Published online Apr 18, 2023. doi: 10.13105/wjma.v11.i4.79
Peer-review started: December 26, 2022
First decision: March 9, 2023
Revised: March 18, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: April 18, 2023
Processing time: 108 Days and 21.7 Hours
Computational psychiatry is an emerging field that not only explores the biolo
Core Tip: This study reviews and integrates the methods and models in the clinical practice of computational psychiatry and constructs a complete and mature Artificial Intelligence ecosystem. The ecosystem for computational psychiatry includes data acquisition, preparation, modeling, application, and evaluation. This approach allows researchers to integrate data from a variety of sources to obtain a more complete understanding of mental health conditions. Despite the continuous development and breakthrough of computational psychiatry, it has not yet influenced routine clinical practice and still faces many challenges, such as data availability and quality, biological risks, equity, and data protection.
- Citation: Liu XQ, Ji XY, Weng X, Zhang YF. Artificial intelligence ecosystem for computational psychiatry: Ideas to practice. World J Meta-Anal 2023; 11(4): 79-91
- URL: https://www.wjgnet.com/2308-3840/full/v11/i4/79.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i4.79
Mental illness is a significant threat to human health, which was especially evident during the coronavirus disease 2019 (COVID-19) pandemic[1,2]. In recent years, artificial intelligence has played an increasingly prominent role in the clinical practice of psychiatry. The birth of computational psychiatry represents not only the inevitable choice to conform to the trend of the fourth industrial revolution but also an important means to solve the real dilemma.
Psychiatry mainly studies the causes, symptoms, and clinical diagnosis of human mental diseases. Computational psychiatry[3] uses computational and mathematical techniques to better understand mental disorders and to develop new treatments. Computational psychiatry is an emerging psychiatry approach that integrates various multidisciplinary approaches, such as psychiatry, neuroscience, machine learning, psychology, statistics, and computer science, to develop quantitative models of mental illness and to assess the effectiveness of different treatments[4]. Specifically, computational psychiatry builds computational models of brain function based on the neurological and cognitive phenomena associated with mental illness, predicts the abnormal degree of mental function, and evaluates the efficacy of treatment by using detailed multidimensional computational models[5,6].
Computational psychiatry includes two approaches: Data-driven computational psychiatry and theory-driven computational psychiatry[6]. Data-driven approaches involve machine learning and big data analytics, and they can improve predictive accuracy in clinical diagnosis, prognosis, and treatment by learning clinical and biological data. The theory-driven approach derives from computational neuroscience and focuses more on constructing models to understand the mechanisms of psychosis[7]. Due to the fact that computational psychiatry is based on mathematics, computer science, biological science, and other deep theories, it has the advantage of multidisciplinary integration[3]. One of the key goals of computational psychiatry is to move beyond the traditional "black box" approach to understanding the brain[8], whereby researchers study the symptoms and behaviors of individuals without fully understanding the underlying mechanisms. By introducing computational and statistical approaches, computational psychiatry has opened the "black box" of pathological mechanisms[9]. Moreover, neural computing functions provide precise algorithmic details for the analysis and solution of specific problems.
Computational psychiatry can identify the pathogenesis of mental diseases from both theory-driven and data-driven aspects, which is the result of the fusion of computational neuroscience and psychiatry[10]; in addition, it has a significant contribution to the diagnosis, treatment, and prevention of mental diseases. Overall, computational psychiatry is a rapidly growing and exciting field that has the potential to revolutionize our understanding of mental illness and to allow for the development of new treatments. By using computational and mathematical techniques to build quantitative models of mental illness, researchers in the field are working to identify the underlying mechanisms of mental illness and to develop more effective treatments.
Although various experimental studies have provided valuable information for understanding and explaining the underlying mechanisms of mental illness[11-13], the development of computational psychiatry is challenged by multiple interactions[14]. For instance, one of the biggest challenges faced by computational psychiatry today is the availability and quality of data. Mental health disorders are complex and multifaceted, and it is difficult to collect data that accurately reflect experiences with the disorders. Another challenge is the interpretability of the results. Many techniques that are used in computational psychiatry are highly complex and even difficult for experts in the field to understand, which makes it difficult for researchers to communicate their findings with others and for clinicians to apply these findings to actual treatment. Many other problems also need to be solved, such as the technical connection between model development and clinical practice and ethical acceptability. Despite these issues, we remain optimistic about the future of computational psychiatry.
Establishing a complete artificial intelligence ecosystem of computational psychiatry is an effective method to solve the challenges in the clinical practice of psychiatry. In this study, we focus on building an artificial intelligence ecosystem for computational psychiatry to better facilitate the elimination of barriers to clinical practice. This review aims to make a fundamental contribution to shaping the ecosystem and for allowing the modules to be smoothly applied. Moreover, it outlines the responsibilities of the different agents and the linkages between them and builds a loop from data collection to modeling, evaluation, and clinical practice. We plan to sort out and integrate the same and different methods and models in the field, overcome the existing limitations, provide full attention to the role of each subject, and eventually form a complete and mature ecosystem. It is believed that as the field continues to evolve, researchers will eventually find ways to overcome the challenges and make greater advances in our understanding and treatment of mental health conditions.
In this review, we used "computational psychiatry", "machine learning", "artificial intelligence", "psy
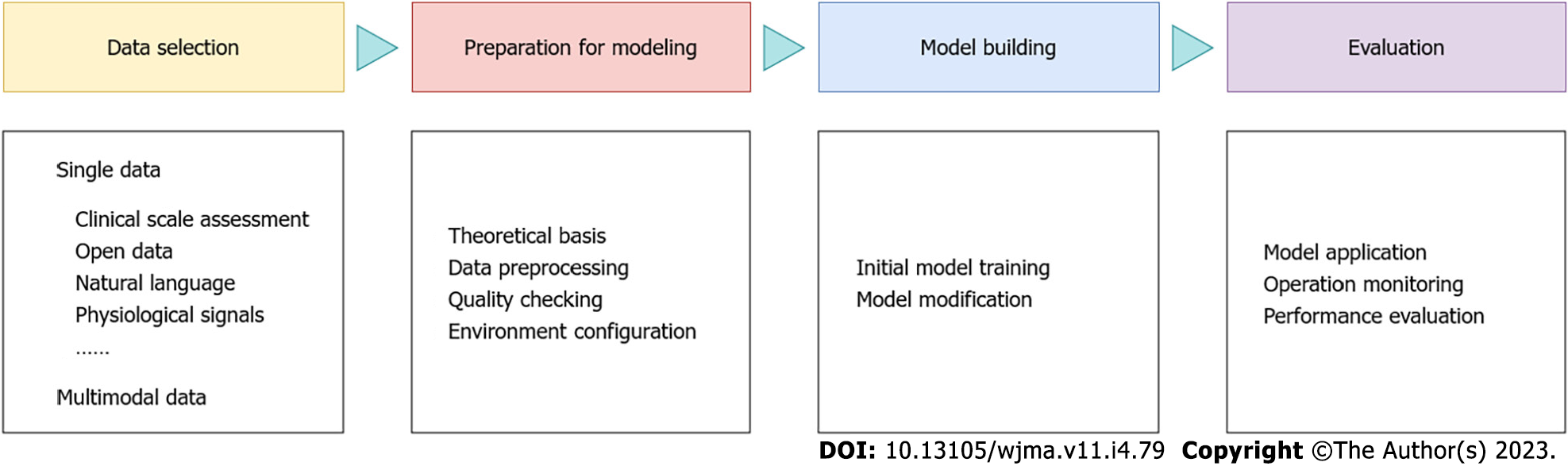
Based on the literature concerning clinical thinking and life cycle management of artificial intelligence projects, we conducted an integrated design of the ecosystem of computational psychiatry. We divided the clinical practice process of computational psychiatry into the following four main stages: data acquisition, modeling preparation, model construction, and application evaluation (Figure 1).
One of the strengths of computational psychiatry is its ability to integrate big datasets of various forms to help researchers gain a more complete understanding of a patient's mental health. Thus, the first and most critical step in the artificial intelligence ecosystem for computational psychiatry is data collection. During this process, researchers can select one or more input data that can be measured according to the relevance of the research problem[15]. Common forms include clinical scales, visual data, voice, physiological signals, and Internet of Things data, etc. Although there are a wide variety of input data, tool selection should be based on a clear understanding of treatment strategies and the realistic evaluation of clinical effectiveness. Moreover, it should consider the mutual limiting effect of different data acquisition methods and technology operations, as well as the quality of original data and the details of processing methods, which will directly affect the reliability of the tools, and thus affect the effectiveness of clinical application[16,17].
Modeling relies on different theoretical traditions[18]. For example, algorithm engineers are required to follow industry practice rules and conference content, articles, or implicit guidelines related to machine learning, and psychiatrists are bound by rules in the legal and medical fields, such as the National Institute for Health and Clinical Excellence guidelines or the American Psychiatric Association Practice guidelines. In addition, judgments are often made differently depending on the unique personality of the model builder. For example, clinicians' decision-making styles and willingness to take risks have a direct impact on their treatment paths and diagnostic strategies, and conservative and adventurous engineers also exhibit differences in aesthetic awareness and modeling styles. Therefore, the theoretical basis is worth fully preparing before building the model. The second is data preprocessing and quality checking. Since the collected data are often incomplete, as well as the fact that data from heterogeneous data sources may need to be collected, the raw data need to be preprocessed and quality checked to ensure the quality of the data. Only after data cleaning, data integration, data reduction, data transformation, and other processing can standardize data for model construction. The establishment of the compilation environment is also one of the preparatory works of model construction. There are several open source platforms that can be used for training, testing, and benchmarking algorithms based on different design requirements, such as OpenAI Gym[19], which provides a range of tasks, including some classic arcade games including Doom, as well as models, tests, and diagnostic paradigms that can be used for mental illness.
The first two steps in the computational psychiatry ecosystem both serve the third step; specifically, after collecting and processing the corresponding data in the theoretical context, the next step involves normative model building[20-22]. This step is divided into two parts: initial model training and model modification. Machine learning[23-27] is often used in model training to recognize emotional states[28], to detect mood swings[29], and to diagnose mental diseases[30]. There are two main types of machine learning: supervised learning and unsupervised learning. Supervised learning uses categorization and regression to learn from examples of existing labels. This method is often used to build classifiers to distinguish healthy people from sick people or to build predictive models. Washington et al[31] designed Guesswhat, which is a smartphone game for emotional data collection. They trained a pediatric emotion classification convolutional neural network classifier to recognize children's expressions, such as sadness, surprise, disgust, happiness, and neutral expressions. Their results demonstrated the value of mobile digital health. Although mood classifiers have made remarkable progress in automatic emotion recognition, the computational cost of existing models is too high. Banerjee et al[32] optimized the design of the machine learning model. The MobileNet-V2 network that they trained on ImageNet achieved a balanced accuracy rate of 65.11% and an F1 score of 64.19% on CAFE. Through optimization techniques, machine learning models can achieve greater accuracy and lighter weight. Unsupervised learning[33,34] is a classification method that does not require human data classification but automatically divides the structure based on the inherent distribution characteristics of datasets. In addition, clustering methods are often used in unsupervised learning. Regardless of which training method is used, professional school education and clinical training are needed. For example, clinicians require training in psychiatric education based on clinical case studies (fictional or nonfictional), and machine learning engineers require systematic schooling and professional experience. Finally, the initial model is reasonably built.
Computational psychiatry seeks to develop quantitative, mechanistic models of psychiatric disorders[35] that can help researchers better understand the biological and cognitive processes that lead to these disorders. A key benefit of computational models is that they can help researchers generate testable hypotheses about the underlying mechanisms of mental disorders. For example, reinforcement learning models of addiction[36,37] can be used to generate hypotheses about specific brain regions and pathways associated with addiction, as well as types of interventions that may be effective in treating addiction. The model, which is based on principles of neuroscience and psychology, suggests that addiction is caused by a disorder in the brain's reward system, which leads to obsessive behavior and a loss of control over behavior. Another benefit of computational models in psychiatry is that they can help researchers assess the effectiveness of different treatment options. For example, the cognitive-affective neural circuit model of depression[38] can be used to evaluate the efficacy of different antidepressants or psychotherapy based on predictions of their effects on basic brain circuits associated with depression. The model, which is based on evidence from neuroscience and psychology, proposes that depression is caused by the disequilibrium of the brain's emotional and cognitive processing systems, which leads to symptoms such as low mood, reduced negative thinking, and reduced motivation. However, regardless of how good the model is, there is room for improvement. As more theoretical background is accumulated in clinical practice, updated data will be incorporated into the model[39,40]. Moreover, the model will expose more practical problems in clinical application; thus, it needs to be constantly adjusted and modified to adapt to new challenges.
Computational psychiatry is not currently used clinically, but it has the potential to inform new clinical interventions and treatments for mental illness to help guide the treatment of mental disorders. For example, clinicians can use computational models to assess an individual's brain activity and symptoms to choose the most appropriate treatment for them. By using computational and mathematical models to better understand the underlying mechanisms of these diseases, researchers in the field can identify potential targets for intervention and assess the likely effects of different treatment options. However, more research is needed to fully understand the clinical potential of computational psychiatry and to develop the necessary tools and techniques to apply it in the clinical setting. Given the complexity of psychiatric disorders, future applications should be subject to enhanced regulatory oversight of clinical practice, as well as the evaluation and post hoc analysis of actual clinical benefits and model performance[41].
Based on the review of the existing studies, we conclude that data sources mainly include the following methods: scales, public data, language, physiological signals, blood, multimodal data, etc. (Table 1). This paper will discuss some of these categories.
| Module | Category |
| Single data | Clinical scales[42,45], electronic medical records[46], and digital scales[47,48] |
| large online sample[49], national population surveys[50], and large multisite public datasets[51] | |
| Images[55] and videos[56,57] | |
| Language[62] and baseline interviews[60] | |
| Emotional faces[63,64], electrocardiogram[65], electroencephalogram[68-70], magnetoencephalogram[71], and magnetic resonance imaging[72,73,75] | |
| Human motion bone data[79] | |
| Blood[81,85] | |
| Multimodal data | Multimodal data[45,86,89] |
Clinical scales[42-45] are one of the most widely used tools in clinical evaluation, and mature scales include the World Health Organization-Quality of Life-Brief (WHO-QoL-Bref), cognitive function test, Hamilton Depression Scale, Autism Diagnostic Observation Schedule, Hamilton Anxiety Scale, Fibromyalgia and Chronic Fatigue Rating Scale, etc. Large datasets accumulated through electronic medical records[46] facilitate the determination of goals by using computational methods. Self-reported digital scales[47,48] are used in the following manner. Researchers load the quantitative list of questions into the app, allow users to answer questions by using smart devices, and ultimately screen for symptoms based on the answers. This approach relies on mobile technology rather than traditional clinical scales.
Dubois et al[49] used a large online sample to demonstrate an association between human exploration strategies and impulse psychiatry, which not only demonstrated that impulsivity is associated with specific forms of exploration but also explored links between impulsivity and other psychiatric dimensions. Moreover, Nam et al[50] used machine learning and web analysis to identify factors associated with depression from national population surveys. Nielsen et al[51] discussed how large multisite public datasets contribute to the application of machine learning in psychiatry. Furthermore, Hu et al[52] collected users’ text expressions on social platforms (such as Weibo) as data sources to predict their depression symptoms. Artificial intelligence (especially big data)[53] plays a vital role in health care, thus demonstrating its significant potential in applications[54].
Research indicates that psychiatric patients have different color vision and are less able to discriminate between colors than ordinary people. Therefore, the color recognition of images can be used to examine the difference between psychiatric patients and control groups or as a prognostic diagnosis. Shen et al[55] reviewed the paintings of 281 patients with chronic schizophrenia and 35 patients with healthy controls and used a series of computational analyses to scan and process the images. The results showed that color paint images have the potential to be used as a clinical diagnostic and prognostic tool for patients with chronic schizophrenia. The video data collected by the camera exhibit a large deviation, which is caused by noise in the natural environment. Moreover, existing studies provide optimized schemes through data collection pipelines, feature engineering, and data expansion strategies. The standard diagnosis for autism spectrum disorders takes several hours and assesses 20 to 100 behaviors (e.g., eye contact, social smiling, etc.). Leblanc et al[56] introduced feature replacement methods to analyze family videos to establish the diagnosis of autism. They rated 140 videos of children on YouTube, filled in missing values by using feature replacement methods, and optimized the performance of the autism detection classifier. Dynamic feature replacement methods are superior to traditional methods in terms of performance and can reduce the impacts of missing values on video diagnosis[56]. Furthermore, Tariq et al[57] used mobile devices to classify videos by machine learning and labeled video features. This method ensures the accuracy of assessment and improves the speed of diagnosis.
Automated speech analysis has been used in psychiatric diagnosis[58,59] and learns baseline interview data through machine learning algorithms to predict mental illness. Carrillo et al[60] conducted baseline autobiographical interviews with patients and transcribed them by using machine learning algorithms to predict the effectiveness of psilocybin for depression. The combination of machine learning with automated speech algorithms[61] contributes new ideas for the prediction and diagnosis of psychiatry. Moreover, the acquisition of language is relatively mild compared to acquiring intrusive data and supports self-testing by users[62]. Computational linguistics combined with artificial intelligence provides a good aid to clinical diagnosis and risk monitoring.
Physiological signals include emotional faces[63,64], electrocardiogram[65], electroencephalogram (EEG)[66-69], magnetoencephalogram[71], and functional magnetic resonance imaging (fMRI)[72-77]. There are two main methods to collect biological signals: invasive and noninvasive methods. Noninvasive data acquisition is commonly used, including electroencephalogram and functional magnetic resonance imaging. In recent years, physiological signals have been increasingly used to measure emotional responses. Compared with audiovisual data, physiological signals provide more detailed and real information. However, there are too many interference factors in the collection process of physiological signals, and the processing mechanism is more complex.
The clinical and scientific value of full body movement assessment has been increasingly recognized, and it is often used in the diagnosis of cerebral palsy. Previous studies were mostly based on computer vision[78]. However, during the process of sorting out the relevant studies, we noticed an interesting experimental study[79]. From the perspective of full body kinematics, the team built a machine-learning model to establish the purpose of automatic recognition and classification of depression. They used Kinect to capture human motion bone data, conducted experiments with four machine learning tools (including a support vector machine, logistic regression, random forest, and gradient lift), and finally utilized the evaluation and classification of patients with depression and without depression. This experimental study allows us to demonstrate the auxiliary role of kinematics in the identification of depression. However, when motion capture equipment is used to record the joint skeleton data of participants in motion, the captured data often contain noise due to the influence of the environment and sensor accuracy, which limits the accuracy of the data.
Biomarkers[80] are a group of proposed markers in recent years related to cell growth, proliferation, and disease occurrence, and they can be used to reflect drug reactions during pathological processes or after therapeutic interventions. Wagh et al[81] reviewed gene expression studies based on peripheral blood to identify gene expression biomarkers for schizophrenia. According to a genome-wide association study (GWAS), C-reactive protein (CRP) which is a biomarker of chronic inflammation, in the blood is likely associated with an increased risk of major depression; however, it is also correlated with a decreased risk of anorexia nervosa, obsessive-compulsive disorder, and schizophrenia[82]. By examining RNA, researchers can determine the patient’s current state of anxiety, depression, and mania[83]. Moreover, despite the differences in population characteristics, analysis methods of gene expression, and nature of the research, the results still proved the validity of blood-based gene expression. Fernandes et al[84] used a machine learning algorithm composed of peripheral blood immunoinflammatory biomarkers and cognitive biomarkers in the diagnosis of bipolar disorder and schizophrenia with clinical effectiveness. The manner in which machine learning is combined with pharmacogenomic data provides a new way to predict patients with major depression. In a systematic review of recent advances in machine learning and pharmacogenomics studies, Bobo et al[85] demonstrated the effectiveness of pharmacogenomics in predicting short-term antidepressant responses and suggested that the prediction of treatment outcomes may depend on background factors that cannot be captured by machine learning algorithms.
In addition to collection methods of single data, multimodal datasets are increasingly used in psychiatry, such as the use of clinical scale evaluation and resting-state functional magnetic resonance imaging (MRI) to establish a prediction model of mood disorders, anxiety, and anhedonia[45], as well as a machine learning framework based on multimodal neuropsychiatric data to predict the responses of patients with schizophrenia to treatment[86]. Chen et al[87] conducted a comprehensive review of the practice of machine learning combined with neuroimaging in psychiatry, which emphasized the importance of multimodal data and the extraction of multimedia information. Data were collected through a combination of electronic questionnaires, standard clinical care record reviews, and device output analysis[88]. Although this statistical method integrating multimodal data demonstrates advantages over the general methods of single data, it is usually prone to overfitting and poor generalization[89]. The method of how to avoid these problems should be further explored in the future.
The building of an AI ecosystem for computational psychiatry currently faces multiple challenges, which can be broadly divided into three categories: technical factors, cost and context, and ethical challenges. In this section, each challenge is explained separately.
It is important to note that computational psychiatry is still in its early stages, and there are many challenges that must be overcome. The most fundamental challenge is technical difficulty. Examples include data availability and quality, data transparency[90], technology openness, and professional integration. The quality of the raw data and the details of the processing are directly related to the interpretation of the results. One primary way to address this challenge is to increase collaboration with experts in other fields, such as computer science and engineering. By combining their expertise, researchers can develop new algorithms and tools to better handle the complex datasets associated with mental health research. Automation, rigor, and standardization of treatment methods[16] is another manner to advance the field of computational psychiatry, which can help to ensure that the results of computational research are replicable and can be generalized to a wider population. Due to the fact that "data-driven" research is based on the analysis and application of data, the transparent presentation of data results without bias and selectivity is the norm that researchers must follow. In response, there is a need to develop an interpretable, transparent, and universally applicable scientific review framework[91] to ensure the feasibility of using AI in psychiatry. Although the rapid development of artificial intelligence approaches has made up for the shortcomings of traditional mental illness research methods, thus identifying increasingly more information related to brain function, it must be stated that mental illness researchers and clinicians know very little about computational technology methods[92,93]. It is recommended that there should be improvements in the computational literacy of neuroscientists and mental health professionals[94] while also leveraging the talent development role of higher education to bring more people with cross-disciplinary professional backgrounds into the field. Another note about computational psychiatry is the importance of ensuring that the field remains accessible and inclusive. As computing becomes more widely used in mental health research, it is important that these technologies are not just reserved for the best-funded or best-known researchers. Instead, an open and inclusive approach should be taken to provide researchers from diverse backgrounds and institutions with the tools and resources that are needed to conduct computational psychiatry research. It is also important for researchers to engage with policy-makers and advocacy groups[95] to ensure that findings from computational psychiatry are translated into practical applications.
The costs mentioned in this section include time costs[18] and labor costs, which are uncontrollable factors that should be considered in clinical modeling. Due to the wide range of projects contained in the ecosystem, the system operation needs to be repeatedly monitored, evaluated, adjusted, and optimized, thus requiring a large amount of time. Moreover, there are many participating roles in each link, and there are cost consumption problems in coordination and communication management. For example, a team of clinicians may accept social and institutional pressures, and there may be conflicts between experienced mature doctors and novice decision-makers. The intersection and unification of viewpoints under different theoretical backgrounds in interdisciplinary cooperation also require coordination and compromise. Second, the reasonable match between professional salary structure and working style will also affect the clinical practice effects. In addition to the abovementioned overt factors, some individuals have raised concerns about the use of computational techniques in studies of mental health conditions, wherein they have argued that these methods may oversimplify complex phenomena and ignore important environment-specific factors. We also need to consider whether the modeling state of computational psychiatry follows the natural trajectory of core neurobiology[3] and whether computational psychiatry is detached from the developmental background of the field of psychiatry. When we discuss the development of psychiatry with sophisticated AI approaches, we must not lose sight of the core purpose of disease treatment.
In addition, there are ethical issues with the use of computing in mental health research[96,97]. The application of AI to psychiatry needs to consider AI ethical issues, including respecting patient autonomy by providing adequate consent[70,98], data ownership, the ignoring of conscious experience, privacy protection[99], and equity[100]. An ethically acceptable manner[101,102] is an obstacle to the transformation of computational psychiatry from theory to practice. Some researchers have argued that the use of these techniques can lead to biased or discriminatory results, especially if the algorithm is not properly trained or verified. In the practice of treating and predicting mental illness, we call upon researchers and health care professionals to approach patients with rigorous optimism concerning the principles of kindness[103], harmfulness, respect for autonomy and justice[42], and prevention of ethical issues from the aspects of communication, consent, and contrast[104]. According to four basic ethical principles (respect, no harm, benefit, and justice), researchers should fully respect the independent will of data providers when collecting and using data, as well as pay attention to the protection of their personal privacy and process data anonymously. In addition, the participants’ rights and interests should be the first priority. Justice and fairness should be adhered to. Moreover, informed consent should be obtained, and the process should be open and fair. Although we are aware that computational AI approaches (such as machine learning) can have a profound impact in psychiatry, there are still no applications that constitute standard clinical practice. The early consideration of these ethical challenges and the establishment of standards and requirements to eventually allow for the early use of the benefits of AI for mental health care should be enacted. Despite these concerns, we remain convinced that the potential benefits of computational psychiatry far outweigh the risks[105]. By properly using AI to study mental health conditions, researchers can gain a more comprehensive and nuanced understanding of mental illness, which could ultimately lead to better treatments.
There were several limitations to this study. First, all of the relevant literature that was analyzed in this paper is in English and does not cover studies in other languages (such as Chinese, Korean, Japanese, and German). Thus, the coverage of the research may still be insufficient. Second, this paper is only a summary of the research in related fields, which cannot be applied to clinical treatment. This review only collates extensive research on data sources, tools, and model frameworks in computational psychiatry and does not use explicit methods, such as systematic reviews, nor does it address substantive clinical outcomes.
This paper builds an artificial intelligence ecosystem for computational psychiatry by reviewing the literature, including the following four stages: data acquisition, preparation for modeling, model building, and application evaluation. In terms of data acquisition, we discussed different data acquisition methods and data forms and summarized single data source methods, such as scale, open data, language, and physiological signals, as well as multimodal data statistical methods combining different types of data. In terms of preparation for modeling, we explored constraints from both the clinician and algorithm engineer industry norms and emphasized the importance of data preprocessing and quality testing. For model building, we proposed two steps of normative modeling (initial model training and model modification) and discussed supervised learning and unsupervised virtual seats in machine learning. Finally, based on the relevant theory and experience, we prospectively assessed the aspect of application evaluation and clarified the complexity and necessity of model performance evaluation and post analysis.
In conclusion, computational psychiatry is a promising field that has the potential to revolutionize our understanding and treatment of mental health conditions. In recent years, research on computational psychiatry has produced many good results. For example, it has made profound theoretical breakthroughs in the integration of computer science, biology, psychiatry, statistics, and other disciplines. In addition, it has allowed for the performance of more in-depth research in the use of computing and mathematical techniques to explain mental diseases and has made many attempts and modifications in data collection, model construction, and other aspects. It is worth mentioning that this field has accumulated a rich amount of data, with data originating from traditional clinical scale evaluations to the application of big data, from language to EEG, and from a single dataset to multimodal data, which provides a solid foundation for future clinical practice.
However, it should not be ignored that computational psychiatry is still in its early stages and experiences of technical challenges, such as data quality and tool openness, cost issues (such as role conflict and development cycle), and ethical challenges (such as data privacy, respect, and equity). More work will need to be performed to realize its full potential to ensure that existing discoveries are eventually translated into clinical applications. Specifically, the need for an artificial intelligence ecosystem for computational psychiatry can help researchers clarify their work, build on it, further develop better algorithms and techniques to analyze complex datasets, establish more rigorous and standardized experimental methods, and collaborate with policy-makers and advocacy groups to ensure that the findings of computational psychiatry are translated into practical applications. When considering that the use of artificial intelligence needs to experience a series of ethical problems caused by computing technology, the establishment of relevant application standards and moral guidelines should be emphasized in the future. Moreover, future research should focus on the integration of computational psychiatry with other disciplines, such as psychology, neuroscience, and genetics. By combining multidisciplinary and multidisciplinary expertise, researchers can gain a more comprehensive understanding of the crux of mental illness and develop more effective treatments and interventions.
| 1. | COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2518] [Cited by in RCA: 3080] [Article Influence: 616.0] [Reference Citation Analysis (0)] |
| 2. | Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 1367] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 3. | Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends Cogn Sci. 2012;16:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 4. | Friston K. Computational psychiatry: from synapses to sentience. Mol Psychiatry. 2023;28:256-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Constant A, Badcock P, Friston K, Kirmayer LJ. Integrating Evolutionary, Cultural, and Computational Psychiatry: A Multilevel Systemic Approach. Front Psychiatry. 2022;13:763380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Huys QJM, Browning M, Paulus MP, Frank MJ. Advances in the computational understanding of mental illness. Neuropsychopharmacology. 2021;46:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Huys QJ, Maia TV, Frank MJ. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci. 2016;19:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 608] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 8. | Sarris J. Disruptive innovation in psychiatry. Ann N Y Acad Sci. 2022;1512:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Macpherson T, Churchland A, Sejnowski T, DiCarlo J, Kamitani Y, Takahashi H, Hikida T. Natural and Artificial Intelligence: A brief introduction to the interplay between AI and neuroscience research. Neural Netw. 2021;144:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (10)] |
| 10. | Kucikova L, Danso S, Jia L, Su L. Computational Psychiatry and Computational Neurology: Seeking for Mechanistic Modeling in Cognitive Impairment and Dementia. Front Comput Neurosci. 2022;16:865805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Khaleghi A, Zarafshan H, Vand SR, Mohammadi MR. Effects of Non-invasive Neurostimulation on Autism Spectrum Disorder: A Systematic Review. Clin Psychopharmacol Neurosci. 2020;18:527-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Mostafavi SA, Khaleghi A, Mohammadi MR. Noninvasive brain stimulation in alcohol craving: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;101:109938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Zarafshan H, Khaleghi A, Mohammadi MR, Moeini M, Malmir N. Electroencephalogram complexity analysis in children with attention-deficit/hyperactivity disorder during a visual cognitive task. J Clin Exp Neuropsychol. 2016;38:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Bhugra D, Tasman A, Pathare S, Priebe S, Smith S, Torous J, Arbuckle MR, Langford A, Alarcón RD, Chiu HFK, First MB, Kay J, Sunkel C, Thapar A, Udomratn P, Baingana FK, Kestel D, Ng RMK, Patel A, Picker L, McKenzie KJ, Moussaoui D, Muijen M, Bartlett P, Davison S, Exworthy T, Loza N, Rose D, Torales J, Brown M, Christensen H, Firth J, Keshavan M, Li A, Onnela JP, Wykes T, Elkholy H, Kalra G, Lovett KF, Travis MJ, Ventriglio A. The WPA-Lancet Psychiatry Commission on the Future of Psychiatry. Lancet Psychiatry. 2017;4:775-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 15. | Rutherford S, Kia SM, Wolfers T, Fraza C, Zabihi M, Dinga R, Berthet P, Worker A, Verdi S, Ruhe HG, Beckmann CF, Marquand AF. The normative modeling framework for computational psychiatry. Nat Protoc. 2022;17:1711-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 16. | Wu C, Ferreira F, Fox M, Harel N, Hattangadi-Gluth J, Horn A, Jbabdi S, Kahan J, Oswal A, Sheth SA, Tie Y, Vakharia V, Zrinzo L, Akram H. Clinical applications of magnetic resonance imaging based functional and structural connectivity. Neuroimage. 2021;244:118649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Janssen RJ, Mourão-Miranda J, Schnack HG. Making Individual Prognoses in Psychiatry Using Neuroimaging and Machine Learning. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:798-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Martin VP, Rouas JL, Philip P, Fourneret P, Micoulaud-Franchi JA, Gauld C. How Does Comparison With Artificial Intelligence Shed Light on the Way Clinicians Reason? Front Psychiatry. 2022;13:926286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Cullen M, Davey B, Friston KJ, Moran RJ. Active Inference in OpenAI Gym: A Paradigm for Computational Investigations Into Psychiatric Illness. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Lanillos P, Oliva D, Philippsen A, Yamashita Y, Nagai Y, Cheng G. A review on neural network models of schizophrenia and autism spectrum disorder. Neural Netw. 2020;122:338-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Loosen AM, Hauser TU. Towards a computational psychiatry of juvenile obsessive-compulsive disorder. Neurosci Biobehav Rev. 2020;118:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Mujica-Parodi LR, Strey HH. Making Sense of Computational Psychiatry. Int J Neuropsychopharmacol. 2020;23:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24:1583-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (13)] |
| 24. | Ruengchaijatuporn N, Chatnuntawech I, Teerapittayanon S, Sriswasdi S, Itthipuripat S, Hemrungrojn S, Bunyabukkana P, Petchlorlian A, Chunamchai S, Chotibut T, Chunharas C. An explainable self-attention deep neural network for detecting mild cognitive impairment using multi-input digital drawing tasks. Alzheimers Res Ther. 2022;14:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Torabi Moghadam B, Etemadikhah M, Rajkowska G, Stockmeier C, Grabherr M, Komorowski J, Feuk L, Carlström EL. Analyzing DNA methylation patterns in subjects diagnosed with schizophrenia using machine learning methods. J Psychiatr Res. 2019;114:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Trinhammer ML, Merrild ACH, Lotz JF, Makransky G. Predicting crime during or after psychiatric care: Evaluating machine learning for risk assessment using the Danish patient registries. J Psychiatr Res. 2022;152:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Watts D, Moulden H, Mamak M, Upfold C, Chaimowitz G, Kapczinski F. Predicting offenses among individuals with psychiatric disorders - A machine learning approach. J Psychiatr Res. 2021;138:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Wang X, Nie D, Lu B. Emotional state classification from EEG data using machine learning approach. Neurocomputing. 2014;129:94-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 29. | Eldar E, Roth C, Dayan P, Dolan RJ. Decodability of Reward Learning Signals Predicts Mood Fluctuations. Curr Biol. 2018;28:1433-1439.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Honnorat N, Dong A, Meisenzahl-Lechner E, Koutsouleris N, Davatzikos C. Neuroanatomical heterogeneity of schizophrenia revealed by semi-supervised machine learning methods. Schizophr Res. 2019;214:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Washington PY, Kalantarian H, Kent J, Husic A, Kline A, Leblanc É, Hou C, Mutlu C, Dunlap K, Penev Y, Varma M, Stockham NT, ChrismanBS, Paskov KM, Sun MW, Jung JY, Voss C, Haber N, Wall DP. Training an Emotion Detection Classifier using Frames from a Mobile Therapeutic Game for Children with Developmental Disorders. ArXiv. 2020;. [DOI] [Full Text] |
| 32. | Banerjee A, Mutlu OC, Kline A, Washington P, Wall D. Training and Profiling a Pediatric Emotion Recognition Classifier on Mobile Devices for Autism Detection and Treatment (Preprint). [DOI] [Full Text] |
| 33. | Dimitri GM, Spasov S, Duggento A, Passamonti L, Lio P, Toschi N. Unsupervised stratification in neuroimaging through deep latent embeddings. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Jacob S, Wolff JJ, Steinbach MS, Doyle CB, Kumar V, Elison JT. Neurodevelopmental heterogeneity and computational approaches for understanding autism. Transl Psychiatry. 2019;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Langley C, Cirstea BI, Cuzzolin F, Sahakian BJ. Theory of Mind and Preference Learning at the Interface of Cognitive Science, Neuroscience, and AI: A Review. Front Artif Intell. 2022;5:778852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Adams RA, Huys QJ, Roiser JP. Computational Psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87:53-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 38. | Barnard PJ, Teasdale JD. Interacting cognitive subsystems: A systemic approach to cognitive-affective interaction and change. Cogn Emot. 1991;5:1-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Donini M, Monteiro JM, Pontil M, Hahn T, Fallgatter AJ, Shawe-Taylor J, Mourão-Miranda J; Alzheimer's Disease Neuroimaging Initiative. Combining heterogeneous data sources for neuroimaging based diagnosis: re-weighting and selecting what is important. Neuroimage. 2019;195:215-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Liu XQ, Guo YX, Zhang WJ, Gao WJ. Influencing factors, prediction and prevention of depression in college students: A literature review. World J Psychiatry. 2022;12:860-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (39)] |
| 41. | Starke G, De Clercq E, Borgwardt S, Elger BS. Computing schizophrenia: ethical challenges for machine learning in psychiatry. Psychol Med. 2021;51:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Kanchanatawan B, Sriswasdi S, Maes M. Supervised machine learning to decipher the complex associations between neuro-immune biomarkers and quality of life in schizophrenia. Metab Brain Dis. 2019;34:267-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Kosmicki JA, Sochat V, Duda M, Wall DP. Searching for a minimal set of behaviors for autism detection through feature selection-based machine learning. Transl Psychiatry. 2015;5:e514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Liu Q, Woo M, Zou X, Champaneria A, Lau C, Mubbashar MI, Schwarz C, Gagliardi JP, Tenenbaum JD. Symptom-based patient stratification in mental illness using clinical notes. J Biomed Inform. 2019;98:103274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Mellem MS, Liu Y, Gonzalez H, Kollada M, Martin WJ, Ahammad P. Machine Learning Models Identify Multimodal Measurements Highly Predictive of Transdiagnostic Symptom Severity for Mood, Anhedonia, and Anxiety. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Edgcomb JB, Zima B. Machine Learning, Natural Language Processing, and the Electronic Health Record: Innovations in Mental Health Services Research. Psychiatr Serv. 2019;70:346-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Bangerter A, Manyakov NV, Lewin D, Boice M, Skalkin A, Jagannatha S, Chatterjee M, Dawson G, Goodwin MS, Hendren R, Leventhal B, Shic F, Ness S, Pandina G. Caregiver Daily Reporting of Symptoms in Autism Spectrum Disorder: Observational Study Using Web and Mobile Apps. JMIR Ment Health. 2019;6:e11365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Jones RM, Tarpey T, Hamo A, Carberry C, Lord C. Smartphone measures of day-to-day behavior changes in children with autism. NPJ Digit Med. 2018;1:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Dubois M, Hauser TU. Value-free random exploration is linked to impulsivity. Nat Commun. 2022;13:4542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 50. | Nam SM, Peterson TA, Seo KY, Han HW, Kang JI. Discovery of Depression-Associated Factors From a Nationwide Population-Based Survey: Epidemiological Study Using Machine Learning and Network Analysis. J Med Internet Res. 2021;23:e27344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Nielsen AN, Barch DM, Petersen SE, Schlaggar BL, Greene DJ. Machine Learning With Neuroimaging: Evaluating Its Applications in Psychiatry. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Hu X, Shu J, Jin Z. Depression tendency detection model for Weibo users based on Bi-LSTM. 2021 IEEE International Conference on Artificial Intelligence and Computer Applications (ICAICA) 2021. [DOI] [Full Text] |
| 53. | Rutledge RB, Chekroud AM, Huys QJ. Machine learning and big data in psychiatry: toward clinical applications. Curr Opin Neurobiol. 2019;55:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 54. | Graham SA, Lee EE, Jeste DV, Van Patten R, Twamley EW, Nebeker C, Yamada Y, Kim HC, Depp CA. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020;284:112732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 55. | Shen H, Wang SH, Zhang Y, Wang H, Li F, Lucas MV, Zhang YD, Liu Y, Yuan TF. Color painting predicts clinical symptoms in chronic schizophrenia patients via deep learning. BMC Psychiatry. 2021;21:522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Leblanc E, Washington P, Varma M, Dunlap K, Penev Y, Kline A, Wall DP. Feature replacement methods enable reliable home video analysis for machine learning detection of autism. Sci Rep. 2020;10:21245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Tariq Q, Daniels J, Schwartz JN, Washington P, Kalantarian H, Wall DP. Mobile detection of autism through machine learning on home video: A development and prospective validation study. PLoS Med. 2018;15:e1002705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 58. | Corcoran CM, Cecchi GA. Using Language Processing and Speech Analysis for the Identification of Psychosis and Other Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:770-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Corcoran CM, Mittal VA, Bearden CE, E Gur R, Hitczenko K, Bilgrami Z, Savic A, Cecchi GA, Wolff P. Language as a biomarker for psychosis: A natural language processing approach. Schizophr Res. 2020;226:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 60. | Carrillo F, Sigman M, Fernández Slezak D, Ashton P, Fitzgerald L, Stroud J, Nutt DJ, Carhart-Harris RL. Natural speech algorithm applied to baseline interview data can predict which patients will respond to psilocybin for treatment-resistant depression. J Affect Disord. 2018;230:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Bedi G, Cecchi GA, Slezak DF, Carrillo F, Sigman M, de Wit H. A window into the intoxicated mind? Neuropsychopharmacology. 2014;39:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Sommer IE, N de Boer J. How to reap the benefits of language for psychiatry. Psychiatry Res. 2022;318:114932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Portugal LCL, Schrouff J, Stiffler R, Bertocci M, Bebko G, Chase H, Lockovitch J, Aslam H, Graur S, Greenberg T, Pereira M, Oliveira L, Phillips M, Mourão-Miranda J. Predicting anxiety from wholebrain activity patterns to emotional faces in young adults: a machine learning approach. Neuroimage Clin. 2019;23:101813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Liu XQ, Guo YX, Xu Y. Risk factors and digital interventions for anxiety disorders in college students: Stakeholder perspectives. World J Clin Cases. 2023;11:1442-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (14)] |
| 65. | Brosschot JF, Thayer JF. Heart rate response is longer after negative emotions than after positive emotions. Int J Psychophysiol. 2003;50:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Greenman DLB, La MAN, Shah S, Chen Q, Berman KF, Weinberger DR, Tan HY. Parietal-Prefrontal Feedforward Connectivity in Association With Schizophrenia Genetic Risk and Delusions. Am J Psychiatry. 2020;177:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Petrantonakis PC, Hadjileontiadis LJ. A novel emotion elicitation index using frontal brain asymmetry for enhanced EEG-based emotion recognition. IEEE Trans Inf Technol Biomed. 2011;15:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Şen B, Peker M, Çavuşoğlu A, Çelebi FV. A comparative study on classification of sleep stage based on EEG signals using feature selection and classification algorithms. J Med Syst. 2014;38:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Taylor JA, Matthews N, Michie PT, Rosa MJ, Garrido MI. Auditory prediction errors as individual biomarkers of schizophrenia. Neuroimage Clin. 2017;15:264-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Howe Iii EG, Elenberg F. Ethical Challenges Posed by Big Data. Innov Clin Neurosci. 2020;17:24-30. [PubMed] |
| 71. | Medina R, Bouhaben J, de Ramón I, Cuesta P, Antón-Toro L, Pacios J, Quintero J, Ramos-Quiroga JA, Maestú F. Electrophysiological Brain Changes Associated With Cognitive Improvement in a Pediatric Attention Deficit Hyperactivity Disorder Digital Artificial Intelligence-Driven Intervention: Randomized Controlled Trial. J Med Internet Res. 2021;23:e25466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Adams RA, Pinotsis D, Tsirlis K, Unruh L, Mahajan A, Horas AM, Convertino L, Summerfelt A, Sampath H, Du XM, Kochunov P, Ji JL, Repovs G, Murray JD, Friston KJ, Hong LE, Anticevic A. Computational Modeling of Electroencephalography and Functional Magnetic Resonance Imaging Paradigms Indicates a Consistent Loss of Pyramidal Cell Synaptic Gain in Schizophrenia. Biol Psychiatry. 2022;91:202-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 73. | Čukić M, López V, Pavón J. Classification of Depression Through Resting-State Electroencephalogram as a Novel Practice in Psychiatry: Review. J Med Internet Res. 2020;22:e19548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Parkes L, Satterthwaite TD, Bassett DS. Towards precise resting-state fMRI biomarkers in psychiatry: synthesizing developments in transdiagnostic research, dimensional models of psychopathology, and normative neurodevelopment. Curr Opin Neurobiol. 2020;65:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 75. | Tolmeijer E, Kumari V, Peters E, Williams SCR, Mason L. Using fMRI and machine learning to predict symptom improvement following cognitive behavioural therapy for psychosis. Neuroimage Clin. 2018;20:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Winterburn JL, Voineskos AN, Devenyi GA, Plitman E, de la Fuente-Sandoval C, Bhagwat N, Graff-Guerrero A, Knight J, Chakravarty MM. Can we accurately classify schizophrenia patients from healthy controls using magnetic resonance imaging and machine learning? Schizophr Res. 2019;214:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 77. | Rosa MJ, Portugal L, Hahn T, Fallgatter AJ, Garrido MI, Shawe-Taylor J, Mourao-Miranda J. Sparse network-based models for patient classification using fMRI. Neuroimage. 2015;105:493-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 78. | Silva N, Zhang D, Kulvicius T, Gail A, Barreiros C, Lindstaedt S, Kraft M, Bölte S, Poustka L, Nielsen-Saines K, Wörgötter F, Einspieler C, Marschik PB. The future of General Movement Assessment: The role of computer vision and machine learning - A scoping review. Res Dev Disabil. 2021;110:103854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 79. | Li W, Wang Q, Liu X, Yu Y. Simple action for depression detection: using kinect-recorded human kinematic skeletal data. BMC Psychiatry. 2021;21:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Loth E, Ahmad J, Chatham C, López B, Carter B, Crawley D, Oakley B, Hayward H, Cooke J, San José Cáceres A, Bzdok D, Jones E, Charman T, Beckmann C, Bourgeron T, Toro R, Buitelaar J, Murphy D, Dumas G. The meaning of significant mean group differences for biomarker discovery. PLoS Comput Biol. 2021;17:e1009477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Wagh VV, Vyas P, Agrawal S, Pachpor TA, Paralikar V, Khare SP. Peripheral Blood-Based Gene Expression Studies in Schizophrenia: A Systematic Review. Front Genet. 2021;12:736483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Reay WR, Geaghan MP, Atkins JR, Carr VJ, Green MJ, Cairns MJ. Genetics-informed precision treatment formulation in schizophrenia and bipolar disorder. Am J Hum Genet. 2022;109:1620-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | IU School of Medicine. Researchers Develop Blood Test For Depression, Bipolar Disorder. EurekAlert 2021. Available from: https://www.eurekalert.org/pub_releases/2021-04/iuso-iso040721.php. |
| 84. | Fernandes BS, Karmakar C, Tamouza R, Tran T, Yearwood J, Hamdani N, Laouamri H, Richard JR, Yolken R, Berk M, Venkatesh S, Leboyer M. Precision psychiatry with immunological and cognitive biomarkers: a multi-domain prediction for the diagnosis of bipolar disorder or schizophrenia using machine learning. Transl Psychiatry. 2020;10:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 85. | Bobo WV, Van Ommeren B, Athreya AP. Machine learning, pharmacogenomics, and clinical psychiatry: predicting antidepressant response in patients with major depressive disorder. Expert Rev Clin Pharmacol. 2022;15:927-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 86. | Ambrosen KS, Skjerbæk MW, Foldager J, Axelsen MC, Bak N, Arvastson L, Christensen SR, Johansen LB, Raghava JM, Oranje B, Rostrup E, Nielsen MØ, Osler M, Fagerlund B, Pantelis C, Kinon BJ, Glenthøj BY, Hansen LK, Ebdrup BH. A machine-learning framework for robust and reliable prediction of short- and long-term treatment response in initially antipsychotic-naïve schizophrenia patients based on multimodal neuropsychiatric data. Transl Psychiatry. 2020;10:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 87. | Chen ZS, Kulkarni PP, Galatzer-Levy IR, Bigio B, Nasca C, Zhang Y. Modern views of machine learning for precision psychiatry. Patterns (N Y). 2022;3:100602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 88. | Sohl K, Kilian R, Brewer Curran A, Mahurin M, Nanclares-Nogués V, Liu-Mayo S, Salomon C, Shannon J, Taraman S. Feasibility and Impact of Integrating an Artificial Intelligence-Based Diagnosis Aid for Autism Into the Extension for Community Health Outcomes Autism Primary Care Model: Protocol for a Prospective Observational Study. JMIR Res Protoc. 2022;11:e37576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 89. | Mihalik A, Ferreira FS, Moutoussis M, Ziegler G, Adams RA, Rosa MJ, Prabhu G, de Oliveira L, Pereira M, Bullmore ET, Fonagy P, Goodyer IM, Jones PB; NeuroScience in Psychiatry Network (NSPN) Consortium, Shawe-Taylor J, Dolan R, Mourão-Miranda J. Multiple Holdouts With Stability: Improving the Generalizability of Machine Learning Analyses of Brain-Behavior Relationships. Biol Psychiatry. 2020;87:368-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | Rüppel J. ["Allowing the Data to 'Speak for Themselves'" - The Classification of Mental Disorders and the Imaginary of Computational Psychiatry]. Psychiatr Prax. 2021;48:S16-S20. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 91. | Chandler C, Foltz PW, Elvevåg B. Using Machine Learning in Psychiatry: The Need to Establish a Framework That Nurtures Trustworthiness. Schizophr Bull. 2020;46:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Tandon N, Tandon R. Using machine learning to explain the heterogeneity of schizophrenia. Realizing the promise and avoiding the hype. Schizophr Res. 2019;214:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Cao XJ, Liu XQ. Artificial intelligence-assisted psychosis risk screening in adolescents: Practices and challenges. World J Psychiatry. 2022;12:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (9)] |
| 94. | Stringaris A. Editorial: Are computers going to take over: implications of machine learning and computational psychiatry for trainees and practising clinicians. J Child Psychol Psychiatry. 2019;60:1251-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 95. | Saha K, Torous J, Ernala SK, Rizuto C, Stafford A, De Choudhury M. A computational study of mental health awareness campaigns on social media. Transl Behav Med. 2019;9:1197-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Brown C, Story GW, Mourão-Miranda J, Baker JT. Will artificial intelligence eventually replace psychiatrists? Br J Psychiatry. 2021;218:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Gauld C, Micoulaud-Franchi JA, Dumas G. Comment on Starke et al.: 'Computing schizophrenia: ethical challenges for machine learning in psychiatry': from machine learning to student learning: pedagogical challenges for psychiatry. Psychol Med. 2021;51:2509-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | McKernan LC, Clayton EW, Walsh CG. Protecting Life While Preserving Liberty: Ethical Recommendations for Suicide Prevention With Artificial Intelligence. Front Psychiatry. 2018;9:650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Wiese W. [From the Ethics of AI to the Ethics of Consciousness: Ethical Aspects of Computational Psychiatry]. Psychiatr Prax. 2021;48:S21-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Wiese W, Friston KJ. AI ethics in computational psychiatry: From the neuroscience of consciousness to the ethics of consciousness. Behav Brain Res. 2022;420:113704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Dluhoš P, Schwarz D, Cahn W, van Haren N, Kahn R, Španiel F, Horáček J, Kašpárek T, Schnack H. Multi-center machine learning in imaging psychiatry: A meta-model approach. Neuroimage. 2017;155:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 102. | Koutsouleris N, Hauser TU, Skvortsova V, De Choudhury M. From promise to practice: towards the realisation of AI-informed mental health care. Lancet Digit Health. 2022;4:e829-e840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 103. | Logan DE, Breazeal C, Goodwin MS, Jeong S, O'Connell B, Smith-Freedman D, Heathers J, Weinstock P. Social Robots for Hospitalized Children. Pediatrics. 2019;144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 104. | Walsh CG, Ribeiro JD, Franklin JC. Predicting suicide attempts in adolescents with longitudinal clinical data and machine learning. J Child Psychol Psychiatry. 2018;59:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 105. | Bzdok D, Meyer-Lindenberg A. Machine Learning for Precision Psychiatry: Opportunities and Challenges. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (4)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Cabezuelo AS, Spain; Cawthorpe DR, Canada; Morya AK, India S-Editor: Liu JH L-Editor: A P-Editor: Yu HG