Published online Dec 18, 2023. doi: 10.13105/wjma.v11.i7.317
Peer-review started: May 18, 2023
First decision: July 28, 2023
Revised: August 23, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: December 18, 2023
Processing time: 210 Days and 3.7 Hours
Modern immunosuppression has led to a decrease in rejection rates and improved survival rates after solid organ transplantation. Increasing the potency of immunosuppression promotes post-transplant viral infections and associated cancers by impairing immune response against viruses and cancer immunoe
Core Tip: Post-transplant malignancy poses a serious threat with increased risk in organ recipients, varying with the intensity of net immunosuppression. Various virus infections are either causative or associative or promote the development of post-transplant malignancies. It is crucial to be aware of different viral infections so as to pre-emptively screen viral infections and survey for post-transplant cancers, helping early diagnosis, thereby favoring improved outcomes and graft survival. Transplant clinicians must be up to date on current management strategies with the vital role of immunosuppression reduction and options like antivirals, rituximab, chemotherapy, adoptive immunotherapy, topical therapy and surgery based on individual case characteristics.
- Citation: Yadav R, El Kossi M, Belal D, Sharma A, Halawa A. Post-transplant malignancy: Focusing on virus-associated etiologies, pathogenesis, evidence-based management algorithms, present status of adoptive immunotherapy and future directions. World J Meta-Anal 2023; 11(7): 317-339
- URL: https://www.wjgnet.com/2308-3840/full/v11/i7/317.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i7.317
Post-transplant infections and malignancies are on the rise with increasing efficacy of immunosuppression[1,2]. Several population-based registries found a 2–5-fold increase in cancer risk after transplantation[3-7].
Although multifactorial, most of these cancers are attributed to a viral cause (known or suspected) and immunosuppression plays a significant role, as it suppresses the immune response to oncoviruses and impairs cancer immunosurveillance[3,8]. Eight to ten percent of kidney transplant recipients’ deaths are due to post-transplant cancers, the third leading cause of mortality after cardiovascular disease and infection in organ recipients[9,10].
Diverse types of malignancies can develop after transplantation, with some incurring a significant increase in incidence (lymphoma, non-melanoma skin cancer, lung, colon and liver) and others are not (ovarian, brain, breast, prostate and cervical malignancy) as mentioned in Table 1[9,11,12]. Table 2 emphasizes the burden of cancer, especially related to viral infections during the post-transplant period.
| Standardized incidence ratio compared to general population | Post-transplant cancers |
| > 5 | NMSC, PTLD, lip, RCC and KS |
| 2-5 | Melanoma, thyroid cancer, leukemia and multiple myeloma |
| < 2 | Breast, brain, lung and prostate cancer |
| Cancers associated with post-transplant viral infections | Meta-analysis SIR |
| EBV-associated | |
| Hodgkin’s lymphoma | 3.89 (2.42-6.26) |
| NHL | 8.07 (6.40-10.2) |
| HHV8-associated | |
| Kaposi’s sarcoma | 208 (114-369) |
| HBV/HCV-associated | |
| Hepatocellular | 2.13 (1.16-3.91) |
| HPV-associated | |
| Cervical | 2.13 (1.37-3.30) |
| Vulva & vagina | 22.8 (15.8-32.7) |
| Penis | 15.8 (5.79-34.4) |
| Anus | 4.85 (1.36-17.3) |
| Oropharynx | 3.23 (2.4-4.35) |
| Non-melanocytic skin cancer | 28.6 (9.39-87.2) |
Currently, there is varied agreement regarding the prevention, diagnosis, treatment and surveillance of post-transplant cancers, especially in relation to viral infections. Additionally, the introduction of adoptive immunotherapy (AI) has resulted in the dilemma of treatment management alternatives.
This article focuses on the up-to-date information of the various post-transplant virus-associated etiologies and their pathogenetic differences compared to the general population with respect to post-transplant malignancy. It also mentions in detail about comprehensive consensus regarding the management of post-transplant malignancy, pertaining to viral infections, in light of recent research findings, including the role of AI. Furthermore, this article highlights the need of future research with the purpose of developing a tailored therapeutic strategy for each patient based on existing risk factors and diagnostic techniques.
Various viruses that have been associated with causing[13-17] or promoting[18-19] post-transplant malignancies as given in Table 3.
| Virus | Associated/related post-kidney transplant tumours/cancers |
| EBV | PTLD, smooth muscle tumours |
| HPV | Squamous cell carcinoma |
| HHV8 | Kaposi’s sarcoma, multiple myeloma |
| HIV | Plasmablastic lymphoma, Merkel cell carcinoma |
| HBV/HCV | Hepatocellular carcinoma |
| BK polyomavirus | Urothelial, renal cell and collecting duct carcinoma |
| CMV | Gastrointestinal tumours, nephrogenic adenoma |
The commonest cancer following kidney transplantation is skin cancer, which is more aggressive than in the general population and nearly affects 50% of post-transplant patients[20]. Non-melanoma skin cancers (NMSCs) are the most common type, reported in up to 82% of patients within 20 years of transplantation[21,22]. Ninety percent of all NMSCs are squamous cell carcinoma (SCC) and basal cell carcinoma (BCC)[23,24]. Post-transplant recipients in comparison to the general population, have a 65–250-fold and 10-fold increased risk of developing SCC and BCC, respectively[20]. Various studies have reported that the ratio of BCC to SCC in the general population (5:1) is reversed in organ recipients (1:4 to 1:5)[23,24]. BCC, SCC, Kaposi’s sarcoma (KS) and malignant melanoma constitute up to 90%–95% of all skin cancers in transplant recipients[25,26]. Rare skin cancers include cutaneous lymphoma, Merkel cell carcinoma, vascular cutaneous tumor (angiosarcoma), mesenchymal cutaneous tumors and adnexal gland carcinoma.
Even though human papilloma virus (HPV) is frequently detected in warts, hair follicles, and keratotic lesions, both in patients with and without skin tumors, there is no conclusive evidence linking HPV to skin tumor development in transplanted patients[27,28]. Oncogenic (HPV types 16 and 18) and non-oncogenic (HPV types 6 and 11) HPV DNA is found in 65%–90% of SCC in organ recipients, but its carcinogenic role is still unclear[27].
Novel polyoma virus has been identified in human Merkel cell carcinoma (hence the name Merkel cell virus or MCV) with possible causation[29].
The skin cancers of organ recipients tend to be more aggressive, present at a younger age, and involve multiple primary sites as opposed to those of the general population.
Multiple factors contribute to the etiology of skin cancer, including immunosuppression, intensity of immunosuppression, UV radiation exposure, white race, older age, a history of skin cancer, human herpes virus (HHV) 8 and possibly HPV 16/18 and MCV[30].
Epstein–Barr virus (EBV) is a member of the gamma herpesvirus family, and is an encapsulated single-stranded DNA virus and ubiquitous. There are two strains infecting humans, EBV-1 and 2 (previously called EBV A and B). In the USA and Europe, EBV-1 predominates, whereas in Africa and New Guinea, both EBV strains are equally prevalent[31]. EBV spreads via saliva (and possible transmission through sexual intercourse), before spreading to circulating B cells through infection of the oropharyngeal epithelium[32]. EBV seroprevalence is 100% by age 4 years and 89% by 19 years in developing and developed nations and varies with socioeconomic status[33,34].
Kidney transplant recipients are susceptible to acute infection or reactivation of a latent virus, with clinical manifestations ranging from non-neoplastic viral replication (asymptomatic viremia, infectious mononucleosis) to neoplastic viral proliferations, like post-transplant lymphoproliferative disorder (PTLD) and smooth muscle tumors[35,36].
Asymptomatic low-level, high-level, or the absence of viremia may exhibit no distinguishable symptoms and usually detected through screening with EBV polymerase chain reaction[37]. In a few studies, renal dysfunction, patient and graft survival are no different between groups (absent, low or high viral loads), whereas others report a higher incidence of opportunistic infections with increasing viral loads[37,38]. EBV seronegative at transplantation, prior history of PTLD and non-Caucasians are risk factors for EBV viremia[37].
Other manifestation of EBV includes EBV-associated Guillain–Barre syndrome[39], gastric carcinoma[40], smooth muscle tumors[41], hemophagocytic syndrome[42] and autoimmune hemolytic anemia[43].
EBV-related PTLD, is the most serious sequel in organ recipients by the virus and cumulative incidence varies with 1%–5%, 2%–10% and 5%-20% in kidney, heart and lung and intestinal and multivisceral transplant recipients[44]. Other manifestations include an 11.8-fold increased risk of non-Hodgkin’s lymphoma in kidney transplant recipient compared to the age-matched non-transplant group[45].
PTLDs, mostly (65%–80%) present as extranodal masses and vary histologically as infectious mononucleosis-like, plasmacytic hyperplasia, florid follicular hyperplasia, polymorphic, monomorphic PTLD (B- and T-/NK-cell types) or classical Hodgkin’s lymphoma PTLD[46]. Risk factors associated with PTLD in kidney transplantation are listed in Table 4. Early PTLD (< 1 year post-transplant) is usually seen in EBV-seronegative recipients, polymorphic, with graft involvement (in 57%) and responds to reduction in immunosuppression (RIS). Late PTLD is usually monomorphic, disseminated and extranodal (graft involvement - only 10%) and resistant to RIS[47-50].
| Risk factors of PTLD in KT | Likely cause/association |
| Recipient age < 10 yr | A greater likelihood of being seronegative for EBV |
| Recipient age > 60 yr | Associated finding in various studies |
| EBV seropositive donor to EBV seronegative negative recipient (EBV D+/R-) | 90% are donor derived and 10–76-fold higher incidence of early PTLD |
| Bimodal peak | First peak (with higher incidence) in first 2 years and 2nd peak between 5 to 10 years post-transplant |
| Intensity of immunosuppression and use of T cell depleting antibodies (ATG and/or OKT3), belatacept | Reduction in cancer immunosurveillance |
| Treated acute rejection within first year after transplantation with depleting antibodies | Reduction in cancer immunosurveillance |
| Simultaneous pancreas–kidney transplantation | Association |
| HLA mismatches (especially HLA B and DR mismatches) | Likely, due to higher associated risk of rejection and use of increased net immunosuppression |
The most common sites of PTLD involvement are the gastrointestinal tract (15%–30%), lungs, skin (5%–10%), liver, central nervous system (CNS) (20%–25%, usually late PTLD) , and the allograft (20%–25%, often culminating in allograft loss)[50]. CNS PTLD often has poor prognosis, and has the highest incidence in kidney transplant recipients[35,51,52].
HPV is a double-stranded DNA virus that can infect the keratinized skin (basal epithelium), mucous membranes, and the cervical transformation zone and spread via direct contact transmission (person to person). HPV types 6, 11, 16 and 18 are implicated in low- and high-grade neoplasia[28,53-55]. HPV has been linked to precancerous lesions (cervical intraepithelial neoplasia and anal intraepithelial neoplasia), lesions with low malignant potential like cutaneous, anogenital warts and certain cancers [cervical, anal, vulvar/vaginal/penile squamous cell cancers, rarely oropharyngeal (head and neck) cancers][56].
