Published online Mar 2, 2023. doi: 10.13105/wjma.v11.i3.55
Peer-review started: December 31, 2022
First decision: January 20, 2023
Revised: January 22, 2023
Accepted: February 13, 2023
Article in press: February 13, 2023
Published online: March 2, 2023
Processing time: 59 Days and 20 Hours
Chronic kidney disease (CKD), especially in advanced stages, is an important cause of infertility. In CKD patients, infertility has been linked to multiple factors. The pathophysiology of infertility related to CKD is complex and forked. Correction of modifiable factors can improve fertility in both genders. In males as well as females, successful kidney transplantation offers good chances of restoration of reproductive function. In female renal allograft recipients, recovery of reproductive functions in the post-transplant period will manifest as restoration of normal menses and ovulation. Owing to this improvement, there is a signi
Core Tip: Chronic kidney disease (CKD) is a major cause of infertility in both sexes. Multiple factors amplify infertility in CKD patients. Kidney transplantation can restore fertility in men and women. Menses will return in the majority of females after kidney transplantation. This improvement increases the risk of accidental pregnancy, so contraception should be discussed in advance. Kidney transplant recipients utilize several contraceptives to plan pregnancy. Preference and tolerability determine contraception choice. If pregnancy occurs, transplanted women experience the same physiologic changes as pregnant women with native kidneys. During pregnancy, immunosuppressive drugs can cause consequences. Breastfeeding kidney transplant recipients should discuss immunosuppressive and other medicines.
- Citation: Habli M, Belal D, Sharma A, Halawa A. Infertility, pregnancy and breastfeeding in kidney transplantation recipients: Key issues. World J Meta-Anal 2023; 11(3): 55-67
- URL: https://www.wjgnet.com/2308-3840/full/v11/i3/55.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i3.55
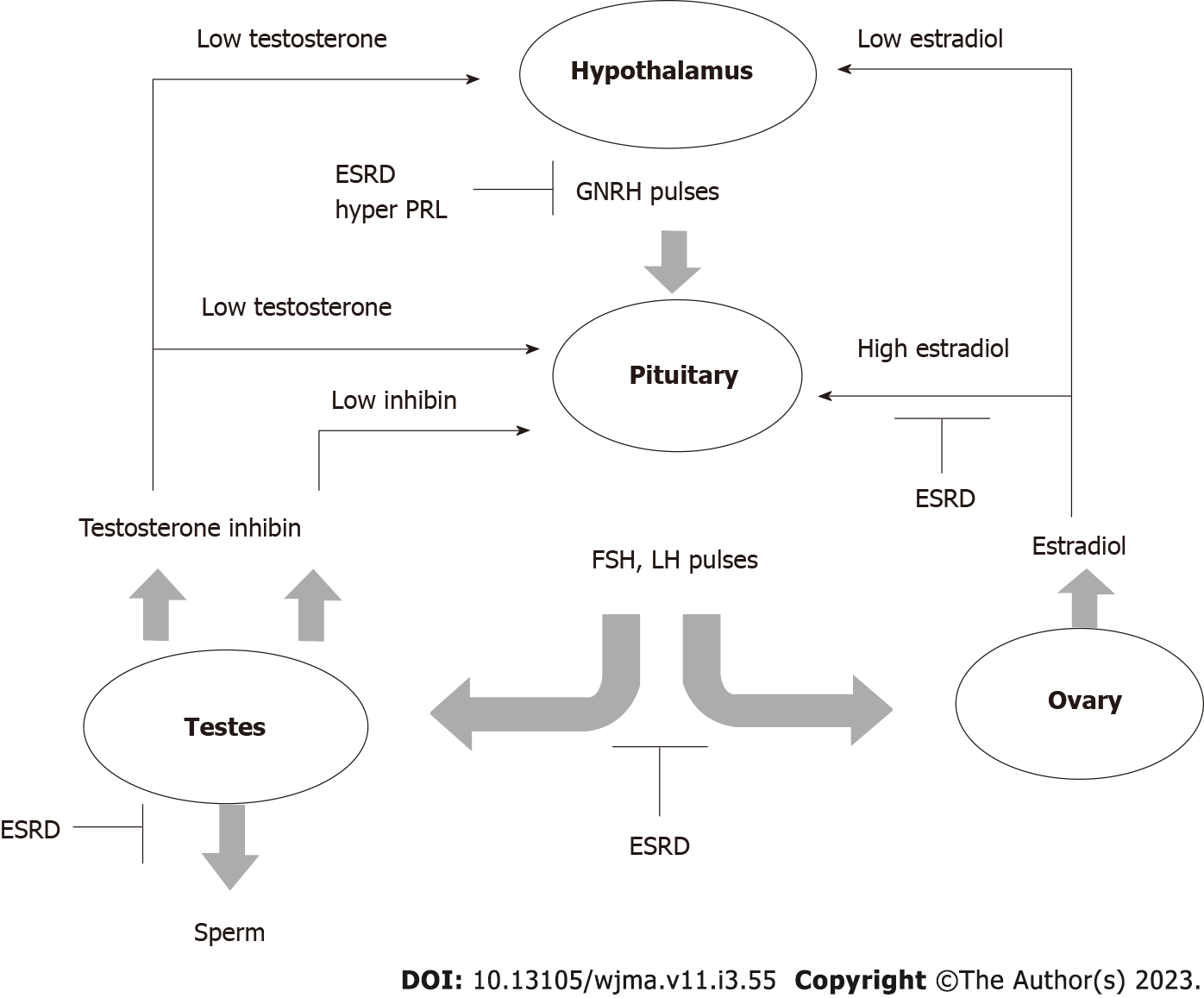
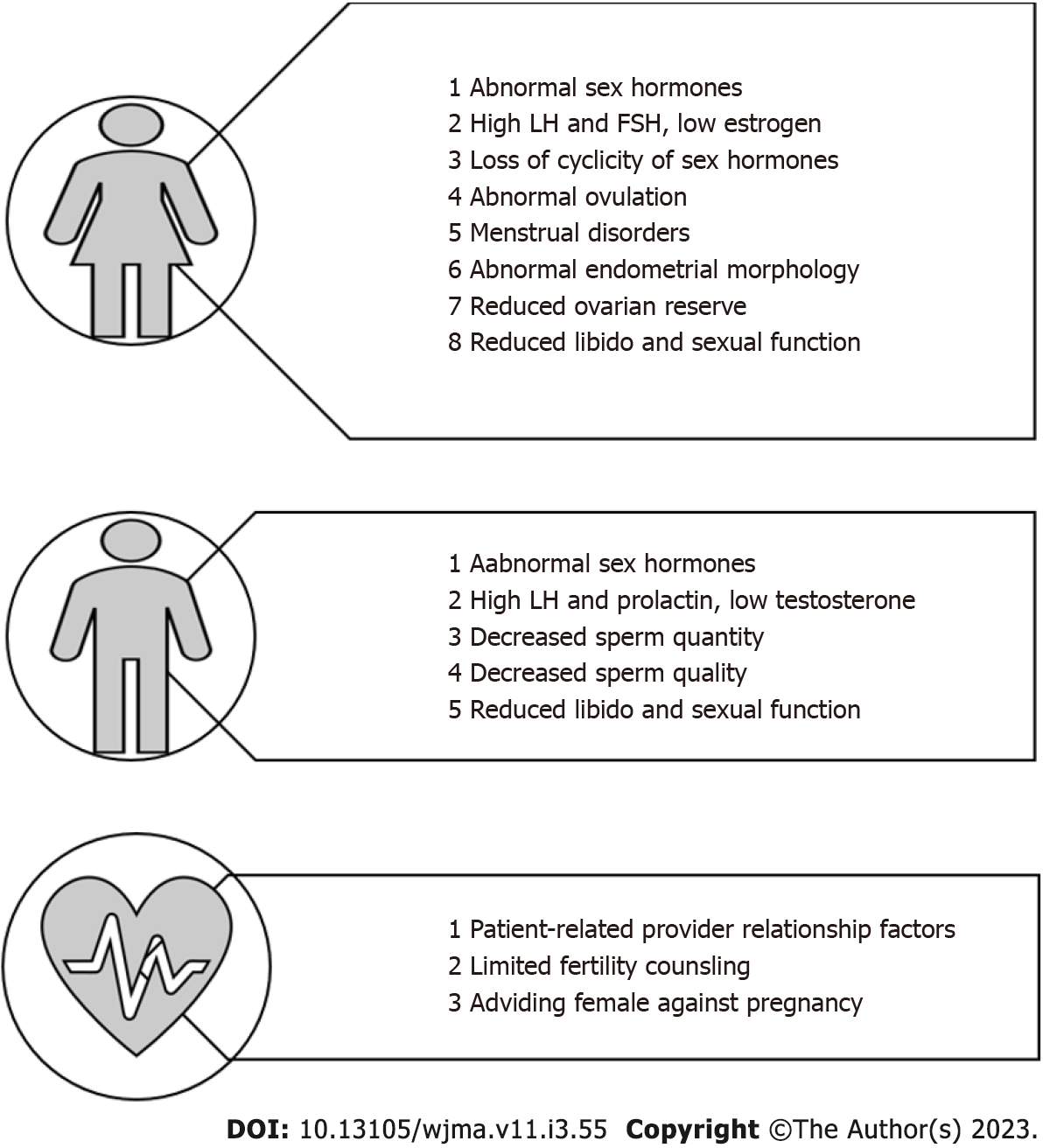
Chronic kidney disease, especially in advanced stages causes fertility and sexual dysfunction in males as well as in females[1] (Figure 1). The male sexual health malfunction manifests as erectile dysfunction in up to 80% of end-stage renal disease (ESRD) patients on hemodialysis[2]. The pathophysiology is due to a combination of vascular calcification, accelerated atherosclerosis, uremic neuropathy, impairment of the hypothalamic-pituitary-testicular axis and secondary hyperparathyroidism[3]. ESRD is also with alteration in the levels of sex hormones which include reduction in testosterone level and elevation in luteinizing hormone (LH) and follicle-stimulating hormone (FSH)[4]. The prevalence of infertility in females, of childbearing age, with ESRD, has been reported as high as 92%[5].
