Published online Sep 26, 2015. doi: 10.5662/wjm.v5.i3.108
Peer-review started: March 18, 2015
First decision: May 13, 2015
Revised: May 22, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 26, 2015
Processing time: 190 Days and 0.6 Hours
Hepatic insufficiency is a fatal liver disease with a significant decrease in functioning hepatocytes. If hepatocytes could be generated from human induced pluripotent stem (hiPS) cells and transplanted into patients with hepatic insufficiency, the disease may become curable. However, a major limitation to this therapeutic strategy is due to the tumorigenicity of hiPS cells and their ability to form cancer. Current methods for eliminating unwanted hiPS cells use genetic manipulation or reagents that are potentially hazardous for hepatocytes; therefore, revised methods are necessary and anticipated. Glucose and arginine are essential cell culture medium ingredients for the survival of most cells, including hiPS cells. However, hepatocytes can produce its own glucose and arginine through galactokinase and ornithine transcarbamylase, respectively. Therefore, it was hypothesized that unwanted hiPS cells could be eliminated in a medium without glucose and arginine, and supplemented with galactose and ornithine instead. This modified medium has been established as hepatocyte selection medium (HSM). So far, attempts to generate a pure colony of mature hepatocytes from hiPS cells have not been successful. After establishment of co-culture in HSM, primary human hepatocytes survive while hiPS cells die within three days. Our latest results regarding a modification of HSM will be introduced in this manuscript.
Core tip: Human induced pluripotent stem (hiPS) cells have the potential to differentiate into mature hepatocytes. If undifferentiated hiPS cells persist among transplanted hepatocytes, hiPS cells may potentially develop into cancer. Glucose and arginine are essential for the survival of most cells; however, mature hepatocytes survive in the media because they can produce glucose and arginine using galactokinase and ornithine transcarbamylase, respectively. Therefore, we created a hepatocyte selection medium (HSM) that lacks glucose and arginine but is supplemented with galactose and ornithine. After establishment of co-culture in HSM, human primary hepatocytes survive while hiPS cells die within three days.
- Citation: Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Hepatocyte selection medium eliminating induced pluripotent stem cells among primary human hepatocytes. World J Methodol 2015; 5(3): 108-114
- URL: https://www.wjgnet.com/2222-0682/full/v5/i3/108.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i3.108
Human induced pluripotent stem (hiPS) cells have pluripotency and potential to differentiate to various types of somatic cells. HiPS cells are established with four reprogramming factors[1].
Hepatic failure is fatal in that numbers of functioning hepatocytes significantly decreases below a safe level to support life. If hepatocytes are generated from hiPS cells and transplanted to patients with hepatic failure, they would be cured[2]. One major problem of transplantation is graft-verses-host disease. The graft-verses-host disease could be avoided, if hepatocytes are differentiated from hiPS cells from the patients.
One problem arises with the transplantation. HiPS cells may reside in the transplanted hepatocytes. If the residual hiPS cells are transplanted to the patients, they potentially form cancer. Therefore, methods for eliminating hiPS cells among differentiated hepatocytes used for transplantation are needed for development. The methods should be non-hazardous to hepatocytes and specific for hiPS cells. As such, a new medium to eliminate hiPS cells “hepatocyte selection medium” (HSM), has been developed.
HiPS cells differentiation to hepatocytes has been investigated[3-8]. They are divided into two categories: growth factors and transcription factors.
Currently, the most common protocols are stepwise addition of growth factors to simulate the process of in vivo hepatocyte differentiation during liver development[3-6]. Transcription factors are sequentially expressed during hepatocyte differentiation[7]. HiPS cells are difficult to transfect with plasmid DNA and as such, adenovirus vectors are more efficiently used to introduce transcription factors to hiPS cells. These transcriptions factors then induce differentiation of hiPS cells into hepatoblast-like or hepatocyte-like cells[6,8]. However, there are limitations using this method and cells exhibit only certain similarities to primary hepatocytes and are therefore called “hepatocyte-like cells”. An organoid of liver is formed after mixing human mesenchymal stem cells, human umbilical vascular endothelial cells, and hepatocyte-like cells differentiated from hiPS cells[9]. One major problem is that most attempts to obtain mature hepatocytes from hiPS cells have not been successful.
Another limitation, as described in further detail below, is the possibility of tumor formation and cancer development due to the presence of undifferentiated hiPS cells among transplanted hepatocyte-like cells.
HiPS cells proliferate rapidly and have active telomerase activity, which are closely associated with tumorigenicity[1,10]. Tumorigenicity is therefore a major concern with the transplantation of somatic cells differentiated from hiPS or other stem cells into patients[11]. In fact, teratoma is formed in the liver transplanted with mouse hepatocytes differentiated from embryonic stem (ES) cells[12]. This phenomenon strongly supports the tumorigenicity of the residual undifferentiated mouse ES cells among the hepatocytes. At an early stage, it was speculated that teratoma was caused by viral vectors integrated to the host genome[13]. To reduce this risk, two methods have been attempted. First one is the Sendai virus and plasmid vectors because they do not integrate to the genome[14,15]. Second one is to omit c-Myc from the four reprogramming factors[16]. With all the above trials, pluripotent stem cells still form teratoma. It is, then, speculated that tumorigenicity is strongly associated with pluripotency[10,17]. Methods should be investigated to eliminate hiPS cells from the transplanted cells.
Until now, several methods are reported on the elimination of unwanted hiPS cells.
First attempt was introduction of thymidine kinase gene followed by addition of ganciclovir. Nanog is a homeodomain protein, and maintains pluripotency of ES cells[18]. Nanog promoter would drive thymidine kinase gene if the promoter is followed by the gene in ES cells. It would be expected that hiPS cell would be eliminated with ganciclovir. As expected, hiPS cells die after the addition of ganciclovir into the media[19]. Similarly, Lim et al[20] introduced the thymidine kinase gene with lentiviral vectors. However, the approach of selectively eliminating hiPS cells in this manner may be problematic because ganciclovir may be hazardous to hepatocytes.
Second attempt was small molecules. A library of small chemicals was screened to find molecules that had the potential of induction of apoptosis for mouse ES cells[21]. Benzethonium chloride and methylbenzethonium successfully induced apoptosis in hiPS.
N-oleoyl serinol (S18) is a ceramide analogue. S18 ablated pluripotent cells in EB differentiating toward neuronal lineage[22]. Unexpectedly, S18 promoted differentiation of embryoid bodies to neural lineage. S18 is unique in two aspects: elimination of hiPS cells and promotion of differentation toward neural lineage.
Altogether, the above methods require genetic modification or reagents that are hazardous to hiPS cells. However, genetic modifications and toxic reagents are not desirable for the transplantation of somatic cells differentiated from hiPS cells. Therefore, a method should be developed to eliminate hiPS cells using non-toxic materials.
Glucose is indispensable for virtually all type of the cells to survive. Glucose is metabolized to pyruvate though glycolysis. Pyruvate produces energy through tricarboxylic acid cycle.
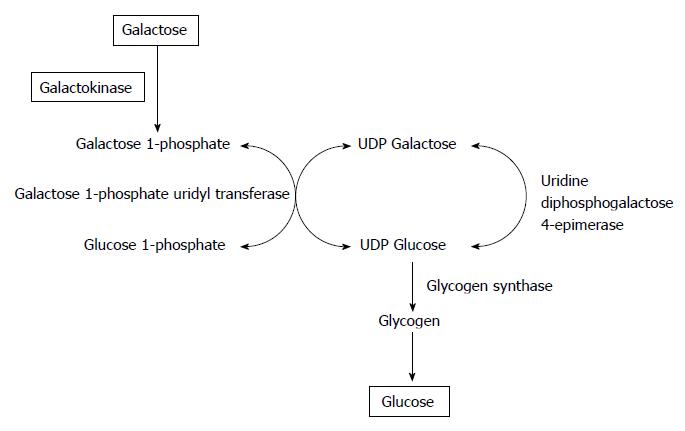
Figure 1 illustrates galactose metabolization to glucose. Galactose is catalyzed to galactose-1-phosphate by galackokinase (GALK). Galatcose-1-phosphate uridyl transferase changed galactose-1-phosphate to glucose-1-phosphate. Through this reaction, glycogen is synthesized. Glycogen enters glycolysis. Finally, glucose is produced from galactose. Glucose-1-phosphate is changed to glucose-6-phosphate. Glucose-6-phosphate is the first metabolite from glucose. Glycolysis follows glucose-6-phosphate. GALK is solely expressed in the liver and kidney[23,24]. In this sense, galactose is the same source of energy as glucose in hepatocytes due to GALK. Therefore, it is expected that hepatocytes survive in a medium without glucose or pyruvate, and added with galactose[25,26]. As expected, hepatocytes survive in a medium without glucose, and added with galactose[27].
Metabolomic profiling has shown that glycolysis is activated, glucose consumption is up-regulated, and lactate accumulation occurs in reprogrammed hiPS cells[28].
Glucose-depleted and lactate-enriched medium eliminated residual undifferentiated hiPS cells from induced differentiated cardiomyocytes[29]. Cardiomyocytes are able to obtain ATP from lactate while hiPS cells are not[30]. Using this non-genetic method, the authors have succeeded in selecting cardiomyocytes differentiated from hiPS cells with great purity and without forming tumors.
Arginine is important in production of nitric oxide polyamine[31]. Arginine is classified as a non-essential amino acid because the amino acid is produced by de novo synthesis. Cells require arginie to survive because its amount of production is not enough[32]. Cells are, therefore, hard to survive without argnine[33]. Culture media contain arginine for cells to survive and proliferate.
Arginine is produced through urea cycle. Arginine is normally produced in hepatocytes because urea cycle is expressed only in hepatocytes.
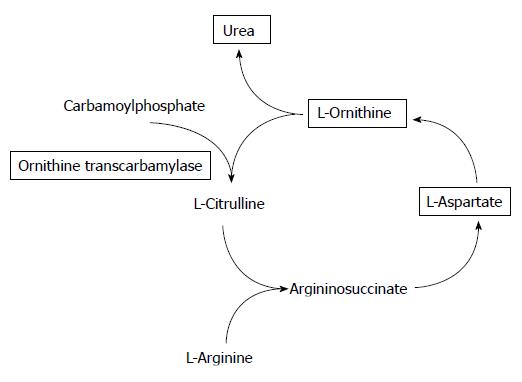
Urea cycle is important to detoxify ammonium ions produced from protein degradation[34]. Figure 2 maps metabolism of urea cycle. L-ornithine and carbamoylphosphate is metabolized to L-citrulline by ornithine transcarbamylase (OTC). OTC deficienty causes hyperammonaemic crises in neonates[35].
Niwa et al[27] cultured a rat hepatoma cell line, H4-IIE, in a medium without arginine. The cells were successfully cultured up to 30 passages. Interestingly, the surviving cells expressed all of the enzymes involved in the urea cycle. We could not find any literature addressing the involvement of the urea cycle in hiPS cells.
In humans, two genes are involved in galactose metabolism: GALK1 (NM_000154) and GALK2 (NM_002044). GALK1 is a major player of galactose metabolism, its deficiency causes cataracts in infants[36]. Whereas, GALK2 was originally discovered to be involved in N-acetylgalactosamine metabolism[37]. However, in conditions with high concentrations of galactose, GALK2 demonstrates galactokinase activity.
HiPS cells express GALK1, GALK2, and OTC at significantly lower levels than fetal or adult liver[38]. It was therefore, expected that hiPS cells were eliminated in a medium without glucose or arginine.
Based on the discussions above, HSM was made to eliminate mouse ES cells among cells differentiating toward hepatocyte lineage[39]. HSM was made from powder to omit glucose and arginine because the two ingredients are included in all the culture media commercially available. Formulation of HSM is based on Leibovits-15 medium that is suitable for the maintenance of function of cultured hepatocytes. HSM is not only deprived of glucose and arginine but also supplemted with galactose and ornithine. In addition, HSM is supplemented with proline for the synthesis of DNA in hepatocytes[40]. When HSM is applicable to human in the future, xeno-free condition is desirable. HSM, therefore, does not contain fetal bovine serum but knockout serum replacement (KSR) (Life Technologies, Grand Island, NY, United States) at 10%.
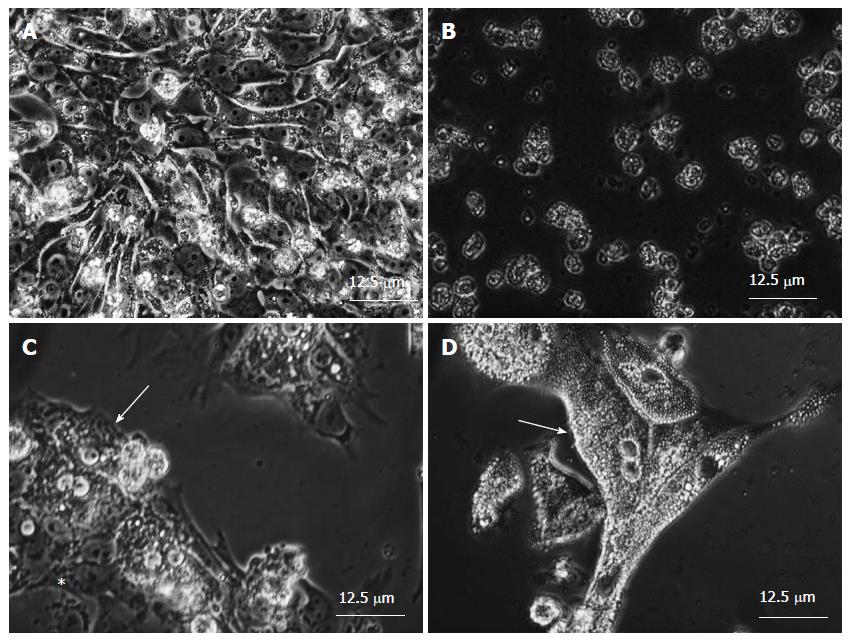
HSM was made as mentioned above. HiPS cells died within three days as expected in HSMs (Figure 3A and B)[38]. One concern arose: KSR. KSR might contain glucose, arginine, or both. To address this possibility, KSR was dialyzed, and compared with non-dialyzed one. HiPS cells died in HSM with or without dialysis. It was confirmed that hiPS cells die in HSM in three days. Theses encouraging results prompted us to culture hepatocytes in HSM. So far, differentiation has not been successful of hiPS cells to mature hepatocytes. Our HSM is not useful for generation of mature hepatocytes because the medium albated hiPS cells in three days culture. It, therefore, has not been successful to enrich mature hepatocytes differentiated from hiPS from undifferentiated hiPS cells. To overcome the limiting situation, primary human hepatocytes are subjected to co-culture experiments in place of hepatocytes successfully differentiated from hiPS cells. Figure 3C shows established co-culture of hiPS cells and primary human hepatocytes. Figure 3D clearly show that all the hiPS cells are eliminated and primary human hepatocytes survive in HSM[38].
There are two ways of application of HSM. One is its initial aim to eliminate hPS cells among hepatocytes for transplantation. Another is application of HSM to hepatocyte differentiation.
One of the characteristics of HSM is that the medium does not have any toxic materials. Another characteristic of HSM is that it does not require genetic manipulation. HSM is, therefore, safe to eliminate unwanted hiPS cells. HSM is potentially necessary when patients with hepatic failure are transplanted with hepatocytes differentiated from hiPS cells to eliminated residual hiPS cells.
Kondo et al[41] report a medium that promotes hepatocyte differentiation from hiPS cells. The formulation of their medium is close to our HSM.
Recently, we have established a new medium based on HSM to initiate hepatocyte differentiation[42]. The medium is supplemented with an apoptosis inhibitor, oncostatin M, and small molecules. The report by Kondo et al[41] and our recent progress suggest that HSM may be a platform medium for differentiation of hiPS cells to hepatocytes.
HSM eliminates hiPS cells. HSM successfully isolates primary human hepatocytes from co-culture of hiPS cells and primary human hepatocytes. HSM may pave a way to a novel protocol to generate mature hepatocytes from hiPS cells.
| 1. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14577] [Article Influence: 809.8] [Reference Citation Analysis (0)] |
| 2. | Chang HM, Liao YW, Chiang CH, Chen YJ, Lai YH, Chang YL, Chen HL, Jeng SY, Hsieh JH, Peng CH. Improvement of carbon tetrachloride-induced acute hepatic failure by transplantation of induced pluripotent stem cells without reprogramming factor c-Myc. Int J Mol Sci. 2012;13:3598-3617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | DeLaForest A, Nagaoka M, Si-Tayeb K, Noto FK, Konopka G, Battle MA, Duncan SA. HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development. 2011;138:4143-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 975] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 5. | Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Takayama K, Inamura M, Kawabata K, Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T, Furue MK. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol Ther. 2012;20:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol. 2008;73:119-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Inamura M, Kawabata K, Takayama K, Tashiro K, Sakurai F, Katayama K, Toyoda M, Akutsu H, Miyagawa Y, Okita H. Efficient generation of hepatoblasts from human ES cells and iPS cells by transient overexpression of homeobox gene HEX. Mol Ther. 2011;19:400-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1568] [Article Influence: 120.6] [Reference Citation Analysis (1)] |
| 10. | Kooreman NG, Wu JC. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J R Soc Interface. 2010;7 Suppl 6:S753-S763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3332] [Cited by in RCA: 3108] [Article Influence: 163.6] [Reference Citation Analysis (12)] |
| 12. | Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc. 2005;37:285-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 648] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 985] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 15. | Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1459] [Cited by in RCA: 1336] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 16. | Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2072] [Cited by in RCA: 1984] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 17. | Pierce GB, Dixon FJ, Verney EL. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab Invest. 1960;9:583-602. [PubMed] |
| 18. | Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 859] [Cited by in RCA: 791] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 19. | Cheng F, Ke Q, Chen F, Cai B, Gao Y, Ye C, Wang D, Zhang L, Lahn BT, Li W. Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials. 2012;33:3195-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Lim TT, Geisen C, Hesse M, Fleischmann BK, Zimmermann K, Pfeifer A. Lentiviral vector mediated thymidine kinase expression in pluripotent stem cells enables removal of tumorigenic cells. PLoS One. 2013;8:e70543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Conesa C, Doss MX, Antzelevitch C, Sachinidis A, Sancho J, Carrodeguas JA. Identification of specific pluripotent stem cell death--inducing small molecules by chemical screening. Stem Cell Rev. 2012;8:116-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Ohira RH, Dipple KM, Zhang YH, McCabe ER. Human and murine glycerol kinase: influence of exon 18 alternative splicing on function. Biochem Biophys Res Commun. 2005;331:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Ai Y, Jenkins NA, Copeland NG, Gilbert DH, Bergsma DJ, Stambolian D. Mouse galactokinase: isolation, characterization, and location on chromosome 11. Genome Res. 1995;5:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Phillips JW, Jones ME, Berry MN. Implications of the simultaneous occurrence of hepatic glycolysis from glucose and gluconeogenesis from glycerol. Eur J Biochem. 2002;269:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Sumida KD, Crandall SC, Chadha PL, Qureshi T. Hepatic gluconeogenic capacity from various precursors in young versus old rats. Metabolism. 2002;51:876-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Niwa A, Yamamoto K, Sorimachi K, Yasumura Y. Continuous culture of Reuber hepatoma cells in serum-free arginine-, glutamine- and tyrosine-deprived chemically defined medium. In Vitro. 1980;16:987-993. [PubMed] |
| 28. | Folmes CD, Arrell DK, Zlatkovic-Lindor J, Martinez-Fernandez A, Perez-Terzic C, Nelson TJ, Terzic A. Metabolome and metaboproteome remodeling in nuclear reprogramming. Cell Cycle. 2013;12:2355-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Hemmi N, Tohyama S, Nakajima K, Kanazawa H, Suzuki T, Hattori F, Seki T, Kishino Y, Hirano A, Okada M. A massive suspension culture system with metabolic purification for human pluripotent stem cell-derived cardiomyocytes. Stem Cells Transl Med. 2014;3:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 784] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 31. | Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2062] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 32. | Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Wheatley DN, Scott L, Lamb J, Smith S. Single amino acid (arginine) restriction: growth and death of cultured HeLa and human diploid fibroblasts. Cell Physiol Biochem. 2000;10:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Walker V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes Metab. 2009;11:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Gallagher RC, Lam C, Wong D, Cederbaum S, Sokol RJ. Significant hepatic involvement in patients with ornithine transcarbamylase deficiency. J Pediatr. 2014;164:720-725.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Davit-Spraul A, Pourci ML, Soni T, Lemonnier A. Metabolic effects of galactose on human HepG2 hepatoblastoma cells. Metabolism. 1994;43:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Ai Y, Basu M, Bergsma DJ, Stambolian D. Comparison of the enzymatic activities of human galactokinase GALK1 and a related human galactokinase protein GK2. Biochem Biophys Res Commun. 1995;212:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Tomizawa M, Shinozaki F, Sugiyama T, Yamamoto S, Sueishi M, Yoshida T. Survival of primary human hepatocytes and death of induced pluripotent stem cells in media lacking glucose and arginine. PLoS One. 2013;8:e71897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Tomizawa M, Toyama Y, Ito C, Toshimori K, Iwase K, Takiguchi M, Saisho H, Yokosuka O. Hepatoblast-like cells enriched from mouse embryonic stem cells in medium without glucose, pyruvate, arginine, and tyrosine. Cell Tissue Res. 2008;333:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Nakamura T, Teramoto H, Tomita Y, Ichihara A. L-proline is an essential amino acid for hepatocyte growth in culture. Biochem Biophys Res Commun. 1984;122:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Kondo Y, Yoshihashi S, Mimori K, Ogihara R, Kanehama Y, Maki Y, Enosawa S, Kurose K, Iwao T, Nakamura K. Selective culture method for hepatocyte-like cells differentiated from human induced pluripotent stem cells. Drug Metab Pharmacokinet. 2014;29:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. An Optimal Medium Supplementation Regimen for Initiation of Hepatocyte Differentiation in Human Induced Pluripotent Stem Cells. J Cell Biochem. 2015;116:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Holan V, Zou ZM S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK