Published online Jun 26, 2015. doi: 10.13105/wjma.v3.i3.151
Peer-review started: July 30, 2014
First decision: December 17, 2014
Revised: April 22, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: June 26, 2015
Processing time: 336 Days and 19.9 Hours
AIM: To investigate the 7-valent pneumococcal conjugate vaccine (PCV7) effectiveness.
METHODS: A systematic literature review of studies which evaluated the effectiveness of PCV7 vaccine was performed searching the keyword “heptavalent pneumococcal conjugate vaccine” in PubMed and Scopus until March 16, 2013. The selection of potential eligible articles was done by two researchers independently on the basis of abstract and title and only post-marketing studies were included in the systematic review. Data extraction was carried out by two researchers with respect to invasive pneumococcal diseases due to both all and vaccine serotypes in pre-vaccine and post-vaccine periods in children less than 5 years. Results of studies which were considered suitable for meta-analysis were combined by means of relative risk (RR) with 95%CI. Vaccine effectiveness was calculated as (1-RR) × 100. Heterogeneity was assessed by I2 and a random effects model was used to combine data in the case of heterogeneity. RevMan 5 was used to pool data.
RESULTS: On the whole, 757 eligible papers were identified from the literature search in PubMed and Scopus. Of them, 62 were finally considered in the systematic review and 38 were included in the meta-analysis. In all post-marketing studies included in the systematic review the incidence of invasive pneumococcal diseases due to vaccine serotypes declined significantly with the exception of few studies showing stability or a slight, but not significant, increase. Furthermore most of studies highlighted also a reduction in the incidence of invasive pneumococcal diseases due to all serotypes. With regards to meta-analysis, a random effects model was used to combine data because of the high heterogeneity. Data combination showed that the effectiveness of PCV7 in reducing invasive pneumococcal diseases due to vaccine serotypes and to all serotypes was 84% (95%CI: 74%-90%) and 53% (95%CI: 46%-59%) respectively. These results are confirmatory with respect to the efficacy of PCV7 against invasive pneumococcal diseases due to vaccine serotypes.
CONCLUSION: PCV7 implementation determines a significant decrease of invasive pneumococcal diseases.
Core tip: This systematic review and meta-analysis was performed with the aim to collect data from post-marketing studies on 7-valent pneumococcal conjugate vaccine (PCV7) and to provide evidence about the impact of the vaccine in the real world. Eligible articles were identified through a search on PubMed and Scopus. The meta-analysis showed that PCV7 is able to reduce invasive pneumococcal diseases due to both vaccine serotypes and to all serotypes. The effectiveness was 84% (95%CI: 74%-90%) and 53% (95%CI: 46%-59%) respectively. These data may be taken into consideration in order to foresee the impact under real conditions of PCV13 which has replaced PCV7 from 2010 onwards.
- Citation: de Waure C, Specchia ML, Capizzi S, Aljicevic M, Dujovic M, Malaj A, Ricciardi W. Effectiveness of 7-valent pneumococcal conjugate vaccine: A meta-analysis of post-marketing studies. World J Meta-Anal 2015; 3(3): 151-162
- URL: https://www.wjgnet.com/2308-3840/full/v3/i3/151.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i3.151
Streptococcus pneumoniae (S. pneumoniae) is a leading cause of severe bacterial infectious disease and World Health Organization has estimated that this bacteria causes 1.4-1.6 million child deaths annually[1,2], in that around 11% of all deaths in children < 5 years[3]. More than 90 serotypes of S. pneumoniae exist. These strains may cause invasive pneumococcal disease (IPD). The highest incidence of IPD is seen in children < 2 years old. In order to prevent disease caused by S. pneumoniae, two types of vaccines, polysaccharide (PPV) and conjugate (PCV) exist, even though the PPV vaccine is ineffective in children < 2 years old[4].
The PCV vaccines consist of capsular PPVs bound to proteins which are highly immunogenic and enhance an immune response by recruiting type 2 helper T cells, which allows for immunoglobulin type switching and production of memory B cells. The main drawbacks of PCV vaccines are that they only provide protection against a subset of serotypes covered by the PPV vaccines[5-7]. In fact, PCV vaccines encompass the 7-valent vaccine (PCV7), the PCV10 and the PCV13. Currently, PCV13 is used in prevention campaigns. Its marketing authorization in the European Union goes back to December 2009[8]. PCV13 has replaced PCV7 from 2010 onward.
The PCV7, providing protection against serotypes 4, 6B, 9V, 14, 18C, 19F and 23F, was introduced into routine childhood immunization program in the United States in 2000 and was shown to reduce the incidence of IPD by all and vaccine-serotypes[9,10]. Notwithstanding, some studies have described significant rises in non-vaccine serotypes after the implementation of universal PCV7 programs[11-14]. Based on the favourable United States experience and the proof of vaccine efficacy[15] a number of countries have introduced PCV7[16]. Worldwide the vaccine has been provided with different schedules. In Europe both the 2 + 1 and 3 + 1 schedules have been used[16].
In the light of monitoring the health impact of technologies and policies, data from the real practice should be collected and analysed. Because of the recent introduction and implementation of PCV13, many data from real practice are only available for PCV7 even though evidence is being produced on PCV13 also[17-23]. Notwithstanding, this evidence should be considered early and is still scant in order to make a meta-analysis. Furthermore, it is mostly related to the transition period between the use of PCV7 and the introduction of PCV13 which took place from 2010 onward with different time schedules across countries. Based on this premises, the objective of this study was to perform a systematic review and a meta-analysis of post-marketing studies on the effectiveness of PCV7 in comparison with no vaccination in preventing IPD in children less than 5 years of age worldwide. The final aim was to provide evidence about PCV7 effectiveness under real conditions and to foresee the potential impact of PCV13 on the basis of results. The systematic review was performed according to PRISMA Statement published by Moher et al[24].
A literature search was conducted using PubMed and Scopus search engines. The following search strategy was used: “heptavalent pneumococcal PCV vaccine” (Substance Name) NOT [“Clinical Trial” (Publication Type) OR “Clinical Trials as Topic” (Mesh) OR “Controlled Clinical Trial” (Publication Type) OR “Clinical Trial, Phase IV” (Publication Type) OR “Clinical Trial, Phase III” (Publication Type) OR “Clinical Trial, Phase II” (Publication Type) OR “Clinical Trial, Phase I” (Publication Type)]. The search covered the period up to March 16, 2013, without starting date, and was limited to English-language publications.
The selection of potential eligible articles was done by two researchers independently on the basis of title and abstract. Full text of eligible articles was collected for the final judgment on inclusion. Disagreements were solved through consensus or the consultation of a third researcher.
We defined a priori criteria for the inclusion of studies in this meta-analysis, selecting studies dealing with the incidence of IPD in children less than 5 years of age in the period before and after the introduction of PCV7. Only articles releasing data on IPD incidence in pre- and post-vaccination periods were included in the quantitative assessment.
The following data were recorded from each study: first author, journal, published year, country, study population, IPD case definition, crude number or incidence of IPD before and after the introduction of PCV7. Data on IPD caused by all serotypes and due to vaccine serotypes, if available, were collected. Data extraction was performed by two researchers independently and disagreements were solved through consensus or the consultation of a third researcher.
Studies were included in the meta-analysis if they provided crude data or if it was possible to get them through computation.
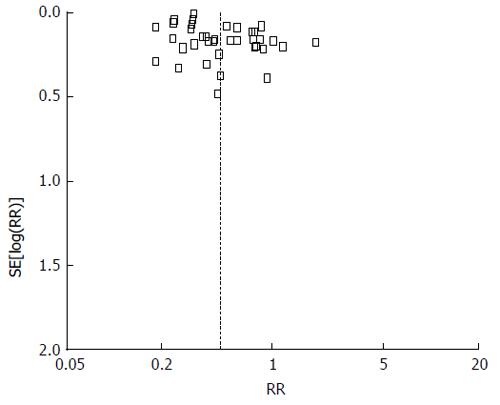
The relative risk (RR) with 95%CI was used to combine data. Vaccine effectiveness was calculated as (1-RR) × 100. RevMan 5 was used to combine data and a fixed effects model was applied in the case of absence of heterogeneity (I2 < 50%). On the other way around, a random effects model was used. Studies which were not considered in the meta-analysis were described qualitatively in Table 1. Finally, publication bias was assessed by means of funnel plots.
| Ref. | Country | Study period | Invasive pneumococcal disease definition | Main results |
| Albrich et al[25] | United States | 1997-2004 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Ampofo et al[26] | United States | 1997-2010 | Isolation of S. pneumoniae from sterile body fluid | The proportion of children younger than 2 yr with IPD decreased (54% vs 43% with respect to all serotypes and 56% vs 43% for vaccine serotypes), while the proportion of disease among children aged 2-4 slightly increased (27% vs 29% with respect to all and vaccine serotypes) |
| Aristegui et al[27] | Spain | 1998-2003 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Barricarte et al[28] | Spain | 2001-2005 | Isolation of S. pneumoniae from sterile body fluid | The overall effectiveness in reducing IPD was 31% (OR = 0.69, 95%CI: 0.37-1.27) and 88% (OR = 0.12, 95%CI: 0.02-0.91) for all serotypes and vaccine serotypes respectively |
| Benito-Fernández et al[29] | Spain | 2000-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Ben-Shimol et al[30] | Israel | 1989-2010 | Isolation of S. pneumoniae from sterile body fluid | In 2009 and 2010, IPD incidence (due to vaccine serotypes) were 15.9 per 100000 and 5.4, per 100000 respectively (a 43% and 81% decrease compared to 2003-2007) |
| Bjornson et al[31] | Canada | 2001-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Calbo et al[32] | Spain | 1999-2004 | Isolation of S. pneumoniae from sterile body fluid | The IPD incidence significantly decreased from 96.9 cases per 100000 person-years to 90.6 cases per 100000 person-years (7% reduction) |
| Carstairs et al[33] | United States | 2000-2002 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Casado-Flores et al[34] | Spain | 2001-2006 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| CDC[35] | United States | 1998-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| De Serres et al[36] | United States | 2001-2009 | Isolation of S. pneumoniae from sterile body fluid | Effectiveness of PCV7 against IPD due to vaccine serotypes was 97% (95%CI: 92%-98%) among healthy children and 88% (95%CI: 78%-94%) among children with comorbid conditions. The incidence of IPD due to non-vaccine serotypes increased from 6.8 per 100000 (1998-1999) to 10.3 per 100000 in 2007 (51% increase) |
| De Wals et al[37] | Canada | 2007-2010 | Isolation of S. pneumoniae from sterile body fluid | A decrease in the frequency of IPD caused by vaccine serotypes was observed |
| Dias et al[38] | Portugal | 1999-2004 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Vestrheim et al[39] | Norway | 2004-2008 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Dubos et al[40] | France | 2000-2005 | Isolation of S. pneumoniae from sterile body fluid | A decrease of 82% (95%CI: 52%-95%) of cases was observed (from 8.9 cases per 100000 in 2001 to 1.8 per 100000 in 2005) in children < 2 yr |
| Fenoll et al[41] | Spain | 1996-2001 2005-2006 | Isolation of S. pneumoniae from sterile body fluid | A decrease of the incidence of IPD due to vaccine serotypes from 5.2 per 100000 in 1996-2001 to 2.4 per 100000 in 2005-2006 was observed |
| Flannery et al[42] | United States | 1998-2002 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Giele et al[43] | Australia | 1996-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Schutze et al[44] | Arkansas | 1998-2003 | Isolation of S. pneumoniae from sterile body fluid | A decrease of IPD from 44.2 per 100000 person-years to 8.30 per 100000 person-years was observed in children < 2 yr |
| Guevara et al[45] | Spain | 2001-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Haddy et al[46] | United States | 1999-2002 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Hanna et al[47] | Queensland | 1999-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Hanquet et al[48] | Belgium | 2002-2008 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Harboe et al[49] | Denmark | 2000-2008 | Isolation of S. pneumoniae from sterile body fluid | In children < 2 yr, the overall incidence decreased from 54 to 23 cases per 100000 (IRR = 0.43, 95%CI: 0.29-0.62) and from 36.7 to 7.7 (IRR = 0.20, 95%CI: 0.09-0.38) for vaccine serotypes. A non-significant increase was observed in children aged 2-4 yr |
| Hennessy et al[50] | United States | 1995-2003 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| CDC[51] | United States | 1998-2003 | Isolation of S. pneumoniae from sterile body fluid | A decrease of IPD due to vaccine serotypes from 80 cases per 100000 to 4.6 per 100000 was observed (decrease of 94% (95%CI: 92%-96%) from 1998-1999 to 2003 |
| Hsu et al[52] | United States | 1998-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Hsu et al[53] | United States | 1990-1991 2001-2003 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Hsu et al[54] | United States | 2001-2007 | Isolation of S. pneumoniae from sterile body fluid | IPD incidence was stable during the 6 yr period, although IPD due to vaccine serotypes decreased |
| Ingels et al[55] | Denmark | 2000-2010 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Wenger et al[56] | United States, Alaska | 1986-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Johnson et al[57] | South Australia | 2002-2009 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Kellner et al[58] | Canada | 1998-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Kyaw et al[59] | United States | 1996-2004 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Leal et al[60] | Alberta | 1998-2010 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Liao et al[61] | Taiwan | 2000-2008 | Isolation of S. pneumoniae from sterile body fluid | The overall incidence of IPD decreased by 33% (95%CI: 0%-72.2%) |
| Messina et al[62] | United States | 1999-2001 2003-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Muñoz-Almagro et al[63] | Spain | 1997-2006 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Patrzalek et al[64] | Poland | 2005-2010 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Pérez et al[65] | Spain | 1998-2008 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Pérez-Trallero et al[66] | Spain | 1996-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Pilishvili et al[67] | United States | 1998-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Poehling et al[68] | United States | 1997-2004 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| [69] | Canada | 2002-2005 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Ramani et al[70] | United States | 1994-2001 | Hospital discharges for IPD | A significant decrease was observed only for children aged < 1 yr (from 40 per 100000 to 23 per 100000 person years). All other age groups did not show a significant change in discharge rates for IPD |
| Rendi-Wagner et al[71] | Austria | 2001-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Rodenburg et al[72] | Netherlands | 2004-2008 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Rückinger et al[73] | Germany | 1997-2003 2007-2008 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| de Sevilla et al[74] | Spain | 2007-2009 | Isolation of S. pneumoniae from sterile body fluid | An increase of 44% of IPD (95%CI: 10%-89%) was shown |
| Shafinoori et al[75] | United States | 1998-2004 | Isolation of S. pneumoniae from sterile body fluid | A significant 68% and 70% decrease of IPD in children < 2 yr and aged 2 to 4 yr respectively was observed |
| Shah et al[76] | United States | 1999-2003 | Hospital discharges for IPD | A significant decrease from 12.03 per 100000 person-years in 1999 to 5.60 per 100000 person-years in 2003 was shown |
| Techasaensiri et al[77] | United States | 1999-2008 | Isolation of S. pneumoniae from sterile body fluid | The incidence of IPD significantly decreased in children < 2 yr |
| Tsai et al[78] | United States | 1994-1999 2001-2004 | Hospital discharges for pneumococcal meningitis | The average annualized rates of hospitalizations decreased from 7.7 per 100000 to 2.6 per 100000 in children < 2 yr and from 0.9 per 100000 to 0.5 per 100000 in children aged 2-4 (a reduction of 66%, 95%CI: 56.3%-73.5% and of 51.5%, 95%CI: 28.9%-66.9% respectively) |
| Tsigrelis et al[79] | United States | 1995-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Tyrrell et al[80] | Canada | 2000-2006 | Isolation of S. pneumoniae from sterile body fluid | IPD due to vaccine serotypes decreased of 61% in children < 2 yr (from 96.7 per 100000 person-years to 25.8 per 100000 person-years) and of 57% in children from 2 to 4 yr (from 24.5 per 100000 person-years to 10.6 per 100000 person-years) |
| Van der Linden et al[81] | Germany | 1997-2010 | Isolation of S. pneumoniae from sterile body fluid | IPD incidence decreased from 2.4 per 100000 to 0.3 per 100000 |
| Vestrheim et al[82] | Norway | 2002-2007 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Weatherholtz et al[83] | United States | 1995-2006 | Isolation of S. pneumoniae from sterile body fluid | Rates of IPD due to vaccine serotypes among children aged < 1 yr, 1-2 yr, and 2-5 yr decreased from 210, 263, and 51 cases per 100000 respectively in to 0 case per 100000 |
| Whitney et al[84] | United States | 1998-2001 | Isolation of S. pneumoniae from sterile body fluid | 1 |
| Winters et al[85] | Canada | 2002-2005 | Isolation of S. pneumoniae from sterile body fluid | The incidence of IPD decreased from 54 per 100000 person-years to 16 per 100000 person-years (decrease of 70%). An even stronger decrease was observed in children < 1 yr, where the incidence decreased from 135 per 100000 to 15 per 100000 person-years (decrease of 89%) |
| Yildirim et al[86] | United States | 2007-2010 | Isolation of S. pneumoniae from sterile body fluid | IPD cases due to vaccine serotypes decreased |
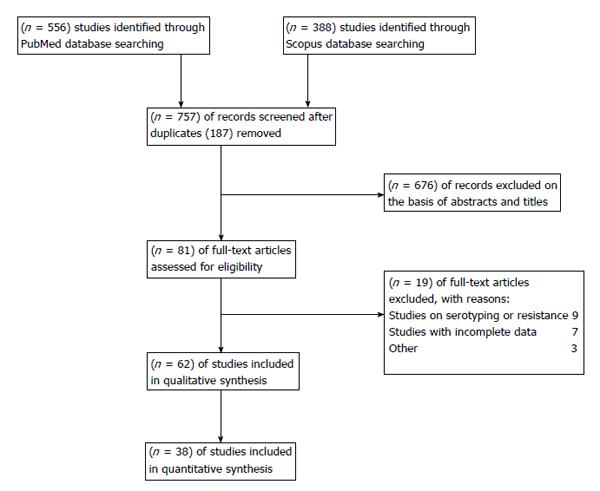
On the whole, 556 articles were yielded from PubMed and 388 from Scopus but 187 papers were shared by the two databases for a total of 757 papers. Of them, 62 were finally considered in the systematic review (Figure 1)[25-86]. Their characteristics and results are shown in Table 1.
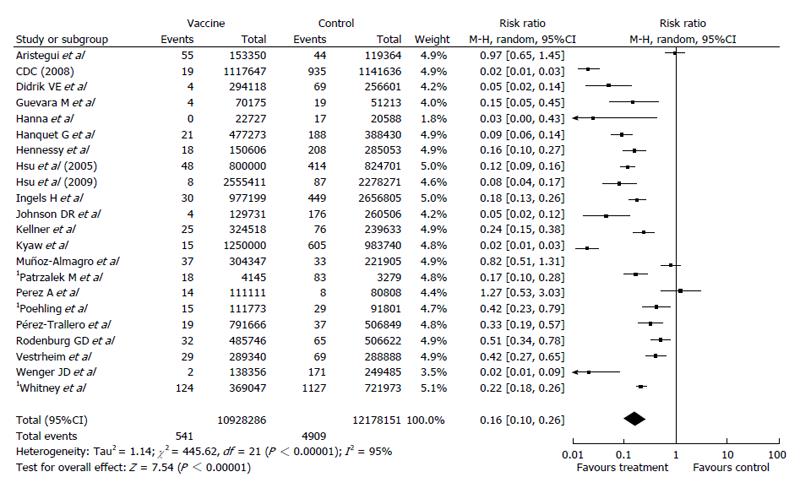
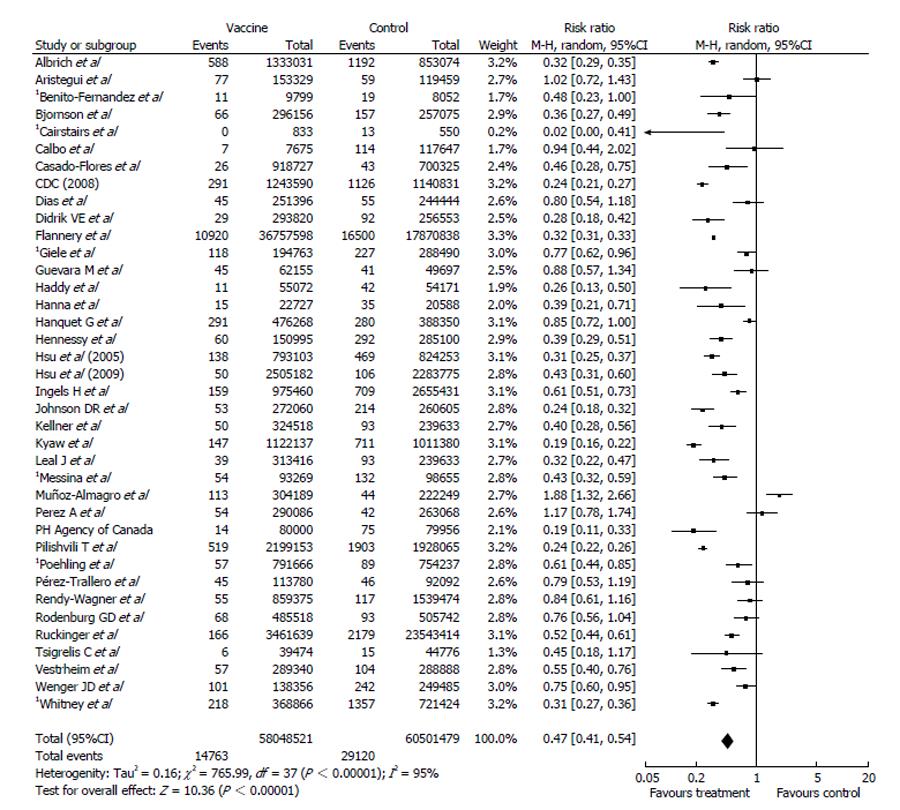
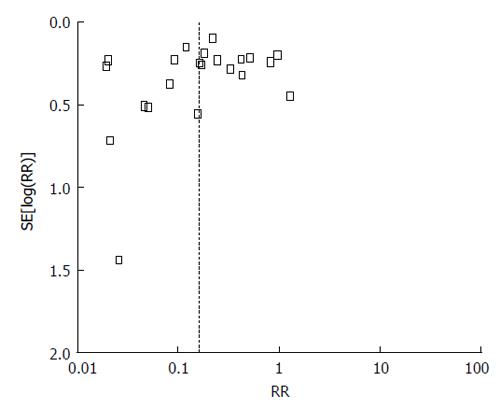
With respect to meta-analysis, 38 articles provided data on IPD due to all serotypes while 22 allowed the collection of data on IPD due to vaccine serotypes. Data combination showed a vaccine effectiveness of 84% for IPD due to vaccine serotypes (RR = 0.16, 95%CI: 0.10%-0.26; I2 = 95%, Figure 2) and 53% (RR = 0.47, 95%CI: 0.41-0.54; I2 = 95%, Figure 3) for IPD related to all serotypes. Publication bias could not be excluded with respect to the assessment of effectiveness against IPD due to vaccine serotypes while may be excluded as regards IPD due to all serotypes (Figures 4 and 5).
Our study aimed to review and combine data of post-marketing studies on PCV7 worldwide.
The analysis and data combination allowed us to investigate the effectiveness of PCV7 and its impact in terms of public health. Results are indeed useful for supporting decision-makers in the field of vaccinations. In particular, findings of the meta-analysis showed that the effectiveness of PCV7 in reducing IPD due to vaccine serotypes is 84%. The effectiveness is estimated to be 53% with respect to IPD due to all serotypes.
The results of our study are aligned with the evidence on the efficacy of PCV7 demonstrated in randomized clinical trials (RCT). In fact, a meta-analysis of RCT conducted by Pavia et al[15] showed an efficacy of 89% in preventing IPD due to vaccine serotypes, and of 63%-74% in preventing IPD due to all serotypes. Indeed, as IPD due to vaccine serotypes, effectiveness data have confirmed efficacy data. With this respect it is important to point out that the assessment of efficacy of interventions is critical in order to decide upon their adoption and is addressed through explanatory clinical trials[87]. Notwithstanding, the proof of efficacy is not always sufficient because it is also important to have evidence about how interventions work under more natural field conditions rather than in controlled clinical trials[87,88]. Indeed, overall effectiveness of interventions should be assessed by different study designs able to maximize external validity[87].
As far as PCV7 is concerned, all post-marketing studies showed that the incidence of IPD due to vaccine serotypes declined significantly after the implementation of vaccination, with the exception of few studies[36,27,49,63,65] showing a stability or a slight increase. As a consequence, the implementation of vaccination has definitively contributed in consistently preventing IPD in children up to 5 years of age with a strong impact on population health and costs due to hospitalizations[89,90]. In fact, a relevant reduction of IPD due to all serotypes was also shown by the meta-analysis even though, comparing with IPD due to vaccine serotypes, more studies highlighted a stability or an increase in the overall incidence of IPD[26,32,38,45,48,52,63,65,66,70-72,74,79]. In particular two studies[64,75] showed a significant increase although due to non-vaccine serotypes and in a context of low vaccination coverage. The increase in the incidence of non-vaccine serotypes is a well-known phenomenon which may be counteracted by the extension of serotypes coverage. In this view the availability and the implementation of PCV13 is useful in order to further reduce the incidence of IPD. In fact, the post-licensure assessment already carried out by Andrews et al[23] estimated that the effectiveness of at least 2 doses of PVC13 before 12 mo of age or of 1 dose from 12 mo onwards was 90% (95%CI: 34%-98%) against PCV7 serotypes. This result is aligned with data from our and Pavia et al[15] meta-analyses. Furthermore, PCV13 was shown to have an effectiveness of 73% (95%CI: 55%-84%) against the additional serotypes included in the vaccine[23]. PCV13 may indeed provide an added value in comparison to PCV7. In fact, already available population-based studies showed that IPD decreased of a percentage from 18% to 42% when PCV13 era is compared to PCV7 one[18,20,21]. The decline is more important in children less than 2 years of age in which the decrease in all IPD varies from 50% to 60%[18,20,21].
This study presents some limitations. The research was limited to only two specialized searching engines and, consequently, selection bias may be not excluded. Papers included in the review were heterogeneous with respect to countries and study design as also highlighted by the test of heterogeneity. Crude data were not obtainable from all the papers selected and only children < 5 years of age, independently by their health status, were considered in the analysis. Furthermore, neither a quality assessment nor stratified analyses in order to investigate heterogeneity were performed.
Strengths of this study are represented by the objective itself, because we focused on effectiveness instead of efficacy, and the large number of papers included in the analysis.
The consistent decrease of IPD due to vaccine serotypes after the PCV7 implementation is important as the new PCV13 is being implemented. In fact, it is expected that it will have the same effectiveness in preventing IPD due PCV7 vaccine serotypes and it will also have an important impact on cases due to new vaccine serotypes[91,92].
Streptococcus pneumoniae (S. pneumoniae) is a leading cause of severe bacterial infectious disease, causing 1.4-1.6 million child deaths annually, in that around 11% of all deaths in children < 5 years. Two types of vaccines against S. pneumoniae exist, polysaccharide (PPV) and conjugate (PCV), even though the PPV vaccine is ineffective in children < 2 years old. PCV vaccines encompass the 7-valent vaccine (PCV7), the PCV10 and the PCV13. Currently, PCV13 is used in prevention campaigns. Its marketing authorization in the European Union goes back to December 2009 and it has replaced PCV7 from 2010 onward. Because of the recent introduction and implementation of PCV13, consistent data from real practice are only available for PCV7 and their assessment is of utmost importance in order to monitor the health impact of the vaccine.
The monitoring of the overall health impact of technologies and policies is a key issue in medicine. It is mainly based on post-marketing studies on the effectiveness of interventions carried out through the collection and analysis of data from the real practice. In this context, the objective of the authors study was to perform a systematic review and a meta-analysis of post-marketing studies on the effectiveness of PCV7 worldwide.
The authors’ findings showed that the effectiveness of PCV7 in reducing invasive pneumococcal disease (IPD) is 84% with respect to IPD due to vaccine serotypes and 53% with respect to IPD due to all serotypes. Concerning IPD due to vaccine serotypes, effectiveness data have confirmed efficacy data previously reported in a meta-analysis of randomized clinical trial conducted by Pavia et al. However, with this respect, it is important to emphasize that efficacy trials test the expected results of an intervention under ideal circumstances whereas effectiveness studies measure the beneficial effects under “real world” clinical settings. Indeed, the results of their meta-analysis represent and advance in the knowledge of PCV7 impact.
Given the consistent decrease of IPD due to vaccine serotypes after the PCV7 implementation, results of their systematic review and meta-analysis allow forecasting that the new PCV13, which is being implemented, will further decrease the number of IPD. In fact PCV13 effectiveness is expected to be the same as PCV7 in preventing IPD due to both PCV7 vaccine serotypes and new vaccine serotypes.
IPD is defined as the isolation of S. pneumoniae from a sterile site/body fluid.
The authors performed an interesting and well-written meta-analysis on a highly relevant topic.
| 1. | World Health Organization. Acute respiratory infections. [Update 2009 Sept]. Available from: http://apps.who.int/vaccine_research/diseases/ari/en/index3.html. |
| 2. | Rosen JB, Thomas AR, Lexau CA, Reingold A, Hadler JL, Harrison LH, Bennett NM, Schaffner W, Farley MM, Beall BW. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis. 2011;53:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Laitinen S, Vaara M, Salo E. Pneumococcal serotype distribution in invasive paediatric disease in Southern Finland before the introduction of vaccine. Scand J Infect Dis. 2010;42:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14:e197-e209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Posfay-Barbe KM, Wald ER. Pneumococcal vaccines: do they prevent infection and how? Curr Opin Infect Dis. 2004;17:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165 Suppl 1:S49-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 248] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Westerink MA, Schroeder HW, Nahm MH. Immune Responses to pneumococcal vaccines in children and adults: Rationale for age-specific vaccination. Aging Dis. 2012;3:51-67. [PubMed] |
| 8. | Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1677] [Cited by in RCA: 1567] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 9. | Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27:1030-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Barricarte A, Castilla J, Gil-Setas A, Torroba L, Navarro-Alonso JA, Irisarri F, Arriazu M. Effectiveness of the 7-valent pneumococcal conjugate vaccine: a population-based case-control study. Clin Infect Dis. 2007;44:1436-1441. [PubMed] |
| 11. | Chibuk TK, Robinson JL, Hartfield DS. Pediatric complicated pneumonia and pneumococcal serotype replacement: trends in hospitalized children pre and post introduction of routine vaccination with Pneumococcal Conjugate Vaccine (PCV7). Eur J Pediatr. 2010;169:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 580] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 13. | Lepoutre A, Varon E, Georges S, Gutmann L, Lévy-Bruhl D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill. 2008;13:pii: 18962. [PubMed] |
| 14. | Moore MR, Gertz RE, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 408] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | Pavia M, Bianco A, Nobile CG, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009;123:e1103-e1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis. 2012;12:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29:9127-9131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Kaplan SL, Barson WJ, Lin PL, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2013;32:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Martinelli D, Pedalino B, Cappelli MG, Caputi G, Sallustio A, Fortunato F, Tafuri S, Cozza V, Germinario C, Chironna M. Towards the 13-valent pneumococcal conjugate universal vaccination: effectiveness in the transition era between PCV7 and PCV13 in Italy, 2010-2013. Hum Vaccin Immunother. 2014;10:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Steens A, Bergsaker MA, Aaberge IS, Rønning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine. 2013;31:6232-6238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 22. | Chacon-Cruz E, Rivas-Landeros RM, Volker-Soberanes ML. Early trends in invasive pneumococcal disease in children following the introduction of 13-valent pneumococcal conjugate vaccine: results from eight years of active surveillance in a Mexican hospital. Ther Adv Vaccines. 2014;2:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 24. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48602] [Article Influence: 2858.9] [Reference Citation Analysis (3)] |
| 25. | Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569-1576. [PubMed] |
| 26. | Ampofo K, Pavia AT, Chris S, Hersh AL, Bender JM, Blaschke AJ, Weng HY, Korgenski KE, Daly J, Mason EO. The changing epidemiology of invasive pneumococcal disease at a tertiary children’s hospital through the 7-valent pneumococcal conjugate vaccine era: a case for continuous surveillance. Pediatr Infect Dis J. 2012;31:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Aristegui J, Bernaola E, Pocheville I, García C, Arranz L, Durán G, Pérez L, Bastida M, Canduela C, Herranz Aguirre M. Reduction in pediatric invasive pneumococcal disease in the Basque Country and Navarre, Spain, after introduction of the heptavalent pneumococcal conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2007;26:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Barricarte A, Gil-Setas A, Torroba L, Castilla J, Petit A, Polo I, Arriazu M, Irisarri F, García Cenoz M. [Invasive pneumococcal disease in children younger than 5 years in Navarra, Spain (2000-2005). Impact of the conjugate vaccine]. Med Clin (Barc). 2007;129:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Benito-Fernández J, Raso SM, Pocheville-Gurutzeta I, SánchezEtxaniz J, Azcunaga-Santibañez B, Capapé-Zache S. Pneumococcal bacteremia among infants with fever without known source before and after introduction of pneumococcal conjugate vaccine in the Basque Country of Spain. Pediatr Infect Dis J. 2007;26:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Ben-Shimol S, Greenberg D, Givon-Lavi N, Elias N, Glikman D, Rubinstein U, Dagan R; Israeli Bacteremia and Meningitis Active Surveillance Group. Rapid reduction in invasive pneumococcal disease after introduction of PCV7 into the National Immunization Plan in Israel. Vaccine. 2012;30:6600-6607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Bjornson G, Scheifele DW, Bettinger J, Patrick DM, Gustafson L, Daly P, Tyrrell GJ. Effectiveness of pneumococcal conjugate vaccine in Greater Vancouver, Canada: 2004-2005. Pediatr Infect Dis J. 2007;26:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Calbo E, Díaz A, Cañadell E, Fábrega J, Uriz S, Xercavins M, Morera MA, Cuchi E, Rodríguez-Carballeira M, Garau J; Spanish Pneumococcal Infection Study Network. Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Carstairs KL, Tanen DA, Johnson AS, Kailes SB, Riffenburgh RH. Pneumococcal bacteremia in febrile infants presenting to the emergency department before and after the introduction of the heptavalent pneumococcal vaccine. Ann Emerg Med. 2007;49:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Casado-Flores J, Rodrigo C, Arístegui J, Martínón JM, Fenoll A, Mendez C. Decline in pneumococcal meningitis in Spain after introduction of the heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008;57:144-148. [PubMed] |
| 36. | De Serres G, Pilishvili T, Link-Gelles R, Reingold A, Gershman K, Petit S, Farley MM, Harrison LH, Lynfield R, Bennett NM. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine. 2012;30:4067-4072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | De Wals P, Lefebvre B, Defay F, Deceuninck G, Boulianne N. Invasive pneumococcal diseases in birth cohorts vaccinated with PCV-7 and/or PHiD-CV in the province of Quebec, Canada. Vaccine. 2012;30:6416-6420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Dias R, Caniça M. Invasive pneumococcal disease in Portugal prior to and after the introduction of pneumococcal heptavalent conjugate vaccine. FEMS Immunol Med Microbiol. 2007;51:35-42. [PubMed] |
| 39. | Vestrheim DF, Høiby EA, Bergsaker MR, Rønning K, Aaberge IS, Caugant DA. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine. 2010;28:2214-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Dubos F, Marechal I, Husson MO, Courouble C, Aurel M, Martinot A. Decline in pneumococcal meningitis after the introduction of the heptavalent-pneumococcal conjugate vaccine in northern France. Arch Dis Child. 2007;92:1009-1012. [PubMed] |
| 41. | Fenoll A, Granizo JJ, Aguilar L, Giménez MJ, Aragoneses-Fenoll L, Hanquet G, Casal J, Tarragó D. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol. 2009;47:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Flannery B, Schrag S, Bennett NM, Lynfield R, Harrison LH, Reingold A, Cieslak PR, Hadler J, Farley MM, Facklam RR. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Giele C, Moore H, Bayley K, Harrison C, Murphy D, Rooney K, Keil AD, Lehmann D. Has the seven-valent pneumococcal conjugate vaccine had an impact on invasive pneumococcal disease in Western Australia? Vaccine. 2007;25:2379-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Schutze GE, Tucker NC, Mason EO. Impact of the conjugate pneumococcal vaccine in arkansas. Pediatr Infect Dis J. 2004;23:1125-1129. [PubMed] |
| 45. | Guevara M, Barricarte A, Gil-Setas A, García-Irure JJ, Beristain X, Torroba L, Petit A, Polo Vigas ME, Aguinaga A, Castilla J. Changing epidemiology of invasive pneumococcal disease following increased coverage with the heptavalent conjugate vaccine in Navarre, Spain. Clin Microbiol Infect. 2009;15:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Haddy RI, Perry K, Chacko CE, Helton WB, Bowling MG, Looney SW, Buck GE. Comparison of incidence of invasive Streptococcus pneumoniae disease among children before and after introduction of conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2005;24:320-323. [PubMed] |
| 47. | Hanna JN, Humphreys JL, Murphy DM. Invasive pneumococcal disease in Indigenous people in north Queensland: an update, 2005-2007. Med J Aust. 2008;189:43-46. [PubMed] |
| 48. | Hanquet G, Lernout T, Vergison A, Verhaegen J, Kissling E, Tuerlinckx D, Malfroot A, Swennen B, Sabbe M; Belgian IPD Scientific Committee. Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine. 2011;29:2856-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Harboe ZB, Valentiner-Branth P, Benfield TL, Christensen JJ, Andersen PH, Howitz M, Krogfelt KA, Lambertsen L, Konradsen HB. Early effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish Childhood Immunization Programme. Vaccine. 2010;28:2642-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Hennessy TW, Singleton RJ, Bulkow LR, Bruden DL, Hurlburt DA, Parks D, Moore M, Parkinson AJ, Schuchat A, Butler JC. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23:5464-5473. [PubMed] |
| 51. | Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54:893-897. [PubMed] |
| 52. | Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, Farley MM, Jorgensen JH, Lexau CA, Petit S. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 359] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 53. | Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24:17-23. [PubMed] |
| 54. | Hsu KK, Shea KM, Stevenson AE, Pelton SI; Massachusetts Department of Public Health. Changing serotypes causing childhood invasive pneumococcal disease: Massachusetts, 2001-2007. Pediatr Infect Dis J. 2010;29:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Ingels H, Rasmussen J, Andersen PH, Harboe ZB, Glismann S, Konradsen H, Hoffmann S, Valentiner-Branth P, Lambertsen L; Danish Pneumococcal Surveillance Collaboration Group 2009-2010. Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine. 2012;30:3944-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Wenger JD, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, Parks D, Rudolph K, Hurlburt D, Ritter T. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J. 2010;29:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Johnson DR, D’Onise K, Holland RA, Raupach JC, Koehler AP. Pneumococcal disease in South Australia: vaccine success but no time for complacency. Vaccine. 2012;30:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis. 2009;49:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455-1463. [PubMed] |
| 60. | Leal J, Vanderkooi OG, Church DL, Macdonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J. 2012;31:e169-e175. [PubMed] |
| 61. | Liao WH, Lin SH, Lai CC, Tan CK, Liao CH, Huang YT, Wang CY, Hsueh PR. Impact of pneumococcal vaccines on invasive pneumococcal disease in Taiwan. Eur J Clin Microbiol Infect Dis. 2010;29:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Messina AF, Katz-Gaynor K, Barton T, Ahmad N, Ghaffar F, Rasko D, McCracken GH. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr Infect Dis J. 2007;26:461-467. [PubMed] |
| 63. | Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 64. | Patrzalek M, Gorynski P, Albrecht P. Indirect population impact of universal PCV7 vaccination of children in a 2 + 1 schedule on the incidence of pneumonia morbidity in Kielce, Poland. Eur J Clin Microbiol Infect Dis. 2012;31:3023-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Pérez A, Giménez M, Sala P, Sierra M, Esteve A, Rodrigo C. Increase in invasive nonvaccine pneumococcal serotypes at two hospitals in Barcelona: was replacement disease to blame? Acta Paediatr. 2011;100:1572-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Pérez-Trallero E, Marimon JM, Ercibengoa M, Vicente D, Pérez-Yarza EG. Invasive Streptococcus pneumoniae infections in children and older adults in the north of Spain before and after the introduction of the heptavalent pneumococcal conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2009;28:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 1028] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 68. | Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, Lexau CA, Thomas AR, Harrison LH, Reingold AL. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 331] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 69. | Incidence of invasive pneumococcal disease after introduction of the Universal Infant Immunization Program, British Columbia (2002-2005). Can Commun Dis Rep. 2006;32:157-161. [PubMed] |
| 70. | Ramani RR, Hall WN, Boulton M, Johnson DR, Zhu BP. Impact of PCV7 on invasive pneumococcal disease among children younger than 5 years: a population-based study. Am J Public Health. 2004;94:958-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Rendi-Wagner P, Paulke-Korinek M, Kundi M, Burgmann H, Georgopoulos A, Vécsei A, Kollaritsch H. National paediatric immunization program of high risk groups: no effect on the incidence of invasive pneumococcal diseases. Vaccine. 2009;27:3963-3968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, Spanjaard L, Sanders EA, van der Ende A. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis. 2010;16:816-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Rückinger S, van der Linden M, Reinert RR, von Kries R, Burckhardt F, Siedler A. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine. 2009;27:4136-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | de Sevilla MF, García-García JJ, Esteva C, Moraga F, Hernández S, Selva L, Coll F, Ciruela P, Planes AM, Codina G. Clinical presentation of invasive pneumococcal disease in Spain in the era of heptavalent conjugate vaccine. Pediatr Infect Dis J. 2012;31:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Shafinoori S, Ginocchio CC, Greenberg AJ, Yeoman E, Cheddie M, Rubin LG. Impact of pneumococcal conjugate vaccine and the severity of winter influenza-like illnesses on invasive pneumococcal infections in children and adults. Pediatr Infect Dis J. 2005;24:10-16. [PubMed] |
| 76. | Shah SS, Ratner AJ. Trends in invasive pneumococcal disease-associated hospitalizations. Clin Infect Dis. 2006;42:e1-e5. [PubMed] |
| 77. | Techasaensiri C, Messina AF, Katz K, Ahmad N, Huang R, McCracken GH. Epidemiology and evolution of invasive pneumococcal disease caused by multidrug resistant serotypes of 19A in the 8 years after implementation of pneumococcal conjugate vaccine immunization in Dallas, Texas. Pediatr Infect Dis J. 2010;29:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis. 2008;46:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 79. | Tsigrelis C, Tleyjeh IM, Huskins WC, Lahr BD, Nyre LM, Virk A, Baddour LM. Incidence of invasive pneumococcal disease among children after introduction of a 7-valent pneumococcal conjugate vaccine: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2009;84:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 80. | Tyrrell GJ, Lovgren M, Chui N, Minion J, Garg S, Kellner JD, Marrie TJ. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae pre- and post-seven valent pneumococcal conjugate vaccine introduction in Alberta, Canada, 2000-2006. Vaccine. 2009;27:3553-3560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | van der Linden M, Weiß S, Falkenhorst G, Siedler A, Imöhl M, von Kries R. Four years of universal pneumococcal conjugate infant vaccination in Germany: impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine. 2012;30:5880-5885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Vestrheim DF, Løvoll O, Aaberge IS, Caugant DA, Høiby EA, Bakke H, Bergsaker MR. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine. 2008;26:3277-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 83. | Weatherholtz R, Millar EV, Moulton LH, Reid R, Rudolph K, Santosham M, O’Brien KL. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1706] [Cited by in RCA: 1624] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 85. | Winters M, Patrick DM, Marra F, Buxton J, Chong M, Isaac-Renton JL, Shaw C, Tyrrell GJ, Lovgren M, Paulus S. Epidemiology of invasive pneumococcal disease in BC during the introduction of conjugated pneumococcal vaccine. Can J Public Health. 2008;99:57-61. [PubMed] |
| 86. | Yildirim I, Stevenson A, Hsu KK, Pelton SI. Evolving picture of invasive pneumococcal disease in massachusetts children: a comparison of disease in 2007-2009 with earlier periods. Pediatr Infect Dis J. 2012;31:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Godwin M, Ruhland L, Casson I, MacDonald S, Delva D, Birtwhistle R, Lam M, Seguin R. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. [PubMed] |
| 88. | Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201:1607-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 89. | Lee KK, Rinaldi F, Chan MK, Chan ST, So TM, Hon EK, Lee VW. Economic evaluation of universal infant vaccination with 7vPCV in Hong Kong. Value Health. 2009;12 Suppl 3:S42-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Marchetti M, Colombo GL. Cost-effectiveness of universal pneumococcal vaccination for infants in Italy. Vaccine. 2005;23:4565-4576. [PubMed] |
| 91. | Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O’Brien KL, Moore MR; Serotype Replacement Study Group. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10:e1001517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 92. | Hanquet G, Kissling E, Fenoll A, George R, Lepoutre A, Lernout T, Tarragó D, Varon E, Verhaegen J. Pneumococcal serotypes in children in 4 European countries. Emerg Infect Dis. 2010;16:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
P- Reviewer: Sadoghi P, Trohman RG S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/