Factors that are implicated in the pathogenesis of infertility in women with ESRD include impairment at the level of the hypothalamus-pituitary-ovarian axis manifesting as high FSH and LH and low estrogen levels[6], menstrual disorders in up to 75% of patients manifesting as amenorrhea, oligomenorrhea or functional menopause[7], and abnormal endometrial atrophy due to reduced estrogen level[8]. Other contributions to infertility include reduced libido and orgasmic impairment, in addition to vaginal dryness or failure of vaginal lubrication[9], as shown in Figure 2[10].
Improvement of fertility in patients with kidney disease is achieved by correction of modifiable factors like anemia, hyperparathyroidism, dialysis adequacy[11], avoidance of toxic medications[12], and hormonal replacement therapies[13], However, kidney transplantation remains the best option for the management of infertility due to ESRD for both genders[14].
Following successful kidney transplantation, the function of the hypothalamic-pituitary-gonadal axis is gradually restored leading to normalization of sex hormone levels in men and women in the majority but not in all patients[15,16]. In males, this recovery manifests as improvement in erectile dysfunction, libido, and spermatogenesis, whereas in women as restoration of menses and ovulation[16].
Owing to the improvement in reproductive functions and sexual health within 3-6 mo, these patients should be counselled about the significant potential of conceiving shortly after successful kidney transplantation. Hence it is imperative to explain contraception during the pre-transplantation assessment so that pregnancy can be planned at a time when the risk to mother and fetus is minimal i.e., after one year of uneventful kidney transplantation.
Intra-renal hemodynamics is reported to be altered, starting from the 1st week of pregnancy, due to a reduction in vascular resistance[17], increase in cardiac output[18], and increase in plasma volume[19], which finally lead to an increase in renal blood flow[20] and subsequently increase in GFR. In normal pregnancy with native kidneys, GFR increases along with an increase in renal size[19,21]. Despite volume expansion and increase in cardiac output, mean arterial blood pressure is reduced by about 10-15 mmHg in the 1st trimester and then returned to normal by the 2nd trimester[22].
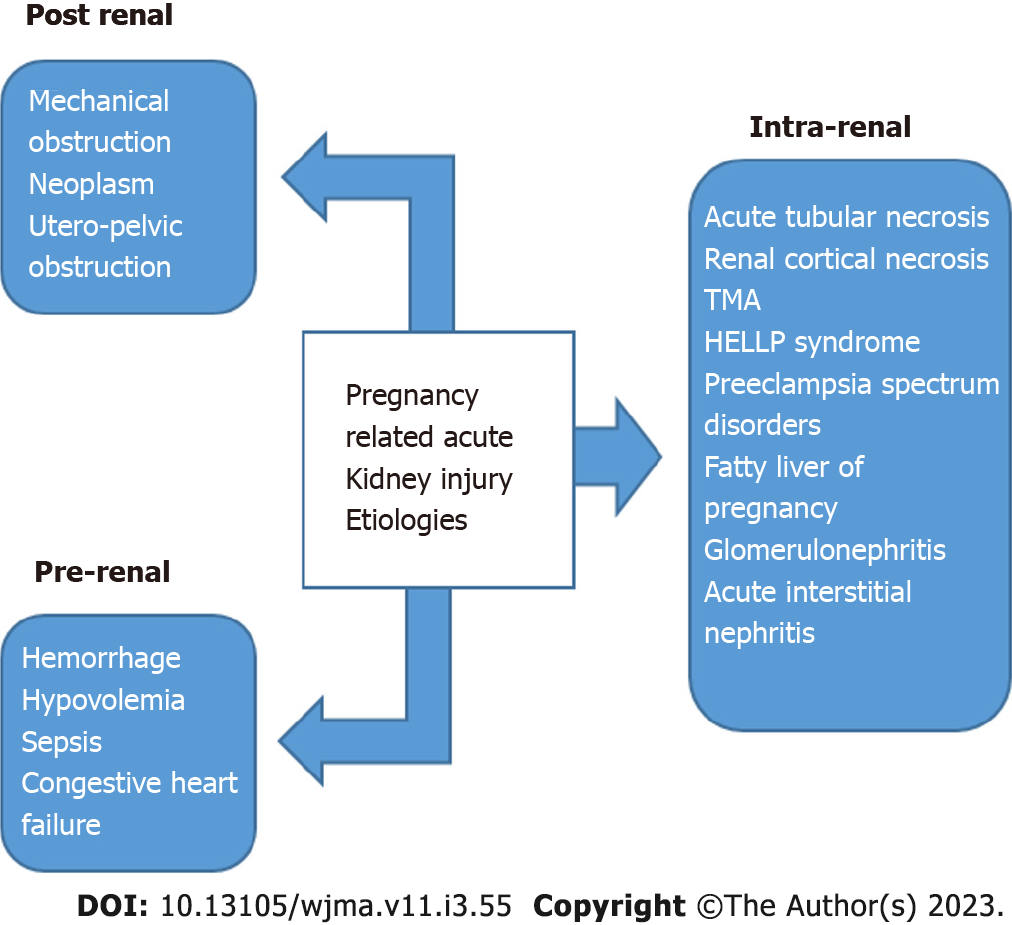
It is well established that acute kidney injury can occur during pregnancy in non-transplant females due to pregnancy itself. Pregnancy-related acute kidney injury can also happen in the transplanted allograft, however, when it occurs it affects not only the pregnant patient but also the fetus. Refer to Figure 3[23].
Several studies evaluated the impact of pregnancy on graft survival. Levidiotis et al[24], using ANZDT Registry data of pregnancy in transplant recipients, demonstrated that the delivery of first live birth was comparable between the study group and control group, and was not associated with worse twenty-year graft survival.
Rahamimov et al[25] evaluated the long-term impact of pregnancy on allograft and patient survival and reported that graft and recipient survival did not differ from the control group in the follow-up.
Shah et al[26] conducted a systemic review and meta-analysis about pregnancy outcomes in kidney transplant recipients. They reported that the rejection rate during pregnancy was 9.4% which is comparable to the United States mean of 9.1%.
To evaluate possible bias in patients' selection that may affect outcomes and interpretation of results, M. Pappias and colleagues evaluated pregnancy outcomes after living kidney donation in a systematic review. In this study, 2 authors used the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) method to evaluate participant selection, exposure, and results. Robvis online software plotted risk-of-bias evaluations. Grading of recommendations, assessment, development, and evaluations method graded study certainty. As a result, authors concluded that after donation, the absolute chance of pregnancy related and associated complications remain minimal, which is comparable to other studies[27].
In conclusion, pregnancy is not associated with worse graft outcomes in kidney transplanted recipients, however, female recipients should be carefully selected before pregnancy planning and should be counseled about possible complications. Stability of kidney function at time of pregnancy detection, should be monitored attentively throughout the course of pregnancy.
Although the majority of female recipients restore their ability to conceive, pregnancy rates are much lower when compared to the general population[24,28,29]. Gill et al[28] demonstrated that the rate of pregnancy in transplanted females was less than 1/3 of the general population in the first 3 years following transplantation surgery.
Shah et al[26], based on a meta-analysis of the outcomes of pregnancy in transplanted patients, reported an increased risk of gestational diabetes and gestational hypertension. Preeclampsia was reported to be sixfold higher in transplant women. Shah et al[26] reported higher rates of preterm delivery, stillbirths, and neonatal death. Other pregnancy-associated complications such as induced abortions, miscarriages and ectopic pregnancies were reported to be more common in kidney transplant recipients.
Deshpande et al[29] reported a live birth rate among pregnant renal transplant recipients comparable to that of the general population. Other retrospective studies have reported live birth rates of up to 79%[30,31]. Preterm delivery was reported to occur in 46% of recipients[30].
Cesarean delivery was significantly more frequent in the transplant population reaching 43%-72%, although no clear evidence to support this practice[29-31]. The United Kingdom Transplant Pregnancy Registry, showed a higher rate of low birth weight in 20% to 50% of cases[30,31].
T-lymphocyte-depleting agents and IL-2 inhibitors (basilixumab) are commonly used as induction therapy for transplant patients. Maintenance therapy is commenced in the hospital and continued to prevent acute rejection. Before conception, modification of immunosuppression is frequently needed, as some drugs have shown to be associated with adverse outcomes in the pregnancy and fetus[32].
In addition to female recipient preparation for pregnancy, male recipients who desires paternity should be also properly counseled about the impact of immunosuppression on fertility. Few studies have reported the negative effect of immunosuppressive drugs, particularly sirolimus, on male fertility. Sirolimus was shown to be linked to reduced fertility following kidney transplantation, due to its toxic effect on the sperm[33,34]. That’s why, unrecovered fertility in male recipients maintained on mammalian target of rapamycin inhibitors (mTORi) following renal transplant surgery, should raise the suspicion of possible drug toxicity.
In the other hand, maintenance immunosuppression in females is modified to avoid teratogenic effect on the fetus. Generally, Mycophenolate Mofetil (MMF)/Mycophenolic Sodium (MPS) is considered unsafe during pregnancy. Kidney transplant recipients who are on MMF during pregnancy are at higher risk of pregnancy loss in the first-trimester first trimester along with severe congenital fetal structural malformations[35-37]. Following exposure to MMF, congenital malformations such as ear, eye, and lip/palate malformations have been reported in 23%-27% of live births[38]. Therefore, MMF should switch over to azathioprine that is, not associated with maternal or fetal risks[39].
Calcineurin inhibitors are the cornerstone of maintenance immunosuppressive therapy in any kidney transplant recipient. Calcineurin inhibitors (CNIs) have been evaluated during pregnancy in renal transplant females. The use of tacrolimus in kidney transplanted pregnant is considered safe. Physiologic changes during pregnancy can alter some pharmacokinetic properties of tacrolimus, that’s why frequent monitoring of tacrolimus levels is recommended[40]. Furthermore, several studies have examined the effect of cyclosporine on the fetus and demonstrated that it is not teratogenic[41,42]. However, the Food and Drug Administration (FDA) categorizes Cyclosporin as category C, which indicates that human risk cannot be excluded. CNIs, in particular tacrolimus, are associated with increased risk of Post-transplant Diabetes Mellitus. It is well established that tacrolimus is more diabetogenic than cyclosporine[43,44]. Increased tacrolimus levels have been strongly linked to altered glucose tolerance, toxic effect on islet cells with subsequent development of diabetes mellitus. In pregnant recipients treated with tacrolimus with new onset hyperglycemia, shifting to safer drug such as cyclosporine could be an option. However, a recent systematic review and meta-analysis compared the impact of cyclosporine and tacrolimus on pregnancy outcomes in liver/kidney transplant recipients, found no significant differences in the incidence of gestational diabetes between them[45].
The use of mammalian target of rapamycin (mTOR) inhibitors is considered a contraindication during pregnancy. Sirolimus should be discontinued at least 12 wk before pregnancy, while everolimus should be discontinued at least 8 wk before conception. Boulay et al[46], and Framarino et al[47]. Reported limited data on the use of mTOR inhibitors in pregnant patients.
With the increased use of co-stimulation blocker, Belatacept, in non-pregnant recipients, there is still no clear evidence on the safety of its use in pregnant recipients[48].
In conclusion, the combination of calcineurin inhibitors, azathioprine and steroids is the mainstay maintenance therapy in pregnant recipients, as no major fetal or maternal effects have been reported.
Hypertension is reported to be more common in pregnant transplant recipients accounting for 20%-70% compared to 1%-5% in pregnant women in the general population[26,30,49]. Hypertension in pregnant transplant recipients, is associated with a higher risk of preeclampsia and eclampsia.
In hypertensive pregnant females, medications such as labetalol[50], calcium channel blockers of dihydropyridine group[51], methyldopa[52], and hydralazine[53] can effectively manage hypertension with a safe profile regarding the pregnant transplant recipient and fetus.
Non-dihydropyridine calcium channel blockers (such as diltiazem and verapamil), should not be administered with calcineurin inhibitors, because of their effect on enzyme CYP3A4 metabolism[54].
Angiotensin-converting enzyme inhibitors, angiotensin receptor inhibitors, and direct renin inhibitors are not acceptable during pregnancy because they are associated with significant fetal risk[55]. Therefore, it is recommended to plan conception at least 6 wk after discontinuation of these drugs.
There is very limited data on the safety of angiotensin-converting enzyme inhibitors in normal lactation. The minimal concentration of angiotensin converting enzyme inhibitors, in breast milk, can cause hemodynamic instability in premature infants and neonates in therapeutic doses. Captopril and Enalapril are excreted in very low doses in breast milk and considered safe with breastfeeding, nonetheless, babies should be monitored for adverse events[56].
Thiazide and loop diuretics use during pregnancy, have not been linked to increased risk of fetal unfavorable outcomes, when prescribed for volume overload and elevated blood pressure. Diuretic usage should be limited, because of major concern about affecting physiologic volume expansion during pregnancy[57].
In the other hand, antibiotics during pregnancy are used more frequently, as the incidence of infections is higher in transplanted patients, owing to the use of potent immunosuppression. Urinary tract infections are prevalent in female transplant patients, and the risk rises by up to 40% during pregnancy, presumably due to physiologic anatomic changes occurring in the urinary tract[58]. The prescription of antibiotics in kidney transplant recipients should always be considered for a potential interaction and possible adverse effects. Antibiotics such as Nitrofurantoin, Amoxicillin, Cephalexin, Cefpodoxime and Fosfomycin are considered safe in pregnancy in kidney transplant recipients with no drug-drug interaction[59]. Ciprofloxacin and Trimethoprim/Sulfamethoxazole are generally not recommended in pregnancy with and without transplantation. Antibiotics that are generally used for the management of upper and lower tract infections include macrolides, quinolones, penicillins and cephalosporins. Clarithromycin, but not azithromycin should be avoided in kidney transplant recipients irrespective of pregnancy, because of its effect on the hepatic/intestinal enzyme CYP3A4 metabolism and subsequent increase in tacrolimus level and possible toxicity. Azithromycin is safe to use during pregnancy in renal transplant recipients, but attention should be paid to the risk of arrythmia as both drugs increase QTc interval. The use of quinolones in pregnancy is still controversial in literature because of concerns on their adverse effects on the fetus formation. However, animal studies did not show an increase in major birth defects, abortion or maternal complications[60]. Hence, quinolones can be prescribed in complicated and life threating infections.
Penicillins and cephalosporins are generally acceptable in kidney transplant recipients in the context of pregnancy.
After kidney transplantation, infection is the second major cause of mortality among transplant patients, behind cardiovascular disease. Up to seventy percent of kidney transplant recipients will encounter an infection episode during the first three years following transplantation, according to estimates[61]. As mentioned earlier, bacterial urinary tract infections are more prevalent during pregnancy in a kidney transplant recipient because of potent immunosuppression used.
Other than urinary tract infection, pregnant transplant recipients are at risk of TORCH infections. TORCH infections are a category of infectious disorders that can be transmitted to a newborn during pregnancy, delivery, or shortly after birth. Toxoplasmosis, rubella, cytomegalovirus, herpes, and others are termed as TORCH. In transplant recipients, the risk of cytomegalovirus infection during pregnancy is minimal, as conception is often planned 1-2 years following transplantation. Congenital cytomegalovirus (CMV) is the leading nongenetic cause of congenital sensorineural hearing loss and neurological impairment[62,63]. Therefore, it is essential that CMV infections be monitored.
Another TORCH virus, Herpes simplex virus can occur during pregnancy in immunocompromised patients as primary infection or activation of latent infection. In case of herpetic infection valacyclovir or acyclovir can be used safely during pregnancy. Caesarean delivery in infected mothers reduces the incidence of newborn herpes 1 or 2. Therefore, caesarean section should be performed if cervical cultures show herpes. To prevent primary varicella-zoster virus (VZV) infection after transplantation, pretransplant screening for past VZV infection should be conducted, and naive patients should be immunized with live attenuated varicella vaccine if possible[58].
Toxoplasmosis in pregnant transplant recipients can be caused by either reactivation of a latent infection or primary infection. In a fitting clinical setting, toxoplasmosis should be evaluated in the differential diagnosis of pneumonia, culture-negative sepsis, and encephalitis. Toxoplasmosis should be screened quarterly in pregnant kidney transplant patients. Sulfadiazine, pyrimethamine and spiramycin should be given to immunosuppressed individuals with growing antibody titers to prevent congenital toxoplasmosis infection[64].
As a conclusion, many illnesses can be avoided or ameliorated by pre- and post-transplant care, pretransplant screening of infections and updated immunization remain the major standard of treatment. Protocol polymerase chain reaction screening of CMV, BK virus and others, in the post-operative period has been also shown to reduce the incidence of infectious complications. Finally, most opportunistic infections occurring in pregnant transplanted patients can be preventable, therefore, transplant nephrologists carry a major responsibility in the delivery of best available medical care for all patients.
Pregnancy in transplant patients with stable kidney function and no risk factors is associated with favorable graft outcomes. The graft failure rate in pregnant transplanted women was comparable to that in non-pregnant allograft recipients at a follow-up of ten years[65]. Renal transplant recipients with hypertension, pre-gestational elevated creatinine, and proteinuria are at higher risk to develop accelerated graft loss.
National Transplantation Pregnancy Registry revealed that recipients who faced graft loss in five years had lower eGFR at baseline before pregnancy, higher serum creatinine after pregnancy, and a higher rejection rate three months after pregnancy[66]. Recurrent acute rejections with renal impairment before and during pregnancy increase the risk of graft failure.
The likelihood of graft failure at five years was significantly higher when serum creatinine was > 1.3 mg/dL pre-pregnancy. Serum creatinine at > 1.6 mg/dL was associated even with a more than 7-fold higher risk of graft failure. Keitel et al[67] reported that pre-pregnancy creatinine was > 1.5 mg/dL in all recipients who experienced graft failure 2 years following childbirth.
Schwarz and colleagues also reported poor graft outcomes in patients with low eGFR before or during pregnancy[68].
Proteinuria before or during pregnancy, especially proteinuria of > 1 g/d, is associated with worse graft survival[69]. Higher the proteinuria, the higher the risk of premature birth, Intrauterine growth retardation, and miscarriages. Hence, it is strongly recommended to achieve low proteinuria levels below 500 mg before pregnancy to avoid adverse events[69].
Despite the fact that end-stage renal disease negatively affects fertility, there is a recovery of reproductive function after a kidney transplant, and pregnancy is common. Fertility can be efficiently reverted in the few months after a kidney transplant. Hence, to guarantee that pregnancies do not occur prior to maternal optimization, it is crucial that women with a history of kidney transplants plan their pregnancies and have access to adequate contraception[70]. Female recipients should be educated about contraceptive methods which could be selected based on old experience, medical history, comorbidities, and preference[71].
Irreversible contraception is usually achieved by surgical procedures like vasectomy[72,73], or tubal ligation[74,75]. Reversible contraception is achieved using an intrauterine device (IUD), hormonal pills/injections or patches and other barrier methods.
Hormonal contraceptives are commonly used and highly effective with a minimal failure rate[76]. Estrogen-based contraceptives are associated with exacerbation of migraines, the risk of venous thromboembolism (VTE), and worsening hypertension control. Depo medroxyprogesterone (DMPA) is an effective and safe contraceptive method, with prolonged effect over 3 mo. The use of DMPA increases the risk of VTE[77]. Other hormonal contraceptives include etonogestrel implant[78], transdermal patch and others.
IUDs are highly effective, easy to insert and low failure rate with no increased risk of VTE. IUDs are not associated with an increased risk of infectious complications[79].
The vaginal ring is another effective method of contraception. It is associated with a lower incidence of adverse events[80].
Barrier methods include condoms, spermicides, diaphragm, cervical caps and sponges are associated with failure rates due to compliance issues. Education of couples on its correct use may reduce the failure rate[81]. In conclusion, there is no study comparing the efficacy of different types of contraception in transplanted females. Therefore, an individualized approach to contraception is recommended, based on comorbidities, associated risk, and preference. A comparison of the effectiveness of contraceptive methods is demonstrated in Figure 4[82].
Pregnancy in a kidney transplant recipient remains to be complicated because of the detrimental effects of immunosuppressive therapy on the renal allograft, fetus and the transplant recipient. The safety of immunosuppression therapy on breastfed babies was addressed in a few studies[83-100]. There is reassuring data on the use of calcineurin inhibitors based regimen in addition to prednisone and azathioprine during lactation. Prednisolone is excreted at very low levels in breast milk. The studied dose of 50 mg/d was not shown to affect growth. The risk of infections or hematological complications in infants is not increased[31].
Regarding azathioprine, its metabolites were undetectable in the breast milk and there was no side effect noted in the infants. Infants of mothers receiving azathioprine did not show any significant increase in infection rate[84-88].
Cyclosporine was reported to have minimal excretion in breast milk. The study showed no nephrotoxic effect, growth retardation or immunosuppressive effects on the baby. Another study demonstrated undetectable Cyclosporine levels in breastfed babies from mothers on Cyclosporine[89-96]. Tacrolimus levels were undetectable in infants. Studies demonstrated that lactation in renal transplant recipients on tacrolimus was safe but needs close monitoring of the infant[97-100].
As mTORi are contraindicated during pregnancy, it is also advised not to initiate mTORi during lactation, as there are no studies that support this practice[101]. MMF usage in breastfeeding was not studied in humans, however, results extrapolated from animal studies demonstrated harm[35]. Belatacept was suggested by transplant experts not to be used while breastfeeding as no study evaluated its effect on infants[102].
