Published online Sep 26, 2015. doi: 10.5662/wjm.v5.i3.122
Peer-review started: April 18, 2015
First decision: June 3, 2015
Revised: June 12, 2015
Accepted: August 13, 2015
Article in press: August 14, 2015
Published online: September 26, 2015
Processing time: 156 Days and 3.3 Hours
There are multiple biases in using observational studies to examine treatment effects such as those from prevalent drug users, immortal time and drug indications. We used renin angiotensin system (RAS) inhibitors and statins as reference drugs with proven efficacies in randomized clinical trials (RCTs) and examined their effectiveness in the prospective Hong Kong Diabetes Registry using adjustment methods proposed in the literature. Using time-dependent exposures to drug treatments yielded greatly inflated hazard ratios (HR) regarding the treatment effects of these drugs for cardiovascular disease (CVD) in type 2 diabetes. These errors were probably due to changing indications to use these drugs during follow up periods, especially at the time of drug commencement making time-dependent analysis extremely problematic. Using time-fixed analysis with exclusion of immortal time and adjustment for confounders at baseline and/or during follow-up periods, the HR of RAS inhibitors for CVD was comparable to that in RCT. The result supported the use of the Registry for performing pharmacoepidemiological analysis which revealed an attenuated low low-density lipoprotein cholesterol related cancer risk with RAS inhibitors. On the other hand, time-fixed analysis with including immortal time and adjustment for confounders at baseline and/or during follow-up periods, the HR of statins for CVD was similar to that in the RCT. Our results highlight the complexity and difficulty in removing these biases. We call for validations of the methods to cope with immortal time and drug use indications before applying them to particular research questions, so to avoid making erroneous conclusions.
Core tip: There are multiple biases in using observational studies to examine treatment effects. These biases include those due to prevalent drug users, immortal time and drug indications that must be taken into consideration. In this regard, we used drugs with proven effects in randomized controlled trials and applied those proposed methods by other groups to estimate their effects in a prospective cohort of patients with type 2 diabetes. Our results highlighted the importance of validating adjustment methods for immortal time and drug use indications before applying them to addressing research questions, so to avoid making erroneous conclusions.
- Citation: Yang XL, Huo XX, Chan JC. Methodological challenges to control for immortal time bias in addressing drug effects in type 2 diabetes. World J Methodol 2015; 5(3): 122-126
- URL: https://www.wjgnet.com/2222-0682/full/v5/i3/122.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i3.122
In pharmacoepidemiological analysis, there are multiple biases in using observational studies to examine treatment effects. These biases may be due to prevalent drug users, immortal time and drug indications[1]. Given these biases, the question to ask is, do we have a way to judge whether the quality of the database of an observational study or the method of analysis is free from these three major biases?
The prevalent user bias is easy to discern and can be readily excluded during data analysis. In type 2 diabetes (T2D), biases due to drug indication depend upon whether the subphenotypes associated with the drug usage (indications) may have selected a patient subgroup inherently at high risk for a clinical outcome, e.g., cardiovascular disease (CVD) or cancer. Immortal time bias is not difficult to detect because we can suspect such a bias as long as immortal time is reported, i.e., non-drug exposure periods have been classified as exposure periods. Several methods have been proposed to control for immortal bias but it is uncertain whether these methods can adequately remove these biases[2-4]. Our recent work has shed light on these important issues[5,6].
Hyperglycemia is the reason why a person is prescribed an antidiabetic regimen which can include various combinations of oral antidiabetic drugs (OAD), insulin and other injectables, such as glucagon like peptide 1[7]. Besides, many factors such as disease severity, predominant disease mechanisms (e.g., insulin deficiency versus insulin resistance), prescribing habits, formulary restrictions, willingness to pay for or accept treatment, referral and volunteer biases can also affect the choices of drug combinations as first, second or third lines of treatments during the clinical course. Of note, some of these factors which can influence drug choices may not be captured in observational studies. Hence, randomized clinical trials (RCT) remain to be the gold standard by evenly distributing these unmeasured confounders in different experimental and control groups to reduce these biases.
Several studies including ours have reported an association of hyperglycemia with cancer in diabetes[8,9]. Our group also reported a linear association between glycated hemoglobin (HbA1c) and all-site cancer in T2D with 1% increase in HbA1c associated with 18% increase in the risk of cancer[10]. These observations were supported by a meta-analysis of RCT data where 0.5% reduction in HbA1c was associated with a non-significant hazard ratio (HR) of 0.91 for cancer risk in T2D[11]. In a recent large randomized trial[12], treatment with saxagliptin, a dipeptidyl peptidase 4, was associated with 0.3% reduction in HbA1c accompanied by a 50% reduction in the risk of pancreatic cancer, albeit short of significance[12]. Although the underlying mechanism linking hyperglycemia and cancer remains to be elucidated, the overarching premise is that users of OADs and insulin are high risk subjects for cancer. Unless these drug indications are captured and removed, these drug users are likely to be found to increase cancer risk, which might be erroneously attributed to drug effects.
In epidemiological analysis, propensity score is often used to control for indications of drug use[13]. The robustness of these scores in removing selection bias is indicated by the area under receiver’s operating characteristics curve (AUC) where values ≥ 0.90, ≥ 0.80 to < 0.90, and ≥ 0.70 to < 0.80 indicate excellent, good and fair performance, respectively[1]. Apart from including propensity score, multivariable analysis with inclusion of subphenotypes associated with a clinical event, e.g., cancer, can also attenuate bias due to drug indications[1]. In prospective cohort analysis of the Hong Kong Diabetes Registry, we had identified a group of subphenotypes for cancer risk in T2D[14], in addition to age and hyperglycemia. These included (1) body mass index ≥ 27.6 kg/m2 and < 24 kg/m2[15]; (2) low-density lipoprotein cholesterol (LDL-C) ≥ 3.8 mmol/L[16]; (3) co-presence of LDL-C < 2.8 mmol/L and albuminuria[17] which was further enhanced in the presence of increased high density lipoprotein cholesterol (HDL-C) ≥ 1.0 mmol/L[18]; (4) co-presence of LDL-C < 2.8 mmol/L and triglyceride < 1.7 mmol/L[18]; and (5) HDL-C < 1.0 mmol/L[19].
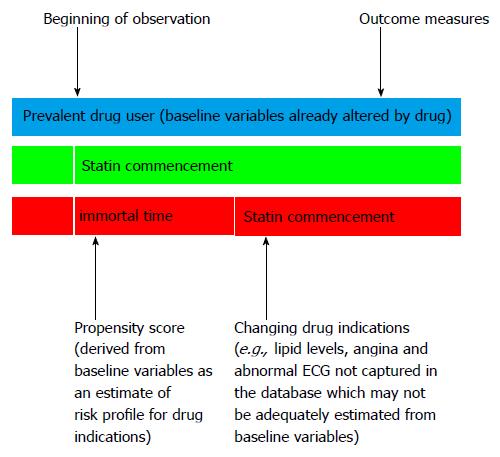
Immortal time refers to a period in cohort studies when non-exposure to a drug treatment from the baseline to the time of initiation of the drug treatment in the “drug exposure group” is misclassified as exposure to the drug treatment[1]. This misclassification may lead to a deflated HR of the treatment for the endpoint due to addition of the non-drug exposure period into the drug exposure period. This can lead to a false conclusion that the drug reduces the risk of the event of interest. Several researchers recommended the use of time-varying or time-dependent drug exposure Cox proportional hazard regression to cope with immortal time bias[3,4]. The use of this method assumes that exposure to the drug treatment or the drug commencement is at random[20]. However, this is rarely the case in real world practice since patients usually start on a drug treatment for a new or changed indication which may not be systematically captured in analysis of cohort study data. If these confounders are not available, the use of a time-dependent model may lead to an increased HR of the treatment for the endpoint (Figure 1).
In order to test the validity of these methods[3,4], we used a referent drug with proven benefits [e.g., statin or renin angiotensin system (RAS) inhibitors] and applied the methods to the Hong Kong Diabetes Registry[5,6] to find out if the estimated effect size fell within the bounds of that reported in RCTs with regard to their associations with CVD. We tested various combinations of exclusion/inclusion of immortal time and adjustment for drug indications at baseline or at the end of immortal time when drug was commenced Consistently, time-dependent drug-exposure Cox models severely inflated the HR of these two drugs for CVD risk[5,6], despite their proven cardioprotective effects in RCTs. In the statin-CVD validation[6], compared to a HR of 0.63 (95%CI: 0.48 to 0.83) in a RCT[21], exclusion of immortal time and adjustment for estimated covariables at the end of the immortal time when statins were commenced, resulted in a 52.3% inflation in the HR of statins for CVD (0.96, 0.72 to 1.27), which was above the higher bound of the 95%CI. On the other hand, inclusion of immortal time, i.e., ignoring immortal time bias, and adjustment for covariables at baseline generated the least inflated HR of 0.64 (0.48 to 0.84) which was within the HR estimates in clinical trial and inflated by 1.59% compared to the absolute HR of 0.63.
In the RAS inhibitors-CVD validation[5], exclusion of immortal time and adjustment for covariables when RAS inhibitors was commenced resulted in a HR of 0.89 (0.68 to 1.17) which was within the estimates of 0.92 (0.84-1.0) reported in RCTs with 3% deflated risk compared to the absolute hazard. By contrast, inclusion of immortal time and adjustment for covariables at baseline yielded a HR of 0.66 (0.51 to 0.86) which was outside the estimates with a 28.3% deflation rate (or inflation rate: -28.3%) compared to the absolute HR.
In most observational or administrative databases, the events preceding the commencement of drugs like statins (e.g., high LDL-C, angina, abnormal imaging) were often not available in the dataset. In a time-dependent model which includes immortal time, inadequate adjustment for indications at the time of drug commencement can lead to overinflated hazards. In this situation, a non-time-dependent analysis but ignoring immortal time and adjusting for propensity score using covariables at baseline might yield the least bias. It is also possible that inflated hazards due to inadequate removal of drug indications and reduced hazards by including the immortal time might have cancelled out one another, giving a HR close to that in a RCT. In the case of drugs with more general indications such as RAS inhibitors, a time-fixed Cox model with exclusion of immortal time and adjustment of covariables at the end of the immortal time, estimated from the baseline variables, might remove most, if not all, of these biases.
Our results highlight the challenges in removing bias from drug indications and immortal time simultaneously if these biases have not been systematically captured. In this new era of big data, clearly, more research is needed to develop methods for removing immortal time bias. This is especially relevant to drugs such as statins and insulin, often prescribed for clinical conditions (e.g., angina, poor glycemic control), the information of which may not be documented in the database. Pending better methodologies, we recommend the use of non-time-dependent model with exclusion of immortal time and adjustment for propensity score or subphenotypes associated with the event of interest to reduce potential biases.
On the other hand, by selecting high quality datasets with documentation of drug usage and prognostic variables, pharmacoepidemiological analysis may uncover novel hypothesis for further testing. In an analysis of the diabetes-cancer link, in light of the phenotypic heterogeneity, we first used multivariable analysis to identify risk factors or subphenotypes associated with cancer. By adjusting for a low LDL-C related cancer-subphenotype at drug commencement, we discovered a novel drug-subphenotype interaction where RAS inhibitors specifically attenuated low LDL-C related cancer risk in T2D[22]. These pharmacoepidemiological findings, coupled with pathophysiological knowledge and evidence from mechanistic investigations, have provided the basis for a hypothesis where the complex cross-talk between the RAS and the insulin-like growth factor 1-cholesterol pathway might explain the diabetes-cancer link, for further testing[14].
