Published online Dec 26, 2015. doi: 10.13105/wjma.v3.i6.295
Peer-review started: May 15, 2015
First decision: June 19, 2015
Revised: August 27, 2015
Accepted: November 30, 2015
Article in press: December 3, 2015
Published online: December 26, 2015
Processing time: 224 Days and 10 Hours
AIM: To determine whether combined transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) improve overall and recurrence-free survival (RFS) compared with RFA alone.
METHODS: We reviewed randomized clinical trials (RCTs) comparing overall survival rate as well as recurrence-free rate for hepatocellular carcinoma (HCC) between TACE-RFA therapy and RFA alone published before April 2015 by conducting a systematic review and meta-analysis. Eligible studies were identified by searching PubMed and EMBASE up to April 2015. Additional studies were retrieved via China Medical Collections, Google Scholar or a hand review of the reference lists of the retrieved articles. The summarized relative risks (RRs) with their 95%CIs were estimated using random-effects model. I2 statistic was calculated to measure the heterogeneity of RRs across studies and Cochran’s Q test was used to test the statistical significance accordingly. Publication bias was assessed primarily based on visual assessment using a funnel plot, and secondly by using Egger’s regression asymmetry test or Begg’s rank correlation test as appropriate. Meta-regression was implemented to examine potential effect modifiers.
RESULTS: Nine single-center RCTs conducted in China and Japan were included, with a total of 618 patients with HCC; 321 of whom (51.9%) received TACE/RFA therapy and 297 received RFA alone. The pooled RRs with corresponding CIs comparing combined TACE/RFA to RFA alone were 1.12 (1.004-1.26) and 1.20 (1.02-1.41) for 1-year and 3-year survival rates, respectively. Similar positive associations were found for 1-year (1.19; 1.05-1.35) and 3-year (1.44; 1.00-2.07) RFS. The beneficial effect was more evident in patients with medium-sized (3-5 cm) tumors and among the Chinese population.
CONCLUSION: Combined TACE/RFA has a beneficial effect on survival and recurrence rates compared with RFA alone, especially for medium-sized HCC and among Chinese patients.
Core tip: A systematic review and meta-analysis were conducted to determine whether combined transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) improve overall and recurrence-free survival compared with RFA alone. Nine single-center randomized controlled trials were included, with a total of 618 patients with hepatocellular carcinoma (HCC), 321 of whom (51.9%) received TACE/RFA and 297 received RFA alone. We found that combined TACE/RFA has a beneficial effect on survival and recurrence rates compared with RFA alone, especially for medium-sized (3-5 cm) HCC and among Chinese patients.
- Citation: Hu MZ, Li SF. Radiofrequency ablation with or without transarterial chemoembolization for hepatocellular carcinoma: A systematic review and meta-analysis. World J Meta-Anal 2015; 3(6): 295-303
- URL: https://www.wjgnet.com/2308-3840/full/v3/i6/295.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i6.295
Hepatocellular carcinoma (HCC) is the fifth most common cancer, and one of the leading causes of cancer-related deaths throughout the world. More than 700000 incident cases are diagnosed and > 600000 deaths are attributed to HCC each year[1]. Surgical resection is the first-line therapeutic option for HCC patients with small solitary nodules without underlying cirrhosis[2-4]; however, its role in treating HCC is limited by strict inclusion criteria. Consequently, various nonsurgical, liver-directed, locoregional therapies, such as radiofrequency ablation (RFA)[5], one of the therapies for HCC, and transcatheter arterial chemoembolization (TACE)[6], which involves administration of chemotherapy directly to the liver tumor via a catheter, have been developed as alternatives, particularly for patients with nonresectable HCC. Although local therapies may have less invasiveness, shorter hospital stay and lower associated mortality, their higher recurrence rates and lower disease-free survival rates are still major concerns. In the past decade, some evidence[7,8] has suggested that combining RFA with TACE improves overall survival (OS) rate and reduces recurrence rate, while other studies have not shown these[9-15].
One recent review found that combination of RFA with TACE increased 1-year survival rate by 114% and 5-year survival rate by 170%[16], which might have been overestimates of the true effect, due to the combination of retrospective cohort studies and randomized clinical trials (RCTs). Three other meta-analyses used odds ratio (OR) as a measure of effect size[17-19], which always exaggerates any “effect”, especially when the survival rate in the control group is very high[20], in addition to its hard interpretability. Of note, RFA is effective in the treatment of small but not surgically resectable HCC (< 3 cm in diameter)[21], so combining RFA with TACE for treating small HCCs may not improve the efficacy compared with using RFA alone. It would be important for clinical practice to establish whether combination therapy improves the survival rate of patients with medium-sized (3-5 cm in diameter) or even large (> 5 cm in diameter) lesions. None of the previous studies has answered this question, and none has discussed whether combined therapy improves the recurrence-free survival (RFS) rate.
Therefore, in this study we investigated whether combined TACE/RFA improved OS and RFS rates, compared with RFA alone, especially for patients with medium-sized (3-5 cm) or large (> 5 cm) HCCs, by conducting a systematic review as well as meta-analysis of RCTs.
This meta-analysis was based on a pre-specified protocol - the PRISMA Statement[22]. PubMed and Embase were systematically searched up to April 2015, with the following terms: “Carcinoma, Hepatocellular”, “Liver Tumor”, “Liver Cell Carcinoma”, “Radiofrequency Ablation”, “Transcatheter Arterial Chemoembolization” and “Clinical Trials”. Studies published in Chinese were searched in Wanfang China Medical Collections (1990 to April 2015) using the corresponding Chinese terms. The references of the retrieved articles were also reviewed. In addition, Google scholar was used to give confirmation of the literature search.
The titles and abstracts of all relevant studies were scanned independently by two authors (Hu MZ and Li SF). Reviews, case reports and letters to the editor were excluded. Studies were included if they met the following criteria: (1) RCTs that involved liver cancer patients; (2) patients were treated with RFA alone or combined with TACE; and (3) OS and/or RFS rate was reported in each group, or these data could be derived from the presented results.
Two authors (Hu MZ and Li SF) independently reviewed the literature and extracted the following data from the included studies: last name of the first author; publication year; country where the study was conducted; number of participants; number of male patients; age; Child-Pugh class; tumor size and number; follow-up time; and 1-, 3- and/or 5-year OS or RFS rates after surgery. If the required data were not available in the primary article, we contacted the authors and requested de novo data. Disagreement on data extraction was resolved by group discussion.
The estimate of the principal effect was defined as the relative risk (RR) of OS rate or RFS rate, comparing the patients that were assigned to combined TACE/RFA therapy to those who received RFA alone. RR > 1 indicates that the combined therapy can benefit the survival/recurrence-free rate. I2 statistic was calculated and used to define low, moderate, and high degrees of heterogeneity with 50% and 75% as the cutoffs. Cochran’s Q test was used to test the statistical heterogeneity of RRs across studies, using 0.10 as the significance level. The summarized RRs with their 95%CIs were estimated using a random-effects model since high heterogeneity was shown in most of the pooled analyses[23].
Potential publication bias was assessed primarily based on visual assessment using a funnel plot and secondarily using Egger’s regression asymmetry test (when the number of studies pooled was ≥ 3) or Begg’s rank correlation test (when the number of studies pooled = 2). Meta-regression was implemented to examine potential effect modifiers that may have affected the observed effects, including tumor size (< 3, 3-5 and > 5 cm) or study location (China or Japan)[24]. Sensitivity analyses were also conducted to evaluate the influence of each study included in the meta-analysis by omitting one study at each time, and statistical model selection in which we repeated the analyses with I2 < 50% and P > 0.10 using a fixed-effects model.
The meta-analysis was performed with STATA statistical software version 13.0 (Stata Corporation, College Station, TX, United States). All statistical tests were two-sided and P≤ 0.05 was considered statistically significant, unless otherwise specified.
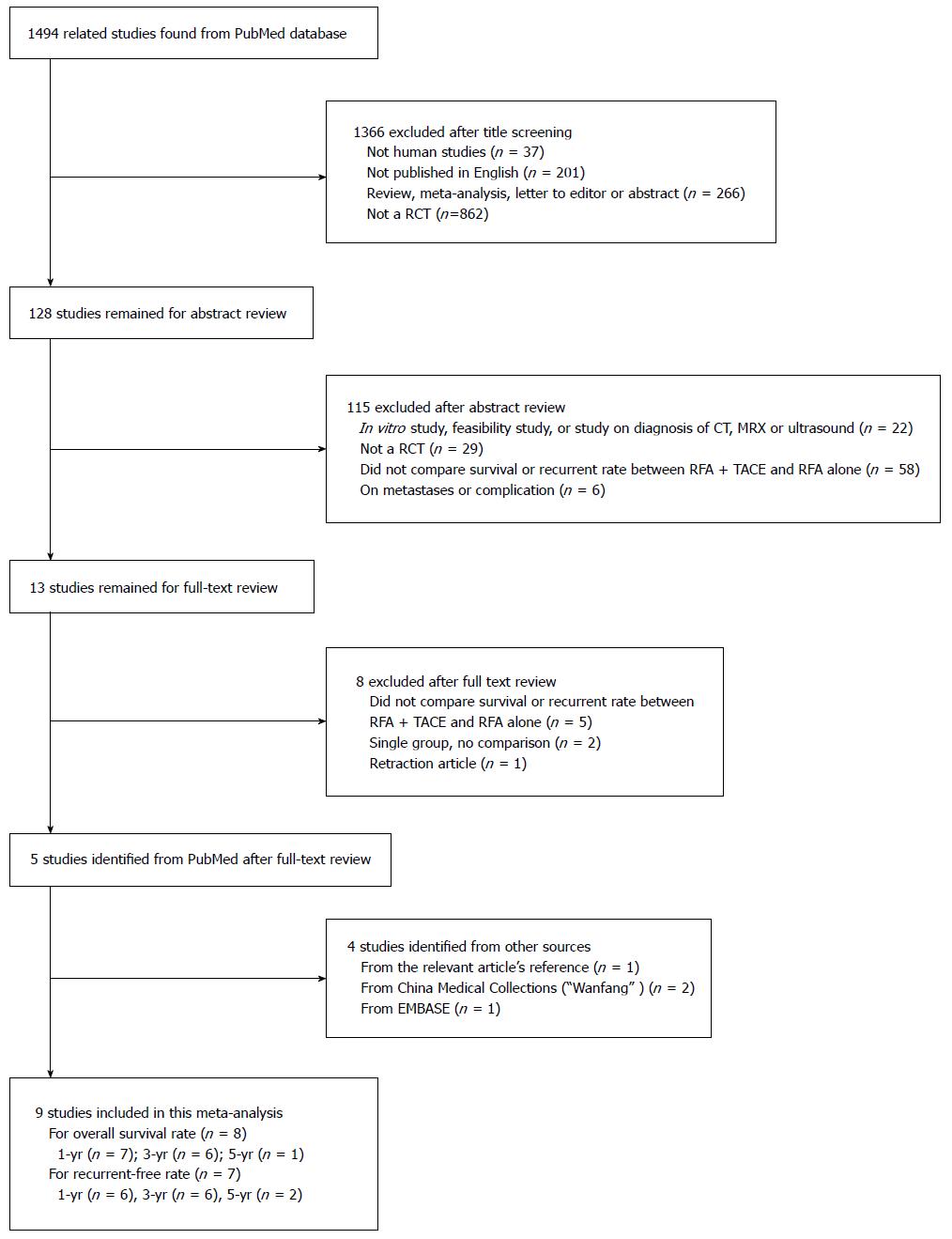
Our literature search resulted in an initial set of 1494 publications from the PubMed database. Of these, 1366 were excluded at the title screening due to at least one of the following reasons: (1) not human studies (n = 37); (2) not original studies, for example, reviews, meta-analyses, letters to editors, or abstracts (n = 467); or (3) not clinical trials (n = 862). Among the remaining 128 studies, 115 were excluded after abstract review because: (1) they were in vitro studies, feasibility studies, or studies of diagnosis with computed tomography, magnetic resonance imaging or ultrasound (n = 22); (2) they were not RCTs (n = 29); (3) they did not compare survival or recurrence rates between RFA plus TACE and RFA alone (n = 58); or (4) they focused on metastases or complications (n = 6). Among the remaining 13 studies, the following were excluded after full-text review: five that did not compare efficacy; two that did not have a control; and one that was retracted by the journal. Four additional studies were found in other resources: one from the references of the relevant articles; two from China Medical Collections (“Wanfang” in Chinese); and one from Embase (Figure 1). Finally, a total of nine RCTs were included in the meta-analysis[7-15].
The main features of the trials included in the meta-analysis are shown in Table 1. Of the nine included studies, seven were published in English[7,8,10,12-15] and two in Chinese[9,11]. All the studies were conducted in Asia: six in China[7-11,13], and three in Japan[12,14,15]. The nine RCTs included 618 HCC patients; Three hundred and twenty-one of whom (51.9%) received combined TACE/RFA therapy and 297 RFA alone. The average follow-up time was 47.1 mo (range: 24-60 mo) among the nine studies.
| Ref. | Arms | No. of patients | Male gender | Age (yr) | Child–Pugh Class (A/B/C) | Tumor size, cm | No. of tumors (1 vs ≥ 2) | Follow-up (mo) | OS rate (%) | RFS rate, % | ||||
| 1-yr | 3-yr | 5-yr | 1-yr | 3-yr | 5-yr | |||||||||
| Zhang et al[9] | RFA + TACE | 15 | 12 | 57.8 ± 11.0 | -- | 4.6 ± 1.3 | -- | 24 | 100 | NA | NA | 92.3 | NA | NA |
| (39-72) | (2.3-7.1) | |||||||||||||
| RFA | 15 | 13 | 58.3 ± 12.7 | -- | 4.2 ± 1.1 | -- | 24 | 80 | NA | NA | -- | NA | NA | |
| (38-78) | (2.4-6.0) | |||||||||||||
| Shen et al[10] | IRFAPA | 18 | 5 | 52.7 | 4/14/0 | 5.6 | 0/18 | 28.3 | 87.5 | 52.2 | NA | 63.9 | 50.0 | NA |
| (20-72) | (2.2-15.8) | (6-38) | ||||||||||||
| RFA | 16 | 3 | 56.1 | 6/10/0 | 5.0 | 3/13 | 19.3 | 73.3 | 20.4 | NA | 30.0 | 18.7 | NA | |
| (36-75) | (2.3-12.3) | (5-36) | ||||||||||||
| Kang et al[11] | RRA + TACE | 19 | 14 | 52.2 | 12/7/0 | 6.7 ± 1.1 | -- | 36 | 84.2 | 36.8 | NA | -- | -- | NA |
| RFA | 18 | 14 | 50.7 | 12/6/0 | 6.2 ± 1.2 | -- | 36 | 61.1 | 16.7 | NA | -- | -- | NA | |
| Kobayashi et al[12] | RFA + AO | 10 | 7 | 67 | 4/5/1 | 1.7 | -- | 48 | -- | -- | NA | 87.5 | 25 | NA |
| (50-76) | (1.0-2.4) | |||||||||||||
| RFA | 10 | 8 | 63 | 3/5/2 | 2.3 | -- | 48 | -- | -- | NA | 70.0 | 20 | NA | |
| (51-75) | (1.0-2.6) | |||||||||||||
| Yang et al[13] | RFA + TACE | 31 | 23 | 60.3 ± 10.9 | -- | 6.5 ± 0.8 | -- | -- | 81.2 | -- | -- | -- | 82.2 | -- |
| RFA | 12 | 8 | 61.0 ± 10.4 | -- | 5.2 ± 0.4 | -- | -- | 57.6 | -- | -- | -- | 65.3 | -- | |
| Shibata et al[14] | RFA + TACE | 46 | 31 | 67.2 ± 8.9 | 32/14/0 | 1.7 ± 0.6 | 43/3 | 60 | 100 | 84.8 | -- | 71.3 | 48.8 | -- |
| (45-83) | (0.9-3.0) | |||||||||||||
| RFA | 43 | 33 | 69.8 ± 8.0 | 33/10/0 | 1.6 ± 0.5 | 42/1 | 60 | 100 | 84.5 | -- | 74.3 | 29.7 | -- | |
| (44-87) | (0.8-2.6) | |||||||||||||
| Morimoto et al[15] | RFA + TACE | 19 | 15 | 70 | 18/1/0 | 3.7 ± 0.6 | -- | 30 (12-46) | 100 | 93 | NA | 67 | -- | 9 |
| (57-78) | ||||||||||||||
| RFA | 18 | 12 | 73 | 16/2/0 | 3.6 ± 0.7 | -- | 32 (15-46) | 89 | 80 | NA | 56 | -- | 28 | |
| (48-84) | ||||||||||||||
| Peng et al[8] | RFA + TACE | 69 | 59 | 57.5 ± 10.0 | 60/9/0 | 2.1 ± 0.5 | 65/4 | 39.2 | 94 | 69 | 46 | 80 | 45 | 40 |
| (19-75) | (0.8-5.0) | (5.0-95.0) | ||||||||||||
| RFA | 70 | 55 | 55.1 ± 9.5 | 59/11/0 | 2.1 ± 0.4 | 65/5 | 33.6 | 82 | 47 | 36 | 64 | 18 | 18 | |
| (22-75) | (0.9-5.0) | (2.0-87.0) | ||||||||||||
| Peng et al[7] | RFA + TACE | 94 | 75 | 53.3 ± 11.0 | 90/4/0 | 3.5 ± 1.4 | 62/32 | 60 | 92.6 | 66.6 | -- | 79.4 | 66.7 | -- |
| RFA | 95 | 71 | 55.3 ± 13.3 | 90/5/0 | 3.4 ± 1.4 | 67/28 | 60 | 85.3 | 59.0 | -- | 60.6 | 44.2 | -- | |
All the studies were single-center trials. The majority of patients (74.1%) were male, ranging from 23.5%[10] to 84.8%[11]. There was no difference in the average proportion of male patients between the two groups (75.1% for the TACE/RFA group vs 73.1% for the RFQ alone group). The average age was 60.0 (range: 50.7-3.0) years, with no difference between the two groups (59.8 years for the TACE/RFA group vs 60.3 years for the RFA alone group), either.
According to the available data, the distribution of Child-Pugh class A, B and C for liver function was 80.6%, 18.9% and 0.5%, respectively. There was no significant difference in this distribution (80.0%/19.6%/0.4% vs 81.1%/18.2%/0.7%) across two comparison groups. About 23.0% of patients had more than two tumors (25.1% for the TACE/RFA group vs 21.0% for the RFA alone group). Patients in three studies each had average tumor size > 5 cm, 3-5 cm, and < 3 cm. The average tumor size among the nine studies was similar between the two groups: approximately 4.0 cm in the combined TACE/RFA group and 3.7 cm in the RFA alone group.
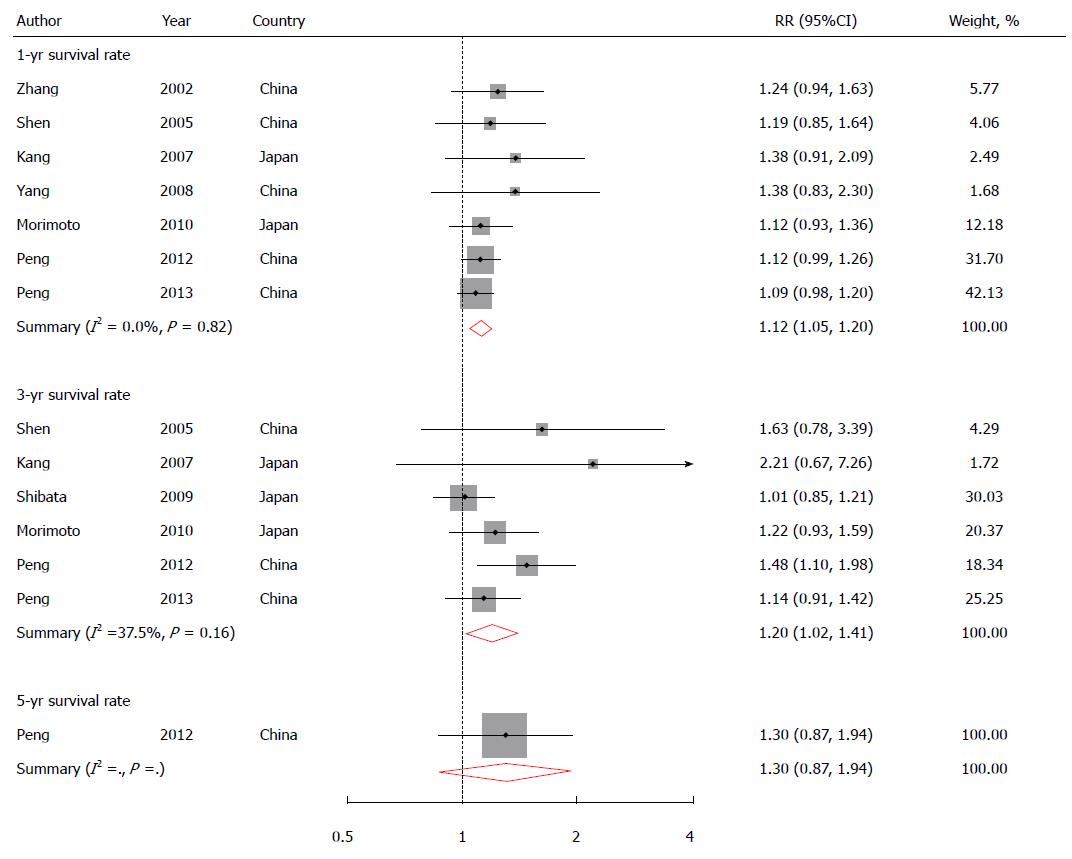
Eight studies reported 1-year OS rate[7-11,13-15]; six presented 3-year OS rate[7,8,10,11,14,15]; and one had 5-year OS rate[8]. The pooled RR (95% CI) for 1-year survival rate comparing combined TACE/RFA to RFA alone was 1.12 (1.004-1.26), with high heterogeneity among eight studies (I2 = 74.6% and P < 0.01) (Figure 2), which could be explained by the fact that one study reported 1-year survival rate of 100% for both groups[14]. After excluding this study, the heterogeneity disappeared among the other seven studies (I2 = 0.0% and P = 0.82). A similar positive association (RR = 1.20; 95%CI: 1.02-1.41) was found for 3-year survival rate without heterogeneity (I2 = 37.5% and P = 0.16). No evidence on publication bias was found (Egger’s test: P = 0.40 for 1-year survival rate; and P = 0.09 for 3-year survival rate).
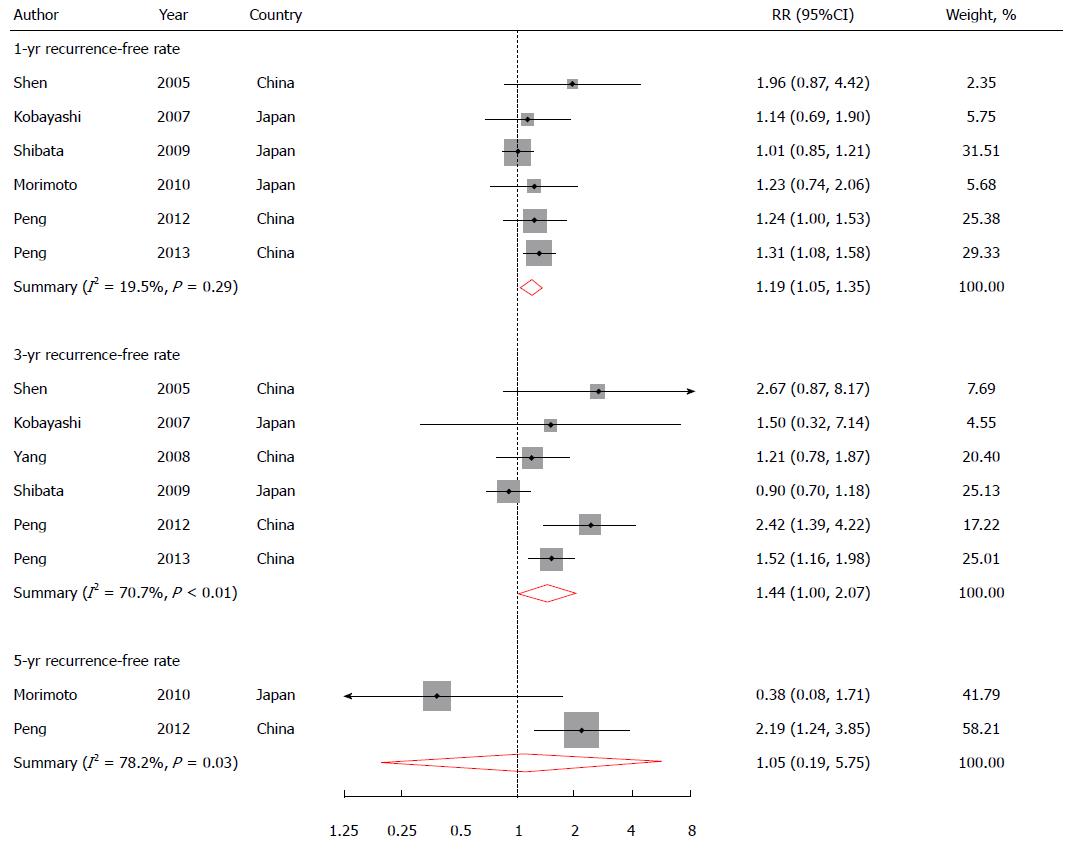
Six studies reported 1-year RFS rate[7,8,10,12,14,15]; six presented 3-year RFS rate[7,8,10,12,14]; and two reported 5-year RFS rate[8,15]. One study reported 1-year RFS rate in the combination treatment group[9], and was not included in the pooling analysis for this outcome. The pooled RR was 1.19 (1.05-1.35) for 1-year RFS rate, 1.44 (1.0-2.07) for 3-year RFS rate, and 1.05 (0.19-5.75) for 5-year RFS rate (Figure 3).
There was no heterogeneity among six studies for 1-year RFS rate (I2 = 19.5% and P = 0.29). High heterogeneity existed among six studies for 3-year RFS rate and in two studies for 5-year RFS rate (I2 = 70.7% and P < 0.01 for 3-year RFS; I2 = 78.2% and P = 0.03 for 5-year RFS). No evidence of publication bias was found for all the three pooled analyses.
Table 2 presents the results from the subgroup analysis stratified by two predetermined factors: tumor size (< 3, 3-5, > 5 cm in diameter) and study location (China vs Japan). The results were more evident in those with medium-sized tumors or among Chinese population.
| Outcome | Potential modifiers | No. of studies | No. of events/patients | Heterogeneity test | RR (95%CI) | ||
| RFA + TACE | RFA | ||||||
| 1-yr OS rate | Tumor size, cm | < 3 | 1 | 65/69 | 59/70 | NA | 1.12 (0.99, 1.26) |
| 3–5 | 3 | 121/128 | 109/128 | I2 = 0.0%, P = 0.66 | 1.11 (1.02, 1.21)1 | ||
| > 5 | 3 | 57/68 | 30/46 | I2 = 0.0%, P = 0.80 | 1.28 (1.02, 1.61)1 | ||
| Study location | China | 5 | 208/227 | 171/208 | I2 = 0.0%, P = 0.77 | 1.12 (1.04, 1.20)1 | |
| Japan | 2 | 35/38 | 27/36 | I2 = 11.2%, P = 0.29 | 1.17 (0.96, 1.43) | ||
| 3-yr OS rate | Tumor size, cm | < 3 | 2 | 87/115 | 69/113 | I2 = 83.2%, P = 0.02 | 1.21 (0.79, 1.83) |
| 3–5 | 2 | 81/113 | 70/113 | I2 = 0.0%, P = 0.68 | 1.17 (0.99, 1.39)1 | ||
| > 5 | 2 | 18/37 | 9/34 | I2 = 0.0%, P = 0.66 | 1.77 (0.95, 3.31) | ||
| Study location | China | 3 | 122/181 | 95/181 | I2 = 18.7%, P = 0.29 | 1.29 (1.05, 1.58)1 | |
| Japan | 3 | 64/84 | 53/79 | I2 = 33.4%, P = 0.22 | 1.12 (0.90, 1.39) | ||
| 1-yr | Tumor size, cm | < 3 | 3 | 102/125 | 88/123 | I2 = 10.7%, P = 0.33 | 1.11 (0.96, 1.28) |
| RFS rate | 3–5 | 2 | 88/113 | 68/113 | I2 = 0.0%, P = 0.83 | 1.30 (1.09, 1.55)1 | |
| > 5 | 1 | 11/18 | 5/16 | NA | 1.96 (0.87, 4.42) | ||
| Study location | China | 3 | 141/181 | 108/181 | I2 = 0.0%, P = 0.55 | 1.29 (1.12, 1.49)1 | |
| Japan | 3 | 60/75 | 53/71 | I2 = 0.0%, P = 0.70 | 1.04 (0.89, 1.23) | ||
| 3-year | Tumor size, cm | < 3 | 3 | 67/128 | 47/123 | I2 = 84.3%, P < 0.01 | 1.45 (0.59, 3.54) |
| RFS rate | 3–5 | 1 | 63/94 | 42/95 | NA | 1.52 (1.16, 1.98)1 | |
| > 5 | 2 | 34/49 | 11/28 | I2 = 51.0%, P = 0.15 | 1.56 (0.70, 3.47) | ||
| Study location | China | 4 | 128/212 | 66/193 | I2 = 41.2%, P = 0.16 | 1.63 (1.19, 2.24)1 | |
| Japan | 2 | 36/59 | 34/53 | I2 = 0.0%, P = 0.52 | 0.90 (0.71, 1.19) | ||
First, we replaced the random-effects model with the fixed-effects model for the pooled analyses. The findings were generally consistent. Second, we omitted one study each time from the pooled analyses and found that no single study substantially influenced the pooled results in the main analyses.
Our meta-analysis showed that RFA with TACE had a beneficial effect on 1- or 3-year OS rate as well as RFS rate, compared with RFA alone, especially for patients with medium-sized (3-5 cm) tumors. Based on the available evidence, there was no difference between the RFA and the TACE + RFA groups in terms of 5-year RFS rate. Whether or not there is difference between groups in 5-year OS rate remains unclear because only a few studies have reported this. A beneficial effect of combined TACE/RFA therapy on RFS rate was more evident among studies conducted in China.
So far, several reviews have been published on this topic. All of them had some flaws in their statistical design. One of them had a combined mixed cohort and RCT design and yielded a biased estimation of the true effects of combined therapy[16]. Three other meta-analyses gave misleading estimation of effect using OR instead of RR[17-19]. The other one pooled seven RCTs and found that RFA with TACE improved survival in patients with HCC > 3 cm[25]. Unfortunately, this review included one article that had already been retracted by the journal due to scientific misconduct[26], in addition to including an abstract[27]. None of these reviews gave a stratified analysis by tumor size and location, or reported results on RFS rate.
Several strengths of this meta-analysis should be mentioned. First, this was a meta-analysis of multiple RCTs, which provided strong evidence for casual inference. Second, this meta-analysis answered whether combined therapy is beneficial to medium-sized tumors in addition to small-sized ones, which is more meaningful in clinical practice. In addition, we found that combined therapy benefited the OS and RFS rates of HCC.
This meta-analysis had some limitations. First, we only included articles published in English and Chinese. Any bias caused by excluding studies published in other languages could not be ruled out. Second, our results were based on unadjusted RRs that were calculated according to the data derived from the original studies. The potential confounding effect could not be completely excluded. However, this meta-analysis included only RCTs, which substantially alleviated this concern. Indeed, there were no significant differences found in age, sex ratio, Child-Pugh class, and tumor size between TACE/RFA and RFA alone. In addition, there was insufficient information to conduct a quality assessment of the included studies. Any inherit limitation in the original studies may have biased our results. Moreover, due to the small number of studies included, we could not comment on the benefit of the combined therapy on 5-year OS and RFS rates.
Surgical resection, liver transplantation and local ablation are currently considered to be the three best and curative treatments for early-stage HCC[28]. However, only 10%-30% of early-stage HCC is suitable for surgery due to poor liver reserve, comorbidity, and shortage of liver donors. Therefore, local ablation plays an important role in the treatment of unresectable or resectable early-stage HCC. Among the various local ablative modalities, RFA is a curative treatment with minimal invasiveness and high efficacy for small HCC that is generally defined as maximal diameter no larger than 3 cm[29]. Efficacy of RFA is reduced as the tumor size increases, which could be due to incomplete ablation and increased blood flow in larger lesions, resulting in heat loss. Therefore, as to the treatment for medium- or large-sized tumors, RFA alone may not be a first choice. Then, combining RFA with TACE may overcome the limitations of each of them used alone and provide better local control of HCCs > 3 cm. In particular, RFA is more efficient when the blood flow is reduced or cells are dying because of chemoembolization and the retained iodized oil after TACE can transfer heat fast.
No strictly designed crossover RCT has been conducted to validate which should be used first in the combination of RFA with TACE. However, it is hypothesized that using TACE before RFA may enhance subsequent RFA because: (1) TACE partially kills some tumor cells through chemotherapy and hypoxic injury, which could reduce tumor size; and (2) TACE decreases arterial blood flow and reduces or even eliminates the heat loss mediated by tissue perfusion[30], which will enlarge ablation and heat coagulation zone. In addition, whether an additional TACE regimen should be used to consolidate the treatment effect after combined TACE/RFA therapy deserves further research.
In this meta-analysis, the beneficial effect of combined TACE/RFA therapy was found to be more pronounced in studies conducted in China than those studies conducted in Japan. This phenomenon might be explained by the fact that HCC etiology in China is different from that in Japan, although the detailed mechanism is not clear. HCC in China is mostly related to hepatitis B virus, while HCC in Japan is associated with alcoholic fatty liver disease.
In conclusion, our meta-analysis found a 12% and 20% increase in 1-year and 3-year OS rate, as well as a 19% and 44% increase in 1-year and 3-year recurrence rate, respectively. The beneficial effect is more evident in medium-sized tumors and among the Chinese population. Further RCTs with large tumors, long-term follow-up, and assessment of the combination model of RFA and TACE, for example, TACE-RFA-TACE, are needed.
We thank Dr. Peng-cheng Xun for his help in verifying our analysis.
Surgical resection is the first-line therapeutic option for hepatocellular carcinoma (HCC) patients with small solitary nodules without underlying cirrhosis; however, its role in treating HCC is limited by strict inclusion criteria. Radiofrequency ablation (RFA), and transcatheter arterial chemoembolization (TACE), therapies for HCC, which involves administration of chemotherapy directly to the liver tumor via a catheter, have been developed as alternatives, particularly for patients with nonresectable HCC. Although local therapies may have less invasiveness, shorter hospital stay and lower associated mortality, their higher recurrence rates and lower disease-free survival rates are still major concerns. In the past decade, some evidence has suggested that combining RFA with TACE improves overall survival (OS) rate and reduces recurrence rate, while other studies have not shown these.
To investigate whether combined TACE/RFA improved OS and recurrence-free survival rates, compared with RFA alone, especially for patients with medium-sized (3-5 cm) or large (> 5 cm) HCCs, by conducting a systematic review as well as meta-analysis of RCTs.
Combined TACE/RFA has a beneficial effect on survival and recurrence rates compared with RFA alone, especially for medium-sized (3-5 cm) HCC and among Chinese patients.
This is a well-written article. The literature search was thorough. The statistical analysis was appropriate. The included studies did not reflect publication bias. The possible limitations of the meta analyses were identified.
| 1. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 249] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, Chang YC, Kohno H, Nakamura T, Yukaya H. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488-494. [PubMed] |
| 4. | Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720-727. [PubMed] |
| 5. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 878] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 6. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2008] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 7. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 8. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 9. | Zhang ZJ, Wu MC, Chen H, Chen D, He J. Percutaneous radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi. 2002;40:826-829. |
| 10. | Shen SQ, Xiang JJ, Xiong CL, Wu SM, Zhu SS. Intraoperative radiofrequency thermal ablation combined with portal vein infusion chemotherapy and transarterial chemoembolization for unresectable HCC. Hepatogastroenterology. 2005;52:1403-1407. [PubMed] |
| 11. | Kang CB, Xu HB, Wang SL, Rui B. Treatment of large hepatoma by TACE in combination with RFA. Zhonghua Gandan Waike Zazhi. 2007;13:828-830. |
| 12. | Kobayashi M, Ikeda K, Kawamura Y, Hosaka T, Sezaki H, Yatsuji H, Akuta N, Suzuki F, Suzuki Y, Arase Y. Randomized controlled trial for the efficacy of hepatic arterial occlusion during radiofrequency ablation for small hepatocellular carcinoma--direct ablative effects and a long-term outcome. Liver Int. 2007;27:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Yang P, Liang M, Zhang Y, Shen B. Clinical application of a combination therapy of lentinan, multi-electrode RFA and TACE in HCC. Adv Ther. 2008;25:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 14. | Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (2)] |
| 15. | Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 251] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 16. | Kong QF, Jiao JB, Chen QQ, Li L, Wang DG, Lv B. Comparative effectiveness of radiofrequency ablation with or without transarterial chemoembolization for hepatocellular carcinoma. Tumour Biol. 2014;35:2655-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872-3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:653-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2012;57:3026-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 20. | Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989-991. [PubMed] |
| 21. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma &lt; or =4 cm. Gastroenterology. 2004;127:1714-1723. [PubMed] |
| 22. | Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the Quality of Reports of Meta-Analyses of Randomised Controlled Trials: The QUOROM Statement. Onkologie. 2000;23:597-602. [PubMed] |
| 23. | Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29:1282-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 492] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 24. | Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 1311] [Article Influence: 57.0] [Reference Citation Analysis (1)] |
| 25. | Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 27. | Aikata H, Shirakawa H, Takaki S. Radiofrequency ablation combined with transcatheter arterial chemoembolizaiton for small hepatocelluar carcinoma. Hepatology. 2006;44:A487. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237-257. [PubMed] |
| 29. | Kitamoto M, Imagawa M, Yamada H, Watanabe C, Sumioka M, Satoh O, Shimamoto M, Kodama M, Kimura S, Kishimoto K. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol. 2003;181:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Pouponneau P, Sollano JDD, Wang DS, Xu WG S- Editor: Yu J L- Editor: A E- Editor: Jiao XK