Published online Dec 26, 2015. doi: 10.5662/wjm.v5.i4.230
Peer-review started: May 11, 2015
First decision: July 6, 2015
Revised: November 10, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: December 26, 2015
Processing time: 219 Days and 21.9 Hours
AIM: To study immunogenicity of outer membrane protein F (OprF) fused with B subunit of LT (LTB), against Pseudomonas aeruginosa (P. aeruginosa).
METHODS: The OprF, a major surface exposed outer membrane protein that is antigenically conserved in various strains of P. aeruginosa, is a promising immunogen against P. aeruginosa. In the present study recombinant OprF and OprF-LTB fusion gene was cloned, expressed and purified. BALB/c mice and rabbits were immunized using recombinant OprF and OprF-LTB and challenged at the burn site with P. aeruginosa lethal dose of 104 CFU. The protective efficacy of rabbit anti OprF IgG against P. aeruginosa burn infection was investigated by passive immunization.
RESULTS: It has been well established that the LTB is a powerful immunomodulator with strong adjuvant activity. LTB as a bacterial adjuvant enhanced immunogenicity of OprF and anti OprF IgG titer in serum was increased. Experimental findings showed significantly higher average survival rate in burned mice immunized with OprF-LTB than immunized with OprF or the control group. Rabbits anti OprF IgG brought about 75% survival of mice following challenge with P. aeruginosa. Post challenge hepatic and splenic tissues of mice group immunized with OprF-LTB had significantly lower bacterial load than those immunized with OprF or the control groups.
CONCLUSION: These results demonstrate that LTB-fused OprF might be a potential candidate protein for a prophylactic measure against P. aeruginosa in burn infection.
Core tip:Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic pathogen that infects hospitalized, burned and immunosuppressed patients. Vaccination of high-risk groups may reduce the incidence and spread of infection. In this study outer membrane protein F (OprF) and fusion genes containing OprF and B subunit of LT were cloned and expressed. The proteins were administered to the experimental mice challenged at the burn site with lethal dose of P. aeruginosa. Significant protection was noted in immunized animals.
-
Citation: Farsani HH, Rasooli I, Gargari SLM, Nazarian S, Astaneh SDA. Recombinant outer membrane protein F-B subunit of LT protein as a prophylactic measure against
Pseudomonas aeruginosa burn infection in mice. World J Methodol 2015; 5(4): 230-237 - URL: https://www.wjgnet.com/2222-0682/full/v5/i4/230.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i4.230
Pseudomonas aeruginosa (P. aeruginosa) is a major cause of morbidity and mortality in hospital acquired infections. The pathogen invades the host tissue of mainly immunocompromised patients. This pathogen is one of the most common organisms isolated in nosocomial pneumonia, urinary tract infection, surgical site infection, burn wounds, the cornea and the lower respiratory tract and those with the cystic fibrosis (CF)[1]. P. aeruginosa infecting strains are initially nonmucoid. The organism converts to mucoid and alginate producing followed by development of biofilm that enhance its antibiotic resistance[2]. The eradication of Pseudomonas frequently proves difficult due to antibiotic resistance and the ability to form a biofilm in case of chronic infection[3]. Antibiotic resistance and biofilm formation on mucosal surfaces further complicates the therapy. Hospital-derived P. aeruginosa strains can become colonized in burn patients that survive the initial burn trauma, are not easily eradicated with antibiotic therapy[4,5]. The initial clinical trials on P. aeruginosa vaccines established vaccine safety, however the limited effectiveness in preventing subsequent infection clearly evidenced the need for reevaluating correlates of vaccine efficacy[6]. Although a significant humoral response was elicited by lipopolysaccharide (LPS) vaccination, it was not able to prevent subsequent infection brought about by P. aeruginosa[7,8]. However, both LPS vaccines did not meet the approval for routine clinical use because of their toxicity associated with their lipid A fraction[9]. This was due in large part to the inability of the vaccine to provide protection against a broad range of P. aeruginosa serotypes[10]. Outer membrane proteins (OMPs), LPS and flagellin have been evaluated as vaccine candidates[11,12]. Conserved region from amino acids of flagellin and two OPMs, i.e., outer membrane protein F (OprF) and OprI in clinical isolates from CF patients have been studied for their protective immunogenicity[6,13]. This sequence was used to create a vaccine preparation that was successfully tested for the ability to protect humans against P. aeruginosa infection[14]. Three fold increase in antigen specific IgG was reported following immunization of CF patients with an OprF-OprI fusion protein[12]. Very high IgG titers were induced in adult mice against OprF, OprI and flagellin following immunization with OprF epitope 8 (amino acid residues 311-341)-OprI-flagellins[15]. As an adjuvant the recombinant flagellin potentially affected the vaccine efficacy[16]. Despite the attractiveness of mucosal vaccination, mucosally administered antigens are frequently not immunogenic. The P. aeruginosa OprF protein, a major outer membrane protein that is surface exposed and antigenically conserved in various strains of P. aeruginosa, is a promising antigen for a vaccine[17]. It is assumed that the most active epitopes of OprF are located in the C-terminal region, because this part of the protein is located on the surface of the bacterial cell[18]. The pure recombinant and synthetic antigens used in modern day vaccines are generally less immunogenic than older style live/attenuated and killed whole organism vaccines. One can improve the quality of vaccine production by incorporating immunomodulators or adjuvants with modified delivery vehicle[19]. It has been well established that the B subunit of LT (LTB) and cholera toxin are powerful immunomodulators with strong adjuvant activity[20,21]. The recombinant LTB is safely and commonly used as an adjuvant to stimulate antibody responses to co-administered protein antigens, and its GM1-binding function is essential for both immunogenicity and adjuvanticity[22]. The aim of this study was to produce a recombinant chimeric protein composed of the OprF fused to LTB in order to evaluate the capacity of this fusion protein to induce a specific immunity in mice burn model against P. aeruginosa.
Plasmid extraction and gel purification kits were from Bioneer (Daejeon, South Korea). The designed primers were synthesized by Bioneer. Nickel-nitrilotriacetic acid (Ni-NTA) agarose was procured from Qiagen (Valencia, United States). Restriction endonucleases were purchased from Cinnagen (Tehran, Iran). T4 DNA ligase (Fermentas, Vilnius, Lithuania), anti-polyhistidine antibodies and anti-mouse IgG conjugated with HRP (RAY Biotech), nitrocellulose membrane (PROTRAN), microtiter plates (Nunc, United States) were used. All other chemical reagents were from Merck (Darmstadt, Germany) or Sigma (Munich, Germany).
E. coli BL21 (DE3) (Invitrogen) and P. aeruginosa (PAO1) were grown in Luria Bertani (LB) medium incubated on a shaker at 37 °C and 150 rpm.
The oprF gene (GenBank accession No.: JX040481.1) was amplified from its genomic DNA by PCR using the OprF-F and OprF-R primers (Table 1). Forward primer was designed to contain a Hind III site and reverse primers carried an Xho I site. The OprF gene was amplified by PCR. Cyclic conditions were initiated at 95 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 58 °C for 1 min, 72 °C for 90 s, and a final extension at 72 °C for 5 min. The amplified fragments were analyzed on 1% agarose gel. The pET28a (+) vector and PCR products were double digested with Hind III and Xho I and were then purified using the Bioneer Gel extraction kit. The ligation of OprF into pET28a (+) was performed using T4 DNA ligase. A helix-forming peptide linkers EAAAK was introduced between OPRF and LTB proteins. For the gene fusion with OprF and LTB, DNA was amplified using the P. aeruginosa chromosome as a template and oligonucleotide pairs Link-EAAAK-F and OprF-R (Table 1) as primers for the LTB-EAAAK-OprF fusion. Forward primer was designed to contain a Hind III site and reverse primers carried an Xho I site. In order to construct LTB-OprF fusion Gene, the OprF gene with a linker was inserted in Hind III and Xho I sites of pET28a (+) vector containing LTB gene in EcoR I and Hind III sites[23]. The recombinant DNA plasmids, OprF- pET28a and LTB-OprF-pET28a were transformed into E. coli strain BL21(DE3). The expression host was grown for 12 h at 37 °C in LB agar containing 70 μg/mL kanamycin.
| Name | Sequence (5’-3’) | Restriction site |
| OprF-F | TTAA AAGCTT ATGAAACTGAAGAACACCTTAG | Hind III |
| OprF-R | TATA CTCGAG TTACTTGGCTTCRGCTTCT | Xho I |
| Linker-EAAAK-F | ATAT AAGCTT GAAGCTGCGGCAAAA ATGAAACTGAAGAAC | Hind III |
| Linker-EAAAK-R | ATAT CTCGAG TTACTTGGCTTCGGCTTCTACTTCGGCTTC | Xho I |
| LTB-F | ATAAGAATTCATGGCTCCGCAAAG | EcoR I |
| LTB-R | ATTAAAGCTTTTAGTTTTCCATCGAGATG | Hind III |
E. coli BL21 cells harboring the OprF-pET28a and LTB-OprF-pET28a constructs were grown at 37 °C under constant shaking at 200 rpm overnight in 10 mL of LB medium containing 70 mg/mL Kanamycin. The culture was then used to inoculate 200 mL of LB medium. 1 mmol/L isopropyl b-D-thiogalactoside (IPTG) was added at the optical density of 0.6 at 600 nm to induce expression. The cells were further incubated for 6 h at 37 °C followed by centrifugation at 10000 ×g for 10 min at 4 °C. The cell pellet resuspended in lysis buffer (100 mmol/L NaH2PO4, 10 mmol/L Tris-Cl, 8 M urea) was sonicated at 200 W at 1 min intervals for five times. The cell suspension was centrifuged at 8000 ×g for 30 min at 4 °C to separate the supernatant from cellular debris. Affinity chromatography was employed to purify the protein from the supernatant using Ni2+-NTA agarose under denaturation condition (Qiagen, CA). A stepwise dialysis was carried out to remove the denaturant (8 mol urea). The fractions were analyzed by SDS-PAGE. Bradford method was used for determination of protein concentration[24].
The purified recombinant OprF, OprF-LTB and bovine serum albumin were electrophoresed on a 12% SDS-PAGE gel to analyze the cross-reactivity. The proteins were blotted onto nitrocellulose membranes. The membrane strips were blocked with 5% nonfat dried milk and washed whit phosphate buffered saline (PBS) (137 mmol/L NaCl, 2.7 mmol/L KCl and 4.3 mmol/L Na2HPO4). The membrane was incubated with rabbit anti-6X His tag antibody (Abcam). This was followed by incubation in 1:10000 dilution of horseradish peroxidase-conjugated (HRP) goat anti-rabbit IgG antibody (Sigma). Detection was carried out using HRP staining solution.
The animal care protocol was approved by Shahed University. Four-six weeks old (16-22 g) BALB/c mice procured from the Razi Institute, Tehran, Iran were housed in clean standard animal care facility of Shahed University. The research was carried out in compliance with the Animal Welfare Act and regulations related to experiments involving animals.
Mice were divided into three groups of five mice each. Ten micrograms of the recombinant protein was injected subcutaneously (sc) per mouse on days 0, 15, 30 and 45. Equal amount of Freund’s complete adjuvant was used at the first dose and incomplete adjuvant in the subsequent doses. Negative control was a group of mice injected with 20 μL of PBS each time simultaneously with the test group.
Five micrograms of the recombinant proteins were coated to the surface of each well of 96-well microtiter plates and incubated overnight at 4 °C. Sera serial dilutions from 1:250 to 1:100000 were added to each well. HRP conjugated anti-mouse IgG (100 μL) diluted to 1:3000 was added to each well as secondary antibody. Orthophenylenediamine (OPD) was added and the reaction was stopped with H2SO4 (2 mol/L) in order to detect the immunoreaction. The plates were analyzed at OD492 by ELISA reader.
The mice burned model was carried out according to Stieritz and Holder[25] with slight modifications. Six-eight-week-old female BALB/c mice (22-25 g; Razi Vaccine and Serum Research Institute, Iran) were anaesthetised intraperitoneally using a mixture of ketamine hydrochloride (100 mg/mL; Alfasan, Woerden-Holand) and xylazine (20 mg/mL; Alfasan, Woerden-Holand) in distilled water. A uniform thermal injury of 120 °C was brought about by exposing the depilated area for 5 s to a round brass probe of 28 mm diameter heated to thermal equilibration with boiling tap water[26]. Sterile saline (2 mL i.p.) was administered to support fluid balance during recovery. Mice were supervised until full recovery. Control mice were shaved but no thermal injury was performed[26].
The mice were inoculated intraperitoneally (i.p.) with P. aeruginosa. Two hundred microliters of the bacterial suspensions at 3 × 104 to 3 × 109 CFU/mL concentrations were administered subeschar at the burn site to six groups of five BALB/c mice per group. Mortality rate was recorded for three consecutive post-challenge days. LD50 was defined as the volume (CFU/mL) of bacterial load that brought about death in half of the population size.
The microbial population was precipitated at 3000 ×g and was then suspended in 0.2 mL PBS. The immunized and control mice were challenged subeschar at the burn site with 104 CFU of P. aeruginosa (PAO1).
Ten immunized and non-immunized mice were sacrificed after 72 h and spleen and liver were removed under aseptic conditions. The surfaces of the samples were washed thoroughly with sterile PBS to remove non adherent bacteria. Tissues were homogenized, and incubated in 1 mL selenite cysteine broth and were subsequently plated on SS agar plates.
Antisera to the OprF and OprF-LTB proteins were raised in a New Zealand White male rabbits (Razi institute, Iran) by injecting 100 μg of OprF and OprF-LTB per animal. Lethal dose (104 CFU) of P. aeruginosa was mixed with 200 μL of immune rabbit serum (diluted to 1:800 in PBS) and was allowed to stand for 30 min at 37 °C. Groups of five mice were injected intraperitoneally with the lethal dose of P. aeruginosa to study the neutralization[27]. In order to verify that natural antibodies in rabbit serum do not offer any resistance to P. aeruginosa in vivo, groups of mice received mixture of lethal dose of P. aeruginosa and normal rabbit serum as control[28]. The animals were monitored for mortality for seven days.
The data derived from the experiments carried out in triplicate were expressed as mean ± SD. Student’s t test was used to calculate the P values in order to determine the significance of differences in the experimental groups. P values of < 0.05 were considered as significant.
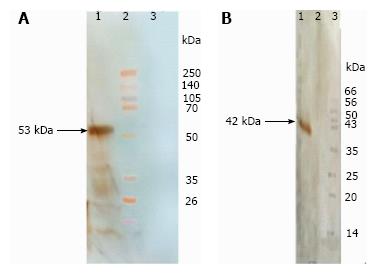
The OprF and Linker-OprF genes of P. aeruginosa (PAO1) were successfully amplified by PCR and the fragments of appropriate size and digestion patterns of amplified genes were observed on 1% agarose gel. The amplified OprF gene was cloned into pET28a (+) and amplified Linker-OprF gene was cloned in frame, at the 3’ end of the LTB gene carried by plasmid pET28a (+) and confirmed by DNA sequencing. The OprF and OprF-LTB were expressed in E. coli BL21 (DE3). The recombinant protein was verified in insoluble pellets by SDS-PAGE. The expression of recombinant proteins was confirmed by reaction with the anti-His-tag antibodies by Western Blotting (Figure 1). Purification of the proteins were carried out under denaturation condition and SDS-PAGE analysis revealed the presence of the approximately 42 kDa (OprF), 53 kDa (OprF-LTB) proteins in the eluted fraction.
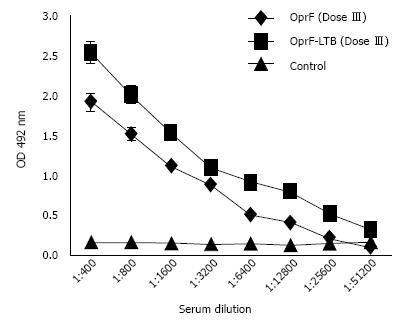
Animals remained healthy and showed no signs of abnormal behavior after vaccination. Following immunization, mice elicited significant IgG antibodies in sera samples compared to control mice (P < 0.05). The antibody titer increased after the second booster, whereas animals received adjuvant and PBS, as a control had no porin-specific antibodies in sera. The combined protein administration had no significant difference with those of the single protein injections (Figure 2).
