Published online Aug 26, 2014. doi: 10.13105/wjma.v2.i3.98
Revised: May 16, 2014
Accepted: June 10, 2014
Published online: August 26, 2014
Processing time: 259 Days and 15 Hours
AIM: To compare the short-term clinical outcomes of robot-assisted gastrectomy (RAG) with laparoscopy-assisted gastrectomy (LAG) in gastric cancer patients.
METHODS: Articles were identified through a literature search of Pubmed, EMBASE, Scopus, Web of Science, Chinese National Knowledge Infrastructure and the Cochrane Library. Weighted mean differences (WMDs) and odds ratios (ORs) were selected as effect sizes for quantitative variables and qualitative variables, respectively. And 95%CIs were also calculated.
RESULTS: A total of 13 studies with 3518 patients were included. RAG was associated with longer operative time (WMD = 46.26 min, 95%CI: 31.89-60.63, P < 0.00001), less blood loss [WMD = -37.19 mL, 95%CI: -60.16-(-14.23), P = 0.002] and shorter postoperative hospital stay [WMD = -0.65 d, 95%CI: -1.24-(-0.05), P = 0.03] than LAG. No significant difference in the numbers of retrieved lymph nodes was found between the two groups (WMD = 1.46, 95%CI: -0.19-3.10, P = 0.08). There was no significant difference in mortality (OR = 1.55, 95%CI: 0.49-4.94, P = 0.45), overall complications (OR = 1.00, 95%CI: 0.80-1.26, P = 0.98), anastomosis leakage (OR = 1.02, 95%CI: 0.62-1.65, P = 0.95) and anastomosis stenosis rates (OR = 0.54, 95%CI: 0.18-1.57, P = 0.25).
CONCLUSION: RAG is effective and safe in the treatment of gastric cancer. RAG is a promising alternative to laparoscopic surgery. Long-term randomized controlled studies with large scale and improved designs are needed to further evaluate the long-term outcomes.
Core tip: A total of 13 studies with 3518 patients were included in this meta-analysis. The results indicated that robot-assisted gastrectomy was associated with longer operative time (WMD = 46.26 min, 95%CI: 31.89, 60.63, P < 0.00001), less blood loss [WMD = -37.19 mL, 95%CI: -60.16-(-14.23), P = 0.002] and shorter postoperative hospital stay [WMD = -0.65 d, 95%CI: -1.24-(-0.05), P = 0.03] than laparoscopy-assisted gastrectomy. Robot-assisted gastrectomy is effective and safe in the treatment of gastric cancer and will be a promising alternative to laparoscopic surgery. Long-term randomized controlled studies with large scale and improved designs are needed to further evaluate the long-term outcomes.
-
Citation: Lin ZD, Liu M, Tang D, Li H, Zhang BM. Robot-assisted
vs laparoscopy-assisted gastrectomy for gastric cancer: A meta-analysis based on 3518 subjects. World J Meta-Anal 2014; 2(3): 98-106 - URL: https://www.wjgnet.com/2308-3840/full/v2/i3/98.htm
- DOI: https://dx.doi.org/10.13105/wjma.v2.i3.98
Gastric cancer is the fourth leading cancer and second leading cause of cancer death in the world[1,2]. At present, radical gastrectomy with lymph node (LN) dissection is still the mainstay of treatment for gastric cancer[3]. Since 1994, the laparoscopy-assisted gastrectomy (LAG) has become widely accepted in Asian countries because it offers less invasiveness and pain, speedier recovery, milder morbidity and shorter hospital stay[4-7]. According to the report of Japanese Society of Endoscopic Surgery, the total number of patients who had undergone LAG for gastric cancer was 34645 until 2013[8]. However, the instruments of LAG have a limited range of motion and are usually associated with a long learning curve, especially in LN dissection[9].
Another minimally invasive approach for gastric cancer seems to be more promising. Hashizume et al[10] had performed distal gastrectomy successfully with the assistance of the da Vinci computer-enhanced surgical system in 2002. They found that the robotic system enhanced visualization of both the operative field and precision of the necessary techniques. It may therefore help surgeons overcome many of the difficulties associated with the endoscopic approach. Since then, several studies[11-15] have been conducted to evaluate the safety and efficacy of robot-assisted gastrectomy (RAG) for gastric cancer. However, most of them were case control studies and their sample sizes were rather small. Therefore, in this study, we conducted a meta-analysis to compare the short-term clinical outcomes of RAG with LAG in gastric cancer patients.
We performed an electronic search of Pubmed, EMBASE, Scopus, Web of Science, Cochrane Library and Chinese National Knowledge Infrastructure from the inception to December 13th, 2013. The following search terms were used: gastric cancer, gastric carcinoma, gastrectomy, robotic, robot, laparoscopy and laparoscopic. Only English and Chinese articles were considered. We also searched additional articles through the reference lists of related papers. Two investigators screened the articles independently.
Two investigators identified appropriate articles and conducted data extraction independently. Eligible studies should match all of the following: (1) study design: prospective or retrospective cohort studies, randomized or nonrandomized controlled studies, case-control studies; (2) study population: gastric cancer patients who received RAG or LAG; (3) grouping: RAG group vs LAG group; and (4) outcomes: intraoperative outcome (operative time, blood loss, number of retrieved LNs, conversion to open gastrectomy) and postoperative outcome (overall complications, anastomosis leakage, anastomosis stenosis, bleeding, intestinal obstruction, mortality and postoperative hospital stay). Meeting abstracts, case reports, editorials and reviews were excluded.
We extracted the study type, country, patient characteristics, age, clinical outcomes, operating cost and the number of cases for each article. The quality of the included studies was evaluated by Newcastle-Ottawa quality assessment (NOS) scale[16]. A study can be awarded a highest score of nine. Data extraction was completed independently by two investigators.
All statistical tests were performed with Review Manager 5.1 software (Cochrane Collaboration, Copenhagen, Denmark). In this study, I2 was used to investigate the heterogeneity. In the analysis process, if I2≥ 50%, we ran a random-effect model. On the other hand, a fixed-effect model was chosen if I2 < 50%. In the analysis of quantitative variables (operative time, blood loss, number of retrieved LNs and postoperative hospital stay), we chose weighted mean difference (WMD) with 95%CI as summary statistics. As for qualitative variables (overall complications, anastomosis leakage, anastomosis stenosis, bleedings, intestinal obstruction, conversion to open gastrectomy and mortality), odds ratios (ORs) with 95% CIs were used accordingly. A value of P < 0.05 (two-tailed test) was considered statistically significant.
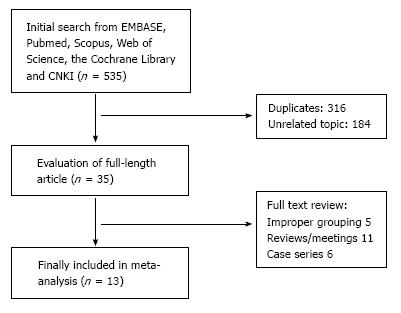
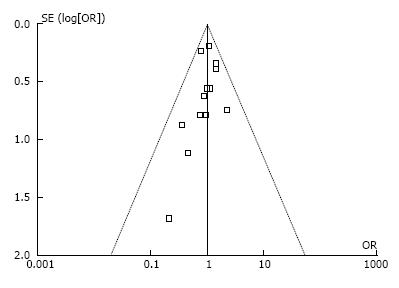
At the beginning of search process, 535 publications were reviewed. After a screening process, 11 retrospective studies[1,3,6,9,11-15,17,18], one nonrandomized prospective study[19] and one randomized control trial[20] were included (Figure 1). Twelve studies[1,3,6,9,11-13,15,17-20] were from Asia and one[14] from Europe. Totally, 3518 patients with gastric cancer were included in this meta-analysis. Among them, 1143 cases were in RAG group, the other 2375 patients received LAG (Table 1). Ten studies[1,3,9,12-15,17-19] were published in English, and three[6,11,20] published in Chinese. No significant publication bias was found (Figure 2).
| Ref. | Country | Group | No. of patients | Age (yr) | Males (%) | BMI (kg/m2) | TNM stage (I/II/III/IV) | NOS score |
| Eom et al[17] | South Korea | RAG | 30 | 52.8 | 70 | 24.2 | 25/3/2/0 | 6 |
| LAG | 62 | 57.9 | 66.1 | 24.1 | 56/6/0/0 | |||
| Huang et al[9] | Taiwan China | RAG | 39 | 65.1 | 48.7 | 24.2 | 29/7/3/0 | 6 |
| LAG | 64 | 65.6 | 67.2 | 24.7 | 55/9/0/0 | |||
| Hyun et al[18] | South Korea | RAG | 38 | 54.2 | 65.8 | 23.8 | 30/5/3/0 | 7 |
| LAG | 83 | 60.3 | 66.3 | 23.8 | 67/9/7/0 | |||
| Kang et al[12] | South Korea | RAG | 100 | 53.2 | 63 | 23.7 | 82/11/7/0 | 6 |
| LAG | 282 | 58.8 | 67.7 | 23.6 | NR | |||
| Kim et al[3] | South Korea | RAG | 436 | 54.2 | 60.8 | 23.6 | 350/51/32/0 | 7 |
| LAG | 861 | 58.8 | 63.9 | 23.5 | 714/96/43/0 | |||
| Kim et al[13] | South Korea | RAG | 16 | 53.8 | 62.5 | 21.3 | NR | 6 |
| LAG | 11 | 57.9 | 90.9 | 25.3 | NR | |||
| Liu et al[6] | China | RAG | 48 | 51.8 | 85.4 | 21.2 | 14/5/27/2 | 7 |
| LAG | 48 | 52.1 | 83.3 | 21 | 16/6/23/3 | |||
| Noshiro et al[19] | Japan | RAG | 21 | 66 | 66.7 | 22.8 | 18/-/-/- | 6 |
| LAG | 160 | 69 | 63.8 | 21.8 | 113/-/-/- | |||
| Pugliese et al[14] | Italy | RAG | 16 | 71 | NR | 28.8 | NR | 7 |
| LAG | 48 | 71 | NR | 28.8 | NR | |||
| Woo et al[1] | South Korea | RAG | 236 | 54 | 57.6 | 23.5 | 236/0/0/0 | 6 |
| LAG | 591 | 58.3 | 61.6 | 23.5 | 591/0/0/0 | |||
| Yoon et al[15] | South Korea | RAG | 36 | 53.9 | 50 | 23.2 | 29/7/0/0 | 7 |
| LAG | 65 | 56.9 | 47.7 | 23.6 | 55/7/3/0 | |||
| Zhang et al[11] | China | RAG | 97 | 56.1 | 68 | 22.5 | 23/22/52/0 | 7 |
| LAG | 70 | 54.8 | 70 | 21.7 | 8/17/45/0 | |||
| Zhao et al[20] | China | RAG | 30 | 71.8 | 73.3 | 23.6 | 2/18/9/1 | 8 |
| LAG | 30 | 72.4 | 76.7 | 23.9 | 1/25/3/1 |
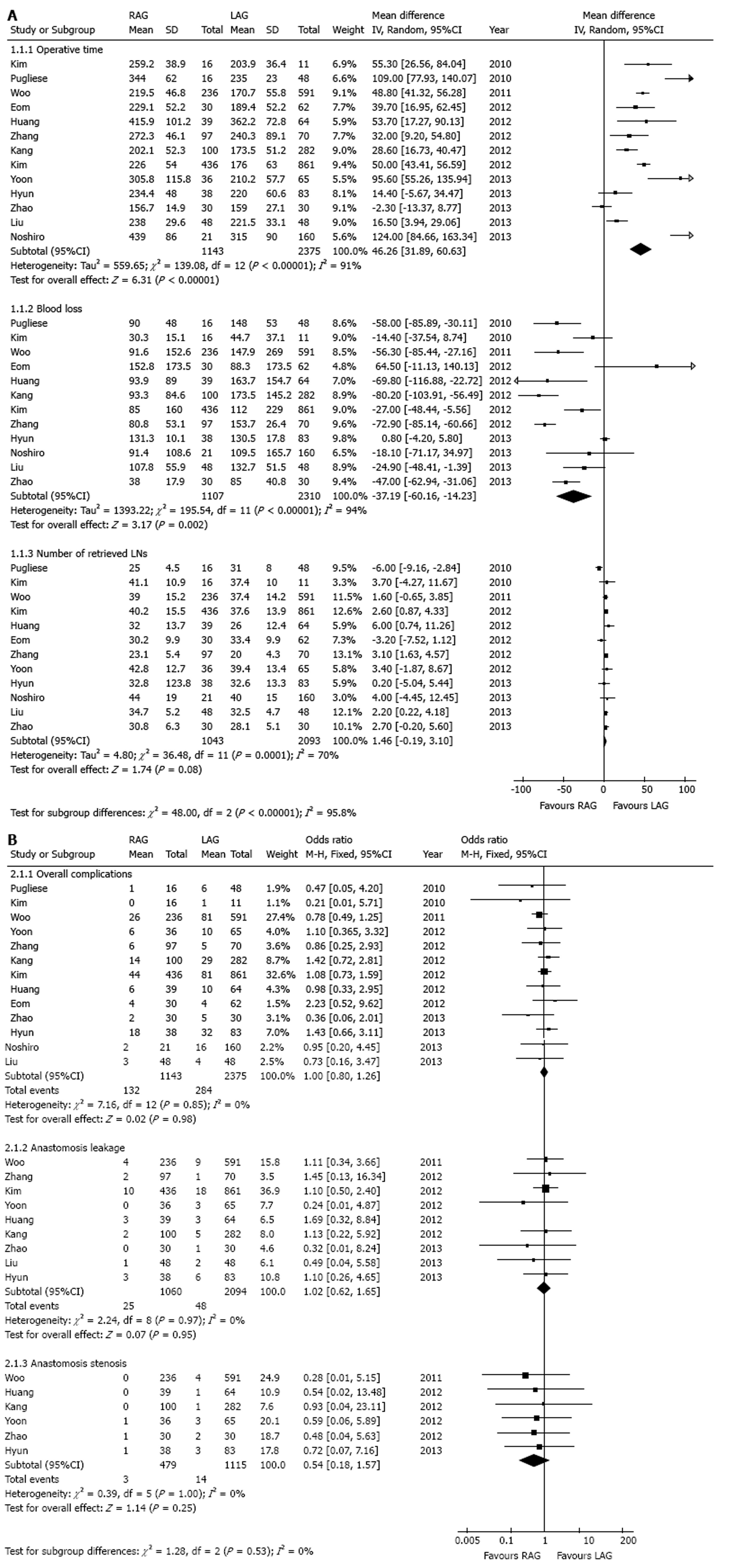
In this pooled analysis, operative time, blood loss and number of retrieved LNs were included. In total, there were 13 studies[1,3,6,9,11-15,17-20] which reported of the operative time, 12 studies[1,3,6,9,11-14,17-20] reported of blood loss and 12 studies[1,3,6,9,11,13-15,17-20] reported of the number of retrieved LNs. In the heterogeneity tests of operative time, blood loss and number of retrieved LNs, I2 were 91%, 94% and 70%, respectively. Accordingly, we chose the random-effect model.
As shown in Figure 3A, the mean operative time for the RAG group was on average 46 min longer than the LAG group (WMD = 46.26 min, 95%CI: 31.89-60.63, P < 0.00001), while mean blood loss was significantly less in the RAG group [WMD = -37.19 mL, 95%CI: -60.16-(-14.23), P = 0.002]. The pooled results also indicated that there was no significant difference in the number of retrieved LNs between the two groups (WMD = 1.46, 95%CI: -0.19-3.10, P = 0.08).
Overall complications, anastomosis leakage and anastomosis stenosis were included for analysis. Information in detail is shown in Figure 3B. Thirteen studies[1,3,6,9,11-15,17-20] which reported of the overall complications were included. No statistical heterogeneity was found in this analysis (I2 = 0%). A fixed-effect model was selected. No significant difference between RAG and LAG group was found in the comparison of the incidences of overall complications (11.5% vs 12.0%, OR = 1.00, 95%CI: 0.80-1.26, P = 0.98).
The incidences of anastomosis leakages were reported in 9 studies[1,3,6,9,11,12,15,18,20]. In total, 1060 patients were treated with RAG and 2094 patients received LAG. No statistical heterogeneity was found (I2 = 0%). There was no significant difference between RAG and LAG group in the comparison of the incidences of anastomosis leakages (2.4% vs 2.3%, OR = 1.02, 95%CI: 0.62-1.65, P = 0.95).
Six studies[1,9,12,15,18,20] involving 1594 subjects were included in the analysis of anastomosis stenosis rates. No statistical heterogeneity was found in this analysis (I2 = 0%) and a fixed-effect model was selected. The results didn’t indicate statistical difference between the two groups in the comparison of the anastomosis stenosis rates (0.6% vs 1.3%, OR = 0.54, 95%CI: 0.18-1.57, P = 0.25).
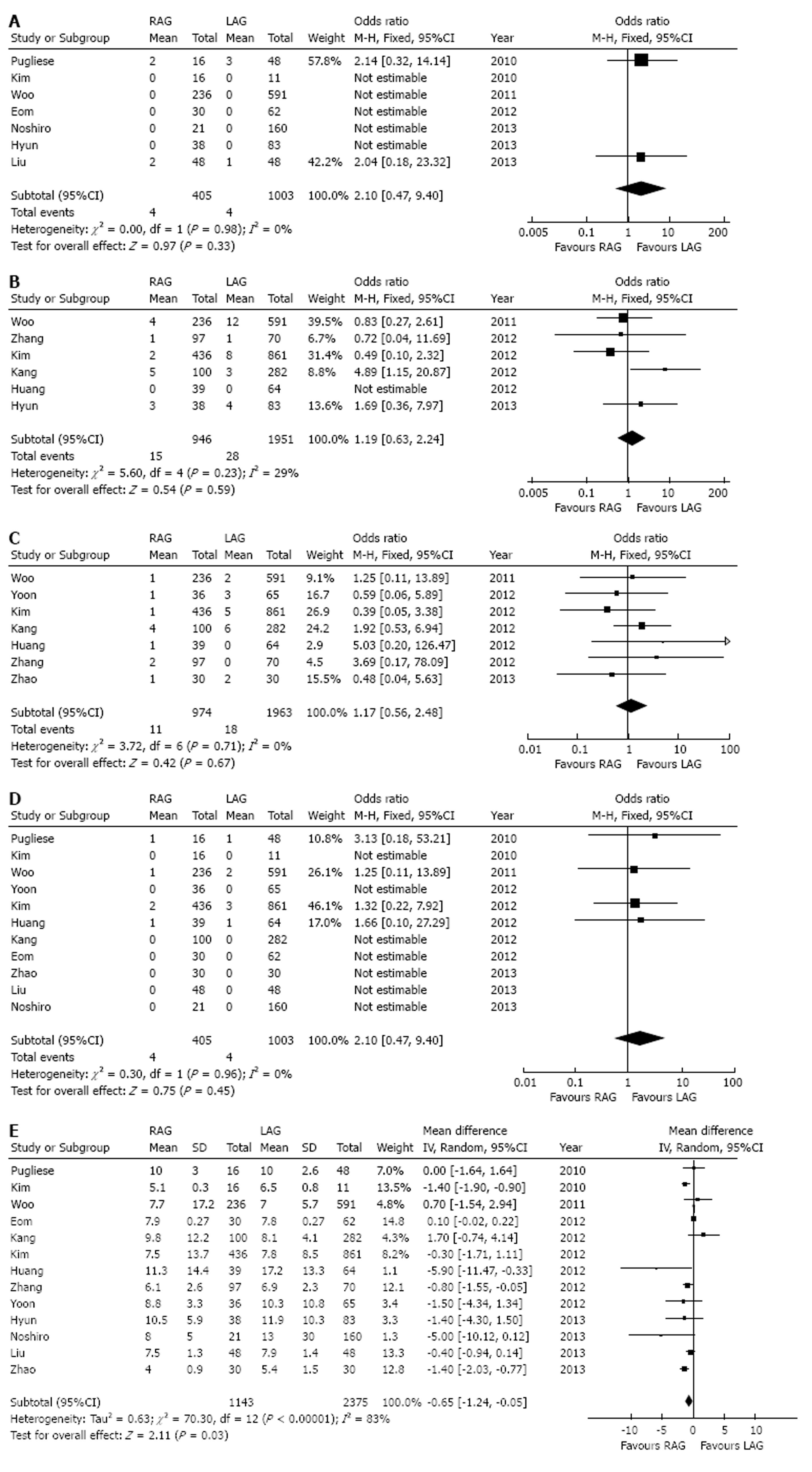
There were 7 studies[1,6,13,14,17-19] which reported of numbers of patients in LAG or RAG group who converted to open gastrectomy (Figure 4A). No statistical heterogeneity was found (I2 = 0%). There was no statistical difference in the comparison of conversion to open gastrectomy between the two groups (OR = 2.10, 95%CI: 0.47-9.40, P = 0.33).
The incidences of bleeding events after operation were reported in 6 studies[1,3,9,11,12,18], involving 2897 subjects. No statistical heterogeneity was found in this analysis (I2 = 29%) and a fixed-effect model was selected (Figure 4B). The results indicated that there was no significant difference in the comparison of bleeding rates (1.6% vs 1.4%, OR = 1.19, 95%CI: 0.63-2.24, P = 0.59).
Seven studies[1,3,9,11,12,15,20] involving 2937 patients were included in the analysis of intestinal obstruction (Figure 4C). A fixed-effect model was selected (I2 = 0%). No significant difference was found in the comparison of intestinal obstruction rates (1.1% vs 0.9%, OR = 1.17, 95%CI: 0.56-2.48, P = 0.67).
Eleven studies[1,3,6,9,12-15,17,19,20] had reported the mortalities (Figure 4D). There were 3230 subjects included (1008 in RAG group and 2222 in LAG group). No statistical heterogeneity was found (I2 = 0%). The results indicated no significant difference of mortality between the two groups (0.5% vs 0.3%, OR = 1.55, 95%CI: 0.49-4.94, P = 0.45).
Thirteen studies[1,3,6,9,11-15,17-20] involving 3518 patients were included in the analysis of postoperative hospital stay (Figure 4E). A random-effect model was selected (I2 = 83%). The RAG group had a shorter mean postoperative hospital stay than the LAG group [WMD = -0.65 d, 95%CI: -1.24-(-0.05), P = 0.03].
The findings from our meta-analysis suggest that RAG is effective and safe for gastric cancer compared to LAG. Overall, combining the available data RAG was associated with longer operative time, less blood loss and shorter postoperative hospital stay than LAG. Moreover, there was no significant difference in mortality, conversion, overall complications, postoperative bleeding events, intestinal obstruction, anastomosis leakage and anastomosis stenosis rates. There was also no significant difference in the numbers of retrieved LNs during the operation between RAG and LAG.
Previous studies have reported the application of RAG for the treatment of gastric cancer. Yoon et al[15] included 36 patients who underwent RAG and 65 patients who underwent LAG at the National Cancer Center in South Korea. The operative data, postoperative morbidity, and pathologic data were analyzed. They found that the mean postoperative hospital stay was 8.8 ± 3.3 d in the RAG group and 10.3 ± 10.8 d in the LATG group (P = 0.416). The mean operative time was 305.8 ± 115.8 min in the RAG group and 210.2 ± 57.7 min in the LAG group (P < 0.001). No significant differences were found in the comparison of mean number of dissected LNs and incidence of postoperative complications. Some other studies[6,18,20] and meta-analysis[21,22] have reported similar results. However, these studies have limited samples and most of them were retrospective. Therefore, we pooled relevant studies and conducted a meta-analysis to compare the short-term clinical outcomes of RAG with LAG systematically. Finally, 13 studies involving 3815 subjects were included. The quality of these studies was relatively high because their NOS scores ranged from 6 to 8. There was a significant heterogeneity among the included studies in the analysis of intraoperative outcomes. This may be explained by the differences in the stage of gastric cancer, resection scope, operation skill, gastric resection approach, extension of LN dissection and the standards for discharge among the studies. Further, according to the funnel plot, the publication bias was acceptable.
According to the results of our analysis, the operative time is much longer in RAG group. It may be related to the increased set-up time to position and the inexpert skill of surgeons. RAG was also associated with less estimated blood loss compared with LAG. It’s more convenient for hemostatic treatment because RAG provides an excellent and stable visualization of the operative field[9,11]. Even though the mean postoperative hospital stay is 0.65 d shorter in RAG, we think that it is of little practical significance because it’s too short. Moreover, there are no differences between RAG and LAG in the comparison of retrieved LNs and postoperative outcomes. Briefly, the results in the current study indicate that RAG is as safe and effective as LAG in the treatment of gastric cancer.
However, the costs of RAG are much higher than those of LAG. The mean cost of RAG is about $6000 to $11400 for gastric cancer, while only $2000 to $6000 in LAG group[9,11,17]. Consequently, before surgeons and patients make the decision, patients’ economic condition should also be taken into consideration.
However, this study had some potential limitations. Firstly, there might be a certain degree of language bias because only publications in Chinese or English were searched in the databases. And then, the number of included subjects was relatively few in this study, which may lead to low statistical power. Moreover, most of them were retrospective designed and long-term outcomes were not reported. More high-quality randomized clinical studies are deserved to better evaluate both short and long-term outcomes of RAG. Further, the end points predetermined in the included studies were different. We can only partly extract the information from these studies. As for study population, most participants were Asian. Studies in Western countries were relatively rare. Lastly, the differences in population characteristics (stage of gastric cancer, age, gender ratio, diabetes mellitus, hypertension, etc.), device and the duration of follow up among the included studies may also lead to a bias in a certain degree.
In conclusion, the synthesis of available evidence indicates that RAG is effective and safe in the treatment of gastric cancer. RAG is a promising alternative to laparoscopic surgery. Long-term randomized controlled studies with large scales and improved designs are needed to further evaluate the long-term outcomes.
The authors wish to thank Dan Huang for assistance with references.
Gastric cancer is the fourth leading cancer and second leading cause of cancer death in the world. Robot-assisted gastrectomy (RAG) is a new approach for gastric cancer and is reported to be safe and efficient. However, most of the studies were case control studies and their sample sizes were rather small.
The purpose of this study was to perform a meta-analysis to compare the short-term clinical outcomes of RAG with laparoscopy-assisted gastrectomy (LAG) in gastric cancer patients systematically.
In the present study, the results indicate that RAG is associated with longer operative time [weighted mean difference (WMD) = 46.26 min, P < 0.00001], less blood loss (WMD = -37.19 mL, P = 0.002) and shorter postoperative hospital stay (WMD = -0.65 d, P = 0.03) than LAG. So far, this is a meta-analysis with most included studies and largest number of included subjects.
RAG is effective and safe in the treatment of gastric cancer. RAG is a promising alternative to laparoscopic surgery.
RAG: Robot-assisted gastrectomy. Robot-assisted surgery is a kind of minimally invasive approaches. It can be used in gastrectomy and may enhance visualization of both the operative field and precision of the necessary techniques. The most popular one is da Vinci computer-enhanced surgical system.
Lin et al in their manuscript present an interesting meta-analysis. This is a well done study.
| 1. | Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, Noh SH. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 472] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Shehzad K, Mohiuddin K, Nizami S, Sharma H, Khan IM, Memon B, Memon MA. Current status of minimal access surgery for gastric cancer. Surg Oncol. 2007;16:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Qiu J, Pankaj P, Jiang H, Zeng Y, Wu H. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Liu C, Tang B, He Y, Shi Y, Zeng D, Luo H, Zhao Y, Qian F, Yu P; Surgical short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy: a case-control study. Disan Junyi Daxue Xuebao. 2013;35:1164-1166. |
| 7. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 8. | Etoh T, Inomata M, Shiraishi N, Kitano S. Minimally invasive approaches for gastric cancer-Japanese experiences. J Surg Oncol. 2013;107:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 9. | Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, Li AF, Chiou SH, Wu CW. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, Koga F, Gotoh N, Konishi K, Maehara S. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. 2002;16:1187-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Zhang X, Jiang Z, Kun Z; Comparative study on clinical efficacy of robot-assisted and laparoscopic gastrectomy for gastric cancer. Zhonhua Weichang Waike Zazhi. 2012;15:804-806. [DOI] [Full Text] |
| 12. | Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer. 2012;12:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Mao L, Jian C, Changzhi L, Dan H, Suihua H, Wenyi T, Wei W. Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: a meta-analysis based on 23,035 subjects. Arch Cardiovasc Dis. 2013;106:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Eom BW, Yoon HM, Ryu KW, Lee JH, Cho SJ, Lee JY, Kim CG, Choi IJ, Lee JS, Kook MC. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol. 2012;38:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, Kim JH, Park SH, Mok YJ, Park SS. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Noshiro H, Ikeda O, Urata M. Robotically-enhanced surgical anatomy enables surgeons to perform distal gastrectomy for gastric cancer using electric cautery devices alone. Surg Endosc. 2014;28:1180-1187. [PubMed] |
| 20. | Zhao K, Pan H, Wang G, Li M, Ruan H, Jiang Z, Li N, Li J; Contrast study of short-term effect between the Da Vinci surgical robot and laparoscopic technology in patients after distal gastric cancer surgery. Zhonguo Shiyong Waike Zazhi. 2013;33:325-327. |
| 21. | Marano A, Hyung WJ. Robotic gastrectomy: the current state of the art. J Gastric Cancer. 2012;12:63-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Xiong J, Nunes QM, Tan C, Ke N, Chen Y, Hu W, Liu X, Mai G. Comparison of short-term clinical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: a meta-analysis of 2495 patients. J Laparoendosc Adv Surg Tech A. 2013;23:965-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
P- Reviewer: Bener A S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