In pharmacoepidemiological analysis, there are methodological challenges in removing biases from immortal time and drug indications simultaneously. Hence, risk associations between drug use and clinical events based on observational studies must be interpreted with great caution. To avoid misinterpretation, researchers should take these biases into consideration at the stage of study design, e.g., by documenting indications or variables at the time when drugs are introduced or changed. Our validation studies indicated that exclusion of immortal time in an analysis testing effects of RAS inhibitors while inclusion of immortal time in an analysis testing effects of statins on CVD, respectively yielded effect sizes in T2D close to those obtained in RCTs. Our findings call for further research in developing methodology to simultaneously remove immortal time bias and drug use indication bias. Meanwhile, in the absence of methods which can address effects of different drugs in multiple databases, it will be prudent to use reference drugs and test the quality of databases and adjustment methods for immortal time and drug indications before testing of other drug associations with clinical outcomes to avoid erroneous conclusions.
| 1. | Yang XL, Ma RC, So WY, Kong AP, Xu G, Chan JC. Addressing different biases in analysing drug use on cancer risk in diabetes in non-clinical trial settings--what, why and how? Diabetes Obes Metab. 2012;14:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 429] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1293] [Article Influence: 71.8] [Reference Citation Analysis (19)] |
| 4. | Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 921] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 5. | Yang X, Kong AP, Luk AO, Ozaki R, Ko GT, Ma RC, Chan JC, So WY. Validation of methods to control for immortal time bias in a pharmacoepidemiologic analysis of renin-angiotensin system inhibitors in type 2 diabetes. J Epidemiol. 2014;24:267-273. [PubMed] |
| 6. | Kong AP, Yang X, So WY, Luk A, Ma RC, Ozaki R, Cheung KK, Lee HM, Yu L, Xu G. Additive effects of blood glucose lowering drugs, statins and renin-angiotensin system blockers on all-site cancer risk in patients with type 2 diabetes. BMC Med. 2014;12:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Stattin P, Björ O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Yang X, Ko GT, So WY, Ma RC, Yu LW, Kong AP, Zhao H, Chow CC, Tong PC, Chan JC. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes. 2010;59:1254-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Yang XL, Ma RC, Chan JC. Meta-analysis of trial data may support a causal role of hyperglycaemia in cancer. Diabetologia. 2011;54:709-710; author reply 711-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2646] [Article Influence: 203.5] [Reference Citation Analysis (7)] |
| 13. | Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327-333. [PubMed] |
| 14. | Yang X, Lee HM, Chan JC. Drug-subphenotype interactions for cancer in type 2 diabetes mellitus. Nat Rev Endocrinol. 2015;11:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Yang X, So WY, Ma RC, Ko GT, Kong AP, Wang Q, Cockram CS, Chow CC, Chan JC, Tong PC. Predicting values of lipids and white blood cell count for all-site cancer in type 2 diabetes. Endocr Relat Cancer. 2008;15:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Yang X, So W, Ko GT, Ma RC, Kong AP, Chow CC, Tong PC, Chan JC. Independent associations between low-density lipoprotein cholesterol and cancer among patients with type 2 diabetes mellitus. CMAJ. 2008;179:427-437. [PubMed] |
| 17. | Yang X, So WY, Ma RC, Ko GT, Kong AP, Zhao H, Luk AO, Lam CW, Ho CS, Tong PC. Low LDL cholesterol, albuminuria, and statins for the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes Care. 2009;32:1826-1832. [PubMed] |
| 18. | Yang X, So WY, Ma RC, Kong AP, Lee HM, Xu G, Ozaki R, Chan JC. Synergistic effects of low LDL cholesterol with other factors for the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Acta Diabetol. 2012;49 Suppl 1:S185-S193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Yang X, So WY, Ma RC, Kong AP, Lee HM, Yu LW, Chow CC, Ozaki R, Ko GT, Chan JC. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care. 2011;34:375-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 2634] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 22. | Yang X, Ma RC, So WY, Wang Y, Kong AP, Ozaki R, Xu G, Chan JC. Renin angiotensin system inhibitors may attenuate low LDL cholesterol-related cancer risk in type 2 diabetes. Diabetes Metab Res Rev. 2014;30:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bugaj AM, Wasko-Czopnik D, Zhou SM S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK