Peer-review started: November 26, 2013
First decision: January 8, 2014
Revised: February 18, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: March 26, 2015
Processing time: 483 Days and 12.2 Hours
In clinical practice obesity is primarily diagnosed through the body mass index. In order to characterize patients affected by obesity the use of traditional anthropometric measures appears misleading. Beyond the body mass index, there are overwhelming evidences towards the relevance of a more detailed description of the individual phenotype by characterizing the main body components as free-fat mass, muscle mass, and fat mass. Among the numerous techniques actually available, bioelectrical impedance analysis seems to be the most suitable in a clinical setting because it is simple, inexpensive, noninvasive, and highly reproducible. To date, there is no consensus concerning the use of one preferred equation for the resting energy expenditure in overweight and/or obese population. Energy restriction alone is an effective strategy to achieve an early and significant weight loss, however it results in a reduction of both fat and lean mass therefore promoting or aggravating an unfavourable body composition (as sarcobesity) in terms of mortality and comorbidities. Therefore the implementation of daily levels of physical activity should be simultaneously promoted. The major role of muscle mass in the energy balance has been recently established by the rising prevalence of the combination of two condition as sarcopenia and obesity. Physical exercise stimulates energy expenditure, thereby directly improving energy balance, and also promotes adaptations such as fiber type, mitochondrial biogenesis, improvement of insulin resistance, and release of myokines, which may influence different tissues, including muscle.
Core tip: There are overwhelming evidences towards the relevance of a more detailed description of the individual phenotype by characterizing the main body components as free-fat mass, muscle mass, and fat mass. Among the numerous techniques actually available, bioelectrical impedance analysis seems to be the most suitable in a clinical setting because it is simple, inexpensive, noninvasive, and highly reproducible. To date, there is no consensus concerning the use of one preferred equation for the resting energy expenditure in overweight and/or obese population.
- Citation: Rotella CM, Dicembrini I. Measurement of body composition as a surrogate evaluation of energy balance in obese patients. World J Methodol 2015; 5(1): 1-9
- URL: https://www.wjgnet.com/2222-0682/full/v5/i1/1.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i1.1
Obesity, defined by excessive adipose tissue, has been shown several deleterious effects on many body organs through thrombogenic, atherogenic, oncogenic, hemodynamic and neurohumoral pathways and has been linked to several chronic diseases, as diabetes, ischemic heart, and musculo-skeletal disorders, together with malignancies. Overweight and obesity represent the fifth leading risk for global death by The World Health Organization on March 2013[1]. At least 2.8 million adults die each year as a result of overweight/obesity. Moreover the 44% of diabetes and 23% of ischemic heart disease burden can be attributable to an abnormal adipose tissue accumulation[1].
Obesity is diagnosed accordingly to the body mass index (BMI). This index has been strongly recommended for use in clinical practice[2,3]. Multiple studies have shown a U or J-shaped relation between BMI, all causes and/or cardiovascular mortality, thus identifying the best survival rate for BMI values in overweight (25-27 kg/m2) followed by a dramatic increase in risk profile out of these values[4-9].
In a cross-sectional study enrolling 13601 subjects (45.5 ± 17 years; 48% men) from the Third National Health and Nutrition Examination Survey (NHANES)[10], the BMI cut-off of ≥ 30 kg/m2 shown an overall low sensitivity and an high specificity to identify obesity such as defined by an excessive body fat percentage at bioelectrical impedance analysis (> 25% in men and > 35% in women, respectively, thus according to the gold standard definition proposed by the World Health Organization[2]. The BMI-based definition of obesity has important limitations on diagnostic performance, particularly in men and elderly, missing more than 50% of people with excessive fat mass. In men, BMI showed a more reliable association with lean mass than with total body fat. In contrast, in women BMI appears to be more accurate to estimate fat-mass, thus explaining why overweight women have been reported as more consistently related to increased mortality compared to overweight male individuals. Furthermore, there is significant inter-subject variability in body fat percentage in individuals with similar BMI values. About 30% of obese subjects do not show any metabolic complications such as diabetes, hypertension, dyslipidemia, etc.), then they can be defined metabolically healthy obese patients[11]. On the other hand, in subgroups of BMI-based normal weight individuals an increased cardiometabolic risk profile and an insulin resistant condition could be described and strictly related to fat mass accumulation[12,13].
Further studies have pointed out the association between visceral adiposity, better than BMI, mortality and cardiovascular disease. Waist circumference as a marker of abdominal adipose tissue, has been placed as one of the main contributors to the Metabolic Syndrome by the National Cholesterol Education Program Adult Treatment Panel III in 2001[14], then as the core feature of the diagnostic criteria proposed by the International Diabetes Federation in 2005.
In the International Day for Evaluation of Abdominal adiposity cross-sectional study, enrolling 168.000 patients in 63 countries, waist circumference showed a higher adjusted odds ratio (95%CI) for diabetes than BMI in overall sample population [1.35 (1.30-1.40) vs 1.23 (1.19-1.27) for men and 1.55 (1.50-1.60) vs 1.23 (1.19-1.27) for women, respectively) such as for cardiovascular disease [1.24 (1.19-1.28) vs 1.13 (1.09-1.17) for men and 1.21 (1.17-1.25) vs 1.20 (1.16-1.24) for women, respectively)[15].
In the NHANES I and NHANES II longitudinal prospective cohort studies, performed in 10169 male subjects a continuous positive relationship between fat-mass and all-cause mortality rate (HR = 1.033, 95%CI: 1.005-1.063, P = 0.0213) have been reported, together with a preponderant significantly negative relationship between free-fat mass and all-cause mortality (i.e., protective; HR = 0.923, 95%CI: 0.906-0.941, P < 0.0001)[16].
Furthermore, BMI and weight loss rate may not be linked to clinical improvement of health-related outcomes, in comparison to different body composition measures by simple and non-invasive methods, which demonstrated a strong relationship between mortality, body lean mass and adipose tissue[17-24].
In conclusion beyond the BMI, there are overwhelming evidences towards a more detailed description of the individual phenotype by characterizing the main body components as free-fat mass (FFM), muscle mass (MM), and fat mass (FM).
Numerous techniques for body composition analysis are currently available: anthropometry, including the 4-skinfold method, hydrostatic weighing, in vivo neutron activation analysis, anthropogammametry from total body 40K, nuclear magnetic resonance, dual-energy X-ray absorptiometry (DEXA), computerized tomography (CT), and bioelectrical impedance analysis (BIA). Evaluating complexity, invasiveness, and cost only DEXA, CT, and BIA may represent the methods of choice to assess body composition in clinical practice whereas the other techniques are limited to scientific purposes.
DEXA estimates body fat and lean mass percentage through a tissue-specific model (fat, lean tissues, and bone) based on X-ray (dose 1-3 mrad) tissue-dependent attenuation[25]. The largest sample (n = 22000 partecipants) have been analyzed by the NHANES[26]. DEXA systems currently available for scanning whole-body tissue composition are capable to analyze a wide range of weights including severe obese subjects (> 150 kg). DEXA scans can be subdivided into different body regions, i.e., trunk, arms, and legs, thus identifying and estimating both android and gynoid fat distribution. DEXA can detect only abdominal adipose tissue accumulation without any distinction between visceral and subcutaneous fat because of their similar X-ray attenuation properties and tissue overlapping[27]. DEXA is the gold standard technique for the evaluation of body composition in clinical research[28-30], although limited in clinical practice by the radiation exposure, availability and cost. The use of the same DEXA instruments and analysis software are of relevant importance for longitudinal studies, since they could influence body composition measurements[31].
In patients affected by malignancies, the analysis at the level of the 3rd lumbar vertebra by CT strongly predicted whole body fat and FFM as compared with DEXA[32]. CT provided an accurate evaluation of body composition, not provided by DEXA or BIA and a X-ray exposition similar to a chest radiography. Although not validated, also CT images of the right thigh halfway showed to be significantly related to overall mortality rate in chronic obstructive pulmonary disease patients[33]. Despite its wide use at diagnosis and follow-up in severely ill patients, CT scan at the 3rd lumbar vertebra cannot be considered a feasible method to assess body composition in obese population requiring expensive equipment, trained operators and exposure to ionizing radiation.
BIA is based on the capacity of hydrated tissues to conduct electrical energy. The BIA methodology have been described in two ESPEN position papers[34,35]. The analysis of total body impedance is based on the estimation of total body water. From total body water, prediction equations allow the calculation of FFM and FM. BIA equations have been validated for chronic obstructive pulmonary disease, AIDS, transplant patients and elderly individuals[36]. A prediction equation for FFM estimation by BIA in adults (age: 20-94 years; BMI: 17.0-33.8 kg/m2) has been proposed[37] as well as reference values of FFM and FM for a Caucasian population[38]. Previously reported data showed no significant differences between DEXA and BIA for the assessment of fat mass in overweight and/or obese patients with a significantly good linear correlation[39,40]. However several factors could limit the validity of BIA in severe obesity[41].
Energy balance is constituted by three major components including energy intake, energy expenditure (EE) and energy storage. Energy expenditure is expressed by resting energy expenditure (REE), as the amount of energy required for the endogenous metabolic activity separated from the metabolic effects of food and physical activity, the food-related thermic effects (TEF), as energy need to absorb and metabolize ingested food, and energy expenditure associated to physical activity (EEPA). EEPA is the most inter-individual variable component of energy expenditure and consists of the amount of physical activity performed multiplied the energy requirement of that activity. The REE is proportional to body mass, particularly to the FFM[42]. The REE prediction equations evaluate the energy expenditure of 2 different body compartments as the adipose tissue or FM and the lean mass or FFM. FFM is the main source for REE and is commonly considered as a surrogate measure of metabolically active tissues. Brain, liver, heart, and kidney account for approximately the 60% of REE, despite a combined weight < 7% of FFM. In comparison to the skeletal muscle, the metabolic rate of heart and kidney is approximately 30-fold higher, and the later approximately 2-fold higher in comparison to brain and liver. The skeletal muscle represents the major contributor to the FFM (50%), but accounts for only the 21% of the REE. Adipose tissue has a low energy expenditure, however its body rate varies significantly according to the dramatic increase of overweight and obesity. Then it is notable that FM represent an important source for the REE in all prediction models. Among adults, REE is lower in elderly, almost in part explained by the change in body composition[27].
Advantage and limitations of currently available non invasive techniques for the estimation of EE are summarized in Table 1.
| Technique | Advantages | Limitations |
| Direct calorimetry | The gold standard method for measure EE in animal models | High complexity, high cost, need to confine the subject for almost 24 h |
| Indirect calorimetry | The gold standard to measure REE in humans. Non invasive, adequately accurate, highly reproducible | High cost, relatively complex, need of trained personnel |
| Bioelectrical impedance analysis | Non invasive, simple, adequately accurate for body composition analysis, relatively inexpensive | The estimation of EE is limited by the need of obesity-specific predictive equations |
| Multi-sensor device | Easy and practical to use | The estimation of EE is limited by the need of obesity-specific predictive equation |
Indirect calorimetry represents the gold standard technique to evaluate REE. This method used the rates of oxygen consumption (VO2) and/or carbon dioxide production (VCO2) to calculate EE according to the Weir predictive equation, derived from studies comparing indirect calorimetry with direct calorimetry[43]. The complexity and cost, together with the requirement of the patient isolation for at least 24 h limit the feasibility of the direct calorimetry in humans.
The indirect calorimetry consists in a gas collector, a canopy and a system that measures the volume and concentrations of O2 and CO2 minute by minute. Through a unidirectional valve located in the ventilated canopy, the calorimeter collect and quantify the volume and concentration of O2 inspired and of CO2 expired. A systematic literature review aimed to determine the optimal conditions for obtaining reliable measures of REE by indirect calorimetry recommends fasting for at least 6 h, avoiding caffeine during the night, nicotine and alcohol for at least 2 h, moderate physical activity for at least 2 h, and vigorous physical activity for 14 h before[44]. Despite methodological, environmental and individual limitations indirect calorimetry represents a non-invasive and very accurate method to estimate REE[45]. However this method is widely limited in most of clinical settings by the requirement of expensive equipment, and trained operators, then many efforts are spent to identify the most accurate predictive equation to determine REE in overweight and obesity.
Predictive equations have generally been calculated from experimental studies performed in healthy subjects on the basis of regression analysis of bodyweight, height, sex, and age as independent variables and REE by indirect calorimetry as a dependent variable. On the basis of a review of available evidences from Harris and Benedict[46], FAO/WHO/UNU weight or weight and height equations[47], and the equations of Mifflin et al[48] and Owen et al[49], Frankenfield et al[50] proposed the use of the Mifflin equation both for overweight and obese subjects. All the available predictive equations have been further validated with indirect calorimetry from adults aged 18-65 years with a BMI of 25 to 40 kg/m2 in order to identify the most accurate and precise REE predictive equation in specific overweight and obese groups of United States and Dutch subjects: the results of this study were similar to the data of Frankenfield et al[50], then supporting the use of Mifflin equation in the United States. However for overweight and obese Dutch adults, there appears to be no accurate equation[51]. This discrepancy for the Dutch than for the United States adults could be explained by the difference about weight and height values, even within sex and BMI subgroups, thus limiting the validity of this equation in similar taller populations. Numerous studies contributed to further evaluate the currently available predictive equations in overweight and obese subjects[52-55] and/or in extremely obese subjects[56-61]. There is some evidences supporting the Mifflin[59], the FAOw[56,58] and the Harris Benedict[60], the Siervo equations[61] in extremely obese subjects. More recently a new equation, using FFM, Horie-Waitzberg, and Gonzalez equations, have been validated and proposed in Brazilian severely obese subjects[62]. The most commonly proposed equation for the measurement of REE have reported in Table 2.
| Age | Sex | Equation | |
| Harris and Benedict (kcal/d) | 15-74 | Male | 66.4730 + 13.7516 (W) + 5.0033 (H) – 6.7550 (A) |
| 15-74 | Female | 655.0955 + 9.5634 (W) + 1.8496 (H) – 4.6756 (A) | |
| Schofield (MJ/die) | 10-17 | Male | 0.074 (W) + 2.754 |
| 10-17 | Female | 0.056 (W) + 2.898 | |
| 18-29 | Male | 0.063 (W) + 2.896 | |
| 18-29 | Female | 0.062 (W) + 2.036 | |
| 30-59 | Male | 0.048 (W) + 3.653 | |
| 30-59 | Female | 0.034 (W) + 3.538 | |
| ≥ 60 | Male | 0.049 (W) + 2.459 | |
| ≥ 60 | Female | 0.038 (W) + 2.755 | |
| FAO/ WHO/ UNU (MJ/d) | 10-17 | Male | 0.0732 (W) + 2.72 |
| 10-17 | Female | 0.0510 (W) + 3.12 | |
| 18-29 | Male | 0.0640 (W) + 2.84 | |
| 18-29 | Female | 0.0615 (W) + 2.08 | |
| 30-60 | Male | 0.0485 (W) + 3.67 | |
| 30-60 | Female | 0.0364 (W) + 3.47 | |
| > 60 | Male | 0.0565 (W) + 2.04 | |
| > 60 | Female | 0.0439 (W) + 2.49 | |
| Mifflin-St Jeor (kcal/d) | 19-78 | Male | 10 × W + 6.25 × H – 5 × A + 5 |
| 19-78 | Female | 10 × W + 6.25 × H – 5 × A – 161 | |
| Owen (kcal/d) | 18-65 | Male | 879 + 10.2 × W |
| 18-65 | Female | 795 + 7.18 × W |
To date there is no consensus about the use of one REE equation compared to others, in overweight and/or obese population. This might be related to differences about group composition, methods, or statistical analysis, at least in part.
In order to estimate EE including EEPA, a practical multi-sensor device, the SenseWear Armband (BodyMedia, Inc., Pittsburgh) has been recently developed. This device contains four sensors to detect heat flux, accelerometry, galvanic skin reaction, skin temperature. Despite excellent results by comparison with energy expenditure measured using the doubly labeled water in overweight/obese children[63] and lactating women[64], other evidences underline the need of obesity-specific equations[65,66].
A meta-analysis of randomized clinical trial suggest that pedometer use is associated with a significant decrease of body weight and blood pressure[67]. However a recent review assessing different accelerometers to measure daily physical activity as a surrogate of EE in comparison with the doubly labeled water only few available devices have been proven to adequately correlate with the reference method[68].
Skeletal muscle plays a critical role in the glucose metabolism and peripheral insulin sensitivity as well as musculoskeletal performance. The importance of lean mass on energy balance has been widely recognized by the rising prevalence of the combination of two condition as sarcopenia and obesity[69]. Two major consensus documents provide different definition of sarcopenia. The European consensus, the Working Group on Sarcopenia in Older People, defines sarcopenia as generalized loss of skeletal muscle mass and strength[70]. The International consensus, the International Working Group on Sarcopenia, requires a decline in muscle mass and walking speed to diagnose sarcopenia[71]. Although sarcopenic obesity is commonly used to define the coexistence of diminished muscle mass and increased adipose tissue, a standard definition of sarcopenic obesity is still lacking. Several clinical studies have indicated that obesity and/or insulin resistance may underlie the development of sarcopenia[72]. A possible role of vitamin D in sarcopenia has been postulated in two studies demonstrating that serum 25-hydroxy vitamin D was negatively correlated with appendicular (legs and arms) fat mass and positively associated with appendicular muscle mass, both evaluated through DEXA analysis[73,74].
Fat mass excess may induce and/or worsen sarcopenia because the increase of lipid depot reduces both amino acid utilization and protein synthesis in muscle fibers. Evaluating 3132 elderly male subjects without diabetes the highest quartile of homeostasis model assessment of insulin resistance showed the highest risk for a decrease in lean body mass and appendicular mass[75]. On the counterpart, skeletal muscle is the main target of insulin, then loss of muscle mass may cause insulin resistance. A previous study identified sarcopenia as a risk factor for exacerbating insulin resistance in obese subject with dysglycemia[76].
Skeletal muscle fibers can be classified into different categories: from slow (type I) fibers with low contractile abilities, numerous mitochondria and high oxidative energy metabolism, to type IIa and type IId/x, and eventually fast (type IIb) fibers able to contract rapidly and predisposed toward glycolytic processes. In obese and diabetic population skeletal muscle commonly contains more type IIb fibers, while slow muscle fiber percentage and skeletal muscle glucose transport are significantly reduced. Sarcopenic individuals have been shown to be associated with a further impairment of muscle fiber content, predominantly of type I[77]. Recent studies have shown that exercise is able to increase the content of type I and type IIa fibers[78]. Furthermore, in skeletal muscle with ageing both the number and morphology of mitochondria are changed with an impaired function and an associated reduced oxidative capacity[79]. In particular since the primary role of mitochondria is to maintain the cellular energy balance, shifts in mitochondria respiratory activity can lead to a reduced maximal capacity of the tricarboxylic acid cycle and the electron transport chain together with an incomplete lipid-induced upregulation of β-oxidation rates, thus inducing a significant accumulation of intramyocellular lipids and further impairing whole body insulin resistance[79]. Physical exercise has been demonstrated to induce mitochondria biogenesis, upregulates skeletal muscle gene expression and protein synthesis, and increases skeletal muscle oxidative capacity both in older and sedentary populations[80].
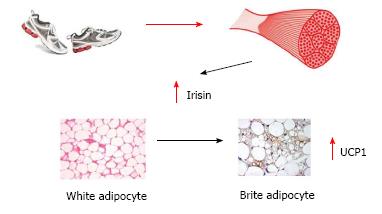
Moreover, recent evidences promote the hypothesis of cross talk between muscle and different tissues mediated by cytokines and other peptides called myokines[81] which are involved both in acute exercise-induced metabolic reactions, as well as in the long-term metabolic benefits induced by exercise[82]. Irisin is one of the most recently identified myokines. Following regular physical activity, Irisin increases two-fold its circulating levels and promotes the shift of white adipocytes into “brite” cells: white adipocytes with a brown-fat-like phenotype. Brown adipocytes activate thermogenesis via the mitochondria uncoupling protein UCP-1 (Figure 1). Overexpression of irisin determined through a gene therapy approach mediated by adenoviral particles, and leading to a modest approximately 3 fold increase in circulating levels, induced browning of subcutaneous white adipose tissue, stimulating a 10-20 fold increase in UCP1, thus increasing energy expenditure and improving glucose tolerance of high fat fed mice[83]. Despite more recent in vitro evidences against the possible translation of the beneficial effects observed in mice to humans, the major role of muscle mass in the energy balance has been definitively established.
In conclusion, physical exercise increases EE and stimulates muscle adaptation mechanisms with potential benefits on different tissues.
Analysis from the NHANES database describes an average daily intake increase of 168 kcal/d for men and of 335 kcal/d for women, from 1971 to 2000[84]. On the counterpart, examining in 2004, the physical activity patterns in a typical agrarian population in the United States through pedometers an average of about 18000 steps per day in men and of about 14000 steps per day have been reported[85], whereas an average American adult walked about 5000 steps per day[86].
An energy balance flipping point between energy intake and energy expenditure could be recognized in United States around 60’ years, thereafter followed by energy intake continuously driving energy expenditure, together with a worldwide burden in the incidence of obesity and type 2 diabetes[87].
The most frequently proposed strategy for treating obesity is food restriction. Energy restriction alone is an effective strategy to achieve a rapid and substantial weight loss, however it results in a reduction of both fat and muscle mass therefore promoting or aggravating an detrimental body composition (as sarcobesity) in terms of mortality and comorbidities. Specifically, approximately 25% of weight loss obtained through short-term low energy diets is lean muscle mass[88-92]. Moreover, the lack of success in weight loss maintenance after low-energy regimen and the subsequent weight regained comprising of up to 80% fat mass compound a further detrimental body composition impairment[93,94]. Weight loss should not be considered the sole focus of therapeutic approach aimed to decrease obesity-related disease risk profile. A systematic review by Chaston et al[95] demonstrates that very low calories regimens (VLCD) result in significant greater loss of FFM (lean) in comparison to low-calories diets[95]. Current evidences sustained that the most beneficial long-term outcomes are achieved with a modest energy deficit (2000-4000 kJ/d)[96]. Therefore increased levels of daily physical activity should be simultaneously promoted. Although resistance training retains or improves the relative percentage of lean mass to total body, current evidence failed to detect a loss of fat mass of a similar magnitude to aerobic training which promotes fat mass loss together with beneficial changes in muscle tissue[97-99].
Studies evaluating efficacy of dietary restriction together with exercise program showed more favorable results (as weight loss rate and body composition improvement) compared to diet or exercise prescription alone[69]. Emerging data suggest that the combination of resistance and aerobic exercise with a modest energy restriction was successful for preserving skeletal muscle concomitantly with a significant decrease of fat mass[100].
In order to characterize patients affected by obesity the use of traditional anthropometric measures appears misleading. A systematic and deep phenotyping of these patients, thus integrating data from body composition analysis and energy expenditure should be used in a dynamic rather than only basal approach to define and periodically verify the efficacy of the therapeutic regimen proposed. Among the numerous techniques evaluated in this paper, BIA seems to be the most suitable in a clinical setting because it is simple, inexpensive, noninvasive, and highly reproducible. Moreover, a recent paper by our research group showed that the FM and MM percentage estimated by BIA at baseline should be considered as predictors of success (weight loss > 5% at 6 mo from baseline) in a individual cognitive-behavioral program[40].
| 1. | World Health Organization. Fact Sheet N° 311. Available from: http: //www.who.int/mediacentre/factsheets/fs311/en/index.html.. |
| 2. | World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [PubMed] |
| 3. | Seidell JC, Kahn HS, Williamson DF, Lissner L, Valdez R. Report from a Centers for Disease Control and Prevention Workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr. 2001;73:123-126. [PubMed] |
| 4. | Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666-678. [PubMed] |
| 5. | Franzosi MG. Should we continue to use BMI as a cardiovascular risk factor? Lancet. 2006;368:624-625. [PubMed] |
| 6. | McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87-97. [PubMed] |
| 7. | Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028-2037. [PubMed] |
| 8. | Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449-460. [PubMed] |
| 10. | Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008;32:959-966. [PubMed] |
| 11. | Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1178] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 12. | Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, Bell JD. Magnetic resonance imaging of total body fat. J Appl Physiol (1985). 1998;85:1778-1785. [PubMed] |
| 13. | Thomas EL, Parkinson JR, Frost GS, Goldstone AP, Doré CJ, McCarthy JP, Collins AL, Fitzpatrick JA, Durighel G, Taylor-Robinson SD. The missing risk: MRI and MRS phenotyping of abdominal adiposity and ectopic fat. Obesity (Silver Spring). 2012;20:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KA, Smith SC, Barter P, Tan CE, Van Gaal L, Wittchen HU. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116:1942-1951. [PubMed] |
| 16. | Allison DB, Zhu SK, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes Relat Metab Disord. 2002;26:410-416. [PubMed] |
| 17. | Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53-59. [PubMed] |
| 18. | Slinde F, Grönberg A, Engström CP, Rossander-Hulthén L, Larsson S. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med. 2005;99:1004-1009. [PubMed] |
| 19. | Futter JE, Cleland JG, Clark AL. Body mass indices and outcome in patients with chronic heart failure. Eur J Heart Fail. 2011;13:207-213. [PubMed] |
| 20. | Segall L, Mardare NG, Ungureanu S, Busuioc M, Nistor I, Enache R, Marian S, Covic A. Nutritional status evaluation and survival in haemodialysis patients in one centre from Romania. Nephrol Dial Transplant. 2009;24:2536-2540. [PubMed] |
| 21. | Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366-2372. [PubMed] |
| 22. | Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, Preux PM, Couratier P. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry. 2011;82:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1822] [Cited by in RCA: 2497] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 24. | Kimyagarov S, Klid R, Levenkrohn S, Fleissig Y, Kopel B, Arad M, Adunsky A. Body mass index (BMI), body composition and mortality of nursing home elderly residents. Arch Gerontol Geriatr. 2010;51:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Leonard CM, Roza MA, Barr RD, Webber CE. Reproducibility of DXA measurements of bone mineral density and body composition in children. Pediatr Radiol. 2009;39:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 812] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 27. | Baracos V, Caserotti P, Earthman CP, Fields D, Gallagher D, Hall KD, Heymsfield SB, Müller MJ, Rosen AN, Pichard C. Advances in the science and application of body composition measurement. JPEN J Parenter Enteral Nutr. 2012;36:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Slosman DO, Casez JP, Pichard C, Rochat T, Fery F, Rizzoli R, Bonjour JP, Morabia A, Donath A. Assessment of whole-body composition with dual-energy x-ray absorptiometry. Radiology. 1992;185:593-598. [PubMed] |
| 29. | Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19:626-631. [PubMed] |
| 30. | Ellegård LH, Ahlén M, Körner U, Lundholm KG, Plank LD, Bosaeus IG. Bioelectric impedance spectroscopy underestimates fat-free mass compared to dual energy X-ray absorptiometry in incurable cancer patients. Eur J Clin Nutr. 2009;63:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66-70. [PubMed] |
| 32. | Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1751] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 33. | Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809-813. [PubMed] |
| 34. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226-1243. [PubMed] |
| 35. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430-1453. [PubMed] |
| 36. | Thibault R, Pichard C. The evaluation of body composition: a useful tool for clinical practice. Ann Nutr Metab. 2012;60:6-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20--94 years. Nutrition. 2001;17:248-253. [PubMed] |
| 38. | Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17:534-541. [PubMed] |
| 39. | Donini LM, Poggiogalle E, Del Balzo V, Lubrano C, Faliva M, Opizzi A, Perna S, Pinto A, Rondanelli M. How to estimate fat mass in overweight and obese subjects. Int J Endocrinol. 2013;2013:285680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Dicembrini I, Pala L, Cresci B, Cremasco F, Rotella CM. Predictors of weight loss in the clinical management of obese patients: the relevance of body composition. Obesity and Metabolism. 2010;6: 29-33. |
| 41. | Deurenberg P. Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am J Clin Nutr. 1996;64:449S-452S. [PubMed] |
| 42. | Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 832] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 43. | Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22:377-388. [PubMed] |
| 44. | Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881-903. [PubMed] |
| 45. | Kee AL, Isenring E, Hickman I, Vivanti A. Resting energy expenditure of morbidly obese patients using indirect calorimetry: a systematic review. Obes Rev. 2012;13:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci USA. 1918;4:370-373. [PubMed] |
| 47. | FAO/WHO/UNU. Energy and protein requirements. Geneva, Switzerland: World Health Organ Tech Rep Ser 1985; . |
| 48. | Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241-247. [PubMed] |
| 49. | Owen OE, Holup JL, D’Alessio DA, Craig ES, Polansky M, Smalley KJ, Kavle EC, Bushman MC, Owen LR, Mozzoli MA. A reappraisal of the caloric requirements of men. Am J Clin Nutr. 1987;46:875-885. [PubMed] |
| 50. | Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105:775-789. [PubMed] |
| 51. | Weijs PJ. Validity of predictive equations for resting energy expenditure in US and Dutch overweight and obese class I and II adults aged 18-65 y. Am J Clin Nutr. 2008;88:959-970. [PubMed] |
| 52. | Frankenfield DC, Rowe WA, Smith JS, Cooney RN. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103:1152-1159. [PubMed] |
| 53. | Siervo M, Boschi V, Falconi C. Which REE prediction equation should we use in normal-weight, overweight and obese women? Clin Nutr. 2003;22:193-204. [PubMed] |
| 54. | Müller MJ, Bosy-Westphal A, Klaus S, Kreymann G, Lührmann PM, Neuhäuser-Berthold M, Noack R, Pirke KM, Platte P, Selberg O. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004;80:1379-1390. [PubMed] |
| 55. | de Luis DA, Aller R, Izaola O, Romero E. Prediction equation of resting energy expenditure in an adult Spanish population of obese adult population. Ann Nutr Metab. 2006;50:193-196. [PubMed] |
| 56. | Das SK, Saltzman E, McCrory MA, Hsu LK, Shikora SA, Dolnikowski G, Kehayias JJ, Roberts SB. Energy expenditure is very high in extremely obese women. J Nutr. 2004;134:1412-1416. [PubMed] |
| 57. | Huang KC, Kormas N, Steinbeck K, Loughnan G, Caterson ID. Resting metabolic rate in severely obese diabetic and nondiabetic subjects. Obes Res. 2004;12:840-845. [PubMed] |
| 58. | Lazzer S, Agosti F, Resnik M, Marazzi N, Mornati D, Sartorio A. Prediction of resting energy expenditure in severely obese Italian males. J Endocrinol Invest. 2007;30:754-761. [PubMed] |
| 59. | Dobratz JR, Sibley SD, Beckman TR, Valentine BJ, Kellogg TA, Ikramuddin S, Earthman CP. Predicting energy expenditure in extremely obese women. JPEN J Parenter Enteral Nutr. 2007;31:217-227. [PubMed] |
| 60. | Boullata J, Williams J, Cottrell F, Hudson L, Compher C. Accurate determination of energy needs in hospitalized patients. J Am Diet Assoc. 2007;107:393-401. [PubMed] |
| 61. | Weijs PJ, Vansant GA. Validity of predictive equations for resting energy expenditure in Belgian normal weight to morbid obese women. Clin Nutr. 2010;29:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Horie LM, Gonzalez MC, Torrinhas RS, Cecconello I, Waitzberg DL. New specific equation to estimate resting energy expenditure in severely obese patients. Obesity (Silver Spring). 2011;19:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Bäcklund K, Sundelin C, Larsson C. Validity of armband measuring energy expenditure in overweight and obese children. Med Sci Sports Exerc. 2010;42:1154-1161. [PubMed] |
| 64. | Slinde F, Bertz F, Winkvist A, Ellegård L, Olausson H, Brekke HK. Energy expenditure by multisensor armband in overweight and obese lactating women validated by doubly labeled water. Obesity (Silver Spring). 2013;21:2231-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Papazoglou D, Augello G, Tagliaferri M, Savia G, Marzullo P, Maltezos E, Liuzzi A. Evaluation of a multisensor armband in estimating energy expenditure in obese individuals. Obesity (Silver Spring). 2006;14:2217-2223. [PubMed] |
| 66. | Bertoli S, Posata A, Battezzati A, Spadafranca A, Testolin G, Bedogni G. Poor agreement between a portable armband and indirect calorimetry in the assessment of resting energy expenditure. Clin Nutr. 2008;27:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304. [PubMed] |
| 68. | Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring). 2007;15:2371-2379. [PubMed] |
| 69. | Parr EB, Coffey VG, Hawley JA. ‘Sarcobesity’: a metabolic conundrum. Maturitas. 2013;74:109-113. [PubMed] |
| 70. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8800] [Article Influence: 550.0] [Reference Citation Analysis (4)] |
| 71. | Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2197] [Cited by in RCA: 2414] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 72. | Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 873] [Cited by in RCA: 794] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 73. | Kim MK, Baek KH, Song KH, Il Kang M, Park CY, Lee WY, Oh KW. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. 2011;96:3250-3256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Seo JA, Cho H, Eun CR, Yoo HJ, Kim SG, Choi KM, Baik SH, Choi DS, Park MH, Han C. Association between visceral obesity and sarcopenia and vitamin D deficiency in older Koreans: the Ansan Geriatric Study. J Am Geriatr Soc. 2012;60:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, Everson-Rose SA, Barrett-Connor E, Orwoll ES. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 76. | Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 461] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 77. | Harrison BC, Leinwand LA. Fighting fat with muscle: bulking up to slim down. Cell Metab. 2008;7:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 79. | Kohara K. Sarcopenic obesity in aging population: current status and future directions for research. Endocrine. 2014;45:15-25. [PubMed] |
| 80. | Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 81. | Pedersen L, Hojman P. Muscle-to-organ cross talk mediated by myokines. Adipocyte. 2012;1:164-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 82. | Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3:1337-1362. [PubMed] |
| 83. | Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3682] [Cited by in RCA: 3680] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 84. | Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available from: http: //www.cdc.gov/nchs/nhanes.htm. |
| 85. | Bassett DR, Schneider PL, Huntington GE. Physical activity in an Old Order Amish community. Med Sci Sports Exerc. 2004;36:79-85. [PubMed] |
| 86. | Bassett DR, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42:1819-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 87. | Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2895] [Cited by in RCA: 3050] [Article Influence: 203.3] [Reference Citation Analysis (0)] |
| 88. | Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 89. | Hoie LH, Bruusgaard D, Thom E. Reduction of body mass and change in body composition on a very low calorie diet. Int J Obes Relat Metab Disord. 1993;17:17-20. [PubMed] |
| 90. | Ballor DL, Katch VL, Becque MD, Marks CR. Resistance weight training during caloric restriction enhances lean body weight maintenance. Am J Clin Nutr. 1988;47:19-25. [PubMed] |
| 91. | Campbell WW, Haub MD, Wolfe RR, Ferrando AA, Sullivan DH, Apolzan JW, Iglay HB. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring). 2009;17:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, McTiernan A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012;20:1628-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 93. | Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872-878; quiz 915-916. [PubMed] |
| 94. | Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr. 2011;94:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 95. | Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007;31:743-750. [PubMed] |
| 97. | Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, Houmard JA, Kraus WE. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985). 2012;113:1831-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 98. | Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 99. | Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433-450. [PubMed] |
| 100. | Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
P- Reviewer: Bertoli S, Niculescu M, Verduci E S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/