clinical Trials Registry: 000005093)[78]. We found that the number of GPC3-peptide specific CTLs in the PBMCs increased after GPC3 vaccination in 9 of 11 cases. In addition, tumor biopsy specimens after vaccination were obtained from 3 patients, and we observed infiltration of CTLs into tumors in all of them. These results confirmed that GPC3 vaccination could induce the infiltration of GPC3 peptide-specific CTLs into tumor. Remarkably, we observed a valuable case in which multiple HCC tissues became inflamed and then necrotic, after 2 times injections of the vaccine. This was a promising result implying that the peptide vaccine therapy could potentially obtain not only immunological response, but also sufficient clinical antitumor effect[79]. We also established several GPC3 peptide-specific CTL clones from tumor biopsy specimens collected after the vaccination[78].
Phase II clinical trial of GPC3 peptide vaccine as a recurrence preventive effect in HCC patients after radical treatment: To evaluate the efficiency of our peptide vaccine in preventing recurrence, we performed a single-arm phase II clinical trial for the GPC3 peptide vaccine as an adjuvant therapy; the trial was performed in 35 patients with HCC after radical treatment (UMIN Clinical Trials Registry: 000002614)[80]. We found that the 1 - and 2-year recurrence rates (the primary endpoints in the trial) in the enrolled patients were 24.4% and 53.7%, respectively. This result showed that the GPC3 peptide vaccine could be useful as adjunctive therapy for HCC after radical therapy. Also, we evaluated the long-term survival prognosis in the enrolled patients. The median PFS and OS were 20.4 and 72.8 mo, respectively, while the 5-year survival rate was 70.6% (in preparation). In addition, the two cases showed recurrence, despite increased peptide-specific CTLs in the peripheral blood after the vaccination. In these HCCs, GPC3 was mostly expressed in the primary tumor before the vaccination; however, its expression was almost absent in the recurrent tumor after vaccination[80]. These results indicate that peptide vaccine therapy targeting tumor-associated antigens could eradicate cancer cells expressing the antigen, but might be ineffective against cancer cells that do not express the antigen or those that have lost antigen expression. Therefore, vaccine therapies targeting multiple cancer-related antigens will be key for future research in this field; combination of immunotherapies with other therapies should also be explored.
As mentioned above, although we could show the safety and immunological response of the peptide vaccine, its anti-tumor effect remains limited. Even if an excellent peptide vaccine could sufficiently induce peptide-specific CTLs, the anti-tumor effect would depend critically on the number of peptides presented to HLA class I molecules on the surface of cancer cells. To improve this defect, we developed an intra-tumor peptide injection therapy, where the peptide vaccine is directly administered into tumor tissues[81]. Direct injection into the tumor allows a sufficient number of peptides to be presented to HLA class I molecules on the tumor cells, thus enhancing the killing capacity of peptide-specific CTLs. In addition, the peptide vaccine itself could induce the infiltration of peptide-specific CTLs into the tumor. Indeed, we showed that intra-tumor peptide injection therapy effectively suppressed tumor growth in a tumor-transplanted mouse model. We also showed that the combination of subcutaneous and intra-tumor injection of the peptide vaccine resulted in higher anti-tumor effect than either of the therapy.
We also found that combining the peptide vaccine with anti-PD-1 antibody[82] or anti-CD4 antibody[83] could enhance the induction of peptide-specific CTLs and result in higher antitumor effects than peptide vaccine therapy alone. The use of anti-CD4 antibodies could remove Tregs and weakly CD4-positive macrophages that suppress the positive immune response against cancers. We expect that these approaches would bring about a breakthrough for peptide vaccine therapy in the future.
We had established multiple GPC3 peptide-specific CTL clones from the peripheral blood and cancer tissues of HCC patients vaccinated in the clinical trials[77,78,84]. Several of these CTL clones had high capability for killing cancer cells that express GPC3 peptides. In collaboration with Mizukoshi et al[64], we generated induced pluripotent stem cell-derived T-cells transduced with GPC3-specific T-cell receptors (TCRs), which were derived from the most suitable of our CTL clones. TCR-engineered T-cell therapy has been said to be superior to peptide vaccine therapy in terms of safety and anti-tumor effect. Thus, TCR-engineered T-cell therapy based on GPC3 might be a promising novel therapy for patients with advanced HCC in the future.
It was recently shown that chimeric antigen receptor (CAR)-transduced T-cell therapy was remarkably effective against blood malignant disease, with a clinical response rate of more than 80%. However, its efficacy against solid cancers has not been established. Phase I clinical trials of anti-GPC3 CAR-modified T-cells based on GC33 are currently underway in China for patients with refractory or relapsed GPC3+ HCC[85]. In collaboration with Ishida et al[86], we are also developing a next-generation CAR-transduced T-cell therapy based on a novel GPC3 antibody. To overcome the disadvantages of the conventional methods of this therapy, we combined FITC-conjugated cancer-specific antibodies with CAR-transduced T-cells that react with FITC. This approach allows us to precisely control the cancer-killing ability of CAR-transduced T-cells by adjusting the dose of FITC-conjugated antibodies; this also helps in the survival of CAR-transduced T-cells.
These studies continue to help us strive toward the goal of establishing a practical clinical application for TCR-engineered T-cells and CAR-transduced T-cells for treating solid cancers.
Previous studies have shown that secreted GPC3 is released into the serum in the patients with HCC. Recently, we developed an assay to quantify serum full-length GPC3 in cooperation with a private company. This assay could measure serum GPC3, which could be useful as a biomarker for early diagnosis, prediction of recurrence, and evaluation of the effect of anti-GPC3 therapy against HCC. In fact, we showed that the vaccinated group (n = 9) had better PFS and OS than the non-vaccinated group (n = 12) in GPC3-positive HCC patients who had high GPC concentration in the peripheral blood after radical surgery (log-rank test, P = 0.075, P < 0.01, respectively) (in preparation). This result suggested that serum GPC3 concentration after radical surgery could be a useful biomarker for predicting the clinical effect of GPC3 vaccination.
The use of immune checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 antibodies has attracted attention as a novel cancer therapy that can provide dramatic and long-term anti-tumor effect through an approach that is different to that of conventional therapies. In 1992, Ishida et al[86] found that the expression of PD-1 was elevated when T-cells underwent apoptosis; after then, they clarified that PD-1 is a receptor that negatively regulates the immune response against cancer cells. In addition, blocking the PD-1/PD-L1 pathway has been shown to cause an anti-tumor effect by excluding the tumor-induced immune suppressive system and promoting the immune response against tumors.
In 1995, Krummel et al[87] found that CTLA-4 was essential for self-tolerance in immunity; its deficiency caused serious autoimmune diseases. In addition, blocking the interaction between CTLA-4 and B7-1/B7-2 induced tumor rejection in mice[88]. These immune checkpoint inhibitor therapies could be another promising option for cancer therapy that ensures safe and efficient therapeutic effects against cancers that are resistant to conventional chemotherapy and radiation therapy. In fact, nivolumab, an anti-PD-1 antibody, and ipilimumab, an anti-CTLA-4 antibody, were the first antibodies to be approved for use against advanced malignant melanomas resistant to conventional chemotherapy and radiation therapy. Since then, based on clinical trials on patients with various types of cancer, these therapies have gradually expanded their application for several cancers[89-91]. However, the response rate for these therapies are still low; for malignant melanomas, excluding Hodgkin’s lymphoma, it was near 30%, while for other cancers, it ranges from 10% to 30%. Thus, despite many favorable signs, these therapies still benefit only a limited number of patients. Therefore, there is an urgent need to establish appropriate biomarkers for predicting the clinical response of patients against immune checkpoint inhibitors. Number of mutations in the patient has been considered as biomarkers to predict the clinical efficacy of these immunotherapies. Also, it has been reported that PD-L1 expression levels could be involved in the clinical effect of immunotherapy in patients with non-small cell lung cancer, but these relevance has not been reported in those with HCC. In May 2017, pembrolizumab was approved by the FDA for patients with malignant diseases who showed high microsatellite instability or incomplete mismatch repair; this was the first approval in which genetic abnormalities, and not the type of cancer, was the adaptation condition[92,93]. Future studies should therefore focus on identifying biomarkers with better accuracy for predicting treatment effects, and on establishing treatment-selective algorithms to use them.
In recent years, against HCC patients, clinical trials for immune checkpoint inhibitor therapy alone and in combination with other conventional therapies have been proposed (Table 1). Originally, there are many immune cells in the liver. In addition, chronic inflammation such as liver cirrhosis and viral hepatitis, which could be the host of HCC, could induce immunosuppression against HCC. These two mechanisms would imply that immune checkpoint inhibitors could have a sufficient therapeutic effect in patients with HCC. In the following sections, we introduce clinical trials for immune checkpoint inhibitor therapy in HCC patients. The development of immune checkpoint inhibitor therapies could trigger a major revolution in conventional hepatocarcinotherapy, and future developments in these therapies look promising.
Nivolumab is the first humanized monoclonal IgG4 antibody against human PD-1. It has been approved for use against many types of cancer, including malignant melanoma, non-small cell lung cancer, renal cell cancer, and gastric cancer. A phase I/II clinical trial for the use of nivolumab against HCC was performed between November 2012 and August 2016 in patients with advanced HCC (Checkmate-040)[94]. The results of this trial confirmed the tolerability of nivolumab in HCC patients. In addition, the results showed an objective response rate (ORR) of 20% (n = 42/214) and a DCR of 64% (n = 138/214). The median duration of response and progression-free survival were 9.9 and 4.0 mo respectively, and OS rate at 9 mo was 74%. A subsequent result published in ASCO 2017 revealed that the median OS was 15.6 mo in 145 cases that were co-treated with sorafenib and 28.6 mo in 80 cases not given sorafenib[95]. In addition, nivolumab therapy had a sustained long-term clinical effect in patients who had partial response (PR) and stable disease (SD)[94]. Based on this promising result, the FDA approved nivolumab for patients with HCC who had previously been treated with sorafenib in September 2017. Currently, phase III clinical trials for the safety and clinical efficacy of the combination of nivolumab and sorafenib as a first-line standard therapy for patients with advanced HCC are being performed, and the results are awaited (Checkmate-459).
Pembrolizumab is another anti-PD1 antibody. Recently, phase II clinical trials for pembrolizumab were performed on 104 patients with advanced HCC who had received sorafenib treatment (KEYNOTE-224)[96]. The results showed 16.3% ORR and 61.5% DCR. The median duration of response and RFS were 2.1 and 4.8 mo, respectively, indicating that pembrolizumab was almost as effective as nivolumab. Currently, phase III clinical trials are underway for evaluating the efficacy of pembrolizumab as a secondary treatment after sorafenib (KEY NOTE-240).
CTLA-4 is a receptor on the cell membrane of T-cells that suppresses antigen presentation by dendritic cells. Treatment with anti-CTLA-4 antibodies could enhance the antitumor effect of cancer-specific T-cells by inhibiting CTLA-4 activity. Currently, ipilimumab, an anti-CTLA-4 antibody, has approval for use in patients with malignant melanoma. Recently, a phase II clinical trial was conducted to evaluate the clinical efficacy of tremelimumab, another anti-CTLA-4 antibody, in patients with HCC[97]. After 8 wk of treatment, 3 and 10 patients (among a cohort of 21 HCC patients) showed PR and SD, respectively. Median PFS and OS were 6.5 and 8.2 mo, respectively.
To achieve a sufficiently strong antitumor immune response, a combination therapy using anti-PD-1 and anti-CTLA-4 antibodies is being developed for HCC treatment. This combination therapy has already been shown to have strong antitumor effects in patients with malignant melanoma[98]. The anti-CTLA-4 antibody increases the number of CTLs that infiltrate into tumor by blocking the CTLA-4/B7 pathway. The anti-PD-1 antibody enhances the anti-tumor immune response of cancer-specific CTLs by blocking the PD-1/PD-L1 pathway. The combination of these two mechanisms of immune responses allows this combination therapy to produce an enhanced antitumor effect.
Also, a phase I/II clinical trial for evaluating the efficacy and safety of a combination therapy using tremelimumab (anti-CTLA-4 antibody) and durvalumab (anti-PD-L1 antibody) is underway in 40 patients with advanced HCC. Interim results of this clinical trial announced at ASCO 2017 revealed that the ORR was 25%[99]. In addition, a phase III clinical trial of these combination therapies as first line therapy in the advanced HCC patients is currently ongoing (NCT 03298451).
In recent years, combination therapies of molecular targeted drugs with immune checkpoint inhibitors have attracted attention. This promising approach could achieve additive therapeutic effects of the two drugs and a synergistic effect by improving the tumor-induced immunosuppressive microenvironment[100]. In fact, clinical trials investigating the efficacy of such combination therapies against HCC have already started in Japan and the United States of America (NCT 03006926).
Several therapies combining local regional methods such as RFA and TAE with immunity checkpoint inhibition therapy have also been reported. Clinical trials for an anti-CTLA-4 antibody as an adjuvant therapy after RFA and TAE showed promising anti-tumor effects, with a PR rate of 26%, a median PFS of 7.4 mo, and a median OS of 12.3 mo[101]. Thus, immune checkpoint inhibitor therapy shows great promise as preventive measure for HCC recurrence. This therapy also has the potential for application to a wide range of treatment strategies, from first-line standard therapy to adjuvant/neoadjuvant therapy.
Immunotherapy is now widely considered a landmark therapeutic strategy that could radically alter conventional cancer therapy. Unfortunately, patients who can benefit from it remain limited, even in the highly promising immune checkpoint inhibitor therapy. Therefore, further understanding of therapeutic effect prediction and resistance mechanism for immunotherapy could be necessary. Immunotherapy, including immune checkpoint inhibition, cannot provide any anti-tumor effect unless T-cells interact with the cancer-associated peptide and the MHC molecules, thus recognizing cancer cells. Therefore, there have been many efforts to identify and develop more promising tumor-associated peptides and their epitopes. Peptide vaccine therapy using cancer-specific antigens such as GPC3 could induce peptide-specific CTLs against several cancers. Thus, peptide vaccine therapy could be a revolutionary therapeutic strategy by combining with other therapies.
Today, advances in next-generation sequencing and bioinformatics have made it possible to catalog all genomic mutations in individual patients. It has been reported that patients who accumulate more genetic mutations show stronger immune response and better anti-tumor effects after adoptive immunotherapy using TILs[102-106] and immune checkpoint inhibitor therapy[92,107,108] than those with fewer mutations. We are currently developing individualized vaccine therapy targeting neo-antigens, which are gene mutation antigens, in several solid tumors, including HCC. Clinical trials are already underway for individualized vaccine therapy in Europe, North America, and China in several cancers, and the preliminary results are beginning to be reported[109-113].
The liver, which contains many immune cells, is unique in that it has a developed immune escape mechanism. The novel immunotherapies could exploit this unique characteristic of the liver and will be ideal candidates for treating HCC in the future. In fact, the widespread acceptance and application of immune checkpoint inhibitor therapy could result in a major paradigm shift in HCC therapy. Cancer immunotherapy and its combination with conventional methods are being rapidly developed worldwide. Meanwhile, immunotherapy has the possibility of causing immune-related adverse events different from conventional therapy, and more severe management may be required. Therefore, for optimization of immunotherapy, we believe that it is urgent to product more strict novel algorithms for treatment selection and management of HCC. We believe that further development of novel cancer immunotherapy can have innovative benefits against many patients with HCC who have been suffering.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 3. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 678] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 4. | Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 403] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 5. | Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, McGlynn KA. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9875] [Article Influence: 759.6] [Reference Citation Analysis (5)] |
| 7. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1336] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 8. | Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573-10583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (7)] |
| 9. | Bruix J. Treatment of hepatocellular carcinoma. Hepatology. 1997;25:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 431] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 319] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-9; discussion 799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 591] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 14. | Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1632-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (9)] |
| 15. | Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol Res. 2015;45:59-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Deng T, Zeng L, Chen W. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2016;46:58-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Ulahannan SV, Duffy AG, McNeel TS, Kish JK, Dickie LA, Rahma OE, McGlynn KA, Greten TF, Altekruse SF. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology. 2014;60:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4736] [Article Influence: 263.1] [Reference Citation Analysis (0)] |
| 19. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10523] [Article Influence: 584.6] [Reference Citation Analysis (9)] |
| 20. | Morimoto M, Numata K, Kondo M, Hidaka H, Takada J, Shibuya A, Kobayashi S, Ohkawa S, Okuse C, Morita S, Taguri M, Tanaka K. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol Res. 2011;41:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol Res. 2015;45:E1-E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Ikemoto T, Shimada M, Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res. 2017;47:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 25. | Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, Okita K, Kumada H. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 26. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4106] [Article Influence: 513.3] [Reference Citation Analysis (5)] |
| 27. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, Mathurin P, Fartoux L, Lin DY, Bruix J, Poon RT, Sherman M, Blanc JF, Finn RS, Tak WY, Chao Y, Ezzeddine R, Liu D, Walters I, Park JW. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 28. | Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res. 2013;19:6678-6685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao K, Uesaka K, Furuse J, Kinoshita T, Nakatsura T. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-3696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Kudo M. Systemic Therapy for Hepatocellular Carcinoma: 2017 Update. Oncology. 2017;93 Suppl 1:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Tsuchiya N, Sawada Y, Endo I, Uemura Y, Nakatsura T. Potentiality of immunotherapy against hepatocellular carcinoma. World J Gastroenterol. 2015;21:10314-10326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Nakamoto Y. Promising new strategies for hepatocellular carcinoma. Hepatol Res. 2017;47:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Li S, Yang F, Ren X. Immunotherapy for hepatocellular carcinoma. Drug Discov Ther. 2015;9:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Yutani S, Shirahama T, Muroya D, Matsueda S, Yamaguchi R, Morita M, Shichijo S, Yamada A, Sasada T, Itoh K. Feasibility study of personalized peptide vaccination for hepatocellular carcinoma patients refractory to locoregional therapies. Cancer Sci. 2017;108:1732-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Sun Z, Zhu Y, Xia J, Sawakami T, Kokudo N, Zhang N. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. Biosci Trends. 2016;10:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Kuang M, Peng BG, Lu MD, Liang LJ, Huang JF, He Q, Hua YP, Totsuka S, Liu SQ, Leong KW, Ohno T. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Peng BG, Liang LJ, He Q, Kuang M, Lia JM, Lu MD, Huang JF. Tumor vaccine against recurrence of hepatocellular carcinoma. World J Gastroenterol. 2005;11:700-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 40. | Bricard G, Bouzourene H, Martinet O, Rimoldi D, Halkic N, Gillet M, Chaubert P, Macdonald HR, Romero P, Cerottini JC, Speiser DE. Naturally acquired MAGE-A10- and SSX-2-specific CD8+ T cell responses in patients with hepatocellular carcinoma. J Immunol. 2005;174:1709-1716. [PubMed] |
| 41. | Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59:3134-3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Cicinnati VR, Zhang X, Yu Z, Ferencik S, Schmitz KJ, Dworacki G, Kaczmarek E, Oldhafer K, Frilling A, Baba HA, Schmid KW, Grosse-Wilde H, Broelsch CE, DeLeo AB, Gerken G, Beckebaum S. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer. 2006;119:2851-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T, Matsui M, Torigoe T, Sato N, Baba H, Nishimura Y. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2689-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332-4341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T, Kaneko S. Identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer. 2006;118:1194-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 487] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 47. | Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 784] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 48. | Perea F, Sánchez-Palencia A, Gómez-Morales V, Bernal M, Concha Á, García MM, González-Ramírez AR, Kerick M, Martin J, Garrido F, Ruiz-Cabello F, Aptsiauri N. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget. 2017;9:4120-4133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, Yamamoto E, Kanaseki T, Hasegawa T, Sato N. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int. 2012;62:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Fujiwara K, Higashi T, Nouso K, Nakatsukasa H, Kobayashi Y, Uemura M, Nakamura S, Sato S, Hanafusa T, Yumoto Y, Naito I, Shiratori Y. Decreased expression of B7 costimulatory molecules and major histocompatibility complex class-I in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Kondo Y, Shimosegawa T. Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs. Int J Mol Sci. 2015;16:3307-3322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4675] [Article Influence: 311.7] [Reference Citation Analysis (1)] |
| 53. | Takata Y, Nakamoto Y, Nakada A, Terashima T, Arihara F, Kitahara M, Kakinoki K, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Kaneko S. Frequency of CD45RO+ subset in CD4+CD25(high) regulatory T cells associated with progression of hepatocellular carcinoma. Cancer Lett. 2011;307:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 487] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 55. | Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 56. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 899] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 57. | Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 58. | Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 666] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 59. | Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 60. | Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 61. | Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ, Yang DL. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Kohga K, Takehara T, Tatsumi T, Ohkawa K, Miyagi T, Hiramatsu N, Kanto T, Kasugai T, Katayama K, Kato M, Hayashi N. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci. 2008;99:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Yamashita T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 65. | Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, Burroughs AK, Meyer T, Behboudi S. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, Ishii H, Furuse J, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T, Komori H, Baba H, Fujiwara T, Nakatsura T. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012;40:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Okumura A, Ishikawa T, Maeno T, Sato K, Ayada M, Hotta N, Yamauchi T, Fukuzawa Y, Kakumu S. Changes in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol Res. 2005;32:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 660] [Article Influence: 33.0] [Reference Citation Analysis (17)] |
| 71. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T, Nakatsura T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 72. | Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M, Furugaki K, Kinoshita Y, Ishiguro T, Hamakubo T, Kodama T, Aburatani H, Yamada-Okabe H, Tsuchiya M. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 74. | Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S, Kobayashi S, Morizane C, Suzuki I, Yamamoto S, Furuse J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci. 2014;105:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, Furukawa Y, Ogawa M, Nakamura Y, Nishimura Y. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin Cancer Res. 2004;10:8630-8640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, Nakatsura T. Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci. 2018;109:531-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Yoshikawa T, Nakatsugawa M, Suzuki S, Shirakawa H, Nobuoka D, Sakemura N, Motomura Y, Tanaka Y, Hayashi S, Nakatsura T. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci. 2011;102:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Tsuchiya N, Yoshikawa T, Fujinami N, Saito K, Mizuno S, Sawada Y, Endo I, Nakatsura T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;6:e1346764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 79. | Sawada Y, Yoshikawa T, Fujii S, Mitsunaga S, Nobuoka D, Mizuno S, Takahashi M, Yamauchi C, Endo I, Nakatsura T. Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican-3-derived peptide vaccination: an autopsy case. Hum Vaccin Immunother. 2013;9:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M, Kinoshita T, Ikeda M, Nakachi K, Yamazaki N, Mizuno S, Takayama T, Yamao K, Uesaka K, Furuse J, Endo I, Nakatsura T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 81. | Nobuoka D, Yoshikawa T, Takahashi M, Iwama T, Horie K, Shimomura M, Suzuki S, Sakemura N, Nakatsugawa M, Sadamori H, Yagi T, Fujiwara T, Nakatsura T. Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy. Cancer Immunol Immunother. 2013;62:639-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2015;46:28-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Fujinami N, Yoshikawa T, Sawada Y, Shimomura M, Iwama T, Sugai S, Kitano S, Uemura Y, Nakatsura T. Enhancement of antitumor effect by peptide vaccine therapy in combination with anti-CD4 antibody: Study in a murine model. Biochem Biophys Rep. 2016;5:482-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Tada Y, Yoshikawa T, Shimomura M, Sawada Y, Sakai M, Shirakawa H, Nobuoka D, Nakatsura T. Analysis of cytotoxic T lymphocytes from a patient with hepatocellular carcinoma who showed a clinical response to vaccination with a glypican‑3‑derived peptide. Int J Oncol. 2013;43:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Jiang Z, Jiang X, Chen S, Lai Y, Wei X, Li B, Lin S, Wang S, Wu Q, Liang Q, Liu Q, Peng M, Yu F, Weng J, Du X, Pei D, Liu P, Yao Y, Xue P, Li P. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front Immunol. 2017;7:690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 86. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1899] [Cited by in RCA: 2283] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 87. | Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1519] [Cited by in RCA: 1678] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 88. | Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2904] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 89. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 12026] [Article Influence: 751.6] [Reference Citation Analysis (0)] |
| 90. | Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2132] [Article Influence: 193.8] [Reference Citation Analysis (0)] |
| 91. | Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4026] [Cited by in RCA: 4611] [Article Influence: 419.2] [Reference Citation Analysis (1)] |
| 92. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7491] [Article Influence: 681.0] [Reference Citation Analysis (2)] |
| 93. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 5173] [Article Influence: 574.8] [Reference Citation Analysis (0)] |
| 94. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J. Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3449] [Article Influence: 383.2] [Reference Citation Analysis (2)] |
| 95. | Crocenzi TS, El-Khoueiry AB, Yau TC, Melero I, Sangro B, Kudo M, Hsu C, Trojan J, Kim TY, Choo SP, Meyer T, Kang YK, Yeo W, Chopra A, Baakili A, Cruz CMD, Lang L, Neely J, Welling T. Nivolumab (nivo) in sorafenib (sor)-naïve and –experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol. 2017;35:4013-4013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 96. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1978] [Article Influence: 247.3] [Reference Citation Analysis (0)] |
| 97. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 772] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 98. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6070] [Cited by in RCA: 6373] [Article Influence: 579.4] [Reference Citation Analysis (12)] |
| 99. | Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M, Xiao F, Morris SR, Sangro B. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol. 2017;35:4073-4073. [RCA] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 100. | Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 101. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 661] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 102. | Wölfel T, Hauer M, Schneider J, Serrano M, Wölfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Büschenfelde KH, Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 782] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 103. | Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, Rosenberg SA. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996;183:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 399] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 104. | Mandruzzato S, Brasseur F, Andry G, Boon T, van der Bruggen P. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J Exp Med. 1997;186:785-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 233] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Saeterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A, Gaudernack G. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98:13255-13260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Linard B, Bézieau S, Benlalam H, Labarrière N, Guilloux Y, Diez E, Jotereau F. A ras-mutated peptide targeted by CTL infiltrating a human melanoma lesion. J Immunol. 2002;168:4802-4808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1611] [Cited by in RCA: 1647] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 108. | Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 692] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 109. | Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3692] [Article Influence: 335.6] [Reference Citation Analysis (1)] |
| 110. | Boisguérin V, Castle JC, Loewer M, Diekmann J, Mueller F, Britten CM, Kreiter S, Türeci Ö, Sahin U. Translation of genomics-guided RNA-based personalised cancer vaccines: towards the bedside. Br J Cancer. 2014;111:1469-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Fritsch EF, Hacohen N, Wu CJ. Personal neoantigen cancer vaccines: The momentum builds. Oncoimmunology. 2014;3:e29311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Vonderheide RH, Nathanson KL. Immunotherapy at large: the road to personalized cancer vaccines. Nat Med. 2013;19:1098-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 113. | Rammensee HG, Singh-Jasuja H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev Vaccines. 2013;12:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Niu ZS, Qi XS S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