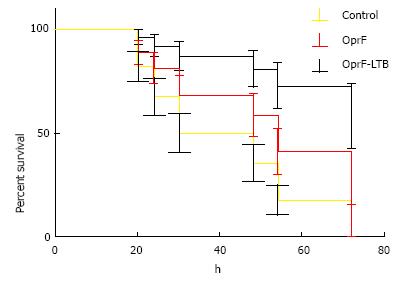
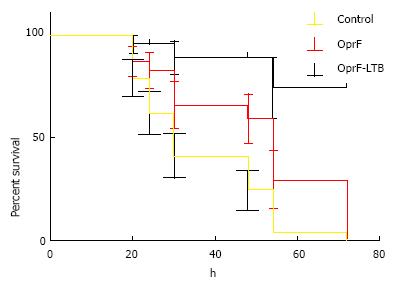
LD50 was determined as 1 × 103 CFU/mL per mouse via injection at the burn site. Percent immunized mice survived is summarized in Figure 3. Control mice died within 24 h of challenge. Mice immunized with OprF-LTB could survive longer than mice immunized with OprF. A significant (P < 0.01) survival was observed in mice group immunized with OprF-LTB. The analysis of variance showed significant differences (P < 0.01) among the treatments.
Mice immunized with OprF-LTB showed high levels of bacteria in outset and reduced gradually to (1.4 ± 0.33) × 102 and (5.2 ± 1.11) × 102 CFU per gram of spleen and liver respectively after nine days. Bacterial loads were detected per gram of spleen and liver of the mice group immunized with OprF (4.13 ± 1.06) × 102 and (6.1 ± 0.41) × 103 CFU respectively. Unimmunized mice exhibited bacterial load of (6.00 ± 1.00) × 106 and (2.00 ± 1.00) × 106 CFU per gram of spleen and liver respectively over the two-week sampling period.
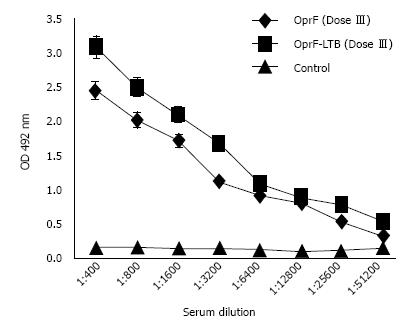
The antibody level raised against OprF and OprF-LTB increased in the vaccinated rabbits (Figure 4). Neutralization test was performed to determine if sera from immunized rabbits could protect naive mice against bacterial challenge. As shown in Figure 5, all experimental mice groups were significantly (P < 0.05) protected. All mice receiving normal rabbit serum succumbed.
P. aeruginosa still remains as a problem in the therapeutic management of nosocomial infections. Today, active and passive immunoprophylaxis against P. aeruginosa infections have been widely considered because antibiotic therapy has provided extensive inherent and acquired resistance. As there is a time limitation for burned patients to respond to infectious agents, passive immunization seems to be the best therapeutic pathway for the prevention and treatment of these patients. In this study OprF and fusion genes containing OprF and LTB were cloned and expressed. The results indicate that the LTB adjuvant may enhance the efficacy of the vaccine candidate. A principal effect of LTB interaction with mammalian cells is the stable cross-linking of GM1 at the cell surface, resulting in the uptake of co administered proteins[29], and enhancement of both humoral and cellular immune responses[30-32]. In order to confirm as to whether or not the OprF-LTB and Oprf were immunogenic in mice, serum IgG antibodies were investigated. The serum IgG titers of mice group immunized with OprF-LTB was significantly increased compared to the group immunized with OprF or control group at the 6th week (P < 0.05). These results indicate that immunogenicity of LTB in mice. Major structural proteins such as VP2 and VP4 of porcine parvovirus were expressed in Lactobacillus casei fused with LTB as a mucosal adjuvant[33,34]. IgG and sIgA levels from mice orally immunized with the fusion proteins were significantly higher than those from mice receiving VP2 or VP4 only without LTB. Our results showed an enhancement the protective efficacy against P. aeruginosa. Experimental findings showed significantly higher survival average rate of 75% in burned mice immunized with OprF-LTB than with OprF as well as the control group. Furthermore, the challenge strain isolated from the hepatic and splenic tissues of mice group immunized with OprF-LTB post challenge was significantly lower than those from the group immunized with OprF. However both immunized groups showed significant reduction of bacterial load in spleen and liver compared to the control group. A divided by eight hundred sera dilution from rabbits immunized with OprF and OprF-LTB protected 95% and 75% mice population when mixed with 10 × LD50 bacterial load. Antibodies alone can provide relative protective immunity against infection that may partly be related to efficiency of opsonization in deracination of infection[35]. OprF-LTB seems to be used as a vaccine candidate where Pseudomonas infections are potential threat in burn patients.
The results demonstrate that LTB-fused OprF might be a potential candidate as a protective immunogen against P. aeruginosa.
The authors wish to thank Shahed University for supporting this work.
Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic pathogen that infects hospitalized, burned and immunosuppressed patients. Vaccination of high-risk groups may reduce the incidence and spread of infection. The outer membrane protein F (OprF), a major surface exposed outer membrane protein that is antigenically conserved in various strains of P. aeruginosa, is a promising immunogen against P. aeruginosa. It has been well established that the B subunit of LT (LTB) is a powerful immunomodulator with strong adjuvant activity. In order to confirm as to whether or not the OprF-LTB and Oprf were immunogenic in mice, serum IgG antibodies were investigated.
A principal effect of LTB interaction with mammalian cells is the stable cross-linking of GM1 at the cell surface, resulting in the uptake of co administered proteins, and enhancement of both humoral and cellular immune responses.
The initial clinical trials on P. aeruginosa vaccines established vaccine safety, however the limited effectiveness in preventing subsequent infection clearly evidenced the need for reevaluating correlates of vaccine efficacy. Although a significant humoral response was elicited by lipopolysaccharide (LPS) vaccination, it was not able to prevent subsequent infection brought about by P. aeruginosa. However, both LPS vaccines did not meet the approval for routine clinical use because of their toxicity associated with their lipid A fraction. Outer membrane proteins, LPS and flagellin have been evaluated as vaccine candidates.
The results demonstrate that LTB-fused OprF might be a potential candidate as a protective immunogen against P. aeruginosa. The oprF gene may be cloned into plasmid vector, and the plasmid vaccines could be delivered to mice to find its immunogenicity as a DNA vaccine.
This is an interesting study regarding the use of recombinant OprF-LTB protein to prevent P. aeruginosa burn infection in mice. The subject is clinically relevant, and the findings of this study are significant.
| 1. | Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 582] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 2. | Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 3. | Elmanama AA, Laham NA, Tayh GA. Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns. 2013;39:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Agnihotri N, Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms--a five-year study. Burns. 2004;30:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. Changes of microbial flora and wound colonization in burned patients. Burns. 2004;30:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Weimer ET, Lu H, Kock ND, Wozniak DJ, Mizel SB. A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa. Infect Immun. 2009;77:2356-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Zuercher AW, Horn MP, Que JU, Ruedeberg A, Schoeni MH, Schaad UB, Marcus P, Lang AB. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol Med Microbiol. 2006;47:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Lang AB, Rüdeberg A, Schöni MH, Que JU, Fürer E, Schaad UB. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr Infect Dis J. 2004;23:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Baumann U, Mansouri E, von Specht BU. Recombinant OprF-OprI as a vaccine against Pseudomonas aeruginosa infections. Vaccine. 2004;22:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | DiGiandomenico A, Rao J, Harcher K, Zaidi TS, Gardner J, Neely AN, Pier GB, Goldberg JB. Intranasal immunization with heterologously expressed polysaccharide protects against multiple Pseudomonas aeruginosa infections. Proc Natl Acad Sci USA. 2007;104:4624-4629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Döring G, Meisner C, Stern M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci USA. 2007;104:11020-11025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Sorichter S, Baumann U, Baumgart A, Walterspacher S, von Specht BU. Immune responses in the airways by nasal vaccination with systemic boosting against Pseudomonas aeruginosa in chronic lung disease. Vaccine. 2009;27:2755-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Arnold H, Bumann D, Felies M, Gewecke B, Sörensen M, Gessner JE, Freihorst J, von Specht BU, Baumann U. Enhanced immunogenicity in the murine airway mucosa with an attenuated Salmonella live vaccine expressing OprF-OprI from Pseudomonas aeruginosa. Infect Immun. 2004;72:6546-6553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mansouri E, Blome-Eberwein S, Gabelsberger J, Germann G, von Specht BU. Clinical study to assess the immunogenicity and safety of a recombinant Pseudomonas aeruginosa OprF-OprI vaccine in burn patients. FEMS Immunol Med Microbiol. 2003;37:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Weimer ET, Ervin SE, Wozniak DJ, Mizel SB. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine. 2009;27:6762-6769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, Hantgan RR, Thomas MJ, Wood J, Bell B. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol. 2009;16:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Stanislavsky ES, Lam JS. Pseudomonas aeruginosa antigens as potential vaccines. FEMS Microbiol Rev. 1997;21:243-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Hedstrom RC, Pavlovskis OR, Galloway DR. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984;43:49-53. [PubMed] |
| 19. | Mohan T, Verma P, Rao DN. Novel adjuvants & amp; delivery vehicles for vaccines development: a road ahead. Indian J Med Res. 2013;138:779-795. [PubMed] |
| 20. | Hur J, Lee JH. Enhancement of immune responses by an attenuated Salmonella enterica serovar Typhimurium strain secreting an Escherichia coli heat-labile enterotoxin B subunit protein as an adjuvant for a live Salmonella vaccine candidate. Clin Vaccine Immunol. 2011;18:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol. 2011;18:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Nazarian S, Gargari SL, Rasooli I, Hasannia S, Pirooznia N. A PLGA-encapsulated chimeric protein protects against adherence and toxicity of enterotoxigenic Escherichia coli. Microbiol Res. 2014;169:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 159186] [Article Influence: 3183.7] [Reference Citation Analysis (1)] |
| 25. | Neely AN, Holder IA, Warden GD. Then and now: studies using a burned mouse model reflect trends in burn research over the past 25 years. Burns. 1999;25:603-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Nichols DP, Caceres S, Caverly L, Fratelli C, Kim SH, Malcolm K, Poch KR, Saavedra M, Solomon G, Taylor-Cousar J. Effects of azithromycin in Pseudomonas aeruginosa burn wound infection. J Surg Res. 2013;183:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Stiles BG, Sexton FW. Immunoreactivity, epitope mapping and protection studies with anti-conotoxin GI sera and various conotoxins. Toxicon. 1992;30:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Bentancor LV, O’Malley JM, Bozkurt-Guzel C, Pier GB, Maira-Litrán T. Poly-N-acetyl-β-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect Immun. 2012;80:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Fingerut E, Gutter B, Goldway M, Eliahoo D, Pitcovski J. B subunit of E. coli enterotoxin as adjuvant and carrier in oral and skin vaccination. Vet Immunol Immunopathol. 2006;112:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Moyle PM, McGeary RP, Blanchfield JT, Toth I. Mucosal immunisation: adjuvants and delivery systems. Curr Drug Deliv. 2004;1:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Williams NA. Immune modulation by the cholera-like enterotoxin B-subunits: from adjuvant to immunotherapeutic. Int J Med Microbiol. 2000;290:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Liu D, Wang X, Ge J, Liu S, Li Y. Comparison of the immune responses induced by oral immunization of mice with Lactobacillus casei-expressing porcine parvovirus VP2 and VP2 fused to Escherichia coli heat-labile enterotoxin B subunit protein. Comp Immunol Microbiol Infect Dis. 2011;34:73-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Qiao X, Li G, Wang X, Li X, Liu M, Li Y. Recombinant porcine rotavirus VP4 and VP4-LTB expressed in Lactobacillus casei induced mucosal and systemic antibody responses in mice. BMC Microbiol. 2009;9:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol. 2003;170:3621-3630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
P- Reviewer: Chen GS, Rogers JV S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
Animal care and use statement: The animals were housed in clean standard animal care facility of Shahed University. The research was carried out in compliance with the Animal Welfare Act and regulations related to experiments involving animals. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/