There is higher risk of HPV-associated malignancies, extensive and treatment-refractory warts on the cutaneous and anogenital areas in transplanted patients (reactivation of old or new infection) compared to age matched non-transplant individuals[3,57].
HPV rarely causes viremia (in immunocompetent as well as immunodeficiency states) but lack of cell-mediated immunity at infected sites, especially in transplant recipients, leads to its persistence, extensive warts that are not responsive to treatment, and increased probability of cancers[58,59].
Persistent infection with HPV 16 and 18 is associated with premalignant and malignant lesions of the cervix, anus, vulva, penis or scrotum. Lesions are typically asymptomatic, may present with abnormal bleeding, ulcer/nodule/wart-like features, local pruritus, pelvic pain, and dyspareunia in some cases[60-62].
There has been links of HPV association with oropharyngeal and lung SCC but with conflicting results[3,63,64].
HHV8, a DNA gamma-herpes virus, has four variants: sporadic or classic (first description by Kaposi), endemic (in sub-Saharan Africa), epidemic (associated with HIV), and iatrogenic (in immunosuppressed transplant recipients)[65].
Virus can be transmitted via saliva (primarily), sexually (semen/vaginal secretion), vertically (breast milk), intravenously (drug use or blood products) or through transplantation.
Like EBV[66], HHV8 invades B cells, macrophages, lymphoepithelial cells and epithelium, can persist lifelong in a latent form, or reactivate when immunosuppressed to enter a lytic form leading to viremia[67,68]. In organ transplant recipients, lytic reactivation of virus due to immunosuppression (iatrogenic) may lead to uncontrolled monoclonal/oligoclonal proliferation of latently infected lymphoepithelial cells or proliferation of post-germinal center where B cell maturation happens.[67,68].
Lymphatic-endothelium-derived cells infected with HHV8 form multicentric neoplasm classically known as KS[69,70]. HHV8 induced neoplastic and non-neoplastic manifestation post-transplant can be derived from latent virus, seroconversion from positive donor to seronegative recipient[71], proliferation of seeded HHV8+ cells[72,73] or KS tumor in transplanted organs[74] while in an immunosuppressed state.
HHV8 is not ubiquitous like EBV, but seroprevalence is higher than 50% in some endemic regions (sub-Saharan Africa, Caribbean, Latin America, Mediterranean, and Middle East) and matches post-transplant KS (PT-KS) herpesvirus-associated pathologies in such regions[75].
KS risk is low in transplant recipients but 200–500-fold higher than in the general population[76,77]. Besides the key risk factor of HHV8 seropositivity, other factors include ethnicity (higher in seroprevalent geographic regions), receipt of lymphocyte depleting agents, HLA-B mismatch, older age and lung transplantation[76,78-82].
PT-KS has a higher incidence in kidney transplant compared to other solid organ transplantations (SOTs) (liver and heart) and rare in hematopoietic stem cell transplantation (HSCT). This condition usually manifests early after transplantation (median 2.5 years) as cutaneous or mucosal lesions, but 25%–50% have visceral manifestations[82] with mortality ranging from 8% to 14%. Disseminated disease is associated with thrombocytopenia, anemia, and abnormalities of bone marrow progenitor cells and widespread involvement (cutaneous, mucosal and visceral). Al-Khader et al[83] proposed clinical staging of PT-KS that assesses extent of disease and guides treatment. Few studies have shown that cytomegalovirus (CMV) infection can reactivate HHV8, and initiate onset and/or recurrence of KS[83,84].
Post-transplantation, HHV8 can also cause other lymphoproliferative disease such as primary effusion lymphoma, multicentric Castleman disease[85,86] and other non-malignant complications like plasmacytic B-cell proliferation, bone marrow failure and hepatitis[82,87].
Observations concerning the impact of HIV infection post-transplantation have been largely based on the experiences of recipients who previously had HIV infection and underwent transplantation. Transplant outcomes in HIV-positive recipients are almost similar to those in non-HIV-positive recipients with few differences[88,89].
KS prevalence in HIV-positive patients on antiretroviral therapy (ART) is 0.18%–0.46%, while it increases to 0.50%–0.66% in transplanted patients[90].
People with HIV [Standardized incidence ratio (SIR) = 4.95%] and organ recipients (SIR = 3.28%) had a greater risk of developing new cancers compared to general population[91].
SOT in HIV-positive patients carries a low risk of recurrence or de novo cancer. HPV-associated neoplasia (cervical, anal and atypia) had a higher risk in a few studies, however, this requires confirmation in future studies[92].
EBV-associated PTLD/lymphoma has similar prevalence in organ recipients with HIV[89].
Compared to non-HIV recipients, incidence of tuberculosis and fungal infections appears to be greater in HIV-infected recipients during the post-transplant period[93].
In a United States registry data (223 660 recipients, 1987–2005), de novo hepatocellular carcinoma (HCC) post-transplantation was evaluated among non-liver (kidney, heart and lung) and liver transplant recipients[94].
In non-liver recipients, the study reported de novo post-transplant HCC incidence of 6.5 per 100 000 person-years. Hepatitis B surface antigenemia [hazard ratio (HR): 9.7], hepatitis C virus (HCV) infection (HR: 6.9), and diabetes mellitus (DM) (HR: 2.8) are risk factors independently linked with HCC incidence. Incidence of HCC was greater in those with HCV (SIR = 3.4) or hepatitis B surface antigenemia (SIR = 6.5), but comparable with general population (SIR = 0.8).
In liver recipients, de novo post-transplant HCC incidence was 25 per 100 000 person-years. Advancing age, male sex (HR: 4.6), HCV infection (HR: 3.1), and DM (HR: 2.7) were independently associated risk factors. Overall, the incidence of HCC was higher (SIR = 3.4), but particularly among individuals with HCV (SIR = 5.0) or DM (SIR = 6.2).
Due to the high endemic prevalence of hepatitis B virus (HBV) infection in Taiwan, HCC is a major malignancy in general as well as in the post-transplant population, favoring hepatitis virus antigenemia as a potential causative factor[95]. HCV infection is also related to post-transplant cirrhosis and thereby increasing the risk of post-transplant HCC[96].
Various other studies of different ethnicities also found that HBV and HCV infection post-kidney transplantation was a significant risk factor for HCC[97,98].
The polyomavirus (BKV) is a ubiquitous polyoma virus that causes asymptomatic infection in childhood and has a seroprevalence of 70%–80% in adults. It develops latency in organs such as the kidneys, ureters, spleen or brain[99]. Its non-oncological manifestations in kidney recipients are ureteral stenosis, vasculopathy, tubulopathy, hemorrhagic cystitis, and interstitial nephritis[100,101]. BKV-related malignancies in kidney recipients include urothelial carcinoma of the renal pelvis, renal cell carcinoma, and collecting duct cancer [99,102-105].
Pathogenesis and transplant specific risk factors for post-transplant malignancies are multifactorial but mainly include immunosuppression and decreased immunosurveillance.
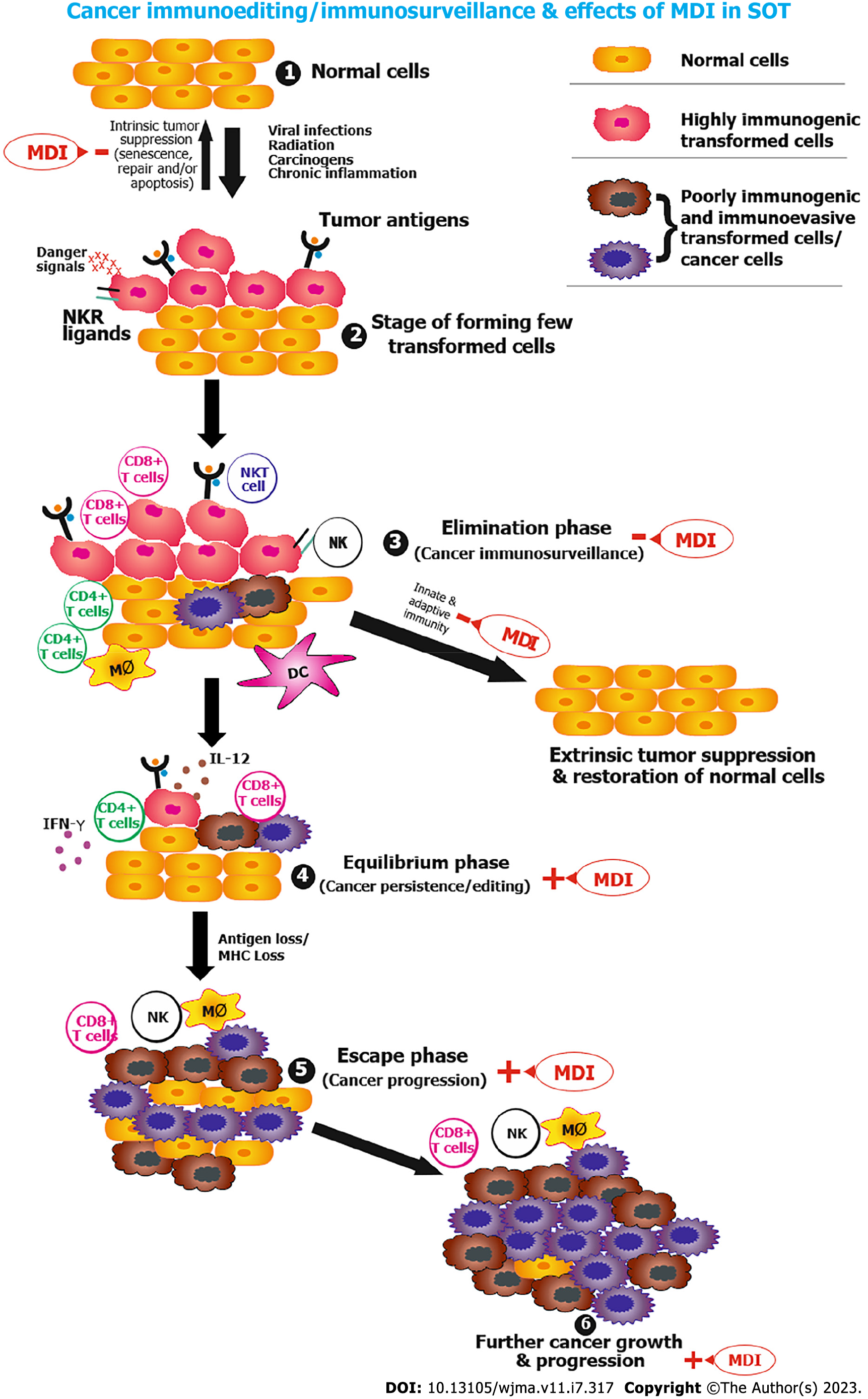
Cancer immunoediting involves three phases (Figure 1)[108-110]: Elimination phase (cancer immunosurveillance); equilibrium phase (cancer persistence/dormancy); and escape phase (cancer progression). Immunosuppression has an impact on all phases.
In post-transplant patients exposed to viral infections, UV radiation, carcinogens or chronic inflammation, some healthy cells transform into highly immunogenic tumor/transformed cells. These tumor cells may revert to normal tissue via a mechanism of intrinsic tumor suppression (repair, apoptosis or senescence), which may become weak due to the effects of modern era immunosuppression.
As soon as these highly immunogenic transformed cells evade the intrinsic tumor suppression mechanism, they enter the elimination phase (cancer immunosurveillance). During the elimination phase, innate and adaptive immunity (NK and T cells) offers protection against the development of cancer (known as extrinsic tumor suppression). If the phase of elimination concludes successfully, the body restores healthy tissue but is weakened by immunosuppression.
When transformed cells escape the elimination phase, they enter an equilibrium state (cancer persistence/dormancy), in which adaptive immunity (T cells, interleukin-2, interferon-) works to maintain such cells in a dormant state. In the event that dormancy occurs efficiently, it prevents outgrowth of transformed cells or occult tumors/cancers throughout life and represents the end stage of cancer immunoediting but is altered by immunosuppression. Tumor immunogenicity is edited during the elimination phase by constant immune selection. Antigen loss variants, flaws in antigen processing or presentation, immune effector cell resistance, and the generation of an immunosuppressive microenvironment within the tumor are some of the editing mechanisms. Genetic instability and tumor heterogeneity increase as editing proceeds, and highly immunogenic tumor cells become less immunogenic and immunoevasive tumor cells.
These less immunogenic and immunoevasive tumor cells escape immunosurveillance and progress to clinically apparent cancer. This phase is designated as the escape phase (cancer progression).
Specific carcinogenic mechanisms of various viral infections post-transplant are listed in Table 5[111].
| Virus | Carcinogenic mechanisms |
| EBV | EBV-infected cells generates more interleukin-6, which promotes the proliferation of B-cells, and interleukin-10, an immunosuppressive cytokine that promotes tumour development |
| HPV | E6 and E7 proteins expressed by HPV suppress p53-mediated apoptosis and increase malignant growth in infected cells |
| HHV8 | Viral proteins encoded by HHV8 inhibit the activation of pro-caspase-8, promotes Ras-PI3K-Akt survival pathway and enhances antiapoptotic Bcl-2 (B-cell lymphoma 2) expression, thereby inhibiting apoptosis and promoting uncontrolled proliferation of infected and endothelial cells |
| HBV | HBx proteins produced by virus activate the Ras-PI3K-Akt survival pathway and change EGFR signalling. In addition, it modifies the transcriptional activity of c-Myc, c-Fos, and c-Jun and promotes the expression of angiogenic factors, including VEGF and angiopoietin-1. Consequently, this stimulates proliferation and angiogenesis |
| HCV | Virus-produced non-structural proteins (NS3 and NS5A) promote the Ras-PI3K-Akt survival pathway. NS5A also modulates the signalling mediated by. Consequently, this stimulates proliferation and angiogenesis |
Multidrug immunosuppression in the transplant setting impacts cancer immune editing by a number of mechanisms, as shown in Table 6.
| Immuno-suppressive agents | Mechanisms in carcinogenesis | Cancer risk |
| Polyclonal lymphocyte depleting agents (OKT3/rATG) | Interfere with T-cells, B-cells, NK and DC functions[143-145] | Increased risk of PTLD |
| Alemtuzumab | Depletes B and T cells | Increased risk[146] |
| NHL (2.5-fold rise) | ||
| Colorectal cancer (2.5-fold rise) | ||
| Thyroid cancer (3-fold rise) | ||
| Mixed results with PTLD association[147,148] | ||
| Cyclosporine A | Downregulate T-bet dependent immunosurveillance[149] | Suppress immune response against melanomas |
| Inhibit antigen presentation by DC[150] | Impairs elimination of oncogenic viruses and overall increased risk of cancer[151] | |
| Tacrolimus | Inhibit antigen presentation by DC[150] | Impairs elimination of oncogenic viruses |
| Overall increase risk of PTLD and reduced trough levels substantially decline the risk[152] | ||
| Azathioprine | selectively depletion of memory T-cells[153] | Linked to late SCC (of skin) and myelodysplastic syndrome [154] |
| Mycophenolate (MMF/MPA) | Antiproliferative and antioncogenic potential[155] | Protective and reduce the risk of PTLD |
| mTOR inhibitors | Promotion of CD8+ central memory T cells[156] | Enhance antiviral immunity |
| Upregulate transcription factor T-bet[157] | T-bet regulates cross-talk of innate and adaptive immune cells and has tumour-suppressive activities[158] | |
| Antioncogenic and antiproliferative role | Overall cancer risk reduction and even regress KS[159] | |
| Belatacept | Inhibitor of T cell proliferation | Unclear though postulated as slight increased risk of oncogenicity[160] |
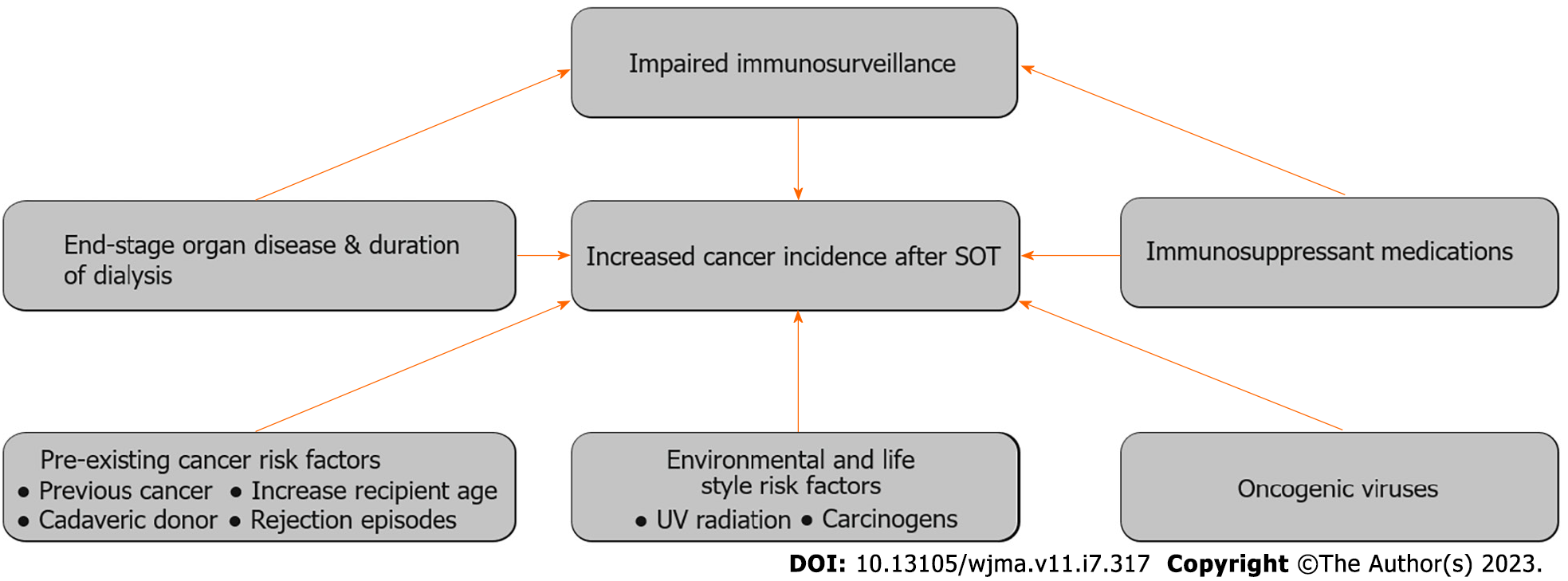
Multifactorial pathogenesis associated with post-transplant malignancy due to decrease immunosurveillance following exposure to viral infections, UV radiation and carcinogens including other related risk factors is summarized in Figure 2[108].
Interaction with a healthy immune system (as in general population) selects tumors devoid of tumor-specific antigens, meaning poorly immunogenic or immunoevasive tumors.
Tumors formed in immunosuppressed hosts are more immunogenic than in the general population (immunocompetent host) as de novo malignancies arise due to permissive effect of immunosuppression by inhibiting cancer immunosurveillance and immunoediting[109,110,112]. RIS and immunotherapy (i.e., adoptive/checkpoint inhibitors) may facilitate immune reconstitution, which can help by clearing immunogenic cancer cells but can raise risk of rejection[113].
Viral etiology is well known and accepted as a probable association or causation (either promoting or inducing) of a wide variety of post-transplant malignancies. Table 7 highlights screening, diagnosis and treatment of post-transplant viral infections.
| Post-transplant virus infections | Screening | Diagnosis | Treatment |
| HPV anogenital/cutaneous manifestation[28,161] | All 9–26-yr: Before transplant, receive 3 doses of HPV vaccine [nine-valent or quadrivalent vaccine (Gardasil 9 or Gardasil; Merck, Whitehouse Station, New Jersey)] or HPV-bivalent vaccine (Cervarix; GlaxoSmithKline, Rixensart, Belgium) in women | Examination and biopsy of atypical lesions | Cutaneous warts: Topicals (patient applied): Salicylic/lactic acid/imiquimod or cryotherapy (provider-applied) |
| Males and females (up to age 45 yr): May also be vaccinated with 3 doses of HPV vaccine (nine-valent) | Anogenital, perianal warts/history of receptive anal intercourse warts: colposcopy/anoscopy | Anogenital warts: topicals (patient applied): podofilox/5% imiquimod cream or cryotherapy/TCA /BCA/podophyllin resin (provider-applied) | |
| Organ recipient’s (15–26 yr): Immunize even if they have anogenital warts | Not responding or extensive or resistant warts: refer to dermatologist | ||
| At each visit: bright light skin examination (including feet) | |||
| Cervical pap smear (with or without HPV PCR co-test): Every 6 mo in first year and then yearly, post-transplant, in females (> 30 yr), irrespective of HPV vaccination status | |||
| If rejection treated with T cell depleting agents, resume above schedule | |||
| Follow in all females irrespective of HPV vaccination status | |||
| EBV viremia/disease | Identify high risk recipients (i.e. EBV D+/R-): EBV viral load once first week, monthly first 3–6 mo, and every 3 mo until the end of the first post-transplant year; Additionally, after treatment of acute rejection[162] | Quantitative EBV load assay [calibrated to World Health Organization IS for EBV DNA) (EBV NAAT) | Reduce immunosuppression with rising EBV loads in EBV-seronegative patients |
| EBV disease precedes detectable or rising EBV loads | Whole blood/lymphocyte samples are preferable to plasma (the EBV viral load is greater and becomes detectable sooner), thereby enhancing sensitivity and early detection/reactivation | ||
| Watch for signs/symptoms: fever, diarrhoea, lymphadenopathy, and allograft dysfunction | Same sample type, assay and laboratory for assessing rise in EBV loads | ||
| HHV8 viremia | Post-transplantation, HHV8 serologic testing is not routinely recommended globally | Serological assays (IFA ELISA) which detect HHV8 antibodies against latent and lytic viral antigens (both)[163]: Issues with such assays are inadequate standardisation, variable sensitivity and specificity among tests (60%–100%), and poor agreement with a predefined reference standard. It is still preferable when compared with quantitative PCR in identifying “at risk” transplant patients in endemic regions | RIS if quantitative PCR elevated/rising and/or absent HHV antibodies in “at risk” post-transplant patient or with non-neoplastic KS diseases |
| Identify “at risk” before transplant, for HHV8 related disease post-transplant, in endemic zone [i.e. R+ (HHV8 reactivation) and D+/R- (HHV8 primary infection)][163,164] | Serological assay which detect HHV8 DNA by quantitative PCR: Its role are: (1) Predicts the occurrence of non-neoplastic HHV8 related diseases (in HHV8 primary infections and high viral loads); | Strictly follow and monitor | |
| (2) Detect active HHV8 replication; and | |||
| And (3) monitor response to treatment in post-transplant patients with HHV8 related diseases | |||
| Issue of serological assays in HHV8 diagnosis: Lack of any serological gold standard assay | |||
| Direct detection of HHV8 (HHV8 immunohistochemical staining) from involved site is still gold standard for diagnosis | |||
| Histopathological confirmation and HHV8 DNAemia confirms the diagnosis | |||
| Plasmacytic B-cell proliferation (HHV8 associated)[82] | Watch for SIS | Biopsy: Shows polyclonal HHV8 B-cell proliferations in lymph nodes/visceral organs | RIS |
| Exclude mimickers of signs/symptoms | HHV8 viral load (quantitative PCR) | Rituximab | |
| Trial of antiviral | |||
| Bone marrow failure/HPS (HHV8 associated)[82,165] | Watch for fever, jaundice, severe pancytopenia, plasmacytosis, hepatosplenomegaly, SIS, rash (maculopapular) | Biopsy confirmation of HHV8 in bone marrow/ lesions | RIS |
| Exclude mimickers of signs/symptoms | HHV8 viral load (quantitative PCR) | Rituximab | |
| Trial of antiviral | |||
| Hepatitis (HHV8 associated) | Elevated liver enzymes, SIS, rash (maculopapular). | HHV8 viral load (quantitative PCR) | RIS |
| Exclude mimickers of signs/symptoms | Biopsy confirmation of lesion/organ affected | Trial of antivirals |
Susceptibility of viral infections post-transplant is proportional to the degree of net immunosuppression and varies greatly due to inherent limitations in the available data. The availability of population registry data for specific viral infections related to the type of organ transplant is insufficient, differs with immunosuppression regimen and geographical distribution, and is, in general, weak worldwide.
After a thorough literature research, we could only find EBV-associated PTLD and HHV8-associated KS risk with different types of organ transplantation as mentioned below. PTLD risk is highest for intestine and multi-organ transplants (12%–17%), followed by lung (6%–10%), heart (3%–5%), liver (2%–3%), and kidney (1.5%–2.5%), being the least[114].
KS incidence varies with organ transplant and is reported as per 100 000 person-years. It was reported as 95.79 [95% confidence interval (95%CI): 42.81–214.31] in kidney, 44.25 (95%CI: 4.78–409.20) in liver, 49.25 (95%CI: 2.48–977.84) in heart and 10.97 (95%CI: 4.12–29.23) in lung [115].
An in-depth detail to diagnose various post-transplant virus associated cancers is outlined in Table 8.
| Post-transplant viral associated malignancy | Diagnosis |
| CIN and cervical cancer and (HPV- associated) | Abnormal cervical Pap test/cytology on screening: Colposcopy biopsy of any suspicious lesion[28,161] |
| AIN and anal cancer (HPV-associated) | Abnormal anal Pap test/cytology on screening: High-resolution anoscopy ± biopsy of any suspicious lesion[28,161] |
| EBV associated PTLD | Identify “B” symptoms (fever, night sweats and weight-loss) |
| Excision biopsy/core biopsy (in allograft PTLD as excision in not practical) is gold standard for diagnosis[46] | |
| Stage PTLD with CT imaging of the chest, abdomen, and pelvis, as well as MRI brain imaging before initiating treatment as in immunocompetent host[166] | |
| PET-CT may help in diagnosing occult PTLD, accurate staging in occult cases and sometime evaluating treatment response[167-169] | |
| PT-KS | Examine for cutaneous or mucosal lesions, visceral involvement and haematological manifestations |
| Diagnostic gold standard: HHV8 confirmation in biopsy of KS lesions[170] | |
| HPE characteristic of PT-KS: Spindle-shaped cells and immunostaining confirmation with latency-associated nuclear antigen and CD34 positive staining[171,172] | |
| Quantitative PCR load of HHV8: Role in supporting diagnosis and monitoring treatment response | |
| Confirmation of diagnosis by HPE and HHV8 DNAemia | |
| Depending on site involved, disease staging by imaging and invasive procedures (e.g., bronchoscopy, esophago-gastroduodenoscopy, colonoscopy)[173] | |
| MCD | Watch for lymph node enlargement, systemic inflammatory symptoms |
| Gold standard for diagnosis: Lymphnode biopsy confirmation of HHV8[170] | |
| HPE: HHV8+ plasmablasts in follicular mantle zone and vascular hyperplasia | |
| Quantitative PCR load of HHV8: Role in supporting diagnosis and monitoring treatment response | |
| Confirmation of diagnosis by HPE and HHV8 DNAemia | |
| PEL | Watch for effusion (pleural, peritoneal, pericardial) |
| Gold standard: Confirmation of HHV8 in pleural/ascitic fluid[170] | |
| HPE characteristic: HHV8+ plasmablasts displaying immunoblastic and anaplastic characteristics | |
| Quantitative PCR load of HHV8: Role in supporting diagnosis and monitoring treatment response | |
| Confirmation of diagnosis by HPE and HHV8 DNAemia |
The literature lacks evidence on how many years of immunosuppression post-transplant increases the risk of cancer. Despite uncertainties, the literature consistently indicates that the overall duration and intensity of immunosuppression, rather than individual drugs in the immunosuppressive regimen, lead to an increased risk of cancer. Table 9 describes treatment and prevention of post-transplant cancers.
| Post-transplant malignancy | Treatment | Prevention |
| CIN (HPV-associated)[28,161] | Loop electrosurgical excision procedure/cryotherapy/cold knife conization of the lesion | Vaccination as mentioned in Table 3 (screening of HPV) |
| Cervical cancer (HPV-associated)[28,161] | Microinvasive disease (< 3 mm): conization[174] | Known previous history: Assess for anogenital lesion for cervical/anal lesions prior to transplant |
| Up to stage IIA: Chemoradiation[175] | Recommend condom use | |
| Locally advanced: Chemoradiation[176] | During laser surgery for HPV lesions, cover skin surface, mask and eye protection to prevent reimplantation of virus in electrocautery fumes | |
| Metastatic: Chemoradiation (palliation and symptoms alleviation)[177] | ||
| AIN (HPV-associated)[28,161] | AIN I (< 1 cm2 at base): Topical 80% TCA[178]/5-fluorouracil[179] or cryotherapy | |
| Larger size AIN I, AIN II and III: Infrared coagulation[180,181] or fulguration (anoscopy guided)[181] | ||
| Anal and penile cancer (HPV-associated)[28,161] | Invasive anal carcinoma: Combined-modality therapy [radiotherapy and chemotherapy (5-fluorouracil and mitomycin/cisplatin)][182] | |
| Penile cancer: Surgical resection ± chemotherapy (as per stage in immunocompetent) | ||
| PTLD[183] | Differentiate allograft dysfunction from PTLD, before initiating treatment using allograft biopsy | EBV viral load surveillance (for EBV D+/R-) as mentioned in screening of EBV |
| RIS: Preferred pre-emptive intervention. Adjust to lowest tolerated immunosuppression, may switch to mTOR inhibitor. Lack of sufficient evidence to suggest any specific RIS protocol or switching to mTOR inhibitor | ||
| Rituximab monotherapy for progressive disease following RIS and CD20+ PTLD | Patients (EBV D+/R-) with fluctuating immunosuppression, episodes of rejection, or who have not established a viral “set point” will be monitored for a period beyond the first year | |
| Cytotoxic chemotherapy if progression after rituximab and RIS. R-CHOP 21 regimen: Four sequential cycles of rituximab/ cyclophosphamide, doxorubicin, oncovin, and prednisone every 3 wK[184,185] | ||
| Children with EBV + PTLD: the low-dose cyclophosphamide and prednisone regimen plus rituximab [186]. | EBV viral loads becomes positive 4 to 16 wk prior to development of PTLD[189] | |
| CD20- Tcell PTLD, B cell, Burkitt and Hodgkin’s lymphoma: same chemotherapy regimen as immunocompetent host | ||
| CNS PTLD: Chemotherapy regimens are same as used to treat primary CNS lymphoma (PCNSL) in general population/ immunocompetent individuals[187,188]. Regimen with systemic rituximab, dexamethasone and antivirals, if unable to tolerate chemotherapy or disease occurring early post-transplant | Monitor viral load in EBV seropositive recipients in re-transplantation after PTLD | |
| Start pneumocystis jirovecii prophylaxis: If PTLD treatment administered beyond RIS | ||
| KS | RIS (30% complete remission in few reports)[190] | Pre transplant “at risk” in endemic areas (D+/R- or R+ HHV8 status): Frequent viral load monitoring for 3–6 months and physical examination of skin and mucosal surfaces as a routine, post-transplant |
| Switch to mTOR if using CNI (mTOR inhibitor is antiangiogenic, inhibit viral replication pathways)[191,192] and helps recovery of HHV-8-specific cytotoxic T cells[78,82] | RIS if viral loads rising while monitoring and switching to mTOR inhibitors early | |
| Antivirals (ganciclovir, foscarnet, cidofovir): Not routinely used, as in vivo efficacy is not demonstrated | ||
| If no response or relapse after above: Oncology consultation and chemotherapy (CHT) (L-anthracyclines) | ||
| If single skin lesion: Surgical excision or intralesional electrocautery or intralesional chemotherapy can be considered | ||
| MCD | RIS (limited evidence) and/or switch to mTOR from CNI (if possible) | |
| Rituximab[193] | ||
| If aggressive disease, no response/relapse: chemotherapy [R-CHOP/R-CVP (rituximab- cyclophosphamide, doxorubicin, vincristine, prednisone)][82] | ||
| PCL | Primary therapy is CHT [cyclophosphamide, doxorubicin, vincristine, prednisone(CHOP)][194] | |
| RIS (limited evidence) | ||
| If CHT contraindicated/no response or relapse: Intracavitary antivirals(cidofovir)[82] |
Due to the rise in the risk of malignancy, monitoring organ recipients post-transplant is vital. Current data suggest that the liver is an immunologically favorable organ and immunosuppression withdrawal is reported in selected patients who underwent liver transplantation (i.e. up to 40% of adults and 60% of pediatric liver recipients)[116]. As data have not been specified in most clinical studies, the usefulness of immunosuppression withdrawal in carefully selected liver transplant recipients has not demonstrated a significant clinical benefit on de novo malignancies post-transplantation[116]. Hence, there is risk of carcinogenesis. The surveillance protocol is provided in Table 10.
| Cancer | Post-transplant surveillance |
| Skin | Self-skin examination monthly; examination by dermatologist: 6 to 12 monthly[162] (expert opinion) |
| PTLD (EBV+) | Routine screening of EBV D+/R- by EBV NAAT: once first week, monthly for next 3–6 mo, and every 3 mo till 1 yr after transplantation[162] (expert opinion) |
| Cervical | Age 25–74 yr: yearly cervical Pap test and pelvic examination[195]; in higher risk category, more frequent Pap test |
| Hepatocellular | Every 6 mo screening with USG ± α-fetoprotein in high risk (i.e. with cirrhosis) (extrapolation from general population) |
| Renal | USG screen every 6–12 mo in high risk (i.e. acquired cystic kidney)[196] |
| Breast | Females < 50 yr: individual decision when to start screening; Females 50–74 yr: every 2 yr screening mammography[197]; [extrapolation from immunocompetent (general) population] |
| Prostate | Men 55–69 yr: individualized screening approach after discussing potential benefits and harm; Men > 70 yr, avoid routine screening[198] [extrapolation from immunocompetent (general) population] |
| Bowel | All 45–75 yr: stool immunochemical testing every 2 yr, 5-yearly FEGD and sigmoidoscopy, or 5–10-yearly colonoscopy[199] |
| Lung | All 55–79 yr who have smoked 1 pack/day for 30 yr or its equivalent (2 packs/day for 15 yr, 3 packs/day 10 yr): yearly low dose CT chest [200] [extrapolation from immunocompetent (general) population] |
Immunosuppression increases the chance of opportunistic infections in the post-transplant period. Limitations of current pharmacological treatment of viral infections in organ recipients include cost, antiviral toxicity, their variable efficacy and even resistance[117]. Most importantly, pharmacotherapies does not aid in pathogen-specific immune reconstitution, and the repeated risk persists after successful cure or eradication of virus. CMV is one potential example of such a pattern[118].
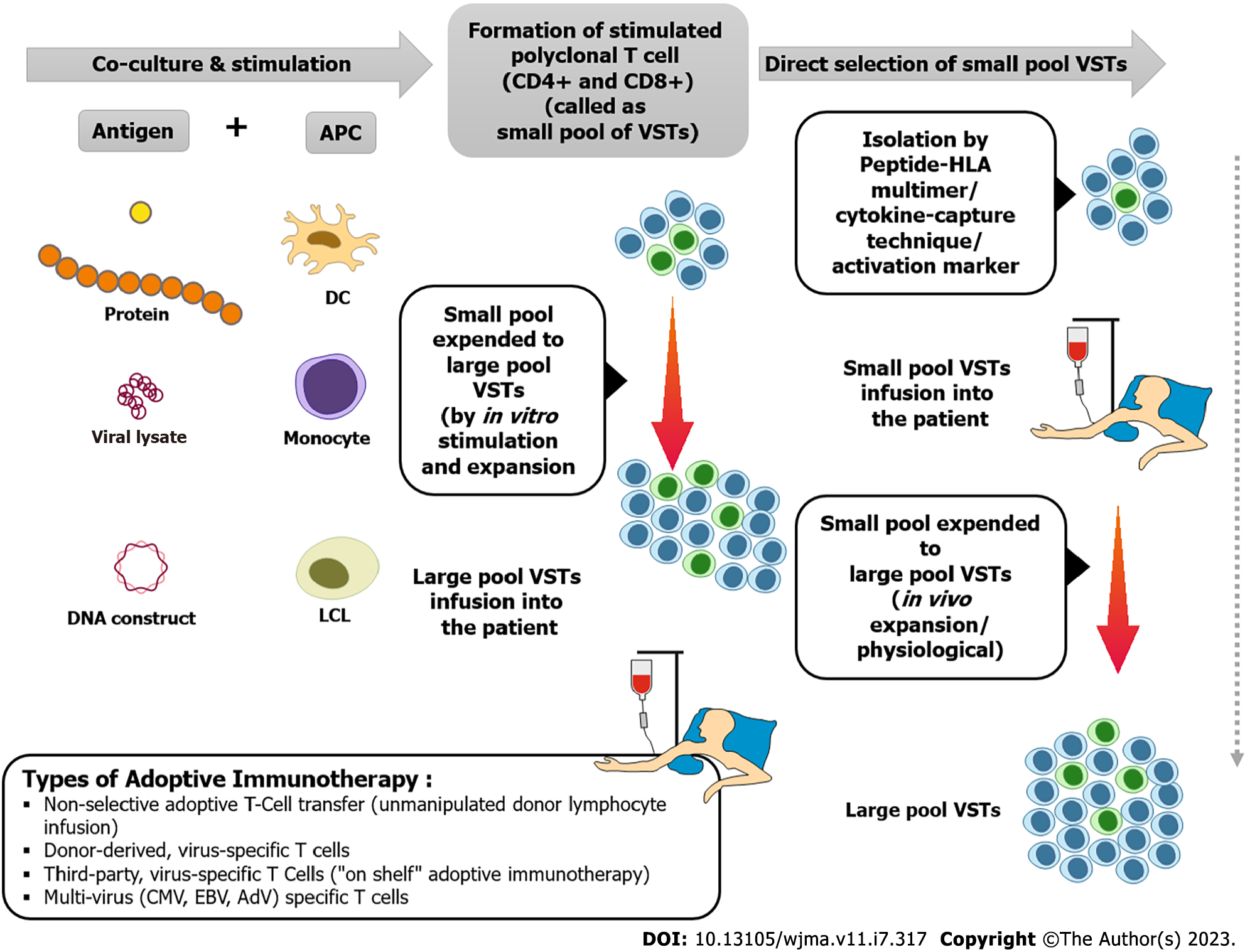
Spiess et al[119] first described the efficacy of AI in murine tumors in 1987, and later demonstrating objective tumor response in metastatic melanoma patients[120].
AI uses pathogen/virus-specific T cells to quickly restore immune responses to infectious pathogens/viruses in organ recipients. Apart from eliciting virus-specific cytotoxic responses, AI has specific advantage over pharmacotherapy by establishing long-term T-cell memory and may help preventing recurrent infections and protects against the organ toxicity/myelosuppression associated with some antivirals.
AI has been explored post-HSCT for CMV, EBV and adenovirus and has weak evidence in SOT. Advancement in immunological techniques has minimized alloreactivity and maximized cytotoxicity with AI, thereby, yielding a targeted approach with good safety profile[121-125].
In EBV-positive PTLD: (1) Failed standard therapy with RIS, rituximab, chemotherapy, and/or radiotherapy[126]; and (2) children failed with RIS and rituximab therapy[127]. Delayed response with AI in such cases is possible due to previous use of rituximab.
In CMV: Refractory and resistant CMV[128-132].
Above indications are inferred from partial/complete response in certain subsets of patients post-transplant after AI therapy when searched within the literature.
Figure 3 illustrates the steps, isolation, and diverse forms of AI[133-137].
AI has been investigated more in HSCT compared to SOT. Most data have come from the variable success of AI in EBV + PTLD disease. Use of AI in CMV disease is sparse and limited to a few cases in SOT. AI needs more evaluation in controlled trials.
Concerns for the widespread use of AI include limitations such as the need for specialized facilities and a specific time to generate, high costs, questionable durability, long-term overall efficacy and safety, the potential for alloreactivity, and reduced ability to mount adequate response with ongoing immunosuppression.
Achievement of complete remission (clinically and radiologically); sustained disease-free status for at least 12–24 mo; presence of seroconversion (virus-specific IgG antibodies); graft nephrectomy in cases of allograft PTLD; and absent or undetectable viral loads after successful treatment of malignancy[50,138,139].
Post-transplant malignancy is a considerable risk and cause of significant morbidity and mortality in organ recipients. Strategically reducing immunosuppression is an important step in the management of post-transplant virus-related cancers. Evidence for prevention, treatment and surveillance in post-transplant viral infections and malignancy are extrapolated from findings in the general population. A multidisciplinary team is vital for successful outcome. An individualized approach is the most effective method and treatment to eradicate or cure might not be the ultimate goal in all cases. AI is currently at an initial stage and has inherent logistic problems. Wait time for re-transplantation following the successful treatment of cancer should be assessed on an individual case basis, taking due consideration of the risks associated with renal replacement therapies. Collaborative efforts among all those engaged in the care of post-transplant patients, observing more extensive care studies and multicenter interventional trials, can enrich the evidence base and long-term, quality care of organ recipients.
| 1. | Vajdic CM, McDonald SP, McCredie MR, Van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA 2006; 296: 2823-2831. [RCA] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 853] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 2. | Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1643] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 3. | Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1131] [Article Influence: 75.4] [Reference Citation Analysis (1)] |
| 4. | Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 363] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 5. | Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 521] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 7. | Li WH, Chen YJ, Tseng WC, Lin MW, Chen TJ, Chu SY, Hwang CY, Chen CC, Lee DD, Chang YT, Wang WJ, Liu HN. Malignancies after renal transplantation in Taiwan: a nationwide population-based study. Nephrol Dial Transplant. 2012;27:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Stallone G, Infante B, Grandaliano G. Management and prevention of post-transplant malignancies in kidney transplant recipients. Clin Kidney J. 2015;8:637-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J. 2018;11:315-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. 2009;9:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. 2004;15:1582-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Turshudzhyan A. Post-renal transplant malignancies: Opportunities for prevention and early screening. Cancer Treat Res Commun. 2021;26:100283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Preiksaitis JK, Keay S. Diagnosis and management of posttransplant lymphoproliferative disorder in solid-organ transplant recipients. Clin Infect Dis. 2001;33 Suppl 1:S38-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Engels EA, Clarke CA, Pfeiffer RM, Lynch CF, Weisenburger DD, Gibson TM, Landgren O, Morton LM. Plasma cell neoplasms in US solid organ transplant recipients. Am J Transplant. 2013;13:1523-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Caillard S, Agodoa LY, Bohen EM, Abbott KC. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation. 2006;81:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Black CL, Foster-Smith E, Lewis ID, Faull RJ, Sidhu SK. Post-transplant plasmablastic lymphoma of the skin. Australas J Dermatol. 2013;54:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | El Hennawy HM, Habhab W, Almutawa A, Shinawi S, Al Ayad A, Fahmy A. Long-term follow-up of post renal transplantation Epstein-Barr virus-associated smooth muscle tumors: Report of two cases and review of the literature. Transpl Infect Dis. 2018;20:e12841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Michel Ortega RM, Wolff DJ, Schandl CA, Drabkin HA. Urothelial carcinoma of donor origin in a kidney transplant patient. J Immunother Cancer. 2016;4:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Kumari K, Pradeep I, Kakkar A, Dinda AK, Seth A, Nayak B, Singh G. BK polyomavirus and urothelial carcinoma: Experience at a tertiary care centre in India with review of literature. Ann Diagn Pathol. 2019;40:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Katabathina VS, Menias CO, Tammisetti VS, Lubner MG, Kielar A, Shaaban A, Mansour J, Surabhi VR, Hara AK. Malignancy after Solid Organ Transplantation: Comprehensive Imaging Review. Radiographics. 2016;36:1390-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Webb MC, Compton F, Andrews PA, Koffman CG. Skin tumours posttransplantation: a retrospective analysis of 28 years' experience at a single centre. Transplant Proc. 1997;29:828-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Piselli P, Busnach G, Fratino L, Citterio F, Ettorre GM, De Paoli P, Serraino D; Immunosuppression and Cancer Study Group. De novo malignancies after organ transplantation: focus on viral infections. Curr Mol Med. 2013;13:1217-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1121] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 24. | Dreno B. Skin cancers after transplantation. Nephrol Dial Transplant. 2003;18:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Mittal A, Colegio OR. Skin Cancers in Organ Transplant Recipients. Am J Transplant. 2017;17:2509-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Greenberg JN, Zwald FO. Management of Skin Cancer in Solid-organ Transplant Recipients: A Multidisciplinary Approach. Dermatol Clin. 2011;29:231-241, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 27. | Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. [PubMed] [DOI] [Full Text] |
| 28. | Chin-Hong PV, Reid GE; AST Infectious Diseases Community of Practice. Human papillomavirus infection in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (4)] |
| 29. | Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2611] [Cited by in RCA: 2333] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 30. | Al-Adra D, Al-Qaoud T, Fowler K, Wong G. De Novo Malignancies after Kidney Transplantation. Clin J Am Soc Nephrol. 2022;17:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 31. | Puchhammer-Stöckl E, Görzer I. Cytomegalovirus and Epstein-Barr virus subtypes--the search for clinical significance. J Clin Virol. 2006;36:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 32. | Thorley-Lawson DA, Edson CM. Polypeptides of the Epstein-Barr virus membrane antigen complex. J Virol. 1979;32:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Balfour HH Jr, Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6-19, 2003-2010. PLoS One. 2013;8:e64921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Caillard S, Lelong C, Pessione F, Moulin B; French PTLD Working Group. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006;6:2735-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, Rosenthal JT, Hakala TR, Shaw BW Jr, Hardesty RL. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 826] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 37. | Morton M, Coupes B, Roberts SA, Johnson SL, Klapper PE, Vallely PJ, Picton ML. Epstein-Barr virus infection in adult renal transplant recipients. Am J Transplant. 2014;14:1619-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Bamoulid J, Courivaud C, Coaquette A, Chalopin JM, Gaiffe E, Saas P, Ducloux D. Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant. 2013;13:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Masajtis-Zagajewska A, Muras K, Mochecka-Thoelke A, Kurnatowska I, Nowicki M. Guillain-Barré syndrome in the course of EBV infection after kidney transplantation--a case report. Ann Transplant. 2012;17:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Lunardi F, Calabrese F, Furian L, Rigotti P, Valente M. Epstein-Barr virus-associated gastric carcinoma 33 years after kidney transplantation. NDT Plus. 2011;4:49-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Tan CS, Loh HL, Foo MW, Choong LH, Wong KS, Kee TY. Epstein-Barr virus-associated smooth muscle tumors after kidney transplantation: treatment and outcomes in a single center. Clin Transplant. 2013;27:E462-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Nanmoku K, Yamamoto T, Tsujita M, Hiramitsu T, Goto N, Katayama A, Narumi S, Watarai Y, Kobayashi T, Uchida K. Virus-associated hemophagocytic syndrome in renal transplant recipients: report of 2 cases from a single center. Case Rep Hematol. 2015;2015:876301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Hamilton AJ, Webb LH, Williams JK, D'Souza RJ, Ngu LS, Moore J. Autoimmune haemolytic anaemia associated with Epstein Barr virus infection as a severe late complication after kidney transplantation and successful treatment with rituximab: case report. BMC Nephrol. 2015;16:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Morscio J, Dierickx D, Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol. 2013;2013:150835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 758] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 46. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5632] [Article Influence: 563.2] [Reference Citation Analysis (1)] |
| 47. | Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, Ansell SM, Gores GJ, Stegall MD, McGregor CG. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Rubinstein J, Toner K, Gross T, Wistinghausen B. Diagnosis and management of post-transplant lymphoproliferative disease following solid organ transplantation in children, adolescents, and young adults. Best Pract Res Clin Haematol. 2023;36:101446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 49. | Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol. 2012;13:122-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Abbas F, El Kossi M, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: Current concepts and future therapeutic approaches. World J Transplant. 2020;10:29-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 51. | Trofe J, Buell JF, Beebe TM, Hanaway MJ, First MR, Alloway RR, Gross TG, Succop P, Woodle ES. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. Am J Transplant. 2005;5:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, Velten M, Moulin B; French Transplant Centers. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 53. | Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 331] [Reference Citation Analysis (0)] |
| 54. | de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 1958] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 55. | Tilston P. Anal human papillomavirus and anal cancer. J Clin Pathol. 1997;50:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Monk BJ, Tewari KS. The spectrum and clinical sequelae of human papillomavirus infection. Gynecol Oncol. 2007;107:S6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Frisch M, Biggar RJ, Engels EA, Goedert JJ; AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 562] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 58. | Euvrard S, Kanitakis J, Chardonnet Y, Noble CP, Touraine JL, Faure M, Thivolet J, Claudy A. External anogenital lesions in organ transplant recipients. A clinicopathologic and virologic assessment. Arch Dermatol. 1997;133:175-178. [PubMed] |
| 59. | Euvrard S, Kanitakis J, Cochat P, Cambazard F, Claudy A. Skin diseases in children with organ transplants. J Am Acad Dermatol. 2001;44:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Dittmer C, Fischer D, Diedrich K, Thill M. Diagnosis and treatment options of vulvar cancer: a review. Arch Gynecol Obstet. 2012;285:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Matoso A, Ross HM, Chen S, Allbritton J, Epstein JI. Squamous neoplasia of the scrotum: a series of 29 cases. Am J Surg Pathol. 2014;38:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Peng W, Feng G, Lu H, Chen J, Chen K, Hao Y, Cao Y. A case report of scrotal carcinoma and review of the literature. Case Rep Oncol. 2012;5:434-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Klein F, Amin Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer. 2009;65:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 64. | Koshiol J, Rotunno M, Gillison ML, Van Doorn LJ, Chaturvedi AK, Tarantini L, Song H, Quint WG, Struijk L, Goldstein AM, Hildesheim A, Taylor PR, Wacholder S, Bertazzi PA, Landi MT, Caporaso NE. Assessment of human papillomavirus in lung tumor tissue. J Natl Cancer Inst. 2011;103:501-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Nagy S, Gyulai R, Kemeny L, Szenohradszky P, Dobozy A. Iatrogenic Kaposi's sarcoma: HHV8 positivity persists but the tumors regress almost completely without immunosuppressive therapy. Transplantation. 2000;69:2230-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 757] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 67. | Schulz TF. The pleiotropic effects of Kaposi's sarcoma herpesvirus. J Pathol. 2006;208:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Riva G, Barozzi P, Torelli G, Luppi M. Immunological and inflammatory features of Kaposi’s sarcoma and other Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-associated neoplasias. AIDS Rev. 2010;12:40-51. [PubMed] |
| 69. | Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 622] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 70. | Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4341] [Cited by in RCA: 4178] [Article Influence: 130.6] [Reference Citation Analysis (3)] |
| 71. | Luppi M, Barozzi P, Santagostino G, Trovato R, Schulz TF, Marasca R, Bottalico D, Bignardi L, Torelli G. Molecular evidence of organ-related transmission of Kaposi sarcoma-associated herpesvirus or human herpesvirus-8 in transplant patients. Blood. 2000;96:3279-3281. [PubMed] |
| 72. | Sarid R, Pizov G, Rubinger D, Backenroth R, Friedlaender MM, Schwartz F, Wolf DG. Detection of human herpesvirus-8 DNA in kidney allografts prior to the development of Kaposi's sarcoma. Clin Infect Dis. 2001;32:1502-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Barozzi P, Bosco R, Vallerini D, Potenza L, Torelli G, Luppi M, Facchetti F, Guaraldi G, Schulz TF. KSHV/HHV-8 infection of tubular epithelial cells in transplantation kidney. Transplantation. 2006;82:851-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Barozzi P, Luppi M, Facchetti F, Mecucci C, Alù M, Sarid R, Rasini V, Ravazzini L, Rossi E, Festa S, Crescenzi B, Wolf DG, Schulz TF, Torelli G. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat Med. 2003;9:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Penn I. Kaposi's sarcoma in transplant recipients. Transplantation. 1997;64:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Mbulaiteye SM, Engels EA. Kaposi's sarcoma risk among transplant recipients in the United States (1993-2003). Int J Cancer. 2006;119:2685-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant. 2013;13:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Barozzi P, Bonini C, Potenza L, Masetti M, Cappelli G, Gruarin P, Whitby D, Gerunda GE, Mondino A, Riva G, Vallerini D, Quadrelli C, Bosco R, Ciceri F, Bordignon C, Schulz TF, Torelli G, Luppi M. Changes in the immune responses against human herpesvirus-8 in the disease course of posttransplant Kaposi sarcoma. Transplantation. 2008;86:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Cahoon EK, Linet MS, Clarke CA, Pawlish KS, Engels EA, Pfeiffer RM. Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int J Cancer. 2018;143:2741-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Francès C, Marcelin AG, Legendre Ch, Chevret S, Dussaix E, Lejeune J, Euvrard S, Bigorie A, Schulz TF, Agbalika F, Lebbé C; Skin and Organ Transplantation Group of the French Society of Dermatology. The impact of preexisting or acquired Kaposi sarcoma herpesvirus infection in kidney transplant recipients on morbidity and survival. Am J Transplant. 2009;9:2580-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Cattani P, Capuano M, Graffeo R, Ricci R, Cerimele F, Cerimele D, Nanni G, Fadda G. Kaposi's sarcoma associated with previous human herpesvirus 8 infection in kidney transplant recipients. J Clin Microbiol. 2001;39:506-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood. 2012;120:4150-4159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Al-Khader AA, Suleiman M, Al-Hasani M, Haleem A. Posttransplant Kaposi sarcoma: staging as a guide to therapy and prognosis. Nephron. 1988;48:165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Marcelin AG, Roque-Afonso AM, Hurtova M, Dupin N, Tulliez M, Sebagh M, Arkoub ZA, Guettier C, Samuel D, Calvez V, Dussaix E. Fatal disseminated Kaposi's sarcoma following human herpesvirus 8 primary infections in liver-transplant recipients. Liver Transpl. 2004;10:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645-656. [PubMed] |
| 86. | Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276-1280. [PubMed] |
| 87. | Mularoni A, Gallo A, Riva G, Barozzi P, Miele M, Cardinale G, Vizzini G, Volpes R, Grossi P, Di Carlo D, Luca A, Trenti T, Luppi M, Conaldi PG. Successful Treatment of Kaposi Sarcoma-Associated Herpesvirus Inflammatory Cytokine Syndrome After Kidney-Liver Transplant: Correlations With the Human Herpesvirus 8 miRNome and Specific T Cell Response. Am J Transplant. 2017;17:2963-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Sawinski D. Kidney Transplantation in Patients with HIV. Kidney360. 2020;1:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Muller E, Botha FCJ, Barday ZA, Manning K, Chin-Hong P, Stock P. Kidney Transplantation in HIV-positive Patients: Current Practice and Management Strategies. Transplantation. 2021;105:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 90. | Charpentier C, Delyon J, Glotz D, Peraldi MN, Rerolle JP, Barrou B, Ducroux E, Coilly A, Legeai C, Barete S, Lebbé C. Kaposi Sarcoma in HIV-positive Solid-Organ Transplant Recipients: A French Multicentric National Study and Literature Review. Transplantation. 2019;103:e22-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Oliveira Cobucci RN, Saconato H, Lima PH, Rodrigues HM, Prudêncio TL, Junior JE, Giraldo PC, Gonçalves AK. Comparative incidence of cancer in HIV-AIDS patients and transplant recipients. Cancer Epidemiol. 2012;36:e69-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Nissen NN, Barin B, Stock PG. Malignancy in the HIV-infected patients undergoing liver and kidney transplantation. Curr Opin Oncol. 2012;24:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Miro JM, Agüero F, Duclos-Vallée JC, Mueller NJ, Grossi P, Moreno A; ESCMID Study Group of Infection in Compromised Hosts. Infections in solid organ transplant HIV-infected patients. Clin Microbiol Infect. 2014;20 Suppl 7:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Hoffmann CJ, Subramanian AK, Cameron AM, Engels EA. Incidence and risk factors for hepatocellular carcinoma after solid organ transplantation. Transplantation. 2008;86:784-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Hsu NW, Chuang FR, Chen YT, Chen CL, Cheng YF. Hepatocellular carcinoma in kidney transplant recipients. Transplant Proc. 2010;42:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Hsiao CY, Lee PH, Ho CM, Wu YM, Ho MC, Hu RH. Post-transplant malignancy in liver transplantation: a single center experience. Medicine (Baltimore). 2014;93:e310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Ridruejo E, Mandó OG, Dávalos M, Díaz C, Vilches A. Hepatocellular carcinoma in renal transplant patients. Transplant Proc. 2005;37:2086-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Chok KS, Lam CM, Li FK, Ng KK, Poon RT, Lo CM, Fan ST. Management of hepatocellular carcinoma in renal transplant recipients. J Surg Oncol. 2004;87:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 99. | Narayanan M, Szymanski J, Slavcheva E, Rao A, Kelly A, Jones K, Jaffers G. BK virus associated renal cell carcinoma: case presentation with optimized PCR and other diagnostic tests. Am J Transplant. 2007;7:1666-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 100. | Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 308] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 101. | Petrogiannis-Haliotis T, Sakoulas G, Kirby J, Koralnik IJ, Dvorak AM, Monahan-Earley R, DE Girolami PC, DE Girolami U, Upton M, Major EO, Pfister LA, Joseph JT. BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345:1250-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Wang HH, Liu KL, Chu SH, Tian YC, Lai PC, Chiang YJ. BK virus infection in association with posttransplant urothelial carcinoma. Transplant Proc. 2009;41:165-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Knöll A, Stoehr R, Jilg W, Hartmann A. Low frequency of human polyomavirus BKV and JCV DNA in urothelial carcinomas of the renal pelvis and renal cell carcinomas. Oncol Rep. 2003;10:487-491. [PubMed] |
| 104. | Monini P, Rotola A, Di Luca D, De Lellis L, Chiari E, Corallini A, Cassai E. DNA rearrangements impairing BK virus productive infection in urinary tract tumors. Virology. 1995;214:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Emerson LL, Carney HM, Layfield LJ, Sherbotie JR. Collecting duct carcinoma arising in association with BK nephropathy post-transplantation in a pediatric patient. A case report with immunohistochemical and in situ hybridization study. Pediatr Transplant. 2008;12:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Adani GL, Baccarani U, Lorenzin D, Gropuzzo M, Tulissi P, Montanaro D, Currö G, Sainz M, Risaliti A, Bresadola V, Bresadola F. De novo gastrointestinal tumours after renal transplantation: role of CMV and EBV viruses. Clin Transplant. 2006;20:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 107. | Hung SY, Tseng HH, Chung HM. Nephrogenic adenoma associated with cytomegalovirus infection of the ureter in a renal transplant patient: presentation as ureteral obstruction. Transpl Int. 2001;14:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 108. | Acuna SA. Etiology of increased cancer incidence after solid organ transplantation. Transplant Rev (Orlando). 2018;32:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 109. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4672] [Article Influence: 311.5] [Reference Citation Analysis (1)] |
| 110. | Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1617] [Cited by in RCA: 1534] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 111. | Balan M, Chakraborty S, Pal S. Signaling Molecules in Posttransplantation Cancer. Clin Lab Med. 2019;39:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 112. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3592] [Article Influence: 149.7] [Reference Citation Analysis (0)] |
| 113. | Maggiore U, Pascual J. The Bad and the Good News on Cancer Immunotherapy: Implications for Organ Transplant Recipients. Adv Chronic Kidney Dis. 2016;23:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 114. | Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 115. | Liu Z, Fang Q, Zuo J, Minhas V, Wood C, Zhang T. The world-wide incidence of Kaposi's sarcoma in the HIV/AIDS era. HIV Med. 2018;19:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 116. | Manzia TM, Angelico R, Gazia C, Lenci I, Milana M, Ademoyero OT, Pedini D, Toti L, Spada M, Tisone G, Baiocchi L. De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature. World J Gastroenterol. 2019;25:5356-5375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 117. | Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ; Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1307] [Cited by in RCA: 1251] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 118. | Servais S, Dumontier N, Biard L, Schnepf N, Resche-Rigon M, Peffault de Latour R, Scieux C, Robin M, Meunier M, Xhaard A, Sicre de Fontbrune F, Le Goff J, Socié G, Simon F, Mazeron MC. Response to antiviral therapy in haematopoietic stem cell transplant recipients with cytomegalovirus (CMV) reactivation according to the donor CMV serological status. Clin Microbiol Infect. 2016;22:289.e1-289.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Spiess PJ, Yang JC, Rosenberg SA. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst. 1987;79:1067-1075. [PubMed] |
| 120. | Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1319] [Cited by in RCA: 1242] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 121. | Bollard CM. Improving T-cell therapy for epstein-barr virus lymphoproliferative disorders. J Clin Oncol. 2013;31:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Cruz CR, Hanley PJ, Liu H, Torrano V, Lin YF, Arce JA, Gottschalk S, Savoldo B, Dotti G, Louis CU, Leen AM, Gee AP, Rooney CM, Brenner MK, Bollard CM, Heslop HE. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy. 2010;12:743-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, Brenner MK, Heslop HE. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 890] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 124. | Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 125. | Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283-4292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 126. | Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, Burns D, McAulay K, Turner M, Bellamy C, Amlot PL, Kelly D, MacGilchrist A, Gandhi MK, Swerdlow AJ, Crawford DH. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 127. | Chiou FK, Beath SV, Wilkie GM, Vickers MA, Morland B, Gupte GL. Cytotoxic T-lymphocyte therapy for post-transplant lymphoproliferative disorder after solid organ transplantation in children. Pediatr Transplant. 2018;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 128. | Kaul DR, Stoelben S, Cober E, Ojo T, Sandusky E, Lischka P, Zimmermann H, Rubsamen-Schaeff H. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant. 2011;11:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Holmes-Liew CL, Holmes M, Beagley L, Hopkins P, Chambers D, Smith C, Khanna R. Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin Transl Immunology. 2015;4:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 130. | Macesic N, Langsford D, Nicholls K, Hughes P, Gottlieb DJ, Clancy L, Blyth E, Micklethwaite K, Withers B, Majumdar S, Fleming S, Sasadeusz J. Adoptive T cell immunotherapy for treatment of ganciclovir-resistant cytomegalovirus disease in a renal transplant recipient. Am J Transplant. 2015;15:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 131. | Smith C, Beagley L, Rehan S, Neller MA, Crooks P, Solomon M, Holmes-Liew CL, Holmes M, McKenzie SC, Hopkins P, Campbell S, Francis RS, Chambers DC, Khanna R. Autologous Adoptive T-cell Therapy for Recurrent or Drug-resistant Cytomegalovirus Complications in Solid Organ Transplant Recipients: A Single-arm Open-label Phase I Clinical Trial. Clin Infect Dis. 2019;68:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | van der Heiden P, Marijt E, Falkenburg F, Jedema I. Control of Cytomegalovirus Viremia after Allogeneic Stem Cell Transplantation: A Review on CMV-Specific T Cell Reconstitution. Biol Blood Marrow Transplant. 2018;24:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Kaeuferle T, Krauss R, Blaeschke F, Willier S, Feuchtinger T. Strategies of adoptive T -cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J Hematol Oncol. 2019;12:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 134. | Houghtelin A, Bollard CM. Virus-Specific T Cells for the Immunocompromised Patient. Front Immunol. 2017;8:1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 135. | Ouellette CP. Adoptive Immunotherapy for Prophylaxis and Treatment of Cytomegalovirus Infection. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 136. | Sutrave G, Gottlieb DJ. Adoptive cell therapies for posttransplant infections. Curr Opin Oncol. 2019;31:574-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Roddie C, Peggs KS. Immunotherapy for transplantation-associated viral infections. J Clin Invest. 2017;127:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 138. | Johnson SR, Cherikh WS, Kauffman HM, Pavlakis M, Hanto DW. Retransplantation after post-transplant lymphoproliferative disorders: an OPTN/UNOS database analysis. Am J Transplant. 2006;6:2743-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 139. | Lim WH, Au E, Krishnan A, Wong G. Assessment of kidney transplant suitability for patients with prior cancers: is it time for a rethink? Transpl Int. 2019;32:1223-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Wong G, Chapman JR. Cancers after renal transplantation. Transplant Rev (Orlando). 2008;22:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 141. | Smith JM, Rudser K, Gillen D, Kestenbaum B, Seliger S, Weiss N, McDonald RA, Davis CL, Stehmen-Breen C. Risk of lymphoma after renal transplantation varies with time: an analysis of the United States Renal Data System. Transplantation. 2006;81:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 142. | Le J, Durand CM, Agha I, Brennan DC. Epstein-Barr virus and renal transplantation. Transplant Rev (Orlando). 2017;31:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 143. | Hardinger KL. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy. 2006;26:1771-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 144. | Zand MS. B-cell activity of polyclonal antithymocyte globulins. Transplantation. 2006;82:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 145. | Midtvedt K, Fauchald P, Lien B, Hartmann A, Albrechtsen D, Bjerkely BL, Leivestad T, Brekke IB. Individualized T cell monitored administration of ATG versus OKT3 in steroid-resistant kidney graft rejection. Clin Transplant. 2003;17:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Hall EC, Engels EA, Pfeiffer RM, Segev DL. Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation. 2015;99:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 147. | Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, Potdar S, Shapiro R, Dharnidharka VR, Kauffman HM. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant. 2007;7:2619-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 148. | Dharnidharka VR, Lamb KE, Gregg JA, Meier-Kriesche HU. Associations between EBV serostatus and organ transplant type in PTLD risk: an analysis of the SRTR National Registry Data in the United States. Am J Transplant. 2012;12:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 149. | Rovira J, Renner P, Sabet-Baktach M, Eggenhofer E, Koehl GE, Lantow M, Lang SA, Schlitt HJ, Campistol JM, Geissler EK, Kroemer A. Cyclosporine A Inhibits the T-bet-Dependent Antitumor Response of CD8(+) T Cells. Am J Transplant. 2016;16:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 150. | Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951-3955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 151. | Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 543] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 152. | Dharnidharka VR, Ho PL, Stablein DM, Harmon WE, Tejani AH. Mycophenolate, tacrolimus and post-transplant lymphoproliferative disorder: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant. 2002;6:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 153. | Ben-Horin S, Goldstein I, Fudim E, Picard O, Yerushalmi Z, Barshack I, Bank I, Goldschmid Y, Meir SB, Mayer L, Chowers Y. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, Burke MM, Sullivan D, Macpherson P, Karran P. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (4)] |
| 155. | Végso G, Sebestyén A, Paku S, Barna G, Hajdu M, Tóth M, Járay J, Kopper L. Antiproliferative and apoptotic effects of mycophenolic acid in human B-cell non-Hodgkin lymphomas. Leuk Res. 2007;31:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1318] [Cited by in RCA: 1308] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 157. | Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818-15823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 158. | Lee K, Min HJ, Jang EJ, Hong JH, Hwang ES. In vivo tumor suppression activity by T cell-specific T-bet restoration. Int J Cancer. 2010;127:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 159. | Di Paolo S, Teutonico A, Ranieri E, Gesualdo L, Schena PF. Monitoring antitumor efficacy of rapamycin in Kaposi sarcoma. Am J Kidney Dis. 2007;49:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 160. | Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 586] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 161. | Chin-Hong PV. Human Papillomavirus in Kidney Transplant Recipients. Semin Nephrol. 2016;36:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 162. | Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3:S1-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 1150] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 163. | Lebbé C, Legendre C, Francès C. Kaposi sarcoma in transplantation. Transplant Rev (Orlando). 2008;22:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 164. | Luppi M, Barozzi P, Guaraldi G, Ravazzini L, Rasini V, Spano C, Riva G, Vallerini D, Pinna AD, Torelli G. Human herpesvirus 8-associated diseases in solid-organ transplantation: importance of viral transmission from the donor. Clin Infect Dis. 2003;37:606-7; author reply 607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 165. | Luppi M, Barozzi P, Rasini V, Riva G, Re A, Rossi G, Setti G, Sandrini S, Facchetti F, Torelli G. Severe pancytopenia and hemophagocytosis after HHV-8 primary infection in a renal transplant patient successfully treated with foscarnet. Transplantation. 2002;74:131-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 166. | Sandlund JT, Guillerman RP, Perkins SL, Pinkerton CR, Rosolen A, Patte C, Reiter A, Cairo MS. International Pediatric Non-Hodgkin Lymphoma Response Criteria. J Clin Oncol. 2015;33:2106-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 167. | Bianchi E, Pascual M, Nicod M, Delaloye AB, Duchosal MA. Clinical usefulness of FDG-PET/CT scan imaging in the management of posttransplant lymphoproliferative disease. Transplantation. 2008;85:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 168. | von Falck C, Maecker B, Schirg E, Boerner AR, Knapp WH, Klein C, Galanski M. Post transplant lymphoproliferative disease in pediatric solid organ transplant patients: a possible role for [18F]-FDG-PET(/CT) in initial staging and therapy monitoring. Eur J Radiol. 2007;63:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 169. | Dierickx D, Tousseyn T, Requilé A, Verscuren R, Sagaert X, Morscio J, Wlodarska I, Herreman A, Kuypers D, Van Cleemput J, Nevens F, Dupont L, Uyttebroeck A, Pirenne J, De Wolf-Peeters C, Verhoef G, Brepoels L, Gheysens O. The accuracy of positron emission tomography in the detection of posttransplant lymphoproliferative disorder. Haematologica. 2013;98:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 170. | Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011;24:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 171. | Pak F, Pyakural P, Kokhaei P, Kaaya E, Pourfathollah AA, Selivanova G, Biberfeld P. HHV-8/KSHV during the development of Kaposi's sarcoma: evaluation by polymerase chain reaction and immunohistochemistry. J Cutan Pathol. 2005;32:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 172. | Cheuk W, Wong KO, Wong CS, Dinkel JE, Ben-Dor D, Chan JK. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 173. | Brambilla L, Boneschi V, Taglioni M, Ferrucci S. Staging of classic Kaposi's sarcoma: a useful tool for therapeutic choices. Eur J Dermatol. 2003;13:83-86. [PubMed] |
| 174. | Bisseling KC, Bekkers RL, Rome RM, Quinn MA. Treatment of microinvasive adenocarcinoma of the uterine cervix: a retrospective study and review of the literature. Gynecol Oncol. 2007;107:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 175. | Stehman FB, Ali S, Keys HM, Muderspach LI, Chafe WE, Gallup DG, Walker JL, Gersell D. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197:503.e1-503.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 176. | Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 177. | van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol. 2011;98:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Singh JC, Kuohung V, Palefsky JM. Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with men. J Acquir Immune Defic Syndr. 2009;52:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 179. | Richel O, Wieland U, de Vries HJ, Brockmeyer NH, van Noesel C, Potthoff A, Prins JM, Kreuter A. Topical 5-fluorouracil treatment of anal intraepithelial neoplasia in human immunodeficiency virus-positive men. Br J Dermatol. 2010;163:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 180. | Stier EA, Goldstone SE, Berry JM, Panther LA, Jay N, Krown SE, Lee J, Palefsky JM. Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. J Acquir Immune Defic Syndr. 2008;47:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 181. | Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Dis Colon Rectum. 2008;51:829-35; discussion 835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 182. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. Anal Carcinoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:852-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 183. | Allen UD, Preiksaitis JK; AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 184. | Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, Morschhauser F, Jaccard A, Lamy T, Leithäuser M, Zimmermann H, Anagnostopoulos I, Raphael M, Riess H, Choquet S; German PTLD Study Group; European PTLD Network. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 185. | Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. 2017;35:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 186. | Gross TG, Orjuela MA, Perkins SL, Park JR, Lynch JC, Cairo MS, Smith LM, Hayashi RJ. Low-dose chemotherapy and rituximab for posttransplant lymphoproliferative disease (PTLD): a Children's Oncology Group Report. Am J Transplant. 2012;12:3069-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 187. | Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb E, Ambrosetti A, Roth A, Hemmaway C, Ferrari A, Linton KM, Rudà R, Binder M, Pukrop T, Balzarotti M, Fabbri A, Johnson P, Gørløv JS, Hess G, Panse J, Pisani F, Tucci A, Stilgenbauer S, Hertenstein B, Keller U, Krause SW, Levis A, Schmoll HJ, Cavalli F, Finke J, Reni M, Zucca E, Illerhaus G; International Extranodal Lymphoma Study Group (IELSG). Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217-e227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 188. | Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, La Rosée P, Binder M, Fabbri A, Torri V, Minacapelli E, Falautano M, Ilariucci F, Ambrosetti A, Roth A, Hemmaway C, Johnson P, Linton KM, Pukrop T, Sønderskov Gørløv J, Balzarotti M, Hess G, Keller U, Stilgenbauer S, Panse J, Tucci A, Orsucci L, Pisani F, Levis A, Krause SW, Schmoll HJ, Hertenstein B, Rummel M, Smith J, Pfreundschuh M, Cabras G, Angrilli F, Ponzoni M, Deckert M, Politi LS, Finke J, Reni M, Cavalli F, Zucca E, Illerhaus G; International Extranodal Lymphoma Study Group (IELSG). Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4:e510-e523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 189. | Rowe DT, Webber S, Schauer EM, Reyes J, Green M. Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis. 2001;3:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 190. | Penn I. Sarcomas in organ allograft recipients. Transplantation. 1995;60:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 191. | Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1306] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 192. | Nichols LA, Adang LA, Kedes DH. Rapamycin blocks production of KSHV/HHV8: insights into the anti-tumor activity of an immunosuppressant drug. PLoS One. 2011;6:e14535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 193. | Lurain K, Yarchoan R, Uldrick TS. Treatment of Kaposi Sarcoma Herpesvirus-Associated Multicentric Castleman Disease. Hematol Oncol Clin North Am. 2018;32:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 194. | Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 195. | US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 939] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 196. | Wong G, Howard K, Webster AC, Chapman JR, Craig JC. Screening for renal cancer in recipients of kidney transplants. Nephrol Dial Transplant. 2011;26:1729-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 197. | Mehta JM, MacLaughlin KL, Millstine DM, Faubion SS, Wallace MR, Shah AA, Fields HE, Ruddy BE, Bryan MJ, Patel B, Temkit MH, Buras MR, Golafshar MA, Kling JM. Breast Cancer Screening: Women's Attitudes and Beliefs in Light of Updated United States Preventive Services Task Force and American Cancer Society Guidelines. J Womens Health (Larchmt). 2019;28:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 198. | Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 199. | Berger BM, Parton MA, Levin B. USPSTF colorectal cancer screening guidelines: an extended look at multi-year interval testing. Am J Manag Care. 2016;22:e77-e81. [PubMed] |
| 200. | Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol. 2019;5:1318-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: The Transplantation Society; The European Society for Organ Transplantation; American Urological Association; Société Internationale d’Urologie; European Association of Urology; American Society of Transplantation; Urological Society of India.
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Mucenic M, Brazil; Pham TTT, Viet Nam; Singh N, United States S-Editor: Lin C L-Editor: Kerr C P-Editor: Yu HG