Published online Dec 26, 2017. doi: 10.13105/wjma.v5.i6.150
Peer-review started: May 22, 2017
First decision: July 6, 2017
Revised: July 29, 2017
Accepted: September 12, 2017
Article in press: September 12, 2017
Published online: December 26, 2017
Processing time: 218 Days and 15.8 Hours
To outline current evidence regarding prevention and treatment of parastomal hernia and to compare use of synthetic and biologic mesh.
Relevant databases were searched for studies reporting hernia recurrence, wound and mesh infection, other complications, surgical techniques and mortality. Weighted pooled proportions (95%CI) were calculated using StatsDirect. Heterogeneity concerning outcome measures was determined using Cochran’s Q test and was quantified using I2. Random and fixed effects models were used. Meta-analysis was performed with Review Manager software with the statistical significance set at P ≤ 0.05.
Forty-four studies were included: 5 reporting biologic mesh repairs; 21, synthetic mesh repairs; and 18, prophylactic mesh repairs. Most of the studies were retrospective cohorts of low to moderate quality. The hernia recurrence rate was higher after undergoing biologic compared to synthetic mesh repair (24.0% vs 15.1%, P = 0.01). No significant difference was found concerning wound and mesh infection (5.6% vs 2.8%; 0% vs 3.1%). Open and laparoscopic techniques were comparable regarding recurrences and infections. Prophylactic mesh placement reduced the occurrence of a parastomal hernia (OR = 0.20, P < 0.0006) without increasing wound infection [7.8% vs 8.2% (OR = 1.04, P = 0.91)] and without differences between the mesh types.
There is no superiority of biologic over synthetic mesh for parastomal hernia repair. Prophylactic mesh placement during the initial surgery significantly reduces parastomal hernia occurrence regardless of the mesh type.
Core tip: This review and meta-analysis outlines all current evidence regarding prevention and treatment of parastomal hernia and compares the use of synthetic and biologic mesh. There is no superiority of biologic over synthetic mesh for parastomal hernia repair concerning parastomal hernia recurrence, wound infection and mesh infection. Prophylactic mesh placement during the initial surgery significantly reduces parastomal hernia occurrence regardless of the mesh type.
- Citation: Knaapen L, Buyne O, van Goor H, Slater NJ. Synthetic vs biologic mesh for the repair and prevention of parastomal hernia. World J Meta-Anal 2017; 5(6): 150-166
- URL: https://www.wjgnet.com/2308-3840/full/v5/i6/150.htm
- DOI: https://dx.doi.org/10.13105/wjma.v5.i6.150
Parastomal hernia is a common complication of stoma formation during colorectal surgery, with incidences up to 50%. The risk of parastomal hernia is highest within the first few years after formation of the stoma but may develop as much as 20 years later[1]. Hernias are often asymptomatic and managed with conservative treatment. However, 11% to 70% of patients undergo surgery due to discomfort, pain, obstructive symptoms and cosmetic dissatisfaction[2]. These treatment percentages vary because surgeons are often reluctant to repair a parastomal hernia due to the high recurrence rate, complicated operation and co-morbidity of patients. Indeed, a parastomal hernia is regarded as a complex incisional hernia by hernia experts[3]. Hence, many patients suffer but never undergo surgery.
The recurrence rate of parastomal hernia is the lowest after mesh repair (0%-33%), whereas primary fascial closure (46%-100%) and relocation of the stoma (0%-76%) result in much higher rates. Although low recurrence rates are reported after synthetic mesh repair, concerns have been raised regarding the safety of synthetic meshes in (potentially) contaminated fields due to the risk of mesh infection and subsequent removal. Other mesh-related complications include chronic infection, bowel stenosis, erosion of the mesh through the bowel and skin and enteroatmospheric fistulisation. These complications led to the development of biologic mesh, which due to its bio-degradable nature, has the potential to ameliorate these problems in infected and contaminated fields.
The high prevalence of parastomal hernias and the difficulty of repair have led to a shift of focus from repair towards prevention using prophylactic mesh reinforcement at the time of stoma formation. However, prophylactic mesh placement coincides with risk of the same mesh-related morbidities of hernia repair.
There are no trials comparing biologic and synthetic mesh repair for parastomal hernias. Available studies show a large range in reported parastomal hernia recurrence rates and no difference in mesh type concerning hernia recurrence or infection resistance[4-7].
No clear answer can be given as to whether there is a difference between the outcomes of synthetic and biologic mesh repair. However, given the financial costs of biologic mesh, the evidence for superiority and more beneficial outcomes compared to synthetic mesh is mandatory to support its use.
There are various approaches regarding the anatomic position of the mesh during parastomal hernia repair. Meshes are implanted in an inlay, onlay, sublay or underlay (intraperitoneal) position. Laparoscopic repair involves the intraperitoneal technique, and open repair may involve any of the anatomical planes of the mesh. The inlay technique places the mesh within the fascial defect and is sutured to the fascial edges. With onlay repair, the mesh is placed subcutaneously and fixed onto the fascia of the anterior rectus sheath and the aponeurosis of the external oblique abdominal muscle. When using a retromuscular or sublay technique, the prosthesis is placed dorsally to the rectus muscle and anteriorly to the posterior rectus sheath after mobilization of the latter. When performing intraperitoneal repair, the choice can be made between the Sugarbaker and keyhole repair techniques. Regarding the Sugarbaker technique, the hernia defect is closed with intraabdominal placement of the prosthetic mesh securely sutured or tacked to the abdominal wall. Between the abdominal wall and the prosthesis, the bowel is lateralized passing from the hernia sac into the peritoneal cavity[8]. During keyhole mesh repair, a 2-3 cm hole is fashioned in the mesh for passage of the stoma, and the rest of the mesh covers the entirety of the hernia orifice, including sufficient overlap (5 cm beyond the edge of the hernia defect is recommended). Both the keyhole and Sugarbaker techniques can be performed open or laparoscopically[9,10].
The primary aim of the current study was to compare biologic and synthetic mesh use for the treatment and prevention of parastomal hernia by systematic review and meta-analysis of available data in the literature. The secondary aim was to evaluate the different anatomical positions and surgical techniques used for parastomal hernia repair. With the absence of rigorous data focused on hernia recurrence in the literature, this review contributes to the increased understanding of parastomal hernias.
Articles for this review were identified by searching the electronic databases PubMed and Medline (January 1946 to present) and by manual cross-reference searches. The last search was performed on 19-4-2016. The search included the following terms: “Parastomal hernia”, “Parastomal”, “Paracolostomy”, “Paraileostomy”, “Stoma” and “Colostomy” to represent the population. These terms were combined with terms relevant to the outcomes, such as “Ventral hernia”, “Defect”, “Mesh”, “Synthetic mesh”, “Biologic mesh”, “Closure”, “Reconstruction”, “Prosthesis”, “Scaffold”, “Prevention”and “Prophylactic”. The full search strategy is provided in Appendix 1. No limitation to date or language was considered. Randomized and non-randomized studies were included. When multiple studies describing the same population were published, the most complete report was used. The systematic review was performed in accordance with PRISMA[11].
All selected papers were evaluated for methodological quality using the Cochrane risk-of-bias tool for randomized controlled trials and the Newcastle-Ottawa Scale (NOS) for all non-randomized and single group studies[12,13]. Assessment using the Cochrane risk-of-bias tool is based on sequence generation, allocation concealment, blinding of participants, personnel, outcomes assessors, incomplete outcomes data, selective outcomes reporting, and other sources of bias, such as baseline imbalance, early stopping bias, academic bias, and source of funding bias. The NOS is an instrument for assessing methodological quality and potential bias in non-randomized studies. A maximum of nine points were assigned to each study. Studies that scored four for selection, two for comparability, and three for assessment of outcomes were regarded as having a low risk of bias. Studies with two or three stars for selection, one for comparability, and two for outcome were considered as having a medium risk of bias. Any study with a score of one for selection or outcome, or zero for any of the three domains, was deemed as having a high risk of bias. A modification in the NOS was made for single group studies, which consisted of excluding the points for comparability with a maximum of six points: three for selection and three for outcome. After screening titles and abstracts, two reviewers (Knaapen L and Slater NJ) independently reviewed full-text articles for eligibility using the critical appraisal approach. Any disagreement was resolved by consensus with a third reviewer (van Goor).
Studies were identified according to the following inclusion criteria: Participants (human adults, minimum of 18 years of age), intervention (parastomal hernia repair with a synthetic or biologic mesh and prophylactic placement of mesh), and sufficient data available (10 or more patients).
The following criteria were used for exclusion: Stoma relocation, primary suture repair, and unspecified surgical technique. Studies published only as abstracts were excluded because quality assessment could not be performed.
The primary outcome measure was the recurrence rates of parastomal hernia as defined by the respective authors. Secondary outcomes were wound infection, mesh infection, mortality, other complications (medical and surgical), anatomic position of the prosthesis and surgical approach (open or laparoscopic).
All full-text articles that met the inclusion criteria were thoroughly reviewed, and the data for primary and secondary outcomes were extracted and recorded in a data form. Year of publication, study period, level of evidence, mean age, gender, number of patients included and evaluated, type of stoma, surgical technique (open or laparoscopic, anatomical mesh position, keyhole or Sugarbaker), type of mesh (biologic or synthetic) and duration of follow-up were also noted. Weighted pooled proportions with a 95%CI were determined for recurrence, wound infection, mesh infection, other complications and mortality using StatsDirect statistical software[14]. The heterogeneity concerning the outcome measures was determined with Cochran’s Q test and quantified using I2. A random-effects model was used unless heterogeneity was 0%, in which case, a fixed-effects model was used. Meta-analysis was performed using Review Manager[15] with the statistical significance set at P < 0.05.
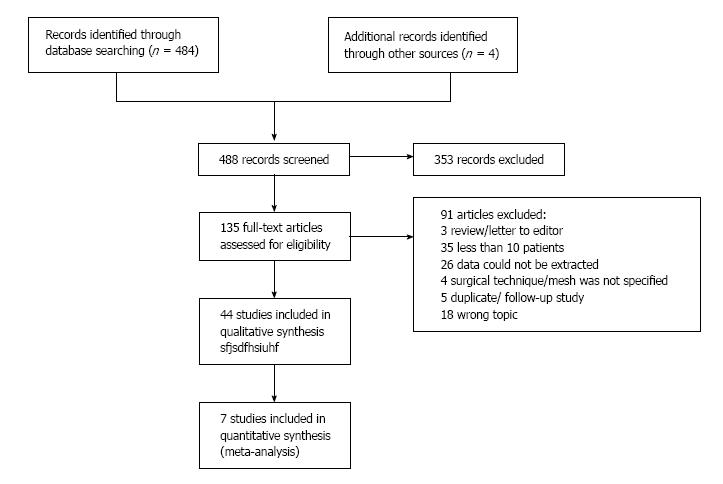
A flowchart overview of the search including reasons for exclusion of studies is shown in Figure 1. A total of 44 studies were included. Five studies provided information on 84 biologic mesh repairs; 21 studies, on 669 synthetic mesh repairs; and 18 studies, on 500 prophylactic mesh placements.
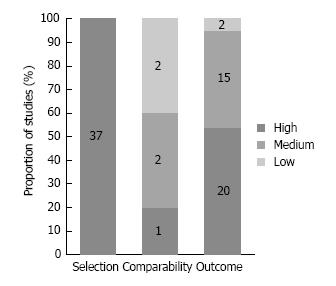
The following were included in the current study: Seven randomized controlled studies (level 1 evidence; all prophylactic mesh repair), 5 non-randomized comparative studies (level 2 evidence) and 32 single-group studies (level 3 evidence). Concerning the risk of bias assessment of seven randomized controlled trials (Figure 2): Sequence generation was unclear in 4 (57%) and low in 3 (43%) studies; allocation concealment was unclear in 1 (14%) and low in 6 (86%) studies; performance bias was high in all 7 (100%) studies; detection bias was low in 3 (43%) and high in 4 (57%) studies; attrition bias was low in all 7 (100%) studies; reporting bias was low in 6 (86%) and high in 1 (14%) study; and other bias was unclear in 2 (29%), low in 3 (43%) and high in 2 (29%) studies.
The Newcastle-Ottawa Scale for quality assessment showed that all 37 non-randomized studies had a low risk of bias for study selection. The five non-randomized two-group studies showed a low risk of bias regarding comparability in 1 study (20%), medium risk in 2 studies (40%), and high risk in 2 studies (40%). The risk of bias for outcome assessment was low in 20 (54%) studies, medium in 15 (41%) studies, and high in two (5%) studies (Figure 3).
Use of funding was not reported in 32 studies (73%). Five studies (11%) reported no funding[2,5,8,16,17]. Industry sponsored 4 biologic mesh studies (9%)[4,18-20]. The manufacturer supplied the mesh material in one biologic and one synthetic mesh study (5%)[21,22]. The state funded one study without financial disclosures reported[23]. Fifty-three percent of patients were female, and the mean age was 64.6 years. The indication for stoma placement was reported in 32 studies: benign disease in 9%, malignant disease in 68%, inflammatory bowel disease or diverticulitis in 19% and other causes in 4%. Patient demographics, study characteristics and critical appraisals are described in Table 1.
| Ref. | Year | Inclusion period | Level of evidence | Mean age, years | Male (%) | Newcastle-Ottawa Scale | Cochrane risk of bias | ||||||||
| Târcoveanu et al[44] | 2014 | 2010-2011 | 1 | NS | NS | ? | ? | - | - | + | - | ? | |||
| Ventham et al[63] | 2012 | 2003-2010 | 2 | I: 69, C: 68 | I: 42%, C: 35% | **** | ** | *** | |||||||
| Hansson et al[8] | 2013 | 2005-2010 | 3 | 63 | 35% | *** | *** | ||||||||
| López-Cano et al[40] | 2012 | 2007-2010 | 1 | I: 72, C: 66 | I: 58%, C: 42% | + | + | - | + | + | + | + | |||
| Hauters et al[16] | 2012 | 2008-2010 | 3 | 69 (median) | 40% | *** | *** | ||||||||
| Fei et al[34] | 2012 | 2008-2010 | 3 | 63 | 45% | *** | *** | ||||||||
| Mizrahi et al[2] | 2012 | 2005-2010 | 3 | 64 | 34% | *** | *** | ||||||||
| Wara et al[5] | 2011 | 1997-2008 | 3 | 62 (median) | 50% | *** | *** | ||||||||
| Janson et al[64] | 2010 | 2003-2007 | 3 | 65 | 40% | *** | ** | ||||||||
| Jänes et al[42] | 2010 | 2003-2006 | 2 | 63 | 66% | **** | ** | ||||||||
| Pastor et al[26] | 2009 | 1999-2006 | 2 | I: 60, C: 54 | I: 42%, C: 54% | **** | * | *** | |||||||
| Lüning et al[65] | 2009 | 1997-2006 | 3 | 65 | 27% | *** | ** | ||||||||
| Serra-Aracil et al[6] | 2009 | 2004-2006 | 1 | I: 68, C: 67 | I: 70%, C: 59% | ? | + | - | + | + | + | - | |||
| Hansson et al[31] | 2009 | 2002-2006 | 3 | 63 | 49% | *** | *** | ||||||||
| Vijayasekar et al[45] | 2008 | 2002-2007 | 3 | 61 | 52% | *** | *** | ||||||||
| Jänes et al[43] | 2009 | 2001-2003 | 1 | I: 70, C: 71 | I: 56%, C: 59% | ? | + | - | - | + | + | - | |||
| Berger et al[35] | 2009 | 2004-2008 | 3 | 69 (median) | NS | *** | *** | ||||||||
| Muysoms et al[27] | 2008 | 2001-2007 | 2 | 70 | 54% | **** | * | *** | |||||||
| Guzmán-Valdivia et al[32] | 2008 | NS | 3 | 67 | 64% | *** | ** | ||||||||
| Berger[39] | 2008 | 2006-2007 | 3 | 72 (median) | 64% | *** | ** | ||||||||
| Craft et al[66] | 2008 | 2004-2006 | 3 | 66 | NS | *** | *** | ||||||||
| Berger et al[7] | 2007 | 1999-2006 | 3 | 70 (median) | 39% | *** | *** | ||||||||
| Mancini et al[29] | 2007 | 2001-2005 | 3 | 60 | 44% | *** | ** | ||||||||
| Marimuthu et al[46] | 2006 | 2002-2005 | 3 | 67 | 44% | *** | ** | ||||||||
| Gögenur et al[22] | 2006 | 2003-2005 | 3 | 71 (median) | 60% | *** | ** | ||||||||
| van Sprundel et al[37] | 2005 | 2000-2003 | 3 | 57 | 31% | *** | *** | ||||||||
| de Ruiter et al[33] | 2005 | 1988-2002 | 3 | NS | NS | *** | *** | ||||||||
| Longman et al[67] | 2005 | 2000-2004 | 3 | NS | NS | *** | ** | ||||||||
| LeBlanc et al[28] | 2005 | NS | 3 | 42-89 | NS | *** | *** | ||||||||
| Stelzner et al[36] | 2004 | 1994-2002 | 3 | 70 (median) | 60% | *** | ** | ||||||||
| Steele et al[30] | 2003 | 1988-2002 | 3 | 64 | 50% | *** | *** | ||||||||
| Hofstetter et al[38] | 1998 | NS | 3 | NS | NS | *** | *** | ||||||||
| Viermaa et al[23] | 2015 | 2010-2013 | 1 | I: 67 | I: 51% | + | + | - | + | + | + | + | |||
| C: 65 | C: 54% | ||||||||||||||
| Asif et al[17] | 2012 | 2004-2011 | 3 | 62 | 60% | *** | ** | ||||||||
| Figel et al[62] | 2012 | 2005-2008 | 3 | 63 | 67% | *** | ** | ||||||||
| Smart et al[4] | 2011 | 2007-2009 | 3 | 72 (median) | 44% | *** | * | ||||||||
| Taner et al[25] | 2009 | 2006-2007 | 3 | NS | 39% | *** | ** | ||||||||
| Hammond et al[68] | 2008 | NS | 1 | I: 43, C: 50 | I: 30%, C: 40% | ? | + | - | - | + | + | ? | |||
| Hammond et al[21] | 2008 | NS | 3 | NS | NS | * | |||||||||
| Aycock et al[18] | 2007 | 2004-2006 | 3 | 56 | 36% | *** | ** | ||||||||
| Araujo et al[24] | 2005 | 3 | 57 | 27% | *** | *** | |||||||||
| Ellis et al[19] | 2010 | 2004-2007 | 3 | 64 | 65% | *** | *** | ||||||||
| Fleshman et al[20] | 2014 | 2010-2012 | 1 | I: 60, C: 59 | I: 55%, C: 50% | + | + | - | + | + | + | - | |||
| Williams et al[41] | 2015 | 2011-? | 2 | I: 49, C: 59 | I: 27%, C: 45% | *** | ** | ||||||||
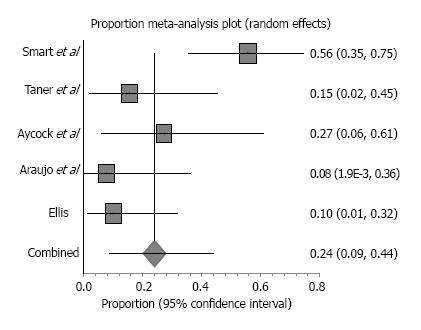
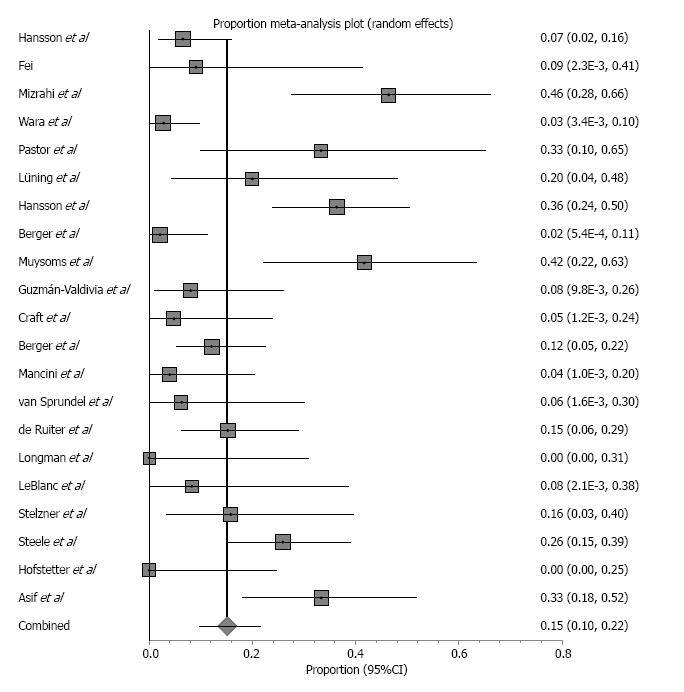
Biological grafts used in the included studies were Surgisis, AlloDerm, Permacol and Peri-Guard (Table 2). Five retrospective studies reported parastomal hernias that were repaired with a biologic mesh and included a combined enrolment of 84 patients. Patient follow-up ranged from 9-50 mo. One case of mortality was reported due to renal failure unrelated to the mesh[4]. Study characteristics and outcomes, including weighted-pooled rates of recurrence and wound-related complications, are shown in Table 3. Five studies reported 23 hernia recurrences with a weighted-pooled proportion of 24% (95%CI: 8.6-44.1) (Figure 4). Only three of these studies reported treatment after recurrence. Araujo et al[24] relocated the stoma and, Ellis et al[19] reported a reoperation using a bioprosthetic not further specified. Taner et al[25] reported two asymptomatic recurrences that were both treated conservatively. There were 4 wound infections that were reported with a weighted-pooled proportion of 5.6% (95%CI: 1.4-12.1)[4,18,25]. One was conservatively treated, one was treated with systemic antibiotics, and two were treated with local wound care[4,18,25]. No mesh infections were reported [0% (95%CI: 0-5.4)]. Other complications [13.4% (95%CI: 1.9-32.7)] were minor complications, including six seroma formations (four treated by drainage and two conservatively treated).
| Name | Material | Coating | Absorbable | Pore size | Weight |
| StomaMesh Surgipro Prolene Central ring enforced polypropylene | Polypropylene | None | No | Small to medium 0.8 mm or large 1.0-3.6 mm | Heavy weight or light weight |
| DUALMESH | Composite multifilament expanded polytetrafluoroethylene | None | No | Very small 3/22 µm | Heavy weight |
| Proceed | Polypropylene Encapsulated in polydioxanone | Oxidized regenerated cellulose | Partially 180 d and 28 d | Large | Light weight |
| Parietex | Composite multifilament Polyester/collagen | Type I collagen, polyethylene glycol, and glycerol layer | Partially 20 d | Large > 3 mm | Medium weight |
| ULTRAPRO | Composite monofilament Polypropylene | Poliglecaprone-25 (monocryl) | Partially 140 d | Large > 3 mm | Light weight |
| VICRYL | Multifilament polyglactin | None | Yes, 60-90 d | Small 0.4 mm | Medium weight |
| Vypro | Polypropylene | PG910 | Partially 42 d | Large > 3 mm | Light weight |
| Composix Parastomal hernia patch | Polypropylene/expanded polytetrafluoroethylene | None | No | Medium 0.8 mm | Light weight |
| DynaMesh | Polypropylene | PVDF | Partially | Large 1-2 mm | Medium weight |
| Surgisis | Porcine small intestine submucosa | None | |||
| AlloDerm | Human acellular dermis | None | |||
| Permacol | Cross-linked acellular porcine collagen | Yes, hexamethylene diisocyanate | |||
| Peri-Guard | Bovine pericardium | Yes; glutaraldehyde | |||
| STRATTICE | Non-crosslinked porcine-derived acellular dermal matrix | None |
| Ref. | No. patients (completed follow-up) | Type of stoma | Material; technique | Recurrence of parastomal hernia1 | Wound infection | Mesh infection | Other3 | Mortality | Follow-up (mo) | ||||
| Mesh | No mesh | Mesh | No mesh | Mesh | No mesh | Mesh | Mesh | No mesh | |||||
| Hansson et al[8] | 61 | - | C: 55 I: 4 U: 2 | L: 55; IPOM: SB; ePTFE | 4 (7) | - | 1 (2) | - | 1 (2) | 21 (34) | - | 12 (2) | 26 |
| Fei et al[34] | 11 | - | C: 6 I: 5 | O: 11 Sublay: K; PP | 1 (9) | - | 0 | - | NS | 3 (27) | - | 0 | 24 |
| Mizrahi et al[2] | 29 (28) | - | C: 18 I: 10 U: 1 | L: 29 IPOM: K; ePTFE | 13 (46) | - | NS | - | 1 (4) | 3 (11) | - | 12 (4) | 28 |
| Wara et al[5] | 72 | - | C: 48 I: 24 | L: 72 IPOM: K; PP+ePTFE | 2 (3) | - | 1 (1) | - | 3 (4) | 20 (28) | - | 22 (3) | 36 |
| Pastor et al[26] | 12 | 13 | C: 10 I: 15 | L: 12 O: 13 IPOM: K 3 SB: 7, lateral slit: 1 e-PTFE | 4 (33) | 7 (54) | 2 (17) | 2 (15) | 0 | 1 (8) | 0 | 0 | 14 |
| Lüning et al[65] | 15 | - | C: 12 I: 3 | O: 16 Onlay PP 7; PE 6; VICRYL 1; CRE-PPM 2 | 3 (20) | - | 0 | - | 1 (7) | 1 (7) | - | NS | 33 |
| Hansson et al[31] | 55 | - | C: 47 I: 5 U: 3 | L 55 IPOM; K ePTFE | 20 (36) | - | 0 | - | 2 (4) | 29 (53) | - | 0 | 36 (median) |
| Berger et al[35] | 47 | - | NS | L: 46 O: 1 Sandwich PVDF-PP | 1 (2) | - | 1 (2) | - | NS | 3 (6) | - | 0 | 20 (median) |
| Muysoms et al[27] | 24 | - | C:20 I: 4 | L: 24 IPOM K:11 non-slit SB 13 Parietex 11; DUALMESH 10; Composix 3 | 10 (42) | - | NS | - | NS | 2 (8) | - | 52 (21) | K: 31 SB: 14 |
| Guzmán-Valdivia et al[32] | 25 | - | C:25 | O: 25; Sublay PP | 2 (8) | - | 2 (8) | - | 0 | 2 (8) | - | 0 | 12 |
| Craft et al[66] | 21 | - | C: 5 I: 7 U: 9 | L: 21; IPOM K: 5 SB: 16 DUALMESH | 1 (5) | - | 1 (5) | - | 2 (10) | 8 (38) | - | 0 | 14 |
| Berger et al[7] | 66 | - | C:58 I:7 U:1 | L: 66; IPOM SB: 41 Sandwich: 25 DUALMESH (until 4-2004) and Polyvinylidene | 8 (12) | - | 1 (2) | - | 2 (3) | 5 (8) | - | 0 | 24 (median) |
| Mancini et al[29] | 25 | - | C: 15 I: 5 U: 6 | L: 25; IPOM SB DUALMESH | 1 (4) | - | 1 (4) | - | 1 (4) | 3 (12) | - | 12 (4) | 19 (median) |
| van Sprundel et al[37] | 16 | - | C: 8 I: 5 U: 4 | O: 16; IPOM K DUALMESH | 1 (6) | - | 0 | - | 0 | 5 (31) | - | 0 | 29 (median) |
| de Ruiter et al[33] | 46 | - | C: 46 | O: 46 Onlay CRE-PPM | 7 (15) | - | 0 | - | 3 (7) | 2 (4) | - | 0 | 51 |
| Longman et al[67] | 10 | - | C: 7 I: 3 | O: 10 Sublay K PP | 0 | - | 0 | - | 0 | 1 (10) | - | 0 | 30 (median) |
| LeBlanc et al[28] | 12 | - | C: 8 I: 2 U: 2 | L: 12 IPOM SB 7, K 5 e-PTFE | 1 (8) | - | 0 | - | 0 | 2 (17) | - | 12 (8) | 20 |
| Stelzner et al[36] | 20 (19) | - | C: 20 | O: 20 IPOM SB e-PTFE | 3 (16) | - | 1 (5) | - | 0 | 3 (16) | - | 0 | 42 |
| Steele et al[30] | 58 | - | C: 31 I: 27 | O: 58 Onlay “Stove pipe hat” PP | 15 (26) | - | 2 (3) | - | 0 | 9 (16) | - | 0 | 51 |
| Hofstetter et al[38] | 13 | - | C: 13 | O: 13 IPOM K e-PTFE | 0 | - | 0 | - | 0 | 0 | - | 0 | NS |
| Asif et al[17] | 33 | C: 12 I: 21 | L: 33 SB:14 K:19 DUALMESH | 11 (33)4 | - | 4 (12) | 0 | 9 (27) | 0 | SB: 7 K: 36 | |||
| Weighted pooled % (95%CI) | 15.1% (9.7-21.6) | 2.8% (1.6-4.4) | 3,1% (1.8-4.6) FE | 17,8% (12.0-24.4) | 1.9 (0.9-3.2) | ||||||||
| Smart et al[4] | 27 | - | C: 20 I:7 | O: 20 Onlay: K; Permacol | 15 (55) | - | 1 (4) | - | 0 | 0 | - | 12 (4) | 17 |
| Taner et al[25] | 13 | - | NS | O: 13 Overlay + Underlay (sandwich) AlloDerm | 2 (15) | - | 1 (8) | - | 0 | 4 (31%) | - | 0 | 10 |
| Aycock et al[18] | 11 | - | C:2 I:9 | O: 11 Inlay 8; Onlay 3; AlloDerm | 3 (27) | - | 2 (18) | - | NS | 1 (9) | - | 0 | 9 |
| Araujo et al[24] | 13 | - | C: 13 | O: 13 Onlay; Peri-Guard | 1 (8) | - | 0 | - | NS | NS | - | 0 | 50 |
| Ellis[19] | 20 | - | C: 17 I: 3 | O: 20 IPOM; SB; Surgisis | 2 (10) | - | 0 | - | 0 | 4 (20) | - | 0 | 18 |
| Weighted pooled % (95%CI) | 24% (8.6-44.1) | 5.6% (1.4-12.1) | 0% (0-5.4) FE | 13.4% (1.9-32.7) | 2.6% (0.3-6.9) FE | ||||||||
Characteristics of the synthetic mesh used in the included studies are given in Table 2. One of the 21 studies was a prospective trial that recruited 12 patients with synthetic mesh repair and 13 control patients without mesh repair. The other 20 studies had a combined enrolment of 669 patients with synthetic mesh repairs[26]. Patient follow-up ranged from 7 to 51 mo. One study did not specify mean or median follow-up. The overall mortality was 1.9% (11 patients, weighted-pooled proportion, 95%CI: 0.9-3.2). None of the deaths were related to the mesh. Four post-operative deaths were due to progressive metastatic disease, two deaths were due to aspiration and subsequent cardiopulmonary arrest, and two deaths were due to secondary cardiopulmonary complications[8,27-29]. Wara et al[5] reported one death due to a neglected bowel injury that resulted in multiorgan failure and another death due to uncontrollable bleeding that resulted from portal hypertension that was unknown prior to surgery. One post-operative death was reported by Mizrahi et al[2] following sepsis that was not further specified and caused by an infected retroperitoneal haematoma, which necessitated a second operation.
Study characteristics and outcomes, including weighted pooled rates of recurrence and wound-related complications, are shown in Table 3. Nineteen studies reported 108 hernia recurrences after mesh repair with a weighted-pooled proportion of 15.1% (95%CI: 9.7-21.6) (Figure 5). From the 19 studies that described hernia recurrence, 10 studies reported treatment. Three studies described 34 reoperations because of symptomatic hernia not further specified[30-32]. Two studies reported 2 patients who required reoperation that involved relocation of their stoma and mesh repairs[27,28]. Van Sprundel et al[33] noted one hernia recurrence due to a wide circle cut in the mesh, and in a second operation, the hernia content was removed, and the circle was narrowed with sutures. Ruiter and co-workers reported 5 patients who had the prosthesis definitively removed (not specified), 1 patient who had a smaller-sized prosthesis implanted and 1 patient who had only the hernia sac closed after midline laparotomy. Muysoms et al[27] noted one patient with a recurrence in whom a second laparoscopy was performed because of obstructive symptoms and was treated with a modified Sugarbaker technique. Another patient needed a laparotomy for a colonic abscess due to Crohn’s disease. After colonic resection and mesh removal, a translocation of the colostomy was performed. Two reoperations for parastomal hernia recurrences were described by Fei et al[34] and Berger et al[35] due to the breakdown of the sutures used for closing and keeping the mesh in place. Berger et al[35] reported three other patients who were treated with the sandwich technique and one with the Sugarbaker technique. All other described hernia recurrences were asymptomatic and treated conservatively.
Surgical wound infection was mentioned in eleven studies reporting 17 patients with a weighted-pooled proportion of 2.8% (95%CI: 1.6-4.4). Four studies reported treatment of wound infection[5,26,29,32]. Two patients were treated by surgical drainage, and five were treated with systemic antibiotics. Pastor et al[26] reported 1 patient with a parastomal abscess and subsequent fistula development repaired by laparotomy, transection of the fistula tract, and re-siting of the ileostomy[26]. Sixteen mesh infections were observed with a weighted-pooled proportion of 3.1% (95%CI: 1.8-4.6), resulting in mesh removal from 14 patients. Other complications [17.8% (95%CI: 12.0-24.4%)] were seroma (31.1%), cardiopulmonary event (8.3%), urinary tract infection (0.8%), cutaneous/fascial dehiscence (0.8%), stoma complications (6.1%), ileus (9.9%), peritonitis (2.3%), post-operative bleeding (3.8%), haematoma (4.5%), bowel stenosis (14.4%), fistula formation (1.5%), renal failure (3%) and other (13.6%). Five of the 41 seromas were treated by surgical drainage, 12 were conservatively treated, and 24 did not have any reported treatment[8,32,34,35].
Comparison of biologic mesh repair and synthetic mesh repair: When comparing the prevalence of hernia recurrence, synthetic mesh repair resulted in a significantly lower rate compared to biologic mesh repair (OR = 1.96; 95%CI: 1.16-3.30; P = 0.01). No significant difference was found concerning wound infection (OR = 1.76; 95%CI: 0.58-5.38; P = 0.32), mesh infection (OR = 0.29; 95%CI: 0.02-4.83; P = 0.39) or other complications (OR = 0.59; 95%CI: 0.29-1.22; P = 0.15) (Table 4).
| Hernia repair | No of studies | No of mesh repairs | Recurrence | Complications | ||
| Wound infection | Mesh infection | Other | ||||
| Biologic mesh | 5 | 84 | 24% (8.6-44.1) | 5.6% (1.4-12.1) | 0% (0-5.4) FE | 13.4% (1.9-32.7) |
| Synthetic mesh | 21 | 669 | 15.1% (9.7-21.6) | 2.8% (1.6-4.4) | 3.1% (1.8-4.6) FE | 17.8% (12.0-24.4) |
| P value | 0.01 | 0.32 | 0.39 | 0.15 | ||
Various mesh positions were applied concerning biologic mesh repair, including inlay, onlay, sublay and underlay (intraperitoneal) placement of the mesh. Two retrospective series reported on 40 cases that involved onlay mesh repairs. Hernias recurred in 31.3% (weighted pooled proportion, 95%CI: 0.9-78.8) of patients. Smart et al[4] placed 16 stomas lateral to the rectus sheath, which showed a high recurrence rate (75%) compared to 11 stomas within the rectus sheath (27%)[4,24]. Ellis et al[19] placed the mesh intraperitoneally using the Sugarbaker technique. Two of 20 (10%) patients had a recurrent hernia after a follow-up of 18 mo. The sandwich technique, which combines the onlay and sublay technique, was reported by Taner et al[25]. After a mean follow-up of 10 mo, two of 13 (15%) patients had a recurrent hernia. One other study reported multiple surgical techniques (including inlay and onlay) and did not allow for stratified outcome extraction[18]. Considering the anatomical position for open synthetic mesh repair, 3 retrospective studies using a series of onlay synthetic mesh repairs, reporting a total of 119 repairs, were included in this study. Hernias recurred in 21.5% (weighted pooled proportion, 95%CI: 14.7-29.3) of patients. In three studies, the mesh was placed in the sublay position, and 3 hernia recurrences with a weighted-pooled proportion of 8.1% (95%CI: 2.1-17.4) were reported.
The mesh was placed intraperitoneally by the open approach in three studies reporting 48 repairs (19 Sugarbaker and 29 keyhole technique repairs)[36-38]. The weighted-pooled proportion of recurrence was 8.8% (95%CI: 1.8-20.2). Seven studies described laparoscopic synthetic mesh repair using the Sugarbaker technique, and the weighted-pooled proportion of hernia recurrence was 10.9% (95%CI: 3.7-21.4). The keyhole technique was used in 8 studies, and hernia recurrence was reported in 35.6% (weighted pooled proportion; 95%CI: 14.6-60.1).
All biologic mesh repairs were via the open approach. Considering the surgical approach used for synthetic mesh repair, 9 studies reported open repairs, 10 studies reported laparoscopic repairs, and 2 studies reported combined open and laparoscopic repairs. Unfortunately, separate data of the different approaches in these last two studies could not be extracted. Within the nine studies that reported 213 open synthetic mesh repairs, hernias recurred in 13.5% (weighted pooled proportion; 95%CI: 8.1-20.2) of patients. Wound infection, mesh infection and other complications were reported in 3% (95%CI: 1.2-5.7), 2.3% (0.7-4.8) and 12.8% (95%CI: 7.4-19.4) of the cases, respectively. Ten studies reported 397 laparoscopic synthetic mesh repairs. The weighted-pooled proportion of hernia recurrence was 18% (95%CI: 8.9-29.5). Wound infection, mesh infection and other complications were reported in 2.4% (95%CI: 0.8-4.8), 3.6% (95%CI: 1.9-5.7) and 23.8% (95%CI: 14.5-34.6) of the cases, respectively.
Comparison of surgical approach: Comparing open vs laparoscopic mesh repair did not result in a significant difference in hernia recurrence (OR = 0.81; 95%CI: 0.51-1.28; P = 0.37), wound infection (OR = 1.17; 95%CI: 0.38-3.62; P = 0.79) or mesh infection (OR = 0.67; 95%CI: 0.21-2.14; P = 0.50). A significantly (OR = 0.39; 95%CI: 0.25-0.63; P ≤ 0.0001) lower occurrence rate of other complications was observed with open repair (Table 5). Regarding laparoscopic synthetic mesh repair, the Sugarbaker technique resulted in a significantly lower recurrence rate of parastomal hernia compared to the keyhole technique (OR = 0.35; 95%CI: 0.21-0.59; P ≤ 0.0001).
| Hernia repair | No. of studies | No. of mesh repairs | Recurrence | Complications | ||
| Wound infection | Mesh infection | Other | ||||
| Open repair | 9 | 213 | 13.5% (8.1-20.2) | 3% (1.2-5.7) FE | 2.3% (0.7-4.8) FE | 12.8% (7.4-19.4) |
| Laparoscopic repair | 10 | 397 | 18% (8.9-29.5) | 2.4% (0.804.8) FE | 3.6% (1.9-5.7) FE | 23.8% (14.5-34.6) |
| P value | 0.37 | 0.79 | 0.5 | ≤ 0.0001 | ||
Eighteen studies reported a total of 500 prophylactic mesh placements, which included 13 studies consisting of 382 patients with synthetic mesh repair and 5 studies consisting of 118 patients with biologic mesh repair. The follow-up ranged from 7-65 mo.
The overall mortality was 2.5% (21 deaths, weighted pooled proportion, 95%CI: 1.3-4.2) None of the deaths were related to the mesh. Two postoperative deaths were due to progressive metastatic disease, one was due to a pulmonary thromboembolism, and two were due to cardiopulmonary complications[22,23,39-41]. Jänes et al[42] reported five deaths due to septic or cardiovascular complications not further specified. Fleshman et al[20] described eleven deaths, none of which were related to the device or treatment not further specified.
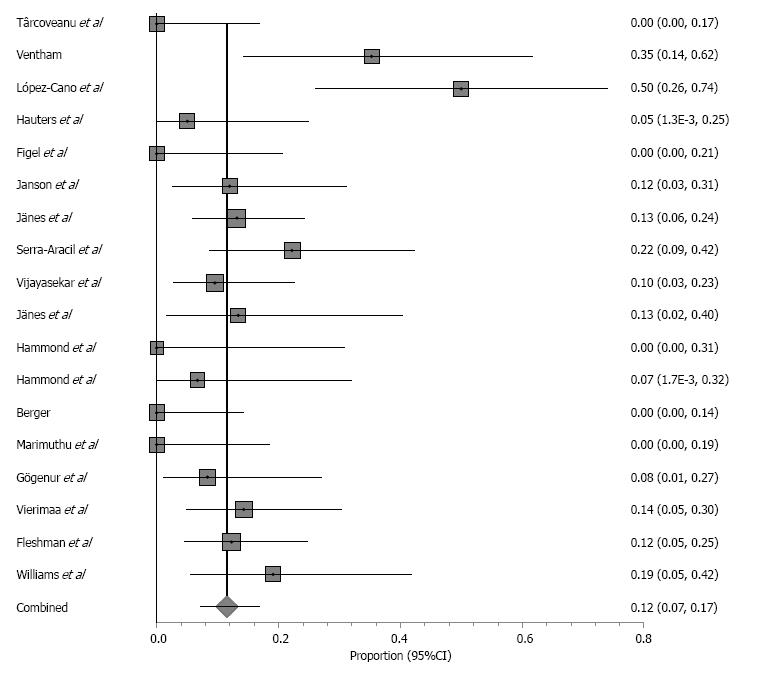
Study characteristics and outcomes, including weighted-pooled rates of hernia occurrence and wound-related complications, are shown in Table 6. When comparing prophylactic placement of biologic mesh with synthetic mesh, there was no significant difference in hernia occurrence (OR = 0.79, 95%CI: 0.40-1.55; P = 0.49) or wound infection (OR = 0.30, 95%CI: 0.07-1.28; P = 0.10). In the mesh group, 58 hernia occurrences were observed with a weighted-pooled proportion of 11.5% (95%CI: 7.1-16.8) (Figure 6) and 31 wound infections with a weighted-pooled proportion of 6.9% (95%CI: 3.6-11.1), and no infections of the prosthesis were reported [0% (95%CI: 0-2.0)].
| Ref. | No. Patients (completed follow-up) | Type of stoma | Material; technique | Parastomal hernia1 | Wound infection | Mesh infection | Other3 | Mor-tality | Follow-up (mo) | ||||
| Mesh | No mesh | Mesh | No mesh | Mesh | No mesh | Mesh | Mesh | No mesh | |||||
| Târcoveanu et al[44] | 20 | 22 | C: 42 | O: 42; Sublay; PP | 0 | 6 (27) | 0 | 2 (9) | 0 | 9 (45) | 11 (50) | 0 | 9 (median) |
| Ventham et al[63] | 17 | 24 | C: 42 | O: 42; Sublay; PP | 6 (35) | 13 (54) | 2 (12%) | 1 (4) | NS | 0 | 0 | 0 | 12 |
| López-Cano et al[40] | 19 (18) | 17 (16) | C: 36 | L: 36; IPOM; SB; Proceed | 9 (50) | 15 (94) | 8 (44) | 3 (19) | 0 | 16 (89) | 5 (31) | 12 (3) | 12 |
| Hauters et al[16] | 20 | - | C: 20 | L: 17 O: 3; IPOM; SB: 20; PCM | 1 (5) | - | 0 | - | 0 | 6 (30) | - | 0 | 24 |
| Figel et al[62] | 16 | - | C: 16 | O: 16; IPOM; SB: 12; K: 4; Surgisis | 0 | - | 0 | - | 0 | NS | - | 0 | 38 (median) |
| Janson et al[64] | 25 | - | C: 25 | L: 25; Sublay; ULTRAPRO | 3 (15) | - | 2 (8) | - | 0 | 1 (4) | - | 0 | 19 |
| Jänes et al[42] | 75 (61) | 18 (12) | C: 79 I: 14 | O: 93; Sublay; ULTRAPRO | 8 (13) | 8 (67) | 6 (8) | 4 (22) | 0 | 0 | 0 | 52 (5) | 15 |
| Serra-Aracil et al[6] | 27 | 27 | C: 54 | O: 54; Sublay; ULTRAPRO | 6 (22) | 12 (44) | 4 (15) | 4 (15) | 0 | 1 (4) | 1 (4) | 0 | 29 |
| Vijayasekar et al[45] | 42 | - | C: 33 I: 9 | O: 42; Sublay; PP | 4 (10) | - | 1 (2) | - | 0 | 1 (2) | - | 0 | 31 |
| Jänes et al[43] | 27 (15) | 27 (21) | C:54 | O: 54; Sublay; Vypro | 2 (13) | 17 (81) | 0 | 0 | 0 | 0 | 0 | 0 | 65 |
| Hammond et al[68] | 10 | 10 | NS | O: 20; Sublay; Permacol | 0 | 3 (30) | 0 | 0 | 0 | 0 | 0 | 0 | 6.5 |
| Hammond et al[21] | 15 | - | NS | O: 15; Onlay: 6; Sublay 9; Permacol | 1 (7) | - | NS | - | NS | NS | - | 0 | 7 (median) |
| Berger[39] | 25 (24) | - | C: 24 I: 1 | L: 6, O: 19; IPOM; K; DynaMesh | 0 | - | 0 | - | 0 | 0 | - | 12 (4) | 11 |
| Marimuthu et al[46] | 18 | - | NS | O: 18; Sublay; Surgipro | 0 | - | 1 (6) | - | 0 | 1 (6) | - | 0 | 16 |
| Gögenur et al[22] | 25 (24) | - | C: 25 | O: 25; Sublay; StomaMesh | 2 (8) | - | 4 (17) | - | 0 | 6 (25) | - | 12 (4) | 12 |
| Vierimaa et al[23] | 42 (35) | 41 (32) | C: 83 | L: 83; IPOM; K; DynaMesh | 5 (14) | 12 (38) | 1 (3) | 2 (6) | NS | 9 (21) | 10 (24) | 12 (1) | 12 |
| Fleshman et al[20] | 55 (49) | 58 (53) | C: I:23/ C:35 I: I:19/ C:36 | O: 113; Sublay; STRATTICE | 6 (12) | 7 (136) | 2 (4) | 3 (6) | 0 | 21 (38) | 30 (52) | 112 (10) | 24 |
| Williams et al[41] | 22 (21) | 11 | C: I:4/ C:7 I: I:11/ C:11 | I: O = 18 L = 4 C: O = 11 SMART Onlay; Permacol | 4 (19) | 8 (73) | NS | NS | 0 | 2 (9) | 0 | 12 (3) | I: 18 C: 9 |
| Weighted pooled %; (95%CI) | 11.5% (7.1-16.8) | 51.5% (33.7-69.1) | 6.90% (3.6-11.1) | 9.30% (4.8-15.1) | 0% (0-2.0) FE | 14.20% (5.5-26.0) | 13.80% (3.0-30.7) | 2.6% (1.3-4.4) | |||||
From the 15 studies reporting hernia occurrence, 9 elaborated on treatment received. Five studies reported 21 reoperations because of a symptomatic hernia not further specified[6,20,23,40,43]. Two studies reported 5 patients who underwent reoperation involving relocation of stoma and mesh repairs[44,45]. All other reported hernia occurrences were asymptomatic and treated conservatively[6,16,22,45]. Six studies reported treatment of a wound infection[6,22,39,42,45,46]. Sixteen patients were treated conservatively, 7 patients were treated by surgical drainage, and 2 patients were treated with systemic antibiotics. Other complications were seroma (7%), cardiopulmonary event (4.7%), urinary tract infection (5.4%), cutaneous/fascial dehiscence (3.9%), stoma necrosis (12.4%), intra-abdominal/pelvic infection (1.6%) stoma-related problems (1.6%), miscellaneous (20.9%) and severe events not further specified (39.5%). All nine reported seromas were treated by surgical drainage[44].
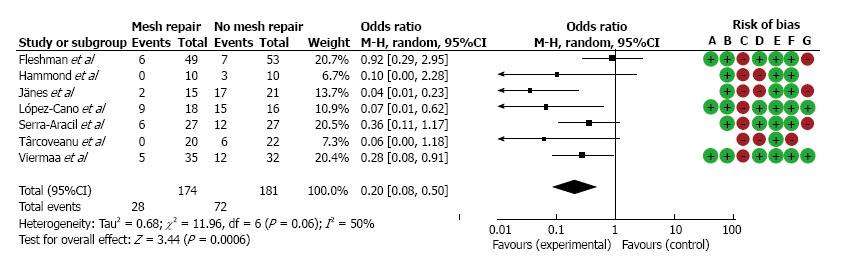
Meta-analysis was performed on the data concerning the incidence of parastomal hernia in the seven randomized controlled trials (Figure 2). Overall, parastomal hernias occurred significantly less in the prophylactic group (weighted-pooled proportion 14.9%; 95%CI: 6.1-26.6) compared to the conventional stoma group (46.8%; 95%CI: 24.7-69.7) (OR = 0.20; 95%CI: 0.08-0.50; P = 0.0006). Concerning the use of prophylactic biologic mesh repair or synthetic mesh repair, there was no significant difference in parastomal hernia occurrence (OR = 0.48; 95%CI: 0.18-1.25; P = 0.13). Additionally, there was no significant difference found between both groups (7.8%; 95%CI: 1.8-17.5 vs 8.2%; 95%CI: 4.2-13.4) regarding wound infection (OR = 1.04 95%CI: 0.53-2.02; P = 0.91 FE).
Anatomic position of the prosthesis: Considering the surgical technique used for prophylactic mesh repair, 12 studies reported open reinforcement, and 3 studies reported laparoscopic reinforcement. Unfortunately, separate data of 2 studies combining open and laparoscopic reinforcement and 1 study combining the onlay and sublay techniques did not allow for stratification of outcomes.
Williams et al[41] used the stapled mesh stoma reinforcement technique (SMART) and reported 21 prophylactic mesh placements and 4 hernia occurrences.
In eleven studies, of which ten reported open and one reported laparoscopic reinforcements, the mesh was placed in the sublay position, and 37 hernia occurrences with a weighted pooled proportion of 11.5% (95%CI: 6.9%-17.1%) were reported. The mesh was placed intraperitoneally in three studies. Figel et al[62] used the open intraperitoneal surgical technique and reported 16 stoma reinforcements without hernia occurrences. Two studies reported the laparoscopic surgical reinforcement technique. Lopez-Cano et al[40] used the Sugarbaker technique and reported 18 mesh placements and 9 (50%) hernia occurences. Vierimaa et al[23] used the keyhole technique and reported 35 mesh placements and 5 (14%) hernia occurrences.
The current study evaluated and compared all the evidence regarding the use of biologic and synthetic mesh for repair and prevention of parastomal hernia. Interestingly, the results of comparing biologic and synthetic mesh repairs showed a comparable or even superior result regarding parastomal hernia recurrence (24% vs 15.1%) and wound infection (5.6% vs 2.8%) in favour of the synthetic mesh repair. Overall, the mesh infection rate was low. Only sixteen mesh infections were reported in 753 repairs (2.1%), which resulted in fourteen mesh removals (all synthetic meshes). However, these observations should be interpreted cautiously because of the low to moderate quality of the studies.
Biologic mesh has gained widespread popularity in the context of infection and a contaminated environment because of their proposed advantages, including bio-compatibility resulting in rapid vascularization and migration of host (immune) cells. It is thought that biologic prostheses are therefore less susceptible to infection than their synthetic counterparts. The ventral hernia working group regards parastomal hernia repair as potentially contaminated (grade 3) and therefore recommends biologic mesh repair[47]. Many authors believe that synthetic mesh should not be used in a contaminated environment or in close proximity to the bowel and stoma due to the risk of erosion and fistula formation. However, studies with high-level evidence are lacking, and the exact origins of these concerns are difficult to identify, are mostly anecdotal or reference old reports using inferior materials and techniques[48-50]. Primus and Harris criticized the surgical literature on the use of biologics in contaminated fields, arguing that cumulative data do not support the claim that biologics are indicated for use in contaminated fields. The primary literature varies widely in terms of sample size, diagnosis of (recurrent) PSH, methods of mesh placement, follow-up period, reported hernia recurrences and surgical site infection[51]. Rosen et al[52] reported a critical review of the surgical literature on biologic mesh repair, which revealed that the majority of the studies evaluating the outcomes of biologic mesh are actually reporting the repair of clean defects. This finding is very surprising given the high costs of biologic mesh, whereas the position of synthetic mesh in “clean”hernia repair has been proven. Despite the lack of high-grade evidence, biologic meshes are still preferred above synthetic mesh in contaminated fields as noted by Bondre et al[53], who conducted a multicentre study about practice patterns in contaminated ventral hernia repair. This review shows a comparable to superior result of synthetic mesh over biologic mesh concerning parastomal hernia recurrence. This finding is confirmed by Lee et al[54] in a systematic review on ventral hernia mesh repair in contaminated fields. Mesh removal due to infection is a much-feared complication. The literature suggest that biologic mesh does not prevent infection but can be more easily salvaged when infection arises[55]. This review challenges the concept that contaminated hernias should be repaired with expensive biologic mesh. Only sixteen mesh infections were seen in this current review, resulting in mesh removal from 14 patients. Concerning parastomal hernia repair, surgeons should carefully balance the risks and costs with the benefits when deciding on the choice of mesh for parastomal hernia repair.
Similar to ventral hernia repair, the prosthesis is placed in either the inlay, onlay, sublay, or underlay (intraperitoneal) position during parastomal hernia repair. None of the included studies used an inlay placement of the prosthesis. Onlay mesh repair showed the highest recurrence rate, whereas the sublay technique showed the lowest in the current study. There was no difference in wound and mesh infection rates between the various anatomic positions. However, firm conclusions cannot be drawn based on this subanalysis because these results were obtained from small groups. Each method of mesh repair has its own theoretical advantages and disadvantages. Laparotomy is avoided with the onlay technique, but it requires extensive dissection of subcutaneous tissue, which predisposes patients for haematoma and seroma formation. Disruption of skin vascularization may lead to impaired wound healing. Additionally, intra-abdominal pressure may lead to lateral detachment of the prosthesis, resulting in the higher recurrence rates. The sublay mesh technique protects the mesh from bacterial contamination while minimizing contact with the bowel because the mesh is enveloped in well-vascularized tissue, whereas the fascia and peritoneum form a natural barrier between prosthesis and abdominal organs. This technique reduces the risk of infection, adhesion or fistulation. The anatomic positions of the sublay and intraperitoneal mesh technique are more attractive because of the benefits from intra-abdominal pressures, which help to keep the mesh in place.
Concerning laparoscopic vs open parastomal hernia repair, this review shows similar results regarding hernia recurrence (18% vs 13.5%; P = 0.37), wound infection (2.4% vs 3%; P = 0.79) and mesh infection (3.6% vs 2.3%; P = 0.50). However, a significantly lower rate of other complications was seen with open repair (23.8% vs 12.8%; P < 0.0001), which was mostly due to the high occurrence of seroma formation in three laparoscopic repair studies[5,8,31] .
When performing laparoscopic intraperitoneal repair there was a significantly lower recurrence rate of parastomal hernia using the Sugarbaker technique compared to the keyhole technique (10.9% vs 35.6%, OR = 0.35; 95%CI: 0.21-0.59; P ≤ 0.0001). Remarkably, it appears that all failures using the keyhole technique were related to the use of an e-PTFE-mesh. As noted by Hansson et al[9], using the keyhole technique estimation of the size of the hole is difficult as mesh shrinkage may result in enlargement of the central hole and reherniation.
Unfortunately, the recurrence rate is still up to one third after mesh repair of parastomal hernias. Our systematic review with meta-analysis shows that prevention of parastomal hernia by the use of mesh at the time of stoma formation reduces the incidence of parastomal hernia significantly compared to the conventional stoma group (14.9% vs 46.8% OR = 0.20; 95%CI: 0.08-0.50; P ≤ 0.0006). Interestingly, placement of preventive mesh did not result in increased wound infection or mesh infection. Recently published reviews also confirm our conclusion that prophylactic insertion of a mesh when forming a stoma prevents parastomal hernia without increasing the incidence of wound infections or other mesh-related complications[56,57].
One point of discussion remains whether universal reinforcement is expedient and cost-effective. Other non-mesh prophylactic measures can be considered, such as lateral rectus abdominis positioned or extraperitoneal positioned stomas[58,59]. Most patients who develop a parastomal hernia are asymptomatic. However, complications due to an untreated parastomal hernia (incarceration, obstruction, strangulation) can be severe and are associated with significant morbidity and mortality. Identification of patients in whom reinforcement is beneficial is essential as the patient can avoid unnecessary longer operative time, costs and possible long-term complications associated with mesh placement. As noted by Hotouras et al[60], risk factors for parastomal hernia formation include abdominal obesity, increasing age, corticosteroid use, poor nutritional status, increased intra-abdominal pressure, connective tissue disorders and other disorders that predispose patients to wound infection such as diabetes. Factors that need to be considered include the reason for the stoma (temporary or permanent stoma), patient co-morbidity, chance of reoperations and risk factors concerning parastomal hernia formation. Patients undergoing stoma formation with short life expectancies will often not survive long enough to develop a parastomal hernia, and patients who are healthy enough to undergo stoma reversal before hernia occurrence would not benefit from prophylactic mesh placement.
Median direct costs for complex ventral hernia repairs with biologic mesh ($16970) is more than twice the amount compared to repairs with synthetic mesh ($7590)[61]. Parastomal hernia repair probably costs less due to the need for smaller meshes; however, a substantial cost difference is expected to remain. Figel et al[62] calculated that by using a bio-prosthetic and considering a 30% incidence of surgical management of parastomal hernia repair, it would be cost-effective if the prosthesis cost less than $4312. The decision to place prophylactic mesh after stoma formation must be patient tailored and may certainly be justified in selected patients. However, standard application in all patients does not seem warranted. More randomized controlled trials with adequate power for risk stratification and subsequent costs of usage of biologic and synthetic mesh are needed.
Most of the studies that were included are retrospective cohorts (level 3 evidence), which could introduce selection and information bias and are affected by heterogeneity. Most study populations were diverse with different types of stomas and indications for the initial surgery. The high recurrence rate regarding biologic parastomal mesh repairs was mostly determined by one study: A 75% recurrence rate of 16 stoma repairs lateral to the rectus sheath compared to a 27% rate when the repair was within the rectus sheath. As noted by Smart et al[4], parastomal hernia repairs where the stoma is lateral to the rectus sheath had a significantly higher risk of recurrence and suggested that this higher risk was likely due to the inherent strength of the tissue onto which the onlay mesh was sutured.
Unfortunately, reporting was insufficient to allow proper stratification for individual risk factors for parastomal hernia. Follow-up time and diagnostic modalities used for determining recurrence rates had a strong impact on the outcome. The longer the follow-up period was, the more recurrences were found. In addition, the diagnostic modalities differ in terms of sensitivity and specificity. Some recurrences found may be of no clinical relevance. Reported follow-up periods within and between studies varied from 7 mo to 51 mo. As recurrence occurs mostly in the first years after operation a minimum follow up of 12 mo seems appropriate.
Definitions of parastomal hernia, wound infection and mesh infection were ill-defined in most studies, and the modality of determining hernia recurrence (e.g., clinical evaluation or CT imaging) was often not clearly stated. Therefore, the results of this review should be interpreted with care.
In an effort to reduce the effect of low quality studies, we excluded the high risk of bias randomized controlled trials for the prophylactic mesh meta-analysis. Only three studies considered of sufficient methodological quality remained, and a second meta-analysis was performed[20,23,40]. No significant difference was found in the occurrence of parastomal hernia when comparing the prophylactic group to the conventional group (OR = 0.33; 95%CI: 0.09-1.20; P = 0.09).
However, provided the large amount of parastomal hernia repairs included in the current report, meaningful conclusions may be drawn regarding optimal surgical management of synthetic and biologic mesh repair in parastomal hernia recurrence.
The current evidence suggests there is no superiority of (more expensive) biologic mesh over synthetic mesh for parastomal hernia repairs after parastomal hernia recurrence, wound infection and mesh infection. In the context of cost-effective healthcare, careful consideration must be taken in choosing the types of materials to use[55]. Sublay seemed to be the most advantageous anatomic position of the mesh, as this position resulted in the lowest recurrence and protects the mesh from bacterial contamination while minimizing contact with the bowel. No difference was found for parastomal hernia recurrence between open or laparoscopic parastomal hernia repairs. When performing laparoscopic repair, the keyhole technique should be abandoned in favour of the Sugarbaker technique when using an ePTFE-mesh because of much higher recurrence rates. As shown by Wara et al[5], the keyhole technique can be considered when using a polypropylene-based mesh or with open parastomal keyhole hernia repairs.
Prophylactic mesh placement at the initial surgery significantly reduced parastomal hernia occurrence on the mid-long term without increasing wound infection or mesh infection. However, it has yet to become clear what the long-term results will be. The number of recurrences will increase over time, though at a slower pace than in the first few years after mesh placement. The same applies to some specific long-term side effects such as mesh infection and mesh-related fistulas. Although their incidence may be low, their impact is disproportionately high.
Identification of patients in whom reinforcement is mandatory is essential, as the patient can avoid unnecessary longer operative time, costs and possible long-term complications associated with mesh placement.
Altogether there is still not enough evidence to recommend the use a biologic mesh over synthetic mesh under contaminated conditions in general and specifically not for parastomal hernia repair. Prophylactic mesh reinforcement during stoma formation significantly reduces parastomal hernia occurrence regardless of mesh type. Yet, a significant number of patients will develop asymptomatic parastomal hernia and there are no data on long term effects of preventive mesh placement. Therefore, it is essential to select the right patient for whom prophylactic reinforcement is mandatory.
Parastomal hernia develops in 50% of patients. Hernias are often asymptomatic and managed with conservative treatments; however, 11% to 70% of patients undergo surgery due to discomfort, pain, obstructive symptoms and cosmetic dissatisfaction. Although standard care is mesh repair, prevention by prophylactic mesh placement is gaining popularity. The use of biologic mesh is becoming more popular as it claims less infections with sustained durability of the repair compared to synthetic mesh. The primary aim of the current study was to compare biologic and synthetic mesh use for the treatment and prevention of parastomal hernia by systematic review and meta-analysis of available data in the literature. The secondary aim was to evaluate different anatomical positions and surgical techniques concerning parastomal hernia repair.
The recurrence rate of parastomal hernia is the lowest after mesh repair (0%-33%), whereas primary fascial closure (46%-100%) and relocation of the stoma (0%-76%) result in much higher rates. Although low recurrence rates are reported after synthetic mesh repair, concerns have been raised regarding the safety of synthetic meshes in (potentially) contaminated fields due to the risk of mesh infection and subsequent removal.
Biologic mesh was first introduced in the 1980s and was developed with the concept that due to its bio-degradable nature, it has the potential to ameliorate problems in infected and contaminated fields. No clear answer can be given as to whether there is a difference in the clinical outcomes between synthetic and biologic mesh repairs. The high prevalence of parastomal hernia and difficulty of repair have led to a shift of focus from repair towards prevention using prophylactic mesh reinforcement at the time of stoma formation.
This review and meta-analysis suggests there is no superiority of biologic over synthetic mesh for parastomal hernia repair after parastomal hernia recurrence, wound infection and mesh infection. Prophylactic mesh reinforcement during stoma formation significantly reduces parastomal hernia occurrence regardless of the mesh type. Identification of patients for whom reinforcement is mandatory is essential, and mesh reinforcement should be reserved for selected patients.
Ostomy formation requires the creation of a full-thickness defect within the abdominal wall. Parastomal hernia is a type of incisional hernia that allows protrusion of abdominal contents through an abdominal wall defect that is created. Both synthetic mesh and biologic mesh (acellular collagen matrix) are used in parastomal hernia repair. There are various approaches regarding the anatomic position of the mesh during parastomal hernia repair. Meshes can be implanted in an inlay (between the fascia), onlay (over the fascia), sublay (below the anterior fascia and muscular level but above peritoneum) or underlay (intraperitoneal) position. Laparoscopic repair involves the intraperitoneal technique, and open repair may involve any of the anatomical planes of the mesh. When performing intraperitoneal repair, the choice can be made between the Sugarbaker and keyhole repair technique.
In this systematic review, the authors have presented a thorough and critical analysis of biologic and synthetic mesh use for the treatment and prevention of parastomal hernias. With a focus on hernia recurrence in the absence of rigorous data in the literature, the current review contributes to the increased understanding of parastomal hernias.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kroese LF, Wara P S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg. 2003;90:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 425] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Mizrahi H, Bhattacharya P, Parker MC. Laparoscopic slit mesh repair of parastomal hernia using a designated mesh: long-term results. Surg Endosc. 2012;26:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Slater NJ, Montgomery A, Berrevoet F, Carbonell AM, Chang A, Franklin M, Kercher KW, Lammers BJ, Parra-Davilla E, Roll S. Criteria for definition of a complex abdominal wall hernia. Hernia. 2014;18:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Smart NJ, Velineni R, Khan D, Daniels IR. Parastomal hernia repair outcomes in relation to stoma site with diisocyanate cross-linked acellular porcine dermal collagen mesh. Hernia. 2011;15:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Wara P, Andersen LM. Long-term follow-up of laparoscopic repair of parastomal hernia using a bilayer mesh with a slit. Surg Endosc. 2011;25:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, Darnell A, Mora-Lopez L, Alcantara-Moral M, Ayguavives-Garnica I, Navarro-Soto S. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg. 2009;249:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Berger D, Bientzle M. Laparoscopic repair of parastomal hernias: a single surgeon’s experience in 66 patients. Dis Colon Rectum. 2007;50:1668-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Hansson BM, Morales-Conde S, Mussack T, Valdes J, Muysoms FE, Bleichrodt RP. The laparoscopic modified Sugarbaker technique is safe and has a low recurrence rate: a multicenter cohort study. Surg Endosc. 2013;27:494-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Hansson BM, Slater NJ, van der Velden AS, Groenewoud HM, Buyne OR, de Hingh IH, Bleichrodt RP. Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg. 2012;255:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Sugarbaker PH. Prosthetic mesh repair of large hernias at the site of colonic stomas. Surg Gynecol Obstet. 1980;150:576-578. [PubMed] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] |
| 12. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The New-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, United Kingdom: Oxford, 2000: 3-5. |
| 13. | The Cochrane Collaboration's tool for assessing risk of bias - Cochrane Handbook for Systematic Reviews of Interventions. United Kingdom: The Cochrane Collaboration, 2011. |
| 14. | StatsDirect statistical software. England: StatsDirect Ltd., 2013. |
| 15. | Copenhagen: The Nordic Cochrane Centre TCC. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre 2014; . |
| 16. | Hauters P, Cardin JL, Lepere M, Valverde A, Cossa JP, Auvray S. Prevention of parastomal hernia by intraperitoneal onlay mesh reinforcement at the time of stoma formation. Hernia. 2012;16:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Asif A, Ruiz M, Yetasook A, Denham W, Linn J, Carbray J, Ujiki MB. Laparoscopic modified Sugarbaker technique results in superior recurrence rate. Surg Endosc. 2012;26:3430-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Aycock J, Fichera A, Colwell JC, Song DH. Parastomal hernia repair with acellular dermal matrix. J Wound Ostomy Continence Nurs. 2007;34:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Ellis CN. Short-term outcomes with the use of bioprosthetics for the management of parastomal hernias. Dis Colon Rectum. 2010;53:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Fleshman JW, Beck DE, Hyman N, Wexner SD, Bauer J, George V; PRISM Study Group. A prospective, multicenter, randomized, controlled study of non-cross-linked porcine acellular dermal matrix fascial sublay for parastomal reinforcement in patients undergoing surgery for permanent abdominal wall ostomies. Dis Colon Rectum. 2014;57:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Hammond TM, Chin-Aleong J, Navsaria H, Williams NS. Human in vivo cellular response to a cross-linked acellular collagen implant. Br J Surg. 2008;95:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Gögenur I, Mortensen J, Harvald T, Rosenberg J, Fischer A. Prevention of parastomal hernia by placement of a polypropylene mesh at the primary operation. Dis Colon Rectum. 2006;49:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Vierimaa M, Klintrup K, Biancari F, Victorzon M, Carpelan-Holmström M, Kössi J, Kellokumpu I, Rauvala E, Ohtonen P, Mäkelä J. Prospective, Randomized Study on the Use of a Prosthetic Mesh for Prevention of Parastomal Hernia of Permanent Colostomy. Dis Colon Rectum. 2015;58:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Araujo SE, Habr-Gama A, Teixeira MG, Caravatto PP, Kiss DR, Gama-Rodrigues J. Role of biological mesh in surgical treatment of paracolostomy hernias. Clinics (Sao Paulo). 2005;60:271-276. [PubMed] [DOI] [Full Text] |
| 25. | Taner T, Cima RR, Larson DW, Dozois EJ, Pemberton JH, Wolff BG. The use of human acellular dermal matrix for parastomal hernia repair in patients with inflammatory bowel disease: a novel technique to repair fascial defects. Dis Colon Rectum. 2009;52:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Pastor DM, Pauli EM, Koltun WA, Haluck RS, Shope TR, Poritz LS. Parastomal hernia repair: a single center experience. JSLS. 2009;13:170-175. [PubMed] |
| 27. | Muysoms EE, Hauters PJ, Van Nieuwenhove Y, Huten N, Claeys DA. Laparoscopic repair of parastomal hernias: a multi-centre retrospective review and shift in technique. Acta Chir Belg. 2008;108:400-404. [PubMed] |
| 28. | LeBlanc KA, Bellanger DE, Whitaker JM, Hausmann MG. Laparoscopic parastomal hernia repair. Hernia. 2005;9:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Mancini GJ, McClusky DA 3rd, Khaitan L, Goldenberg EA, Heniford BT, Novitsky YW, Park AE, Kavic S, LeBlanc KA, Elieson MJ, Voeller GR, Ramshaw BJ. Laparoscopic parastomal hernia repair using a nonslit mesh technique. Surg Endosc. 2007;21:1487-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Steele SR, Lee P, Martin MJ, Mullenix PS, Sullivan ES. Is parastomal hernia repair with polypropylene mesh safe? Am J Surg. 2003;185:436-440. [PubMed] |
| 31. | Hansson BM, Bleichrodt RP, de Hingh IH. Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surg Endosc. 2009;23:1456-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Guzmán-Valdivia G, Guerrero TS, Laurrabaquio HV. Parastomal hernia-repair using mesh and an open technique. World J Surg. 2008;32:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | de Ruiter P, Bijnen AB. Ring-reinforced prosthesis for paracolostomy hernia. Dig Surg. 2005;22:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Fei Y. A modified sublay-keyhole technique for in situ parastomal hernia repair. Surg Today. 2012;42:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Berger D, Bientzle M. Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! A prospective, observational study with 344 patients. Hernia. 2009;13:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Stelzner S, Hellmich G, Ludwig K. Repair of paracolostomy hernias with a prosthetic mesh in the intraperitoneal onlay position: modified Sugarbaker technique. Dis Colon Rectum. 2004;47:185-191. [PubMed] |
| 37. | van Sprundel TC, Gerritsen van der Hoop A. Modified technique for parastomal hernia repair in patients with intractable stoma-care problems. Colorectal Dis. 2005;7:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Hofstetter WL, Vukasin P, Ortega AE, Anthone G, Beart RW Jr. New technique for mesh repair of paracolostomy hernias. Dis Colon Rectum. 1998;41:1054-1055. [PubMed] |
| 39. | Berger D. Prevention of parastomal hernias by prophylactic use of a specially designed intraperitoneal onlay mesh (Dynamesh IPST). Hernia. 2008;12:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | López-Cano M, Lozoya-Trujillo R, Quiroga S, Sánchez JL, Vallribera F, Martí M, Jiménez LM, Armengol-Carrasco M, Espín E. Use of a prosthetic mesh to prevent parastomal hernia during laparoscopic abdominoperineal resection: a randomized controlled trial. Hernia. 2012;16:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Williams NS, Hotouras A, Bhan C, Murphy J, Chan CL. A case-controlled pilot study assessing the safety and efficacy of the Stapled Mesh stomA Reinforcement Technique (SMART) in reducing the incidence of parastomal herniation. Hernia. 2015;19:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Jänes A, Cengiz Y, Israelsson LA. Experiences with a prophylactic mesh in 93 consecutive ostomies. World J Surg. 2010;34:1637-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Jänes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study. World J Surg. 2009;33:118-121; discussion 122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 44. | Târcoveanu E, Vasilescu A, Cotea E, Vlad N, Palaghia M, Dănilă N, Variu M. Parastomal hernias -- clinical study of therapeutic strategies. Chirurgia (Bucur). 2014;109:179-184. [PubMed] |
| 45. | Vijayasekar C, Marimuthu K, Jadhav V, Mathew G. Parastomal hernia: Is prevention better than cure? Use of preperitoneal polypropylene mesh at the time of stoma formation. Tech Coloproctol. 2008;12:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Marimuthu K, Vijayasekar C, Ghosh D, Mathew G. Prevention of parastomal hernia using preperitoneal mesh: a prospective observational study. Colorectal Dis. 2006;8:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Ventral Hernia Working Group. Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 747] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 48. | Houck JP, Rypins EB, Sarfeh IJ, Juler GL, Shimoda KJ. Repair of incisional hernia. Surg Gynecol Obstet. 1989;169:397-399. [PubMed] |
| 49. | Jones JW, Jurkovich GJ. Polypropylene mesh closure of infected abdominal wounds. Am Surg. 1989;55:73-76. [PubMed] |
| 50. | Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr. Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg. 1981;194:219-223. [PubMed] |
| 51. | Primus FE, Harris HW. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia. 2013;17:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Rosen MJ. Biologic mesh for abdominal wall reconstruction: a critical appraisal. Am Surg. 2010;76:1-6. [PubMed] |
| 53. | Bondre IL, Holihan JL, Askenasy EP, Greenberg JA, Keith JN, Martindale RG, Roth JS, Liang MK; Ventral Hernia Outcomes Collaborative. Suture, synthetic, or biologic in contaminated ventral hernia repair. J Surg Res. 2016;200:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Lee L, Mata J, Landry T, Khwaja KA, Vassiliou MC, Fried GM, Feldman LS. A systematic review of synthetic and biologic materials for abdominal wall reinforcement in contaminated fields. Surg Endosc. 2014;28:2531-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Slater NJ, Hansson BM, Buyne OR, Hendriks T, Bleichrodt RP. Repair of parastomal hernias with biologic grafts: a systematic review. J Gastrointest Surg. 2011;15:1252-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Cross AJ, Buchwald PL, Frizelle FA, Eglinton TW. Meta-analysis of prophylactic mesh to prevent parastomal hernia. Br J Surg. 2017;104:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | López-Cano M, Brandsma HT, Bury K, Hansson B, Kyle-Leinhase I, Alamino JG, Muysoms F. Prophylactic mesh to prevent parastomal hernia after end colostomy: a meta-analysis and trial sequential analysis. Hernia. 2017;21:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Kroese LF, de Smet GH, Jeekel J, Kleinrensink GJ, Lange JF. Systematic Review and Meta-Analysis of Extraperitoneal Versus Transperitoneal Colostomy for Preventing Parastomal Hernia. Dis Colon Rectum. 2016;59:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Stephenson BM, Evans MD, Hilton J, McKain ES, Williams GL. Minimal anatomical disruption in stoma formation: the lateral rectus abdominis positioned stoma (LRAPS). Colorectal Dis. 2010;12:1049-1052. [PubMed] [DOI] [Full Text] |
| 60. | Hotouras A, Murphy J, Thaha M, Chan CL. The persistent challenge of parastomal herniation: a review of the literature and future developments. Colorectal Dis. 2013;15:e202-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Reynolds D, Davenport DL, Korosec RL, Roth JS. Financial implications of ventral hernia repair: a hospital cost analysis. J Gastrointest Surg. 2013;17:159-166; discussion 166-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 62. | Figel NA, Rostas JW, Ellis CN. Outcomes using a bioprosthetic mesh at the time of permanent stoma creation in preventing a parastomal hernia: a value analysis. Am J Surg. 2012;203:323-326; discussion 326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Ventham NT, Brady RR, Stewart RG, Ward BM, Graham C, Yalamarthi S, Jones M, Daniel T. Prophylactic mesh placement of permanent stomas at index operation for colorectal cancer. Ann R Coll Surg Engl. 2012;94:569-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Janson AR, Jänes A, Israelsson LA. Laparoscopic stoma formation with a prophylactic prosthetic mesh. Hernia. 2010;14:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Lüning TH, Spillenaar-Bilgen EJ. Parastomal hernia: complications of extra-peritoneal onlay mesh placement. Hernia. 2009;13:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Craft RO, Huguet KL, McLemore EC, Harold KL. Laparoscopic parastomal hernia repair. Hernia. 2008;12:137-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Longman RJ, Thomson WH. Mesh repair of parastomal hernias--a safety modification. Colorectal Dis. 2005;7:292-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Hammond TM, Huang A, Prosser K, Frye JN, Williams NS. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia. 2008;12:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |