Published online Nov 26, 2014. doi: 10.13105/wjma.v2.i4.135
Revised: June 30, 2014
Accepted: August 27, 2014
Published online: November 26, 2014
Processing time: 283 Days and 0.7 Hours
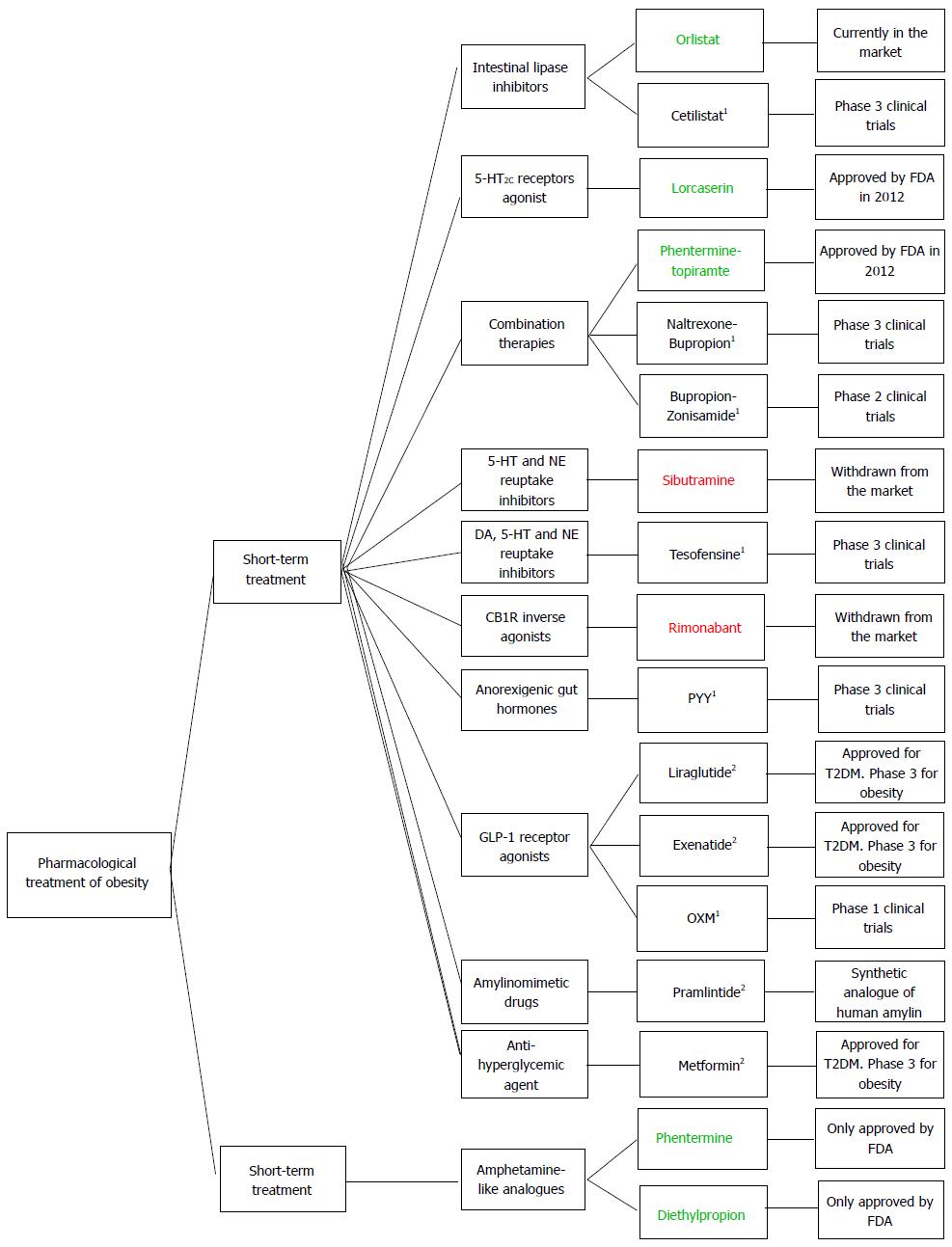
Obesity is a chronic disease which requires treatment. As lifestyle interventions alone hardly ever result in long-term weight loss, pharmacotherapy is an important adjunct to lifestyle measures to improve the induction and maintenance of weight loss. Owing to the limited options currently available for the pharmacological treatment of obesity, it is imperative to develop new safe compounds. This study aims to review the current medications approved by European Medicines Agency and United States Food and Drug Administration (FDA) for the treatment of obesity, focusing essentially on their benefits and risks, as well as on the new drugs which are presently under clinical trials. Moreover, it lists the anti-obesity agents that have been recently withdrawn from the market. A revision of the scientific literature was carried out, through a search on Pubmed for papers published from January 2010 to January 2013. Orlistat (Xenical®) is currently the only long-term pharmacotherapy for obesity available in the European market, as rimonabant and sibutramine were withdrawn in 2008 and 2010, respectively, due to serious psychiatric and cardiovascular adverse effects. Lorcaserin (Belviq®) and the association of phentermine and topiramate (QsymiaTM) were recently approved by FDA. Orlistat suppresses appetite inhibiting gastrointestinal lipase, being its adverse effects mostly gastrointestinal. Lorcaserin activates 5-HT2C receptors, phentermine is a norepinephrine releasing drug, and topiramate is an anticonvulsivant drug with weight loss properties.
Core tip: Obesity is a chronic disease which requires treatment. As lifestyle interventions alone hardly ever result in long-term weight loss, pharmacotherapy is an important adjunct to lifestyle measures to improve the induction and maintenance of weight loss. This study reviews the current medications approved by European Medicines Agency and United States Food and Drug Administration for the treatment of obesity, focusing essentially on their benefits and risks, as well on the new drugs under clinical trials. An original algorithm for assessment and treatment of overweight and obese patients in clinical practice was also developed.
- Citation: Martins A, Morgado S, Morgado M. Anti-obesity drugs currently used and new compounds in clinical development. World J Meta-Anal 2014; 2(4): 135-153
- URL: https://www.wjgnet.com/2308-3840/full/v2/i4/135.htm
- DOI: https://dx.doi.org/10.13105/wjma.v2.i4.135
Obesity is a global epidemic, which is increasing not only in Western cultures, but also in Mexico, Brazil, China and parts of Africa, due to increasing urbanization[1,2], and underlying sedentary lifestyles and overconsumption of calories. According to the most recent estimates by the World Health Organization, more than 1 billion people in the world are overweight or obese, accounting for up to 7% of the health care cost in most developed countries[3]. It is estimated that obesity affects 10%-30% of adults in the European Union (EU) countries and 46.3% in the United States[4]. This chronic disease increases morbidity and mortality, largely due to associated comorbidities, including type 2 diabetes mellitus (T2DM), cardiovascular diseases, premature atherosclerosis, metabolic syndrome, liver disease and cancer[5-9]. About 90% of cases of T2DM are due to excess weight, so professional societies recommend weight loss as a key initial step in the management of overweight or obese T2DM patients[10]. Furthermore, there is a five-to-six times increase in hypertension among obese individuals compared to those with normal weight[11,12].
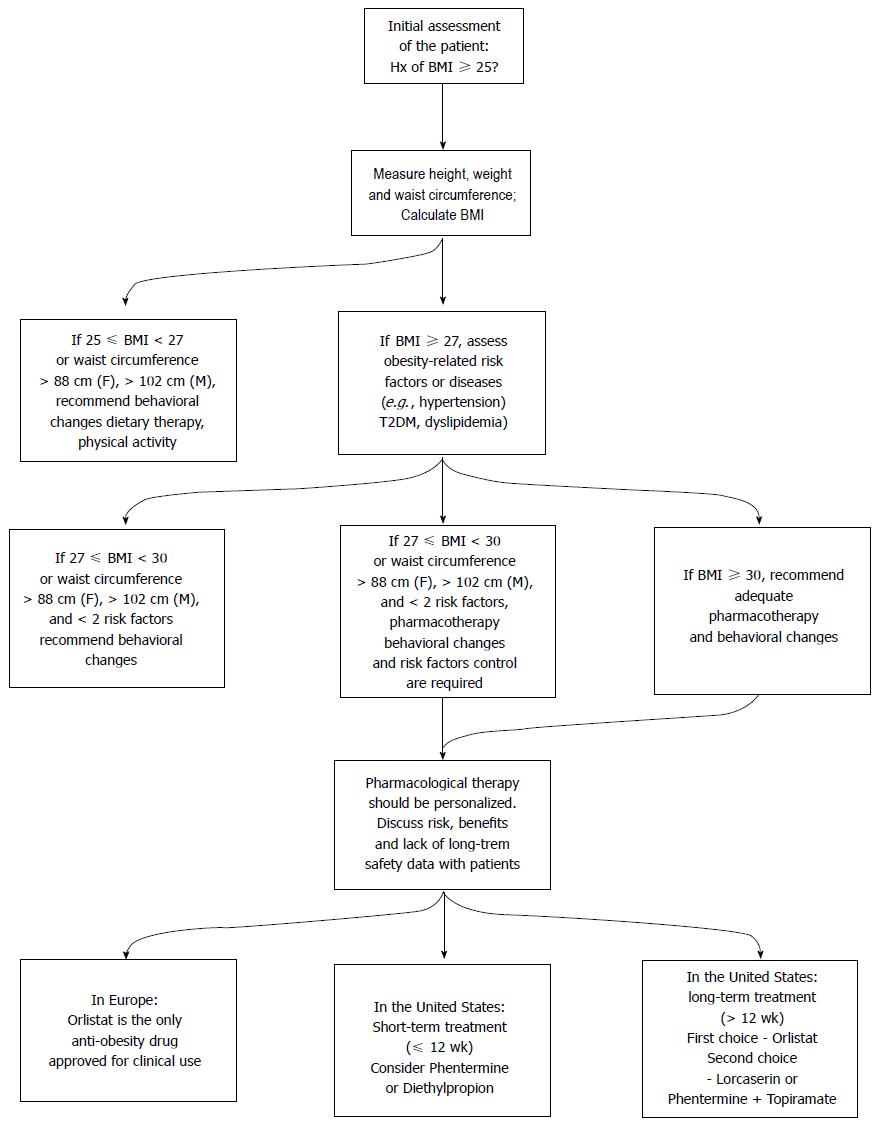
The relation between obesity and several of its comorbidities appears to be mediated by risk factors characterized by the metabolic syndrome, which is closely associated with abdominal obesity[13-15]. Weight loss achieved by changes in eating behavior, diet and physical activity are the cornerstones in the treatment of the metabolic syndrome. However, weight control methods often produce short-term success, while sustained weight maintenance is difficult to achieve[16,17]. Therefore, pharmacological therapy has been proposed as an adjunct to diet and lifestyle changes to improve maintenance of weight loss[18]. Drug therapy is considered for individuals with a body mass index (BMI) greater than 30 kg/m2 or ranging from 25 to 30 kg/m2 if they have concomitant obesity-related risk factors or diseases[19].
Improving diet (i.e., reduction in caloric intake) and increasing physical activity can address obesity. Nevertheless, adherence to lifestyle changes can be challenging for many reasons, such as a lack of will for change on the part of the patient, physical restrictions that limit activity and the need for a multidisciplinary health care team for effective treatment. In severe cases of obesity, with possibly evident comorbidities, pharmacological treatment or bariatric surgery are considered in addition to lifestyle changes[20].
In contrast to orlistat, a gastric and pancreatic lipase inhibitor that inhibits the absorption of fat from the intestines, all of the other mentioned drugs act on the central nervous system (CNS) to suppress appetite, thus reducing food intake, and/or stimulate energy expenditure, thereby reducing body weight. Orlistat is the first prescription treatment for obesity that does not act as an appetite suppressant, incapacitating lipase to break triglycerides down into fatty acids and monoglycerides. It also improves cardiovascular risk factors, including serum triglycerides levels[21-23].
There are currently several drugs towards the pharmacological treatment of obesity under clinical trials. An anti-obesity drug that is already on an advanced stage of clinical trials is Contrave®, a sustained-release formulation of bupropion and naltrexone, which significantly reduced body weight in a phase 3 trial[24]. The FDA has required an additional clinical trial prior to the approval of the drug, because of concern over the cardiovascular safety profile[25].
Phentermine hydrochloride (Adipex-P®) is a sympathomimetic anorectic drug of the phenethylamine class, with pharmacology similar to amphetamine. It is thought to act on the hypothalamus stimulating the adrenal glands to release norepinephrine, thus reducing appetite[26], although it has not been established that the primary action of amphetamine anorectics in treating obesity is appetite suppression, since other CNS actions or metabolic effects may also be involved[27]. It is approved by FDA since 1959 for short-term (≤ 12 wk) treatment of obesity as monotherapy (15-37.5 mg/d) in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction, in the management of exogenous obesity in patients with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of other risk factors (e.g., hypertension, diabetes, dyslipidemia)[28]. In 2005, it was still the most prescribed anti-obesity drug in the United States[29]. However, it was definitely withdrawn from the EU market in May 2001, after a recommendation by European Medicines Agency (EMA) due to an unfavourable risk to benefits ratio, including tolerance and possible abuse potential[30]. Because phentermine has sympathomimetic properties, some reported adverse effects were insomnia, tremor, elevation in blood pressure and pulse rate, headache, palpitation and constipation[31].
Diethylpropion hydrochloride (Tenuate®), also known as amfepramone, is another amphetamine-like analogue that was also approved by FDA in 1959 as a short-term (≤ 12 wk) adjunct in the treatment of exogenous obesity as monotherapy only (one 25 mg tablet three times daily or one 75 mg controlled-release tablet once a day), in a regimen of weight reduction based on caloric restriction in patients with a BMI ≥ 30 kg/m2[32,33]. Diethylpropion, as an amphetamine-like analogue, stimulates neurons to release or maintain high levels of catecholamines, namely dopamine and norepinephrine. High levels of these catecholamines tend to suppress hunger signals and appetite. Diethylpropion, through catecholamine elevation, may also indirectly affect leptin levels in the brain. It is theorized that diethylpropion can raise levels of leptin, which signal satiety. It is also theorized that increased levels of the catecholamines are partially responsible for halting neuropeptide Y. This peptide initiates eating, decreases energy expenditure, and increases fat storage[34]. The most common side effects are dry mouth and insomnia[35]. In 2001, EMA also recommended its withdrawal from the EU market, for the same reasons as phentermine[30].
Orlistat: Orlistat was until recently the only pharmacological weight loss drug on the international market. Xenical® (orlistat 120 mg) was approved as a prescription drug by FDA in 1999 for obesity management and to reduce the risk of regaining weight after prior weight loss. In 2007, Alli (orlistat 60 mg) was approved for over-the-counter use for weight loss in overweight adults (≥ 18 years), along with a reduced-calorie and low-fat diet[36]. Orlistat reversibly blocks the gastrointestinal lipase activity and thus the absorption of about 25% of ingested fat. The 60 mg dose achieves a weight loss of about 5% of total body weight over a 16 wk period, improving insulin resistance and lipid profile[37]. Weight loss with orlistat is about 3% (placebo-subtracted), but meets FDA categorical standard (FDA categorical weight loss is the proportion of subjects who lose at least 5% of baseline body weight in the active-product vs placebo-treated group.)[38]. It is indicated for obese individuals aged 18 or over with an initial BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of other risk factors. The safety and efficacy of Xenical® were evaluated in obese adolescent patients aged 12 to 16 years old. It was concluded that the use of Xenical® in this age group is supported by evidence from adequate and well-controlled studies of Xenical® in adults, from a 54-wk efficacy and safety study and a 21-d mineral balance study in obese adolescent patients aged 12 to 16 years. However, unlike FDA, EMA did not approve the use of orlistat in patients below 18 years of age. With respect to pediatric patients below the age of 12, safety and effectiveness have not been established yet and its use is not approved in this population[39]. The Royal Pharmaceutical Society recommends that BMI of individuals requesting orlistat is always checked and that patients should be advised to take an additional multivitamin supplement, to counteract the loss of fat-soluble vitamins[40]. As it is a lipases inhibitor, orlistat has several potential adverse effects patients need to be aware of, most of them involving the gastrointestinal system.
When combined with a reduced-calorie diet, orlistat has proved to be more effective than diet alone[41]. However, another study, carried out in order to compare a low-carbohydrate, ketogenic diet with orlistat therapy combined with a low fat diet over 48 wk, showed a statistically significant mean weight loss, although similar in the two groups (i.e., -9.5% vs -8.5%)[42]. This intervention also had similar beneficial effects on most measures of cardiovascular disease risk, including waist circumference (which decreased as well), fasting serum lipid profiles and C-reactive protein. Weight loss was significantly greater in participants who attended group sessions regularly, which may indicate the usefulness of these sessions in motivating patients. Efforts should hence be made to incorporate similarly intensive weight loss programs into medical practice[42].
Another study was conducted to compare the effects of one year treatment with orlistat plus L-carnitine and orlistat alone on body weight, glycemic and lipid control and insulin resistance in uncontrolled T2DM[43]. This study led to the conclusion that the association of orlistat plus L-carnitine was more effective than orlistat monotherapy in improving body weight, glycemic and lipid profile, insulin resistance and inflammatory parameters. A weight loss was observed from baseline of 9.5% in the orlistat group and of 11.3% in the orlistat + L-carnitine group. No significant adverse effects were reported. These positive effects can be due to synergism between these two drugs[43].
A Portuguese study, carried out by de Castro et al[44], compared the efficacy and safety of orlistat vs placebo in obese patients with mild to moderate hypercholesterolemia, concluding that treatment with orlistat, plus a reduced calorie diet for 6 mo, achieved significant reductions in weight, BMI and in total and low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol changes being similar in both treatment groups. The mean difference in weight from baseline was 5.9% in the orlistat group and 2.3% in the placebo group. By the end of the study, 49.1% of patients in the orlistat group had lost 5%-10% of their baseline weight, compared to 22.4% of the placebo group and 8.8% had lost over 10% (4.7% more than the placebo group). The frequency of gastrointestinal adverse effects was slightly higher in the orlistat than in the placebo group[44].
Cetilistat: Cetilistat is a new, highly lipophilic benzoxazinone inhibitor of gastrointestinal and pancreatic lipases, which is currently under development for the management of weight loss in obese patients. In a 12-wk, Phase 2 clinical trial in obese patients without medicated comorbidities, administration of cetilistat three times daily combined with a hypocaloric diet produced significantly greater weight loss than placebo[45]. Furthermore, it was well tolerated, the adverse effects being predominantly gastrointestinal and mild (i.e., aware of signs or symptoms, easily tolerated) or moderate (i.e., sign or symptom causes discomfort but does not interfere with normal activities) in severity[45].
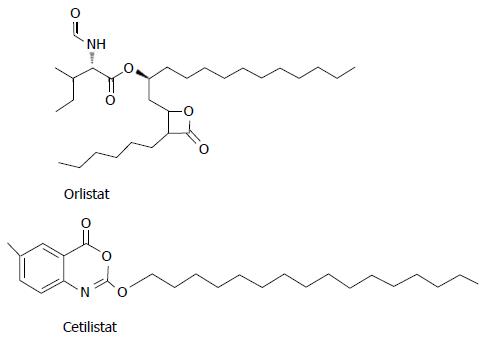
A randomized, double-blind study was carried out to determine the efficacy and safety of cetilistat and orlistat relative to placebo in obese patients with T2DM, treated with metformin[46]. Twelve weeks of treatment with cetilistat 80 or 120 mg three times daily significantly reduced body weight and improved glycemic control in obese T2DM patients. Absolute weight loss was significantly greater in the cetilistat 80 and 120 mg dose groups than in the placebo group (P = 0.01 and P = 0.0002, respectively), but in the group treated with cetilistat 40 mg weight loss was similar to placebo. Cetilistat was well tolerated, having withdrawals due to adverse effects been similar to placebo and markedly less frequent than with orlistat. Discontinuation in the orlistat group was entirely due to gastrointestinal adverse effects. The high level of tolerability to cetilistat led to an increase in compliance and could be clinically significant in the management of this patient population. The difference in adverse effects between cetilistat and orlistat could be attributable to structural differences between them (Figure 1). Although both molecules are lipase inhibitors, the chemical structural differences concerning hydrophilic and lipophilic components may influence the way they interact with fat micelles in the intestine. Orlistat, unlike cetilistat, may hence promote coalescence of micelles, leading to oils and increased gastrointestinal adverse effects[46].
A Bryson et al[47] article, published in 2009, described three phase 1 studies, which demonstrated that cetilistat effectively inhibits gastrointestinal lipases, substantially increasing the amount of faecal fat excreted in healthy male volunteers receiving a controlled calorie diet. It also stated that faecal fat excretion in the cetilistat groups was at least comparable to the orlistat 120 mg three times daily group[47].
In December 2008, phase 2 clinical trials data showing that cetilistat 80 mg and 120 mg promoted significant weight loss compared with placebo (3.85 kg and 4.32 kg vs 2.86 kg, respectively) and was well tolerated in clinically obese patients encouraged Takeda Pharmaceutical Company Limited to start a phase 3 clinical trial in Japan[48].
Lorcaserin: Lorcaserin is a potent and selective serotonin 2C receptor agonist, which has been recently (June 2012) approved by FDA for chronic weight management as Belviq® (lorcaserin hydrochloride), in addition to a hypocaloric diet and exercise. The drug is approved for use in obese (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) adults who have at least one weight related condition, such as hypertension, T2DM or dyslipidemia[49].
Lorcaserin activates 5-hidroxytryptamine (5-HT or serotonin) receptors 5-HT2C, suppressing appetite through the pro-opiomelanocortin (POMC) neurons. This anti-obesity drug reduces body weight both in rodents and humans, although it is not clear whether weight loss is a result of reduced energy intake only or also of enhanced energy expenditure. When gauged by the standards of the Division’s 2007 draft guidance for Developing Products for Weight Management, the mean weight loss associated with the lorcaserin 10 mg once daily and twice daily dose was 3%-4% greater than the mean weight loss with placebo. Therefore lorcaserin did not satisfy the guidance’s mean efficacy criterion. However, the lorcaserin 10 mg twice a day dose did, by a slim margin, satisfy the categorical efficacy criterion[49,50].
Martin et al[51] investigated the effect of lorcaserin on energy intake and energy expenditure, having concluded, after 7 d of weight maintenance (consisting of a diet and exercise plan), that lorcaserin significantly reduced energy intake and appetite. However, it did not alter either energy expenditure or respiratory quotient (RQ) (The ratio of the volume of carbon dioxide produced to the volume of oxygen consumed per unit of time by the body; usually corresponding to the volumes given off and taken up by the lungs. It varies with the source of food energy used.). In combination with a healthy lifestyle program, lorcaserin treatment (10 mg twice daily) resulted in significantly larger weight loss than placebo (lorcaserin, -3.8 ± 0.4 kg; placebo, -2.2 ± 0.5 kg; P < 0.01) over 56 d[51]. Furthermore, treatment with lorcaserin improved the risk factors of cardiovascular disease, namely total and LDL cholesterol, which were significantly reduced, as well as diastolic blood pressure[51]. Concerning adverse effects, lorcaserin was generally well tolerated, headache being the most common adverse effect[51].
Another 2-year trial, carried out by Smith et al[52], and known as Behavioral Modification and Lorcaserin for Overweight and Obesity Management trial, led to the conclusion that lorcaserin, along with behavioral modification (consisting of a nutrition and physical exercise program), produced significant mean weight loss and improved maintenance of weight loss, when compared with placebo (5.8% vs 2.2%, P < 0.001). It was also observed that, at the end of year 1, 47.5% of patients who received lorcaserin lost, at least, 5% of their initial body weight, compared to 20.3% of patients who received placebo (i.e., the proportion of patients in the lorcaserin group was more than twice that in the placebo group) (P < 0.001). The average weight loss achieved in the lorcaserin group after 1 year was associated with an improvement in plasma lipid levels, insulin resistance, and blood pressure. Furthermore, lorcaserin produced a reduction in waist circumference and in the levels of markers of inflammation, such as high-sensitivity C-reactive protein (hs-CRP), thus reducing the risk of future cardiovascular events. Total and LDL cholesterol, as well as triglyceride levels, at the end of year 1 were considerably lower in the lorcaserin group than in the placebo group, but it was increased at the end of year 2 in both groups. Concerning adverse effects, more patients in the lorcaserin group than in the placebo group withdrew from the study because of headache (2.0% vs 0.8%) and dizziness (0.8% vs 0.1%). At the end of year 1, 2.3% of patients in the placebo group and 2.7% of patients in the lorcaserin group developed serotonin-associated valvulopathy (P = 0.70) (relative risk with lorcaserin, 1.1; 95%CI: 0.69-1.85). On the other hand, in year 2, the rate of heart valvulopathy was 2.7% in the placebo group and 2.6% in patients who received lorcaserin during year 1 and year 2[52].
Belviq® should not be used during pregnancy. Treatment may lead to serious adverse effects, including serotonin syndrome, particularly when administered with drugs commonly used to treat depression and migraine, and disturbances in attention or memory. However, the most common adverse effects of Belviq® are headache, dizziness, fatigue, nausea, dry mouth and constipation in non-diabetic patients; and hypoglycemia, headache, back pain, cough and fatigue in diabetic patients. Belviq® should be used with caution in patients with congestive heart failure, due to a possible increase in the number of 5-HT2B receptors in this pathology[49].
Phentermine/topiramate: Topiramate, used as anticonvulsivant and for prevention of migraine headaches, has shown weight loss properties but is not currently approved as a sole therapy for obesity[53]. The mechanism of action for topiramate is unknown, although it is known that it blocks repetitive firing by acting on sodium channels, may enhance GABAA-mediated chloride ions flux, and seems to be an antagonist of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and kainate receptors, blocking the effect of glutamate (an excitatory neurotransmitter)[54].
Qsymia™, an extended-release formulation of phentermine hydrochloride and topiramate, has been approved by FDA, in July 2012, as weight loss therapy, in combination with a reduced-calorie diet and exercise. The drug is approved for use in obese (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) adults, who have at least one weight-related comorbidity, such as hypertension, T2DM or dyslipidemia. Qsymia™ is available in four different extended-release formulations: 3.75 mg of phentermine and 23 mg of topiramate; 7.5 mg of phentermine and 46 mg of topiramate; 11.25 mg of phentermine and 69 mg of topiramate; and a higher dose containing 15 mg of phentermine and 92 mg of topiramate for specific patients. Qsymia™ should be taken once a day in the morning, with or without food. The dose of Qsymia™ should be increased if the patient does not lose a certain amount of weight within the first 12 wk of treatment at the recommended dose. If, after an additional 12 wk period of treatment on a higher dose, the patient has not lost a certain amount of weight, treatment should be discontinued[55]. With respect to pharmacological interactions, Qsymia™ should not be used with: birth control (contraceptive) medications, diuretics (e.g., hydrochlorotiazide), carbonic anhydrase inhibitors, anticonvulsivant medicines (e.g., valproic acid) and medicines that impair or decrease thinking, concentration or muscle coordination[55].
Qsymia™ must not be used in patients with glaucoma or hyperthyroidism and can increase heart rate 0.6 to 1.6 beats per minute (bpm)[56]. Its use in patients with recent or unstable heart disease or stroke is, therefore, not recommended. Heart rate should be regularly monitored in these patients, especially if they are starting or increasing the dose. The FDA approved Qsymia™ with a Risk Evaluation and Mitigation Strategy, the aim of which is to educate prescribers and their patients about the increased risk of birth defects associated with first 3 mo of exposure to Qsymia™, the need for pregnancy prevention and the need to discontinue therapy in pregnancy[57]. The most common adverse effects of Qsymia™ are paresthesia of hands and feet, dizziness, altered taste sensation, insomnia, constipation and dry mouth[57].
Allison et al[58] conducted a 56-wk randomized controlled trial to evaluate the safety and efficacy of a controlled-release formulation of phentermine and topiramate (PHEN/TPM CR) for weight loss and metabolic improvements. In this study, men and women with class II and III obesity (BMI ≥ 35 kg/m2) were randomized to placebo, PHEN/TPM CR 3.75/23 mg or PHEN/TPM CR 15/92 mg, in addition to a low-energy diet. PHEN/TPM CR demonstrated dose-related beneficial effects on weight and metabolic variables, having not been reported serious adverse effects resulting from treatment. Concerning mean weight loss, patients in the placebo, PHEN/TPM 3.75/23, and PHEN/TPM 15/92 groups lost 1.6%, 5.1%, and 10.9% of baseline body weight (BW), respectively, at 56 wk (P < 0.0001). In categorical analysis, 17.3% of placebo patients, 44.9% of PHEN/TPM 3.75/23 patients, and 66.7% of PHEN/TPM 15/92 patients, lost at least 5% of baseline BW at 56 wk (P < 0.0001)[58].
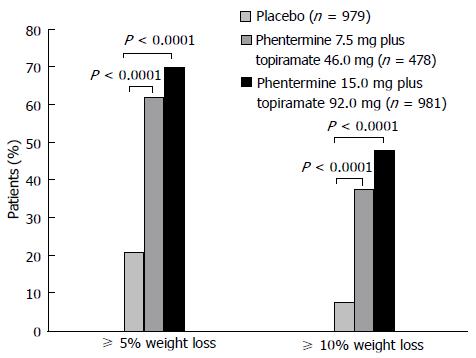
The same authors developed a 56-wk, randomized, placebo-controlled, Phase 3 trial, known as the CONQUER study[59], in order to evaluate the efficacy and safety of two doses of Phentermine/Topiramate CR, in combination with diet and lifestyle modification in overweight and obese adults (Figure 2). These authors demonstrated that both doses of the controlled formulation of phentermine and topiramate, along with lifestyle interventions, are useful for the treatment of obesity, both in reducing weight and improving cardiometabolic variables (blood pressure, waist circumference, concentrations of lipids, glycemia, adiponectin)[59]. It was obtained a mean weight loss (placebo-subtracted) of 6.6% and 8.6% with PHEN/TPM 7.5/46 mg and PHEN/TPM 15/92 mg, respectively, and a categorical weight loss of 62% and 70% with PHEN/TPM 7.5/46 mg and PHEN/TPM 15/92 mg, respectively. These results satisfy the FDA mean and categorical efficacy draft guidance criteria[38]. Furthermore, improvements in risk factors were most pronounced in patients with pre-existing comorbidities. Psychiatric and cognitive adverse dose-dependent effects occurred mainly during the early phase of treatment, and were resolved on drug discontinuation. It is important to mention that patients with clinically significant depression were excluded from this study[59].
The SEQUEL study, a double-blind placebo controlled, 52-wk extension of the CONQUER study[53], was designed to evaluate the longer-term efficacy and safety of lifestyle modification and two doses of PHEN/TPM CR for an additional 52-wk period (in patients who had completed the CONQUER study). Mean weight losses from baseline were -1.8%, -9.3% and -10.5% for placebo, PHEN/TPM 7.5/46 mg and PHEN/TPM 15/92 mg, respectively. A weight loss of 10% was achieved by more than 50% of subjects treated with PHEN/TPM CR. Phentermine/Topiramate CR improved cardiovascular and metabolic variables (e.g., hyperglycemia, dyslipidemia, hypertension), despite reduced use of concomitant medications to control blood pressure, lipid variables, and glycemic variables and decreased the rate of progression to T2DM. The greatest benefic effects were seen in patients receiving PHEN/TPM CR 15 mg/92 mg[53]. However, a mean increase was reported in heart rate of 0.4 bpm in placebo subjects, 1.3 bpm in PHEN/TPM CR 7.5/46 subjects, and 1.7 bpm in PHEN/TPM CR 15/92 subjects. Despite this, PHEN/TPM CR was generally well tolerated, the most commonly reported adverse effects being upper respiratory infection, constipation, paraesthesia, sinusitis and dry mouth. During the extension study, discontinuation rates due to adverse effects were similar between placebo (3.1%) and patients treated with PHEN/TPM CR 7.5/46 (4.5%) and with PHEN/TPM CR 15/92 (4.4%). Therefore, PHEN/TPM CR constitutes a well-tolerated and effective option for the sustained treatment of obese individuals with cardiometabolic disease[53].
Naltrexone/bupropion: Naltrexone is a non-selective μ-opioid receptor antagonist, but is considered rather preferential for μ receptors. It is a synthetic analogue of oxymorphone and naloxone, a white crystalline compound soluble in water. Bupropion is an amino-ketone antidepressant, inhibiting dopamine reuptake. Its structure is closely related to that of diethylpropion, an anorexigenic and sympathomimetic agent previously mentioned. Bupropion is highly soluble in water and is related to the phenylethylamines, which are known for their stimulant effects[60].
Combined treatment with sustained-release (SR) naltrexone and bupropion (Contrave®) was developed to produce complementary actions in CNS pathways regulating body weight: on the one hand, by stimulating hypothalamic pro-opiomelanocortin (POMC) neurons with bupropion; on the other hand, by simultaneously blocking opioid-mediated POMC autoinhibition with naltrexone[24]. POMC producing neurons in the hypothalamus release α-melanocyte-stimulating hormone (α-MSH) and β-endorphin. α-MSH mediates the anorexigenic effect of POMC, whereas β-endorphin performs an autoinhibitory feedback through activation of opioid receptors on POMC neurons, thus inactivating the anorexigenic effect[61-63].
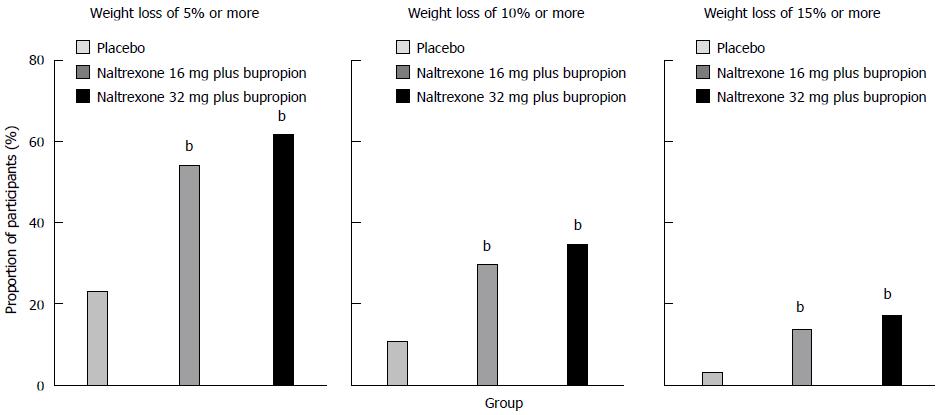
In February 2011, the FDA declined the approval of Contrave® due to concerns related to the long-term cardiovascular safety profile in overweight and obese individuals. The FDA advised the company responsible for the development of Contrave® (Orexigen Therapeutics Inc.) that they would have to conduct a randomized, double-blind, placebo-controlled trial in order to demonstrate that the risk of major cardiovascular adverse effects in overweight and obese patients receiving the drug does not negatively affect the risk-benefit profile[60,64]. One of the conducted trials was the Contrave Obesity Research-I (COR-I) study[24], a randomized, double-blind, placebo-controlled, phase 3, 56-wk trial, which examined the effect of such treatment on body weight in overweight and obese subjects from 34 locations in the United States. The participants were men and women aged between 18 and 65 years with: (1) a BMI ranging from 30 to 45 kg/m2 and uncomplicated obesity; or (2) a BMI ranging from 27 to 45 kg/m2 and dyslipidemia or hypertension. Participants were submitted to a mild hypocaloric diet and exercise and were randomly assigned in a 1:1:1 ratio to receive: (1) naltrexone 32 mg plus bupropion 360 mg; (2) naltrexone 16 mg plus bupropion 360 mg; and (3) a placebo (Figure 3).
The combination treatment produced greater improvements in waist circumference, insulin resistance, and concentrations of HDL cholesterol, triglycerides and hs-CRP compared with placebo[24]. Moreover, naltrexone 32 mg plus bupropion 360 mg originated a better improvement in fasting insulin and glucose concentrations[24]. The groups treated with naltrexone plus bupropion revealed a better weight-related quality of life than the placebo group did, mainly as a result of increased physical function and self-esteem[24]. Treatment with naltrexone 32 mg plus bupropion 360 mg provided greater weight loss as well as greater improvements in cardiometabolic risk factors and quality of life than the naltrexone 16 mg plus bupropion 360 mg formulation. In participants who completed 56 wk of treatment, weight loss was greater in the combination treatment groups (naltrexone 32 mg plus bupropion, -8.1%; naltrexone 16 mg plus bupropion, -6.7%) than in the placebo group (-1.8%). Due to the effects of bupropion, combination treatment originated a small and transient increase in mean systolic and diastolic blood pressure. However, it was subsequently observed that greater weight loss correlated with larger decreases in systolic and diastolic blood pressure in all treatment groups. Although more adverse effects were reported in the groups receiving naltrexone plus bupropion than in the placebo group, those adverse effects were mostly mild to moderate and transient, having been consistent with the known safety and tolerability profiles of the individual drugs. It was then concluded that treatment with sustained-release naltrexone plus bupropion provides a new approach to manage obesity that might improve the ability to control eating behavior and response to food images[24].
The effects of bupropion and naltrexone in addictive disorders, along with evidence suggesting synergism of these drugs in reward pathways, suggest that this combination improves control of eating and response to food cravings. Since many overweight adults report food cravings to be an important obstacle to their ability to adhere to a diet, these actions could add to the utility of naltrexone plus bupropion in treatment of obesity[65].
Another study was the COR-behavior modification (BMOD)[66], a 56-wk, randomized, placebo-controlled trial, aiming to evaluate the efficacy and safety of naltrexone plus bupropion, in addition to an intensive BMOD. The participants were randomly assigned, in a 1:3 ratio, to receive either placebo or naltrexone SR 32 mg/d plus bupropion SR 360 mg/d. The main positive endpoint in the patients receiving treatment was change in body weight, a decrease in weight of 5% or more from baseline at week 56 having been achieved. The larger weight losses produced by naltrexone 32 mg plus bupropion 360 mg were also associated with greater reductions in markers of cardiometabolic risk, such as waist circumference, triglyceride and fasting insulin concentrations. Furthermore, this drug combination, along with BMOD, produced an increase in HDL cholesterol concentration, compared with placebo + BMOD[66]. Despite the reduction in systolic and diastolic blood pressure being higher in the placebo group, additional analyses revealed a relationship between greater blood pressure reductions and greater weight loss in patients treated with naltrexone 32 mg plus bupropion 360 mg + BMOD. The most common adverse effect in the groups treated with naltrexone SR/bupropion SR was mild-to-moderate transient nausea, a well-known side effect of naltrexone, which was dose-dependent. Other reported adverse effects were constipation, dizziness, dry mouth, urticaria, anxiety, disturbance in attention, increase in blood pressure and vomiting. In conclusion, the combination of naltrexone SR/bupropion SR, along with an intensive behavioral modification program, achieved a greater weight loss than BMOD alone, having generally been well tolerated.
Bupropion/zonisamide: The sustained-release combination of bupropion with the anti-epileptic agent zonisamide (Empatic™), developed by Orexigen Therapeutics Inc., has been evaluated in three Phase 2 trials, having produced more weight loss than treatment with either drug given alone[67-69]. The second Phase 2b clinical trial was designed to assess the safety and efficacy of EmpaticTM in healthy, nondiabetic, obese patients[70].
Bupropion was approved by FDA in 1985 as antidepressant and in 1997 for smoking cessation. An immediate release formulation of zonisamide was approved in the United States in 2000 for the adjunctive treatment of partial seizures, a form of epilepsy. It is believed to exert its anticonvulsivant activity by blocking calcium and sodium channels[71]. The mechanism by which it decreases appetite is not known. Zonisamide increases dopamine and serotonin activities, possibly leading to a reduction in appetite and an increase in energy expenditure, and it is believed to suppress the neuropeptide Y/agouti-related protein (NPY/AgRP) neuron, further stimulating the POMC neurons[68]. It also inhibits carbonic anhydrase activity, which possibly alters the perception of taste[72].
A 24-wk Phase 2 study conducted by Orexigen Therapeutics Inc., demonstrated a weight loss of 7.5%, 6.1% and 1.4% from baseline weight with bupropion plus zonisamide 360 mg, bupropion plus zonisamide 120 mg and a placebo, respectively[73]. The most frequently reported adverse effects of zonisamide SR/bupropion SR combination were headache, nausea and insomnia[71].
Tesofensine: Tesofensine was initially developed for the treatment of Parkinson’s disease or Alzheimer’s disease, although it demonstrated a limited efficacy for this application[74]. However, it is a very promising development drug candidate for obesity management, having produced twice the weight loss seen with currently marketed drugs (-7.2 ± 6.7 kg, P < 0.0001)[75]. It simultaneously inhibits three monoaminergic neurotransmitters (dopamine, norepinephrine and serotonin) presynaptic reuptake, thereby increasing their neurotransmission. Dopamine acts in the nucleus accumbens of the forebrain, modulating reward and the “pleasure” feeling of food. Serotonin and norepinephrine act on the hypothalamus, increasing metabolism and reducing appetite. Tesofensine is under development by NeuroSearch for the potential treatment of obesity[76].
Sjödin et al[77] investigated the mechanisms by which tesofensine produces weight loss, measuring energy expenditure and appetite sensations in overweight and obese individuals. Tesofensine induced a 1.8 kg weight loss above placebo after 2 weeks’ treatment (P < 0.0001). It was concluded that tesofensine has a significant effect on appetite sensations and a slight effect on energy expenditure at night and that both effects can contribute to the strong effect of tesofensine in weight reduction[77].
Phase 2 clinical trials with tesofensine were conducted in obese individuals and it was observed that tesofensine produced dose-related reductions in body weight, body fat and waist circumference, as well as improvements in other obesity-related endocrine factors. At the highest dose tested, tesofensine caused dose-dependent elevations in heart rate, with significant increases in blood pressure. However, the findings from these studies suggest that tesofensine may be a well-tolerated long-term treatment for obesity, with minimal cardiovascular effects. FDA appears to share this view, having authorized the Phase 3 trial program for this agent[78].
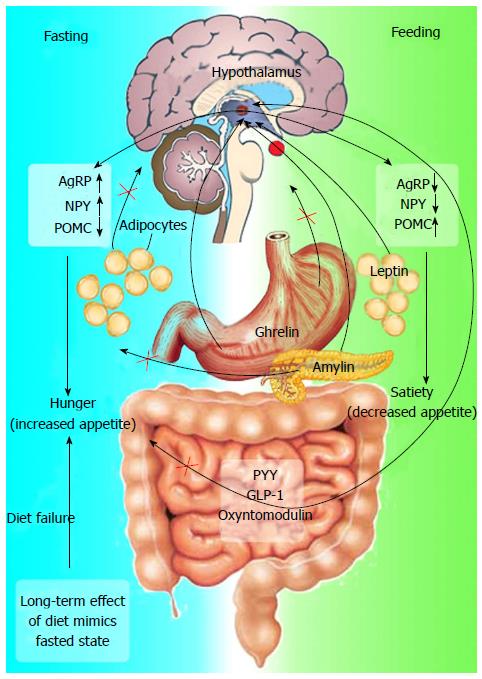
In the last few years, knowledge concerning the mechanisms involved in appetite control has increased. The immediate postprandial state is accompanied by hormonal changes that include: (1) a reduction in the concentrations of the orexigenic gut hormone ghrelin; and (2) an increase in the anorexigenic peptides, such as peptide tyrosine-tyrosine (PYY), glucagon-like peptide-1 (GLP-1) and oxyntomodulin[79].
All of these hormones act on the hypothalamus, playing an important regulatory role in the mediation of hunger, satiety and energy intake (Figure 4)[79]. GLP-1 also exerts an incretin effect on insulin synthesis and secretion, inhibiting glucagon secretion, which may be responsible for postprandial insulin response[80].
GLP-1 is an incretin hormone secreted by enteroendocrine L-cells, which increases insulin secretion after oral ingestion of a meal[80]. Carbohydrates and fat are the most potent secretagogues for GLP-1 release[81].
The incretin response after meals is impaired in many patients with T2DM, owing to resistance to glucose-dependent insulinotropic peptide (GIP) that cannot be compensated for by endogenous GLP-1. Two classes of incretin therapies have been developed, in order to overcome the native GLP-1 short half-life (1-2 min): dipeptidyl peptidase-4 (DPP-4) inhibitors (gliptins), which raise endogenous levels of plasma GLP-1 and GIP, and DPP-4-resistant GLP-1 receptor agonists (liraglutide, exenatide)[82]. In Phase 3 clinical trials, agents from both of these classes of incretin-based therapies produced improvements in glycemic control in patients with T2DM[82]; however, efficacy is greater with GLP-1 RAs, which additionally promote weight loss, reducing systolic blood pressure and improving β-cell function. These effects are particularly advantageous to T2DM patients, as most are affected by β-cell dysfunction, obesity and hypertension[82].
The glucagon-like peptide-1 receptor agonists (GLP-1 RAs) liraglutide and exenatide (or exendin-4) improve glycemic control by stimulating insulin release through pancreatic β-cells, depending on glucose. GLP-1 receptors do not exist only in the pancreas.
Liraglutide (Victoza®) is currently approved by FDA and EMA as an anti-hyperglycemic agent in T2DM adults. Treatment with the 1.8 mg dose resulted in weight loss of 2.0 ± 0.3 kg from baseline after 26 wk, while weight change with placebo varied from a weight loss of 1.5 kg to a 0.6 kg weight gain over that time[83]. Nevertheless, liraglutide causes dose-dependent thyroid C-cell tumors in rodents, being thus contraindicated in patients with a personal or family history of medullary thyroid carcinoma, although it is unknown whether liraglutide causes C-cell tumors in humans[84]. Gastrointestinal adverse events, namely nausea, diarrhea and vomiting, were the most frequently reported events during treatment with liraglutide[84]. Other reported adverse effects were: pancreatitis; hypoglycemia, when liraglutide was used with an insulin secretagogue or insulin; renal impairment; hypersensitivity reactions; headache; and anti-liraglutide antibody formation[85].
At the present, the main adverse effects of GLP-1 and GLP-1 RAs seem to be transient gastrointestinal events. A cardiovascular protective role has also been suggested for GLP-1 in Phase 3 clinical studies, raising the possibility that GLP-1 RAs may lower the risk of cardiovascular events in T2DM patients. In those studies, T2DM patients treated with liraglutide exhibited reduced lipid levels as well as decreased levels of cardiovascular risk biomarkers, including plasminogen activator inhibitor-1 (PAI-1), brain natriuretic peptide and hs-CRP (-7.6%, -11.9% and -23.1%, respectively)[82].
Exenatide (Byetta®) is approved by both FDA and EMA as an adjunct to diet and exercise to improve glycemic control in adults with T2DM[86,87]. A study was conducted to assess the effects of exenatide on body weight and glucose tolerance in nondiabetic obese individuals with normal or impaired glucose tolerance or impaired fasting glucose, submitting obese patients to exenatide or placebo, with simultaneous lifestyle intervention, for 24 wk[88]. Patients treated with exenatide lost 5.1 ± 0.5 kg from baseline versus 1.6 ± 0.5 kg with placebo. Placebo-subtracted difference in percent weight reduction was -3.3% ± 0.5% (P < 0.001). It was observed that exenatide plus lifestyle modification reduced caloric intake, having resulted in greater weight loss than lifestyle modification alone (treatment difference, -3.3%), in nondiabetic obese subjects, with improved glucose tolerance in individuals with impaired glucose tolerance and impaired fasting glucose. Thus, some authors have speculated that exenatide, along with lifestyle modification, has potential as a treatment for obesity in individuals at high risk for developing T2DM[89]. However, the efficacy of weight loss for GLP-1 agonists does not meet FDA standards and they are not well-tolerated for long-term use because they result in nausea and diarrhea. Indeed, nausea was experienced by 25% and 4% and diarrhea by 14% and 3% of exenatide and placebo-treated subjects, respectively[88].
Oxyntomodulin is another gut hormone and GLP-1 receptor agonist, which is released from the small intestine after each meal in proportion to the calories intake. Early studies showed that administration of synthetic oxyntomodulin reduces appetite, suggesting that endogenous oxyntomodulin signals to the brain a feeling of “fullness” or “satiety” after meals[90]. Wynne et al[91] demonstrated that oxyntomodulin reduces energy intake and increases energy expenditure, resulting in negative energy balance. These data support its role as a potential anti-obesity therapy. Field et al[92] carried out a study aiming to investigate whether the anorectic effects of PYY, a Y2 receptor agonist, and oxyntomodulin, a GLP-1 receptor agonist, can be additive. Likewise PYY, oxyntomodulin is secreted by intestinal L-cells after meals. It was observed that a combined infusion of PYY and oxyntomodulin produces an additive anorectic effect in overweight and obese humans, reducing food intake. Furthermore, this and other studies suggest that coadministration of a Y2 receptor agonist and a GLP-1 receptor agonist may be an important strategy for treating obesity, although further studies are necessary[92].
Amylin is cosecreted with insulin from pancreatic β-cells and, like GLP-1, it has been reported to inhibit glucagon secretion, delay gastric emptying, reduce appetite and, therefore, food intake in humans (Figure 4)[93,94]. Amylin may, thus, act as an important regulator of postprandial glucose homeostasis[95]. In vitro, it forms amyloid and undergoes a change in conformation, which leads to loss of biological activity[96,97]. However, the study of analogues of human amylin showed that substitution of alanine and serine at positions 25, 28 and 29, originating pramlintide, promotes stability in solution and allows preservation of biological activity[98]. Thus, responses to the new drug pramlintide provided knowledge of the biology of amylin, as it has the same mechanism of action. Pramlintide, a synthetic analogue of amylin, reduces appetite and food intake, thus producing body weight loss[99]. Pramlintide (Symlin®) is currently approved in the United States as an anti-hyperglycemic agent for patients with diabetes mellitus as an adjunctive therapy to mealtime insulin, being under investigation as a potential treatment for obesity[100].
A 2010 randomized, placebo-controlled trial investigated the efficacy and safety of pramlintide monotherapy or in association with either phentermine or sibutramine, in addition to lifestyle modification[101]. A greater weight loss was reported with both pramlintide plus phentermine (11.3% ± 0.9%) and pramlintide plus sibutramine (11.1% ± 1.1%) than with pramlintide (3.7% ± 0.7%) or placebo alone (2.2% ± 0.7%), confirming the potential of both combinations in treating obesity. With respect to categorical weight loss, ≥ 5% weight loss was achieved by 78% of pramlintide + sibutramine and 82% of pramlintide + phentermine evaluable subjects. These percentages for both combination treatments were significantly greater (P < 0.001) than the percent of subjects achieving ≥ 5% weight loss with placebo (28%) or pramlintide alone (36%). Nevertheless, a greater reduction in blood pressure was observed with both placebo and pramlintide than with combination treatments, which generally produced an elevation in diastolic blood pressure and heart rate[101]. This is a matter of concern, owing to typically elevated cardiometabolic risk profile in obese individuals. An increase in systolic blood pressure was not observed in the group treated with pramlintide plus sibutramine, in contrast to what had been previously reported with sibutramine monotherapy. Both combination treatments improved total and HDL cholesterol, as well as triglycerides levels. On the other hand, pramlintide plus phentermine improved LDL cholesterol levels. The most frequent adverse effect seen with pramlintide monotherapy was nausea, which generally resolved over time. The incidence of psychiatric adverse effects was low, the combination having produced a reduction in the Hospital Anxiety and Depression Scale depression subscore, a self-assessment mood questionnaire[101].
Smith et al[102] demonstrated that pramlintide treatment reduces daily food intake (including fast food intake) and meal sizes, besides improving perceived control of eating in obese individuals and reducing binge eating tendencies. Binge eating disorder is a psychopathology characterized by frequent and persistent episodes of binge eating with the feeling of losing the control due to the absence of regular compensatory behaviors[103].
Leptin is a neurohormone mainly secreted by adipocytes and plays an important role in regulating insulin action and long-term energy homeostasis (Figure 4). Obesity is associated with resistance to many of the metabolic effects of leptin, leading to the notion that resistance to leptin is a cause of insulin resistance associated with obesity[104]. Leptin deficiency produces an increase in body weight and insulin resistance, with consequences to glucose metabolism[105]. It is known that replacement treatment with leptin improves insulin sensitivity in people with leptin deficiency[106,107].
As the neurohormonal control of body weight involves an interaction between long-term adiposal signals (e.g., leptin, insulin) and short-term satiation signals (e.g., amylin, PYY, GLP-1, cholecystokinin), Ravussin et al[108] examined the effect on weight loss of the agonism amylin/leptin, using pramlintide and metreleptin. A 24-wk, randomized, double-blind, active-drug controlled study was developed in obese or overweight patients[108]. The combination pramlintide plus metreleptin produced a greater weight loss (-12.7% ± 0.9%) in the first 20 wk of the study than monotherapy with pramlintide (-8.4% ± 0.9%; P < 0.001) or metreleptin (-8.2% ± 1.3%; P < 0.01), demonstrating synergism between the two drugs. The most frequent adverse effects were mild to moderate nausea and reactions at the injection site. However, this study did not elucidate the mechanism(s) for the observed increase in weight loss, even though the results support further development of pramlintide/metreleptin, towards obesity pharmacotherapy[108].
There are currently no medications approved by FDA to treat obesity in children aged under 12 years. Metformin is approved by FDA for the treatment of T2DM in children aged 10 years or over, having received attention for its potential to be used with weight control purposes in children[109]. It suppresses hepatic glucose production and, at higher doses, improves peripheral insulin sensitivity[110,111]. It is proved that metformin decreases food consumption, stabilizes weight and can induce small weight losses in diabetic and nondiabetic adults[112-114]. Yanovski et al[115] studied the effects of metformin in obese insulin-resistant children aged between 6 and 12 years old, having concluded that treatment with metformin produces a modest reduction in body weight and adiposity, although it improves glucose homeostasis in obese children. Metformin may also be helpful in counteracting the weight gain observed in children taking atypical psychotropic medications. Nevertheless, cessation of metformin therapy may lead to rebound hyperinsulinemia and rapid weight gain[116]. Concerning side effects of metformin, the most common are nausea, flatulence, bloating and diarrhea, all of them usually resolved. Metformin should not be used in renal failure or with intravenous contrast. Multivitamin supplementation is strongly recommended[109].
Ghandi et al[117] carried out a study to compare the effects of metformin and orlistat in obese women with polycystic ovary syndrome and concluded that both metformin and orlistat produce similar reductions in weight, BMI and waist circumference. Furthermore, metformin caused a significant reduction in serum triglyceride and a higher ovulation rate than orlistat, although the difference was not statistically significant.
On the other hand, Wiegand et al[118] studied the effect of metformin therapy in obese insulin-resistant adolescents, having reached the conclusion that metformin and lifestyle modification improved insulin sensitivity, although it did not produce weight loss. Curiously, a higher prevalence of side effects was reported in the placebo group than in the group receiving metformin, showing how important the belief in therapy effects and self-efficacy may be, as it increases the motivation to adopt a healthy lifestyle[118].
Baclofen, a γ-aminobutiric acid B (GABAB) agonist, was demonstrated to reduce body weight more than 2% (baseline: 93.3 ± 9.8 kg; 12 wk: 91.7 ± 10.3 kg, P < 0.05) and waist circumference more than 2 cm in 5 out of 10 subjects, without observed adverse effects in obese patients[119]. Although the reported anti-obesity effects were mild, baclofen may constitute a new anti-obesity drug in humans.
Lactoferrin is a Fe-binding glycoprotein which is present at high concentrations in mammalian milk. It is multi-functional, having antibacterial, antiviral, immunostimulatory, antioxidant and cancer-preventive potential[120-123]. As lactoferrin is ingested by infants in breast milk, it is considered to be safe, having been approved as a food additive in Japan. Ono et al[124] demonstrated that the ingestion of enteric-coated lactoferrin during 8 wk can reduce visceral fat, body weight (-1.5 kg, + 1.0 kg than placebo), BMI and waist circumference both in men and women without the need for lifestyle modifications. Thus, it has potential to prevent obesity and decrease the risk of metabolic syndrome.
Sibutramine: Sibutramine is a norepinephrine and serotonin reuptake inhibitor that was approved in 1997 for weight management and was withdrawn from the market by FDA and EMA in 2010, based on the Sibutramine Cardiovascular OUTcome (SCOUT) trial, which demonstrated a 16% increase in risk of major adverse cardiovascular events (non-fatal heart attack, non-fatal stroke, resuscitation after cardiac arrest and cardiovascular death). The long-term effects of sibutramine treatment in combination with diet and exercise on the rates of cardiovascular events and cardiovascular death among individuals with high cardiovascular disease risk were evaluated in the SCOUT trial[125,126].
James et al[125] investigated the effect of sibutramine on cardiovascular outcomes in overweight and obese subjects, 55 years of age or older, with preexisting cardiovascular disease, T2DM, or both, to examine the cardiovascular consequences of weight management with or without sibutramine in individuals at high risk for cardiovascular events. In the SCOUT trial, event rates in the sibutramine group were lower than expected, and the sibutramine group lost significantly more weight than the placebo group (P = 0.001) and maintained the weight loss[125]. However, the risk of a primary outcome event was 16% higher in the sibutramine group than in the placebo group (P = 0.02). It was concluded that individuals with a history of cardiovascular conditions who were receiving long-term sibutramine treatment had an increased risk of nonfatal myocardial infarction (P = 0.02) and nonfatal stroke (P = 0.03), but not of cardiovascular death (P = 0.90) or death from any cause (P = 0.54). On the basis of these results, the authors of the SCOUT trial concluded that sibutramine should continue to be excluded from use in patients with preexisting cardiovascular disease[125]. These results also led to the withdrawal of sibutramine from the market.
The cannabinoid-1 receptors (CB1Rs) are highly expressed in the CNS, autonomic gastric endings of the peripheral nervous system, and other key cells involved in body energy metabolism, such as adipocytes, hepatocytes and myocytes[127-129]. It has been hypothesized that CB1R inverse agonists act directly on adipocytes, hepatocytes, pancreatic islets and skeletal muscle to mediate metabolic effects[130].
Rimonabant: Rimonabant was the first selective cannabinoid-1 receptor blocker tested in large clinical trials, having been used to reduce body weight and improve cardiometabolic risk factor in obese patients. Rimonabant (Acomplia® in Europe) received marketing approval from the EMA in June 2006. However, it was withdrawn from the market by EMA in 2008, due to safety concerns, namely psychiatric adverse effects. In June 2007, FDA unanimously voted against approval of rimonabant (Zimulti® in the United States) because of increased risk of neurological and psychiatric side effects, namely seizures, depression, anxiety, insomnia, aggressiveness and suicidal thoughts, having never approved its use[131,132].
A prospective, double-blind, placebo-controlled, randomized, two-arm parallel group trial (Atherosclerosis Underlying Development assessed by Intima-media Thickness in patients On Rimonabant/AUDITOR[133] was carried out to determine whether treatment with 20 mg rimonabant would reduce progression of carotid intima-media thickness over 30 mo of treatment in abdominally obese patients with metabolic syndrome. The results of this study demonstrate that rimonabant correlates with an increase in psychiatric symptoms, nervous system disorders (e.g., depression, anxiety) and gastrointestinal disorders. Furthermore, the study suggests that a 5% loss of body weight over a 30-mo period with rimonabant is not sufficient to modify atherosclerosis progression in the carotid artery in obese individuals with the metabolic syndrome[133].
Motaghedi et al[134] observed that rimonabant administration may be efficacious for weight loss in adults with Prader Willi syndrome, characterized by increased appetite and decreased energy expenditure with lower insulin-like growth factor-1, leading to adiposity. Nevertheless, it causes high risk psychiatric adverse effects.
Horder et al[135] findings were the first to show that rimonabant inhibits the neural process of rewarding food stimuli in humans. Thus, it may not only promote weight loss, but it may also indicate a mechanism for inducing anhedonia (i.e., loss of the capacity to experience pleasure), which could increase the risk of depressive symptomatology observed in clinical use.
Clinical trials did not support further development of both taranabant and CP 945598, because of psychiatric adverse effects, similar to those of rimonabant.
Figure 5 provides an overview of all drugs currently approved for both long-term and short-term treatment of obesity, the new drugs presently registered in ongoing clinical trials and those recently withdrawn from the market.
Figure 6 summarizes assessment of overweight (25 kg/m2≤ BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2) patients and pharmacological and non-pharmacological treatment measures, taking into account currently approved anti-obesity drugs both in Europe (by EMA) and in the United States (by FDA).
Table 1 summarizes the subtracted drug/placebo mean weight loss (expressed in terms of percentages or kilograms, as available) of the currently available drugs for long-term treatment of obesity, and of the candidate drugs that have shown more efficacy in clinical trials. It also mentions the main beneficial and adverse effects of each anti-obesity drug, allowing to compare their efficacy and safety.
| Drug | Drug/Placebo subtracted weight loss | Mechanism of action | Other positive effects | Adverse effects |
| Orlistat (Xenical®) | 3.6% | Decreases fat absorption | Improvement in most risk factors of cardiovascular disease and CRP | Increased flatulency Fecal incontinence Diarrhea |
| Lorcaserin (Belviq®) | 3.6% | 5-HT2C receptors agonist | Reduction in energy intake and appetite. Improvement in the markers of cardiovascular disease and inflammation | Headache, fatigue, dizziness, nausea, dry mouth, constipation. Disturbances in attention or memory. Risk of birth defects |
| Phentermine 7.5 + Topiramate 46 (QsymiaTM) | 6.6% | Amphetamine analogue + Anticonvulsivant | Improvement of cardiometabolic variables | Psychiatric and cognitive adverse effects. Risk of birth defects |
| Phentermine 15 + Topiramate 92 | 8.6% | |||
| Cetilistat | 1.46 kg | Decreases fat absorption | Improvement in glycemic control | Mild or moderate gastrointestinal effects |
| Naltrexone 16 + Bupropion (Contrave®) | 4.9% | µ-opioid antagonist + Stimulator of the hypothalamic POMC neurons | Improvement in cardiometabolic risk factors and in insulin resistance | Nausea, constipation, anxiety, increase in blood pressure |
| Naltrexone 32 + Bupropion | 6.3% | |||
| Zonisamide 120 + Bupropion (EmpaticTM) | 4.7% | Increases monoamines (e.g., DA) and POMC neurons activity | Reduction in appetite. Increase in energy expenditure | Headache, nausea, insomnia |
| Zonisamide 360 + Bupropion | 6.1% | |||
| Tesofensine | 5.4 ± 6.7 kg | Inhibits reuptake of DA, 5-HT and NE | Reduction in appetite, body fat and waist circumference | Increase in heart rate and blood pressure |
| Liraglutide (Victoza®) | 0.5 ± 0.3 kg | GLP-1 agonist; increases insulin secretion after meals | Improvement in glycemic control and systolic blood pressure | Nausea, diarrhea, vomiting |
| Exenatide (Byetta®) | 3.5 ± 0.5 kg | GLP-1 agonist; increases insulin secretion after meals | Improvement in glycemic control and systolic blood pressure | Nausea, diarrhea |
| Pramlintide (Symlin®) | 1.5% ± 0.7% | Anti-hyperglycemic agent; synthetic analogue of human amylin | Reduction in binge eating tendencies and blood pressure | Nausea |
Obesity is a chronic disease and an important risk factor for cardiovascular and metabolic diseases, such as T2DM. As lifestyle interventions alone hardly ever result in long-term weight loss, pharmacotherapy is an important adjunct to lifestyle measures to improve the induction and maintenance of weight loss.
Orlistat was until recently the only pharmacological long-term treatment for obesity available on the market, as rimonabant and sibutramine had been withdrawn in 2008 and 2010, respectively, due to serious psychiatric and cardiovascular adverse effects. Lorcaserin (Belviq®) and phentermine plus topiramate (Qsymia™) were recently approved by FDA, in June and July 2012, respectively. Considering the drugs currently available on the market, orlistat is the only whose mechanism of action does not involve the CNS, but the inhibition of gastrointestinal lipase. Lorcaserin activates 5-HT2C receptors, thus suppressing appetite. Qsymia™ is an association of phentermine, a norepinephrine releasing drug, and topiramate, an antiepileptic drug which also exhibits weight loss properties. All these drugs require a combination with lifestyle changes (hypocaloric diet, physical activity) in order to show efficacy. Among the drugs under Phase 3 clinical trials, which have not been approved yet, the most promising ones appear to be cetilistat, which mechanism of action is identical to that of orlistat; Contrave®, an association of naltrexone and bupropion which acts on hypothalamic POMC neurons; tesofensine, a monoamine reuptake inhibitor; and GLP-1 agonists, such as liraglutide and exenatide, which exert an anorexigenic effect and may be helpful to T2DM obese patients. However, Contrave® and tesofensine have shown cardiovascular side effects in clinical trials, which led FDA not to approve them yet. Pramlintide, a synthetic analogue of human amylin currently approved for T2DM management, also produced significant weight loss in clinical trials, having shown a low incidence of psychiatric adverse effects. Enteric coated lactoferrin appears to be a promising treatment, as it does not require lifestyle changes. Baclofen may also constitute a new anti-obesity therapy, since it reduces body weight without reported adverse effects. Regarding the short-term treatment of obesity, phentermine and diethylpropion are currently the two available pharmacological options in the United States, having been withdrawn from the EU market.
In conclusion, owing to the limited options currently available for the pharmacological treatment of obesity, it is imperative to develop new safe compounds. The rapid growth of biomedical science in the last decade and the increasing understanding of the energy metabolism may provide new and better therapies for this pathologic condition.
| 1. | Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 526] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 2. | Abubakari AR, Lauder W, Jones MC, Kirk A, Agyemang C, Bhopal RS. Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health. 2009;123:602-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | WHO. Facts about overweight and obesity (2012 July 2012). Available from: http://www.who.int/mediacentre/factsheets/fs311/en/index.html. |
| 4. | WHO. WHO Global Infobase, data for saving lives. Available from: http://apps.who.int/infobase/Index.aspx.. |
| 5. | Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4734] [Cited by in RCA: 4493] [Article Influence: 280.8] [Reference Citation Analysis (0)] |
| 6. | Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 821] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 7. | Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2823] [Cited by in RCA: 3177] [Article Influence: 167.2] [Reference Citation Analysis (0)] |
| 8. | Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1112] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 9. | Nguyen T, Lau DC. The obesity epidemic and its impact on hypertension. Can J Cardiol. 2012;28:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | American Diabetes Association. Executive summary: Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 Suppl 1:S4-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Haslam DW, James WP. Obesity. Lancet. 2005;366:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 3244] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 12. | Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;240:1607-1610. [PubMed] |
| 13. | Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070-1077. [PubMed] |
| 14. | Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709-2716. [PubMed] |
| 15. | Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud’homme D. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179-184. [PubMed] |
| 16. | Ayyad C, Andersen T. Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999. Obes Rev. 2000;1:113-119. [PubMed] |
| 17. | Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 Suppl:151S-162S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Cannon CP, Kumar A. Treatment of overweight and obesity: lifestyle, pharmacologic, and surgical options. Clin Cornerstone. 2009;9:55-68; discussion 69-71. [PubMed] |
| 19. | Khan A, Raza S, Khan Y, Aksoy T, Khan M, Weinberger Y, Goldman J. Current updates in the medical management of obesity. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:117-128. [PubMed] |
| 20. | Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;CD003641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Kiortsis DN, Filippatos TD, Elisaf MS. The effects of orlistat on metabolic parameters and other cardiovascular risk factors. Diabetes Metab. 2005;31:15-22. [PubMed] |
| 22. | Tzotzas T, Samara M, Constantinidis T, Tziomalos K, Krassas G. Short-term administration of orlistat reduced daytime triglyceridemia in obese women with the metabolic syndrome. Angiology. 2007;58:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Didangelos TP, Thanopoulou AK, Bousboulas SH, Sambanis CL, Athyros VG, Spanou EA, Dimitriou KC, Pappas SI, Karamanos BG, Karamitsos DT. The ORLIstat and CArdiovascular risk profile in patients with metabolic syndrome and type 2 DIAbetes (ORLICARDIA) Study. Curr Med Res Opin. 2004;20:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 650] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 26. | Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapies for obesity: past, current, and future therapies. J Obes. 2011;2011:179674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | FDA (Reference ID: 3115694. 2012 October 2012). Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/088023s037lbl.pdf. |
| 28. | Maher W. Phentermine Labeling: MAO Inhibition. Available from: http://www.fda.gov/ohrms/dockets/dailys/00/Sep00/091500/pdn0001.pdf. |
| 29. | Melnikova I, Wages D. Anti-obesity therapies. Nat Rev Drug Discov. 2006;5:369-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Melnikova I; MHRA. European withdrawal of anorectic agents/appetite suppressants: new legal developments, no new safety issues: Licences for phentermine and amfepramone being withdrawn May 2001. Available from: http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON019540. |
| 31. | Kang JG, Park CY. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab J. 2012;36:13-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 32. | Kang JG; FDA. FDA approved obesity drugs. Available from: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4068B1_05_Approved-Drugs.pdf. |
| 33. | Kang JG; FDA. Tenuate (R). Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/11722s029,12546s032lbl.pdf. |
| 34. | Arias HR, Santamaría A, Ali SF. Pharmacological and neurotoxicological actions mediated by bupropion and diethylpropion. Int Rev Neurobiol. 2009;88:223-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Cercato C, Roizenblatt VA, Leança CC, Segal A, Lopes Filho AP, Mancini MC, Halpern A. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond). 2009;33:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Cercato C; FDA. Orlistat (marketed as Alli and Xenical) Information. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm180076.htm. |
| 37. | Anderson JW. Orlistat for the management of overweight individuals and obesity: a review of potential for the 60-mg, over-the-counter dosage. Expert Opin Pharmacother. 2007;8:1733-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Anderson JW; FDA. Guidance for Industry Developing Products for Weight Management. 2007; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071612.pdf. |
| 39. | Anderson JW; FDA. Reference ID: 3074639. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020766s029lbl.pdf. |
| 40. | Royal_Pharmaceutical_Society_of_Great_Britain. Practice guidance: OTC Orlistat. Available from: http://www.youngsure.com/images/html/practice.pdf. |
| 41. | Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 416] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Yancy WS, Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, Chalecki A, Oddone EZ. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170:136-145. |
| 43. | Derosa G, Maffioli P, Ferrari I, D’Angelo A, Fogari E, Palumbo I, Randazzo S, Cicero AF. Orlistat and L-carnitine compared to orlistat alone on insulin resistance in obese diabetic patients. Endocr J. 2010;57:777-786. [PubMed] |
| 44. | de Castro JJ, Dias T, Chambel P, Carvalheiro M, Correia LG, Guerreiro L, Marques O, Medina JL, Nobre E, Nunes JS. A randomized double-blind study comparing the efficacy and safety of orlistat versus placebo in obese patients with mild to moderate hypercholesterolemia. Rev Port Cardiol. 2009;28:1361-1374. [PubMed] |
| 45. | Kopelman P, Bryson A, Hickling R, Rissanen A, Rossner S, Toubro S, Valensi P. Cetilistat (ATL-962), a novel lipase inhibitor: a 12-week randomized, placebo-controlled study of weight reduction in obese patients. Int J Obes (Lond). 2007;31:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 46. | Kopelman P, Groot Gde H, Rissanen A, Rossner S, Toubro S, Palmer R, Hallam R, Bryson A, Hickling RI. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity (Silver Spring). 2010;18:108-115. [PubMed] |
| 47. | Bryson A, de la Motte S, Dunk C. Reduction of dietary fat absorption by the novel gastrointestinal lipase inhibitor cetilistat in healthy volunteers. Br J Clin Pharmacol. 2009;67:309-315. [PubMed] |
| 48. | Drugdevelopment-technology. com (Cetilistat - Investigational Drug for Obesity (2011 August 2012). Available from: http://www.drugdevelopment-technology.com/projects/cetilistat/#.UCaiod1u38o. |
| 49. | Bryson A; FDA. FDA approves Belviq to treat some overweight or obese adults. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm309993.htm. |
| 50. | Bryson A; FDA. FDA Briefing Document NDA 22529 Lorqess (lorcaserin hydrochloride) Tablets, 10 mg. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM225631.pdf. |
| 51. | Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR, Ravussin E. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab. 2011;96:837-845. [PubMed] |
| 52. | Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256. [PubMed] |
| 53. | Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 54. | Lemke TL, Williams DA, Roche VF, Zito SW. Foye’s Principles of Medicinal Chemistry. 6ed, USA: Lipipincott Williams & Wilkins 2008; . |
| 55. | Lemke TL; FDA. Medication Guide Qsymia™ (2012 October 2012). Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM312590.pdf. |
| 56. | Lemke TL; Avvo. Qsymia- Phenteramine/ Topiramate for weight reduction (2012 October 2012). Available from: http://www.avvo.com/health-guides/qsymia--phenteramine-topiramate-for-weight-reduction. |
| 57. | Lemke TL; FDA. FDA approves weight-management drug Qsymia (2012 July 2012). Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312468.htm. |
| 58. | Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342. [PubMed] |
| 59. | Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352. [PubMed] |
| 60. | Ornellas T, Chavez B. Naltrexone SR/Bupropion SR (Contrave): A New Approach to Weight Loss in Obese Adults. P T. 2011;36:255-262. [PubMed] |
| 61. | Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 Suppl 5:S63-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 393] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 62. | Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1748] [Article Influence: 69.9] [Reference Citation Analysis (11)] |
| 63. | Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199-211. [PubMed] |
| 64. | Saper CB; Orexigen. FDA Issues Complete Response to New Drug Application for Contrave(R) for the Management of Obesity (2011 July 2012). Available from: http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1522207&highlight. |
| 65. | Pelchat ML. Food cravings in young and elderly adults. Appetite. 1997;28:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120. [PubMed] |
| 67. | Gadde KM, Yonish GM, Foust MS, Wagner HR. Combination therapy of zonisamide and bupropion for weight reduction in obese women: a preliminary, randomized, open-label study. J Clin Psychiatry. 2007;68:1226-1229. [PubMed] |
| 68. | Gadde KM; Orexigen. Product candidates - Empatic™ 2012 August 2012. Available from: http://www.orexigen.com/candidates/candidates_empatic.php. |
| 69. | Greenway F, Atkinson JAR. Bupropion and zonisamide for the treatment of obesity [abstract 52-OR]. Obesity Research. 2006;14:A17. |
| 70. | Greenway F; Orexigen. Clinical Trials (cited Accessed August 2012). Available from: http://www.orexigen.com/trials/default.php. |
| 71. | Faria AM, Mancini MC, Melo ME, Cercato C, Halpern A. Recent progress and novel perspectives on obesity pharmacotherapy. Arq Bras Endocrinol Metabol. 2010;54:516-529. [PubMed] |
| 72. | Klonoff DC, Greenway F. Drugs in the pipeline for the obesity market. J Diabetes Sci Technol. 2008;2:913-918. [PubMed] |
| 73. | Fujioka K, Apovian C, Hill J. The Evolution of Obesity Therapies: New Applications for Existing Drugs (MedscapeCME Diabetes Endocrinology, 2010). Available from: http://cme.medscape.com/viewarticle/722366. |
| 74. | Astrup A, Meier DH, Mikkelsen BO, Villumsen JS, Larsen TM. Weight loss produced by tesofensine in patients with Parkinson’s or Alzheimer’s disease. Obesity (Silver Spring). 2008;16:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Gilbert JA, Gasteyger C, Raben A, Meier DH, Astrup A, Sjödin A. The effect of tesofensine on appetite sensations. Obesity (Silver Spring). 2012;20:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Gilbert JA; NeuroSearch. Tesofensine - Mode of Action. August 2012]. Available from: http://neurosearch.com/Default.aspx?ID=8174.. |
| 77. | Sjödin A, Gasteyger C, Nielsen AL, Raben A, Mikkelsen JD, Jensen JK, Meier D, Astrup A. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes (Lond). 2010;34:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Bello NT, Zahner MR. Tesofensine, a monoamine reuptake inhibitor for the treatment of obesity. Curr Opin Investig Drugs. 2009;10:1105-1116. [PubMed] |
| 79. | Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2055] [Cited by in RCA: 2465] [Article Influence: 129.7] [Reference Citation Analysis (1)] |
| 81. | Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, Smout AJ, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2007;293:E743-E753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 82. | Vilsbøll T, Garber AJ. Non-glycaemic effects mediated via GLP-1 receptor agonists and the potential for exploiting these for therapeutic benefit: focus on liraglutide. Diabetes Obes Metab. 2012;14 Suppl 2:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 656] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 84. | Zinman B; FDA. Nda 22-341 victoza risk evaluation and mitigation strategy (REMS). 6ed, USA: Lipipincott Williams & Wilkins 2012; [cited Accessed July 2012] Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM202063.pdf. |
| 85. | Zinman B; FDA. Highlights of prescribing information - Victoza (2012 July 2012). Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022341s007s009s013lbl.pdf. |
| 86. | Zinman B; FDA. Exenatide (marketed as Byetta) Information (2009 August 2012). Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm113705.htm. |
| 87. | Zinman B; EMA. Byetta exenatide. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000698/human_med_000682.jsp&jsenabled=true. |
| 88. | Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, Trautmann M. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173-1175. [PubMed] |
| 89. | Bradley DP, Kulstad R, Racine N, Shenker Y, Meredith M, Schoeller DA. Alterations in energy balance following exenatide administration. Appl Physiol Nutr Metab. 2012;37:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Wynne K, Bloom S. Oxyntomodulin: a new therapy for obesity? Science in School. 2007;25-29. |
| 91. | Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond). 2006;30:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 92. | Field BC, Wren AM, Peters V, Baynes KC, Martin NM, Patterson M, Alsaraf S, Amber V, Wynne K, Ghatei MA. PYY3-36 and oxyntomodulin can be additive in their effect on food intake in overweight and obese humans. Diabetes. 2010;59:1635-1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13-23. [PubMed] |
| 94. | Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci. 2006;361:1219-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 95. | Weyer C, Maggs DG, Young AA, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des. 2001;7:1353-1373. [PubMed] |
| 96. | Goldsbury C, Goldie K, Pellaud J, Seelig J, Frey P, Müller SA, Kistler J, Cooper GJ, Aebi U. Amyloid fibril formation from full-length and fragments of amylin. J Struct Biol. 2000;130:352-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 97. | Higham CE, Jaikaran ET, Fraser PE, Gross M, Clark A. Preparation of synthetic human islet amyloid polypeptide (IAPP) in a stable conformation to enable study of conversion to amyloid-like fibrils. FEBS Lett. 2000;470:55-60. [PubMed] |
| 98. | Janes S, GL Beaumont K, Beeley N, Rink T. The selection of pramlintide for clinical evaluation. Diabetes. 1996;45:p. 235A (Abstract). |
| 99. | Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Aronne L, Fujioka K, Aroda V, Chen K, Halseth A, Kesty NC, Burns C, Lush CW, Weyer C. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 101. | Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity (Silver Spring). 2010;18:1739-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Smith SR, Blundell JE, Burns C, Ellero C, Schroeder BE, Kesty NC, Chen KS, Halseth AE, Lush CW, Weyer C. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am J Physiol Endocrinol Metab. 2007;293:E620-E627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 103. | Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010;67:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 104. | Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973S-979S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (2)] |
| 105. | Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980S-984S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 106. | Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 872] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 107. | Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 108. | Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring). 2009;17:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 109. | August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH, Silverstein JH, Speiser PW, Styne DM, Montori VM. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93:4576-4599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 110. | DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294-1301. [PubMed] |
| 111. | Cigolini M, Bosello O, Zancanaro C, Orlandi PG, Fezzi O, Smith U. Influence of metformin on metabolic effect of insulin in human adipose tissue in vitro. Diabete Metab. 1984;10:311-315. [PubMed] |
| 112. | Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47-53. [PubMed] |
| 113. | Munro JF, MacCuish AC, Marshall A, Wilson EM, Duncan LJ. Weight-reducing effect of diguanides in obese non-diabetic women. Br Med J. 1969;2:13-15. [PubMed] |
| 114. | Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854-865. [PubMed] |
| 115. | Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, Reynolds JC, Brady SM, Calis KA. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477-85. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 116. | Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry. 2002;159:655-657. [PubMed] |
| 117. | Ghandi S, Aflatoonian A, Tabibnejad N, Moghaddam MH. The effects of metformin or orlistat on obese women with polycystic ovary syndrome: a prospective randomized open-label study. J Assist Reprod Genet. 2011;28:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Wiegand S, l’Allemand D, Hübel H, Krude H, Bürmann M, Martus P, Grüters A, Holl RW. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study. Eur J Endocrinol. 2010;163:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 119. | Arima H, Oiso Y. Positive effect of baclofen on body weight reduction in obese subjects: a pilot study. Intern Med. 2010;49:2043-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Tomita M, Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci. 1991;74:4137-4142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 302] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Harmsen MC, Swart PJ, de Béthune MP, Pauwels R, De Clercq E, The TH, Meijer DK. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172:380-388. [PubMed] |
| 122. | Zimecki M, Właszczyk A, Cheneau P, Brunel AS, Mazurier J, Spik G, Kübler A. Immunoregulatory effects of a nutritional preparation containing bovine lactoferrin taken orally by healthy individuals. Arch Immunol Ther Exp (Warsz). 1998;46:231-240. [PubMed] |
| 123. | Shoji H, Oguchi S, Shinohara K, Shimizu T, Yamashiro Y. Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr Res. 2007;61:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Ono T, Murakoshi M, Suzuki N, Iida N, Ohdera M, Iigo M, Yoshida T, Sugiyama K, Nishino H. Potent anti-obesity effect of enteric-coated lactoferrin: decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br J Nutr. 2010;104:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 125. | James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 637] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 126. | Torp-Pedersen C, Caterson I, Coutinho W, Finer N, Van Gaal L, Maggioni A, Sharma A, Brisco W, Deaton R, Shepherd G. Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. Eur Heart J. 2007;28:2915-2923. |
| 127. | Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond). 2005;29:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 128. | Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrié P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908-914. [PubMed] |
| 129. | Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Bátkai S, Marsicano G, Lutz B, Buettner C. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 130. | Pagotto U, Pasquali R. Fighting obesity and associated risk factors by antagonising cannabinoid type 1 receptors. Lancet. 2005;365:1363-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 131. | Pagotto U; FDA. What is rimonabant and what are the associated risks? Available from: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/QuestionsAnswers/ucm136187.htm. |
| 132. | Pagotto U; FDA. FDA Briefing Document NDA 21-888 Zimulti (rimonabant) Tablets, 20 mg. Available from: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounder.pdf. |
| 133. | O’Leary DH, Reuwer AQ, Nissen SE, Després JP, Deanfield JE, Brown MW, Zhou R, Zabbatino SM, Job B, Kastelein JJ. Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: the AUDITOR Trial. Heart. 2011;97:1143-1150. [PubMed] |
| 134. | Motaghedi R, Lipman EG, Hogg JE, Christos PJ, Vogiatzi MG, Angulo MA. Psychiatric adverse effects of rimonobant in adults with Prader Willi syndrome. Eur J Med Genet. 2011;54:14-18. [PubMed] |
| 135. | Horder J, Harmer CJ, Cowen PJ, McCabe C. Reduced neural response to reward following 7 days treatment with the cannabinoid CB1 antagonist rimonabant in healthy volunteers. Int J Neuropsychopharmacol. 2010;13:1103-1113. [PubMed] |
P- Reviewer: Gómez-Sáez J, Masaki T, Papazoglou D S- Editor: Song XX L- Editor: A E- Editor: Wu HL