As ESRD is associated with infertility, kidney transplantation offers the best option to restore sexual health and the ability to conceive. Proper contraception and pregnancy planning are mandatory, to avoid unwanted pregnancy and the toxic effects of immunosuppression on the fetus. Modifications in immunosuppression are essential before conception. Normal lactation is the best feeding for babies, but patients on immunosuppressive drugs should be counseled about their possible side effects. Normal delivery is considered the normal way of delivery, although practice patterns may differ.
Primary care physicians and nephrologists should make a greater effort to discuss menstrual and reproductive issues with women who have received a kidney transplant. The transplant team should provide complete information and counseling to women of childbearing age who are considering pregnancy.
Finally, pregnancy is generally considered safe in the setting of kidney transplant, however, a team approach to care that includes the primary care physician, a transplant nephrologist, and a qualified obstetrician in high-risk pregnancies, is crucial for a successful pregnancy and better outcomes.
| 1. | Salvadori M, Tsalouchos A. Fertility and Pregnancy in End Stage Kidney Failure Patients and after Renal Transplantation: An Update. Transplantology. 2021;2:92-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Türk S, Karalezli G, Tonbul HZ, Yildiz M, Altintepe L, Yildiz A, Yeksan M. Erectile dysfunction and the effects of sildenafil treatment in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2001;16:1818-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Billups KL. Erectile dysfunction as a marker for vascular disease. Curr Urol Rep. 2005;6:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Reinhardt W, Kübber H, Dolff S, Benson S, Führer D, Tan S. Rapid recovery of hypogonadism in male patients with end stage renal disease after renal transplantation. Endocrine. 2018;60:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Wiles KS, Nelson-Piercy C, Bramham K. Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol. 2018;14:165-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Ahmed SB, Vitek WS, Holley JL. Fertility, Contraception, and Novel Reproductive Technologies in Chronic Kidney Disease. Semin Nephrol. 2017;37:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 598] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 8. | Matuszkiewicz-Rowinska J, Skórzewska K, Radowicki S, Niemczyk S, Sokalski A, Przedlacki J, Puka J, Switalski M, Wardyn K, Grochowski J, Ostrowski K. Endometrial morphology and pituitary-gonadal axis dysfunction in women of reproductive age undergoing chronic haemodialysis--a multicentre study. Nephrol Dial Transplant. 2004;19:2074-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Holley JL, Schmidt RJ. Sexual dysfunction in CKD. Am J Kidney Dis. 2010;56:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Dumanski SM, Ahmed SB. Fertility and reproductive care in chronic kidney disease. J Nephrol. 2019;32:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Hladunewich M, Schatell D. Intensive dialysis and pregnancy. Hemodial Int. 2016;20:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Leroy C, Rigot JM, Leroy M, Decanter C, Le Mapihan K, Parent AS, Le Guillou AC, Yakoub-Agha I, Dharancy S, Noel C, Vantyghem MC. Immunosuppressive drugs and fertility. Orphanet J Rare Dis. 2015;10:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Lambertini M, Boni L, Michelotti A, Gamucci T, Scotto T, Gori S, Giordano M, Garrone O, Levaggi A, Poggio F, Giraudi S, Bighin C, Vecchio C, Sertoli MR, Pronzato P, Del Mastro L; GIM Study Group. Ovarian Suppression With Triptorelin During Adjuvant Breast Cancer Chemotherapy and Long-term Ovarian Function, Pregnancies, and Disease-Free Survival: A Randomized Clinical Trial. JAMA. 2015;314:2632-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Pietrzak B, Wielgos M, Kaminski P, Jabiry-Zieniewicz Z, Bobrowska K. Menstrual cycle and sex hormone profile in kidney-transplanted women. Neuro Endocrinol Lett. 2006;27:198-202. [PubMed] |
| 15. | Chakhtoura Z, Meunier M, Caby J, Mercadal L, Arzouk N, Barrou B, Touraine P. Gynecologic follow up of 129 women on dialysis and after kidney transplantation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2015;187:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Akbari F, Alavi M, Esteghamati A, Mehrsai A, Djaladat H, Zohrevand R, Pourmand G. Effect of renal transplantation on sperm quality and sex hormone levels. BJU Int. 2003;92:281-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, Pivarnik J, Spillman T, DeVore GR, Phelan J. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 288] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 18. | Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Guyton AC, Hall JE. Textbook of Medical Physiology (11 ed). Philadelphia: Saunders. 2005; pp. 103g. ISBN 81-8147-920-3. [DOI] [Full Text] |
| 20. | Frederiksen MC. Physiologic changes in pregnancy and their effect on drug disposition. Semin Perinatol. 2001;25:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Rasmussen PE, Nielsen FR. Hydronephrosis during pregnancy: a literature survey. Eur J Obstet Gynecol Reprod Biol. 1988;27:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Hayes M, Larson L. "Chapter 220. Overview of Physiologic Changes of Pregnancy". Principles and Practice of Hospital Medicine. The McGraw-Hill. . [DOI] [Full Text] |
| 23. | Szczepanski J, Griffin A, Novotny S, Wallace K. Acute Kidney Injury in Pregnancies Complicated With Preeclampsia or HELLP Syndrome. Front Med (Lausanne). 2020;7:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Levidiotis V, Chang S, McDonald S. Pregnancy and maternal outcomes among kidney transplant recipients. J Am Soc Nephrol. 2009;20:2433-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Rahamimov R, Ben-Haroush A, Wittenberg C, Mor E, Lustig S, Gafter U, Hod M, Bar J. Pregnancy in renal transplant recipients: long-term effect on patient and graft survival. A single-center experience. Transplantation. 2006;81:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Shah S, Venkatesan RL, Gupta A, Sanghavi MK, Welge J, Johansen R, Kean EB, Kaur T, Grant TJ, Verma P. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol. 2019;20:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 27. | Pippias M, Skinner L, Noordzij M, Reisaeter AV, Abramowicz D, Stel VS, Jager KJ. Pregnancy after living kidney donation, a systematic review of the available evidence, and a review of the current guidance. Am J Transplant. 2022;22:2360-2380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant. 2009;9:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Deshpande NA, James NT, Kucirka LM, Boyarsky BJ, Garonzik-Wang JM, Montgomery RA, Segev DL. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11:2388-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 30. | Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK Transplant pregnancy registry. Transplantation. 2007;83:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Bramham K, Nelson-Piercy C, Gao H, Pierce M, Bush N, Spark P, Brocklehurst P, Kurinczuk JJ, Knight M. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol. 2013;8:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | TOXNET Toxicology Data Network. U.S. National Library of Medicine. Available from: www.toxnet.nlm.nih.gov. |
| 33. | Skrzypek J, Krause W. Azoospermia in a renal transplant recipient during sirolimus (rapamycin) treatment. Andrologia. 2007;39:198-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Zuber J, Anglicheau D, Elie C, Bererhi L, Timsit MO, Mamzer-Bruneel MF, Ciroldi M, Martinez F, Snanoudj R, Hiesse C, Kreis H, Eustache F, Laborde K, Thervet E, Legendre C. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant. 2008;8:1471-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009:S1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1081] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 37. | López LF, Martínez CJ, Castañeda DA, Hernández AC, Pérez HC, Lozano E. Pregnancy and kidney transplantation, triple hazard? Transplant Proc. 2014;46:3027-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Perez-Aytes A, Marin-Reina P, Boso V, Ledo A, Carey JC, Vento M. Mycophenolate mofetil embryopathy: A newly recognized teratogenic syndrome. Eur J Med Genet. 2017;60:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Natekar A, Pupco A, Bozzo P, Koren G. Safety of azathioprine use during pregnancy. Can Fam Physician. 2011;57:1401-1402. [PubMed] |
| 40. | Nevers W, Pupco A, Koren G, Bozzo P. Safety of tacrolimus in pregnancy. Can Fam Physician. 2014;60:905-906. [PubMed] |
| 41. | Durst JK, Rampersad RM. Pregnancy in Women With Solid-Organ Transplants: A Review. Obstet Gynecol Surv. 2015;70:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, da Silva J, Nelson-Piercy C, Cetin I, Costedoat-Chalumeau N, Dolhain R, Förger F, Khamashta M, Ruiz-Irastorza G, Zink A, Vencovsky J, Cutolo M, Caeyers N, Zumbühl C, Østensen M. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 742] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 43. | Sulanc E, Lane JT, Puumala SE, Groggel GC, Wrenshall LE, Stevens RB. New-onset diabetes after kidney transplantation: an application of 2003 International Guidelines. Transplantation. 2005;80:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N; DIRECT (Diabetes Incidence after Renal Transplantation: Neoral C Monitoring Versus Tacrolimus) Investigators. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 465] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 45. | Gong X, Li J, Yan J, Dai R, Liu L, Chen P, Chen X. Pregnancy outcomes in female patients exposed to cyclosporin-based versus tacrolimus-based immunosuppressive regimens after liver/kidney transplantation: A systematic review and meta-analysis. J Clin Pharm Ther. 2021;46:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Boulay H, Mazaud-Guittot S, Supervielle J, Chemouny JM, Dardier V, Lacroix A, Dion L, Vigneau C. Maternal, foetal and child consequences of immunosuppressive drugs during pregnancy in women with organ transplant: a review. Clin Kidney J. 2021;14:1871-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Framarino dei Malatesta M, Corona LE, De Luca L, Rocca B, Manzia TM, Orlando G, Tisone G, Iaria G. Successful pregnancy in a living-related kidney transplant recipient who received sirolimus throughout the whole gestation. Transplantation. 2011;91:e69-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Combs J, Kagan A, Boelkins M, Coscia L, Moritz M, Hofmann RM. Belatacept during pregnancy in renal transplant recipients: Two case reports. Am J Transplant. 2018;18:2079-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | McKay DB, Josephson MA, Armenti VT, August P, Coscia LA, Davis CL, Davison JM, Easterling T, Friedman JE, Hou S, Karlix J, Lake KD, Lindheimer M, Matas AJ, Moritz MJ, Riely CA, Ross LF, Scott JR, Wagoner LE, Wrenshall L, Adams PL, Bumgardner GL, Fine RN, Goral S, Krams SM, Martinez OM, Tolkoff-Rubin N, Pavlakis M, Scantlebury V; Women's Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant. 2005;5:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 312] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 50. | Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, Watt-Coote I, Khalil A, Wiesender C, Seed PT, Chappell LC. Labetalol Versus Nifedipine as Antihypertensive Treatment for Chronic Hypertension in Pregnancy: A Randomized Controlled Trial. Hypertension. 2017;70:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84:1906-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 52. | Magee LA; CHIPS Study Group, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Gafni A, Gruslin A, Helewa M, Hutton E, Koren G, Lee SK, Logan AG, Ganzevoort JW, Welch R, Thornton JG, Moutquin JM. Do labetalol and methyldopa have different effects on pregnancy outcome? BJOG. 2016;123:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237-e260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 1716] [Article Influence: 286.0] [Reference Citation Analysis (0)] |
| 54. | Tantisattamo E, Molnar MZ, Ho BT, Reddy UG, Dafoe DC, Ichii H, Ferrey AJ, Hanna RM, Kalantar-Zadeh K, Amin A. Approach and Management of Hypertension After Kidney Transplantation. Front Med (Lausanne). 2020;7:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 55. | Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 635] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 56. | Parish RC, Miller LJ. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf. 1992;7:14-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Drugs for hypertension. Med Lett Drugs Ther. 2020;62:73-80. [PubMed] |
| 58. | Shah S, Verma P. Overview of Pregnancy in Renal Transplant Patients. Int J Nephrol. 2016;2016:4539342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 59. | EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant. 2002;17 Suppl 4:50-55. [PubMed] |
| 60. | Yefet E, Schwartz N, Chazan B, Salim R, Romano S, Nachum Z. The safety of quinolones and fluoroquinolones in pregnancy: a meta-analysis. BJOG. 2018;125:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients--an analysis of USRDS data. Am J Transplant. 2007;7:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Leruez-Ville M, Foulon I, Pass R, Ville Y. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol. 2020;223:330-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 63. | Ponticelli C, Zaina B, Moroni G. Planned Pregnancy in Kidney Transplantation. A Calculated Risk. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Wulf MW, van Crevel R, Portier R, Ter Meulen CG, Melchers WJ, van der Ven A, Galama JM. Toxoplasmosis after renal transplantation: implications of a missed diagnosis. J Clin Microbiol. 2005;43:3544-3547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Kim HW, Seok HJ, Kim TH, Han DJ, Yang WS, Park SK. The experience of pregnancy after renal transplantation: pregnancies even within postoperative 1 year may be tolerable. Transplantation. 2008;85:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Coscia LA, Constantinescu S, Moritz MJ, Frank AM, Ramirez CB, Maley WR, Doria C, McGrory CH, Armenti VT. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2010;65-85. [PubMed] |
| 67. | Keitel E, Bruno RM, Duarte M, Santos AF, Bittar AE, Bianco PD, Goldani JC, Garcia VD. Pregnancy outcome after renal transplantation. Transplant Proc. 2004;36:870-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Schwarz A, Schmitt R, Einecke G, Keller F, Bode U, Haller H, Guenter HH. Graft function and pregnancy outcomes after kidney transplantation. BMC Nephrol. 2022;23:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 69. | Dimou S, Georgiou X, Sarantidi E, Diallinas G, Anagnostopoulos AK. On the Evidence Supporting That AN11127 Encodes an Aspergillus Nidulans Sec12 Orthologous Protein. Reply to Bravo-Plaza et al. Comment on "Dimou et al. Profile of Membrane Cargo Trafficking Proteins and Transporters Expressed under N Source Derepressing Conditions in Aspergillus nidulans. J. Fungi 2021, 7, 560". J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 70. | Karkar A. Pregnancy and contraceptive issues in renal transplant recipients. Saudi J Kidney Dis Transpl. 2008;19:165-173. [PubMed] |
| 71. | Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation. 2013;95:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Peterson HB, Curtis KM. Clinical practice. Long-acting methods of contraception. N Engl J Med. 2005;353:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Ostrowski KA, Holt SK, Haynes B, Davies BJ, Fuchs EF, Walsh TJ. Evaluation of Vasectomy Trends in the United States. Urology. 2018;118:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 74. | United Nations Department of Economic and Social Affairs, Population Division. Contraceptive Use by Method 2019: Data Booklet 2019. [DOI] [Full Text] |
| 75. | Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Pietrzak B, Kaminski P, Wielgos M, Bobrowska K, Durlik M. Combined oral contraception in women after renal transplantation. Neuro Endocrinol Lett. 2006;27:679-682. [PubMed] |
| 77. | van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol. 2010;30:2297-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Guida M, Visconti F, Cibarelli F, Granozio G, Troisi J, Martini E, Nappi R. Counseling and management of patients requesting subcutaneous contraceptive implants: proposal for a decisional algorithm. Gynecol Endocrinol. 2014;30:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Burkman RT. Intrauterine devices. Curr Opin Obstet Gynecol. 1991;3:482-485. [PubMed] |
| 80. | Pandit SN, Chauhan AR, Anagani M, Reddy S, Birla A, Ray SK. Multicenter Study of Contraceptive Vaginal Ring (NuvaRing®) in Normal Daily Practice in Indian Women. J Obstet Gynaecol India. 2014;64:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Mclure Z. Failure rates of contraceptive methods. Fam Plann Inf Serv. 1981;1:59-61. [PubMed] |
| 82. | Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60. [PubMed] |
| 83. | Thiagarajan KM, Arakali SR, Mealey KJ, Cardonick EH, Gaughan WJ, Davison JM, Moritz MJ, Armenti VT. Safety considerations: breastfeeding after transplant. Prog Transplant. 2013;23:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Gardiner SJ, Gearry RB, Roberts RL, Zhang M, Barclay ML, Begg EJ. Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br J Clin Pharmacol. 2006;62:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Moretti ME, Verjee Z, Ito S, Koren G. Breast-feeding during maternal use of azathioprine. Ann Pharmacother. 2006;40:2269-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Sau A, Clarke S, Bass J, Kaiser A, Marinaki A, Nelson-Piercy C. Azathioprine and breastfeeding: is it safe? BJOG. 2007;114:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 87. | Christensen LA, Dahlerup JF, Nielsen MJ, Fallingborg JF, Schmiegelow K. Azathioprine treatment during lactation. Aliment Pharmacol Ther. 2008;28:1209-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Angelberger S, Reinisch W, Messerschmidt A, Miehsler W, Novacek G, Vogelsang H, Dejaco C. Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J Crohns Colitis. 2011;5:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Lewis GJ, Lamont CA, Lee HA, Slapak M. Successful pregnancy in a renal transplant recipient taking cyclosporin A. Br Med J (Clin Res Ed). 1983;286:603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Ziegenhagen DJ, Crombach G, Dieckmann M, Zehnter E, Wienand P, Baldamus CA. [Pregnancy during cyclosporin medication following a kidney transplant]. Dtsch Med Wochenschr. 1988;113:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 91. | Behrens O, Kohlhaw K, Günter H, Wonigeit K, Niesert S. [Detection of cyclosporin A in breast milk--is breast feeding contraindicated? Geburtshilfe Frauenheilkd. 1989;49:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Ostensen M. Treatment with immunosuppressive and disease modifying drugs during pregnancy and lactation. Am J Reprod Immunol. 1992;28:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Thiru Y, Bateman DN, Coulthard MG. Successful breast feeding while mother was taking cyclosporin. BMJ. 1997;315:463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Munoz-Flores-Thiagarajan KD, Easterling T, Davis C, Bond EF. Breast-feeding by a cyclosporine-treated mother. Obstet Gynecol. 2001;97:816-818. [PubMed] |
| 95. | Osadchy A, Koren G. Cyclosporine and lactation: when the mother is willing to breastfeed. Ther Drug Monit. 2011;33:147-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Morton A. Cyclosporine and lactation. Nephrology (Carlton). 2011;16:249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | French AE, Soldin SJ, Soldin OP, Koren G. Milk transfer and neonatal safety of tacrolimus. Ann Pharmacother. 2003;37:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Gardiner SJ, Begg EJ. Breastfeeding during tacrolimus therapy. Obstet Gynecol. 2006;107:453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Gouraud A, Bernard N, Millaret A, Bruel M, Paret N, Descotes J, Vial T. Follow-up of tacrolimus breastfed babies. Transplantation. 2012;94:e38-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Zheng S, Easterling TR, Hays K, Umans JG, Miodovnik M, Clark S, Calamia JC, Thummel KE, Shen DD, Davis CL, Hebert MF. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol. 2013;76:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol. 2013;8:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol. 2014;28:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Favi E, Italy; Jovandaric MZ, Serbia; Tlili G, Tunisia; Wishahi M, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH