Published online Sep 26, 2014. doi: 10.5662/wjm.v4.i3.189
Revised: June 17, 2014
Accepted: September 6, 2014
Published online: September 26, 2014
Processing time: 201 Days and 4 Hours
The prevalence of dyspepsia is up to 40% in population-based study. Functional dyspepsia is an exclusion diagnosis and it is classified as a chronic abdominal pain-related functional disorder, characterized by the presence of persistent or recurrent pain or discomfort centered in the upper abdomen, neither relief by defecation, nor association with the onset of a change in stool frequency or form. Celiac disease (CD) is a common autoimmune enteropathy, with a prevalence around 1% in the general population. Its diagnosis includes a serological screening and an upper gastrointestinal endoscopy with multiple biopsies. Gluten-free diet is the only effective treatment. CD diagnosis is often delayed in asymptomatic patients or in individuals with less clinical gastrointestinal symptoms. Several studies performed coeliac disease screening in patients with symptoms suggestive of dyspepsia, showing a biopsy-proved prevalence that ranged from 0.5% to 2%. The typical endoscopic markers of villous atrophy are not sufficiently sensitive, so some endoscopic techniques, such as “water immersion” and confocal endomicroscopy were proposed to improve the diagnostic sensitivity and target biopsies. A recent meta-analysis estimated that the prevalence of CD was higher in patients with dyspepsia, but not in a statistically significant way. However this assumption should be confirmed further larger studies.
Core tip: Dyspepsia is classified as a chronic abdominal pain-related functional disorder that affects almost 40% of the population. It can be also a manifestation of celiac disease, an immuno-mediated enteropathy, caused by the ingestion of gluten in genetically predisposed patients. The prevalence of celiac disease among dyspeptic patients has been investigated, with results ranging from 0.5% to 2%. Celiac disease diagnosis requires histological evaluation of villous atrophy on duodenal biopsies specimens. Screening for celiac disease in dyspeptic patients and routinely performing of biopsies during upper gastrointestinal endoscopy, may be useful as part of the diagnostic flow-chart of these patients.
- Citation: Petrarca L, Nenna R, Mastrogiorgio G, Florio M, Brighi M, Pontone S. Dyspepsia and celiac disease: Prevalence, diagnostic tools and therapy. World J Methodol 2014; 4(3): 189-196
- URL: https://www.wjgnet.com/2222-0682/full/v4/i3/189.htm
- DOI: https://dx.doi.org/10.5662/wjm.v4.i3.189
Dyspepsia is one of the most common gastrointestinal disorders to be faced in clinical practice, with prevalence up to 40% in population-based study[1] so that the economic impact is very high.
When dyspepsia is not a manifestation of an organic pathology, such as gastroesophageal reflux disease or peptic ulcer disease, then it is classified as functional dyspepsia (FD).
FD markedly reduces patients’ quality of life, similarly to mild heart failure and menopause[2]. However FD is an exclusion diagnosis and on the basis of Rome III criteria[3], it is defined as the presence of gastroduodenal symptom without evidence of structural disease able to explain the syptoms. Often patients refer to suffer from early satiation or postprandial fullness (postprandial distress syndrome), epigastric pain/discomfort or burning (epigastric pain syndrome).
Pathophysiology of FD is not completely understood yet and several pathophysiological mechanisms have been proposed to underlie symptoms. Central processing of visceral stimuli, low-grade inflammation in the duodenum and genetic factors are the main emerging hypothesis investigated[4]. FD is difficult to manage, because no medication is currently approved in the United States, Canada or the European Union. Many treatments have been proposed (diet, eradication of H. pylori and drugs such as prokinetic agents or protonic pump inhibitors)[5] but no one was satisfactory.
Celiac disease (CD) is an auto-immune enteropathy, whose diagnosis is often delayed in asymptomatic patients or in individuals with less clinical gastrointestinal symptoms, such as abdominal bloating, nausea and vomiting. CD diagnosis, according to the American Gastroenterology Association, consists of a serological screening (including anti-transglutaminase, anti-endomisium and anti-deamidated gliadin antibodies) and an upper gastrointestinal endoscopy with multiple duodenal biopsies. Gluten-free diet is the only effective treatment for the disease.
However, although dyspepsia may be a manifestation CD, most of FD patients do not perform serological screening for CD or duodenal biopsies and there are few data about the prevalence of CD in patients with dyspepsia.
Recent studies[6-10] demonstrates that the prevalence of silent CD in patients with dyspepsia is slightly higher than that of the general population, however in one study it resulted rather low[11].
The 40%-60% of subjects with dyspepsia resulted macroscopically normal when performing upper gastrointestinal endoscopy[12]. Unfortunately, the practice of performing biopsies, even in absence of endoscopic alteration of intestinal mucosa, is quite uncommon.
The typical endoscopic markers of villous atrophy include mosaic pattern, scalloping of folds, and a decrease of duodenal folds. However, mostly in less severe cases, CD diagnosis cannot only be performed on these parameters. So, considering that many authors describe these markers as not sufficiently sensitive, some endoscopic techniques, such as “water immersion” and confocal endomicroscopy (CEM) were proposed to improve the diagnostic sensitivity and target biopsies in most damaged mucosal areas[13,14].
Recent studies demonstrate that the prevalence of CD in patients with dyspepsia is higher than that of the general population[6-10].
Bardella et al[6] prospectively enrolled 517 patients suffering from dyspeptic symptoms. All patients were submitted to upper gastrointestinal endoscopy, and six were diagnosed to be celiac (1.2%). Interestingly three patients (50%) had a normal duodenal endoscopic pattern and five of the six celiac patients were young women aged between 20 to 37 years. The authors suggest to perform serological screening for celiac disease especially in young women suffering from dyspepsia.
Lima et al[7] reported a CD prevalence of 1.4% in a small series of patients with dyspepsia, both were young women, aged 19 and 25 years respectively. In the paper of Ozaslan et al[8] among the 196 investigated patients three were diagnosed to be celiac (1.5%). All were female younger than 52 years, and only two showed abnormal endoscopic findings.
In the manuscript by Giangreco et al[9], published in 2008, the role of upper gastrointestinal endoscopy in CD diagnosis was evaluated in patients suffering from FD. The prevalence of CD was 2% (15 patients out of 726 enrolled), higher than the general population one, also considering that patients with an increased risk for CD (such as first degree relatives) were excluded from the study. Among the 15 CD patients (age ranged 20 to 56): 10 were female and only 8 patients presented endoscopic findings suggestive for CD.
Keshavarz et al[10] investigated the prevalence of CD among 170 patients with FD. Twelve patients (10 female), suffering form dysmotility-type dyspepsia, tested positive for CD related antibodies, however only two of them showed villous atrophy at the histological evaluation.
Only in the paper by Heikkinen et al[11] published in 1995, among the 400 uselected dyspeptic patients enrolled to perform upper gastrointestinal endoscopy, serological evaluation and abdominal ultrasound, CD was diagnosed in 2 patients (both aged less than 64 years). The low prevalence (0.5%) could be due, maybe, to the eterogeneity of the population study, with a higher percentage of aged patients (77% were more than 44 years old) while the most frequent diagnosis in younger patients was lactose intolerance (9%).
In a recent meta-analysis by Ford et al[15], the authors provided a pooled prevalence of biopsy-proven CD of 1.0%, similar to that in the general population, when duodenum biopsy was performed as first-line investigation. However when the authors pooled the data from the studies that used the Rome II criteria for dyspepsia, the biopsy proved CD was 2%, significantly higher.
CD is a chronic, immuno-mediated enteropathy, caused by ingestion of gluten in susceptible individuals, carrying DQ2 and/or DQ8 HLA. It is characterised by a chronic inflammatory state of the small intestine that recover after gluten withdrawal. The typical changes of the duodenal mucosa include: raised intra-epithelial lymphocyte, crypt hyperplasia and various degree of villous atrophy as classified by Marsh and modified by Oberhuber et al[16] in 1999, that decreased digestion of food and micro- and macronutrients absorption.
CD is common, with a prevalence around 1% in the general population of Western countries[17,18], more frequent in females than males.
The pathogenesis is multifactorial, including the interactions between environmental, genetic and immune factors. Gluten, a protein derived from wheat, barley and rye, represents the trigger factor of CD. The alcohol-soluble fraction of gluten, the alpha-gliadin, is rich in prolamine and glutenine that could trigger an immune response, mediated by both innate and adaptative arms of CD patients’ mucosal immune system. Genetic susceptibility plays a crucial role in CD pathogenesis, as demonstrated by the increased prevalence in first-degree relatives (9.5%) and siblings (11%)[19]; in the homozygous twins it arises to 75%[20,21]. The genetic basis of celiac disease can be divided between HLA and non-HLA gene variants[22].
The HLA DQ2 heterodimer is present in 90% of celiac patients, in 5% of the cases the HLA DQ8 heterodimer is present. The HLA DQ2 heterodimer, present in 90% of celiac patients[23], is formed by a beta chain (β) encoded by the allele HLA DQB1 * 02 (HLA DQB1 * 0201 or * 0202) and by an alpha chain (α) encoded by the allele HLA DQA1 * 05. The heterodimer HLA DQ8 is formed by a β chain and an α chain encoded by HLA DQB1 * 0302 and HLA DQA1 * 03 respectively[24].
Genes of the HLA complex can contribute in only 36% of the increased risk of celiac disease in siblings[22], indicating the need for assistance from other non-HLA genes[25].
The frequent association of celiac disease with other monogenic diseases may demonstrate the existence of a link with other genes on chromosome 7 (short arm) implicated in Williams syndrome and on chromosome 21 involved in Down syndrome[26].
A fundamental role in the pathogenesis is carried out by an ubiquitous calcium-dependent enzyme, the transglutaminase type 2 (TG2). The TG2 catalyzes the acyl transfer between the γ-carboxamide group of glutamine and the ε-amino group of lysine or primary amine soluble. This mechanism forms gliadin-gliadin macromolecular complexes, which are considered neoepitopes, therefore non-self antigens against which the immune system reacts.
In the presence of a low pH, an abundance of glutamminic residues and scarcity of proteins that bind lysine, TG2 catalyses the deamidation of glutamine[27-29]. Some of these peptides of “deamidated” gluten, because of their negative charge, show a high affinity for the HLA-DQ2 or-DQ8 heterodimer. Once bound to these molecules they activate intestinal mucosa T cells[30-32] and they cause the cytokine production and the begins of the intestinal damage.
The first modern description of CD is due to Samuel Gee, an English paediatrician, published in the St. Bartholomew’s Hospital Reports of 1888. He recognised CD as a chronic indigestion, occurring in people of all ages, presenting as diarrhoea.
Nowadays clinical presentations of CD may vary from silent to severe malabsorption symptoms (celiac crisis).
Didactically, CD manifestations are divided in: (1) typical: including gastrointestinal symptoms, such as diarrhoea, weight loss, abdominal pain, failure to thrive, abdominal distension and vomiting; (2) atypical: that is for example short stature, iron-deficiency anaemia, dermatitis herpetiformis, delayed puberty; and (3) silent: completely asymptomatic.
A delayed gastric empty and a slow oro-caecal transit has been observed in celiac patients on a gluten containing diet, probably due to abnormal exposure of small bowel unabsorbed starch and fats and to altered neuroimmunomodulation and hormonal deregulation (low levels of cholecystokinin and high levels of peptide YY)[33]. Some authors investigated the transit disorders in patients with untreated CD using the video-capsule endoscopy. Urgesi et al[34] found that there was no difference in gastric empting and small bowel transit time between CD patients and control group. However, Ciaccio et al[35] observed changes in motility of the small bowel and they speculated that the reduced folds can cause more rapid changes in the position and in the width of the luminal centre.
CD diagnosis, according to the American Gastroenterology Association, consists of a serological screening and an upper gastrointestinal endoscopy[36].
Nowadays, CD serological screening is recommended for symptomatic patients, or for those people who are at high risk of CD (such as first degree relatives). It encompasses the total serum IgA, the IgA anti-transglutaminase antibodies (AbTG2), IgA anti-endomisium antibodies (EMA) and IgA anti-deamidated gliadin antibodies (DGP).
AbTG2 proved to have a very high sensitivity (98%-100%) and a very good specificity (94%-98%)[37], they are the most widely used for CD screening, even if they can be found in patients affected by other autoimmune diseases[38]. They can be determined both by ELISA or RIA, the latter technique showing a so high sensitivity[39] that it has been used to detect AbTG2 in saliva[40], demonstrating a correlation with CD histological grading and diffusion of duodenal lesions[41].
EMA have a very high specificity (100%), but a lower sensitivity than AbTG2[38]. They are determined by indirect immunofluorescence, using monkey oesophagus sections as substrate.
DGP have been demonstrated to be more sensitive and specific than the old antigliadin antibodies and they are useful especially in children younger than two years of age[42].
In IgA deficient patients, it is recommended to perform the IgG antibodies, particularly IgG anti-deamidated gliadin antibodies.
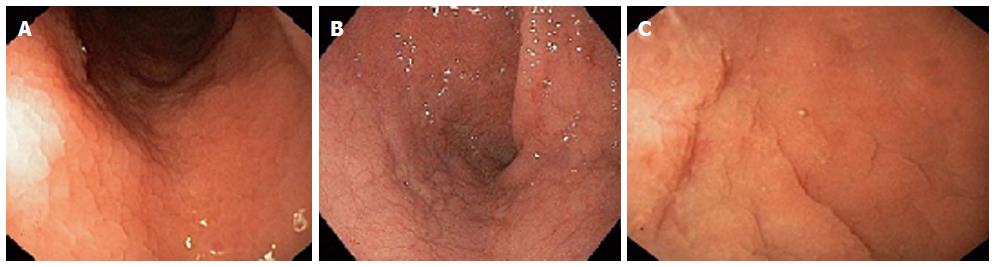
The upper gastrointestinal endoscopy with multiple biopsies, both from duodenal bulb and distal duodenum, is the gold standard for diagnosis[36]. The standard endoscopy does not permit the visualization of villous atrophy, even if several macroscopic markers has been related to CD (Figure 1), such as reduction or absence of duodenal folds, scalloping, nodular appearance and mosaic pattern. However the power of these endoscopic markers to predict the villous atrophy is still debated[43,44]. This variability could be due to the absence of macroscopic sign in case of patchy or partial villous atrophy.
In the last few years, new methods have been developed to evaluate with more accuracy the macroscopic appearance of villous pattern during upper gastrointestinal endoscopy.
The water immersion technique (Figure 1C) is an easy procedure that can emphasize the villous pattern. It consist in a first phase of air suction from the lumen, then a second phase of injection of 90-150 mL of water[45]. It has the potential to target biopsies and, eventually, reduce the number of specimen, thanks to its capability of enhancing areas of villous atrophy. An alternative technique is represented by the chromoendoscopy, that uses the dye staining with indigo carmine in enhancing the visualization of the mucosal surface. This endoscopic tool has showed a better accuracy when combined with magnification endoscopy[14].
Narrow band imaging (NBI) is another technology that improves the visualization of the surface of the superficial mucosa and its vascular architecture.
NBI with optical magnification assists in detecting patients with villous atrophy without determining the level of intraepithelial lymphocytosis and crypt hyperplasia[46,47].
Cammarota et al[48] reported their experience in the use of I-scan technology during endoscopy for the evaluation of the duodenal villous pattern. It works in real time and permits to switch from standard endoscopy to I-scan view very quickly. The authors reported an accuracy of 100% in detecting total villous atrophy, and suggested a possible role of this technique in targeting biopsies in patchy distribution of lesions. However in the reported study, all the enrolled patients underwent upper gastrointestinal endoscopy for suspicion of malabsorption, so they had a high pre-test probability of duodenal atrophy.
Rokkas et al[49] recently published a meta-analysis about the role of video capsule endoscopy in CD diagnosis and reported a pooled sensitivity of the tool of 89%. A normal capsule endoscopy cannot exclude CD, however it could provide information on the extent of the disease, allowing the visualization of not accessible portion of small bowel, even thou the histological evaluation of bioptic samples still remain the gold standard for the diagnosis.
Biopsies taken during endoscopy must be oriented on filter paper, fixed in formalin and embedded in paraffine. After the cut and haematoxylin-eosin staining, an expert pathologist assesses the sections under light microscopy, evaluating the intraepithelial lymphocytes (IEL) count, the villo/crypta ratio and the villous atrophy using the Marsh modified by Oberhuber classification[16]: (1) type 0: normal mucosa with less than 40 IEL/100 enterocites (EC); (2) type 1: infiltrative, that is characterised by normal villous architecture, normal crypt height, but high IEL counts (> 40/100 EC); (3) type 2: hyperplastic, with a normal villous architecture, but but high IEL counts (> 40/100 EC) and crypt hyperplasia; (4) type 3: destructive, in which besides a high IEL counts (> 40/100 EC) and acrypt hyperplasia, it can be also observed a villous atrophy (3a: mild villous atrophy, 3b moderate villous atrophy, 3c: total villous atrophy).
Recently a new classification has ben proposed by Corazza et al[50], in order to reduce the inter and itra-observer disagreements and to facilitate the relationship between pathologists and gastroenterologist. It consists of two degrees: (1) A: non-atrophic lesions of the duodenum; and (2) B: atrophic lesions. It is divided into grade B1, that include mild and moderate villous atrophy, and grade B2, with a total villous atrophy.
The intestinal involvement, however, is not always confined to the duodenum. It has been demonstrated that other portions of the gastrointestinal tract are involved, such as the gastric[51], oral[52] and colonic mucosa[53].
The chronic superficial gastritis has been described as the most frequent form of gastritis that occurs in non treated celiac patients[54], followed by lymphocytic gastritis, a form of gastritis of uncertain pathogenesis[55].
Gluten-free diet is, at this moment, the only effective treatment for coeliac disease, allowing the healing of intestinal mucosa, the improvement of symptoms and prevents the onset of long-term complications, such as osteoporosis[56] and autoimmune disorders[57,58].
However, many efforts have been made to find an alternative therapy for CD, involving the biotechnology field, which led to a better understanding of the molecular mechanisms of coeliac disease and the identification of pathogenetic pathways that could be targeted by new drugs. Currently the main targets under investigation are[27]: (1) endopeptidases capable to detoxify gluten in order to decrease its immunogenic power; (2) modulation of permeability by the pill AT-1001; (3) block of antigen presentation made by inhibitors of TG2 and HLA-DQ2; (4) inflammation modulation using monoclonal antibodies directed against inflammatory cytokines; (5) block of the recruitment of lymphocytes by molecules that inhibit the migration to the intestinal mucosa; and (6) immunomodulation and induction of gluten tolerance.
In the last few years a new gluten- related syndrome is increasing awareness: the non celiac gluten sensitivity (NCGS). NCGS often overlaps with irritable bowel disease syndrome and for both conditions the diagnosis is based on clinical symptoms.
However it is a still poorly defined syndrome, characterized by the presence of gastrointestinal symptoms, such as bloating, abdominal pain, nausea, gastroesophageal reflux disease, and/or extraintestinal manifestation, tiredness, headache, anxiety, foggy mind and peripheral numbness[59]. The clinical presentation of these symptoms has been associates to the ingestion of gluten, however it has been hypothesize that other wheat proteins, such as amylase trypsin inhibitors, could play a role[60]. Also fermentable oligosaccharides, monosaccharides and disaccharides, contained in wheat, rye, but also in milk, legumes, honey and some vegetables (fennel, beetroot, and chicory) has been proposed to be important in NCGS[61]. NCGS is an exclusion diagnosis, so CD and wheat allergy should be rouled out. Its prevalence is still uncertain, ranging from 3.19%[59] in Italy, to 6% in United States[62] and it is more frequent in female than in male. Further study, including possibly a double blind gluten challenge, should be performed to assess the real prevalence of NCGS.
CD diagnosis is often delayed in asymptomatic patients or in individuals with less clinical gastrointestinal symptoms, such as abdominal bloating, nausea and vomiting, despite the many benefits deriving from a prompt identification.
Based on these assumptions, several studies performed coeliac disease screening in patients with symptoms suggestive of dyspepsia, showing a biopsy-proved prevalence that ranged from 0.5% to 2%[6-11]. Interestingly, the subgroup of dyspeptic patients at highest risk comprised young women, aged from 20 to 37 years (RR of CD 3.22)[6].
The 40%-60% of subjects with dyspepsia resulted macroscopically normal when performing upper gastrointestinal endoscopy[63,64]. Unfortunately, the practice of performing biopsies, even in absence of endoscopic alteration of intestinal mucosa, is quite uncommon.
The typical endoscopic markers of villous atrophy include mosaic pattern, scalloping of folds, and a decrease of duodenal folds (Figure 1). However, mostly in less severe cases, CD diagnosis cannot only be performed on these parameters. So, considering that many authors describe these markers as not sufficiently sensitive, some endoscopic techniques, such as “water immersion” and CEM were proposed to improve the diagnostic sensitivity and target biopsies in most damaged mucosal areas[12-14].
A recent meta-analysis by Ford et al[15] evaluated the yield of diagnostic testing for CD in patients affected by dyspepsia. The pooled prevalence of positive celiac serology ranged from 6% to 8%. The author pooled the data from literature and estimated that the prevalence of positive celiac serology ranged from 6% to 8%, the biopsy-proved CD prevalence was also higher in patients with dyspepsia, approximately 2%, than controls, but not in a statistically significant way. However, due to several limits that affected the paper, such as presence of study based only in tertiary care, this assumption should be confirmed further larger and, possibly, case-control studies.
In conclusion screening for CD in patients suffering from dyspeptic symptoms, as defined by Rome III criteria, and routinely performing of biopsies during upper GI endoscopy, may be useful as part of the diagnostic flow-chart of these patients, considering the benefits of a promptly beginning of a gluten-free diet, even thou further, well-defined and case-control studies on a larger population could definitively assess if CD prevalence is higher in dyspeptic patients.
| 1. | Camilleri M, Dubois D, Coulie B, Jones M, Kahrilas PJ, Rentz AM, Sonnenberg A, Stanghellini V, Stewart WF, Tack J. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Moayyedi P, Mason J. Clinical and economic consequences of dyspepsia in the community. Gut. 2002;50 Suppl 4:iv10-iv12. [PubMed] |
| 3. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1493] [Article Influence: 74.7] [Reference Citation Analysis (1)] |
| 4. | Tack J, Masaoka T, Janssen P. Functional dyspepsia. Curr Opin Gastroenterol. 2011;27:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Lacy BE, Talley NJ, Locke GR, Bouras EP, DiBaise JK, El-Serag HB, Abraham BP, Howden CW, Moayyedi P, Prather C. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Bardella MT, Minoli G, Ravizza D, Radaelli F, Velio P, Quatrini M, Bianchi PA, Conte D. Increased prevalence of celiac disease in patients with dyspepsia. Arch Intern Med. 2000;160:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Lima VM, Gandolfi L, Pires JA, Pratesi R. Prevalence of celiac disease in dyspeptic patients. Arq Gastroenterol. 2005;42:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ozaslan E, Akkorlu S, Eskioğlu E, Kayhan B. Prevalence of silent celiac disease in patients with dyspepsia. Dig Dis Sci. 2007;52:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Giangreco E, D’agate C, Barbera C, Puzzo L, Aprile G, Naso P, Bonanno G, Russo FP, Nicoletti A, Incarbone S. Prevalence of celiac disease in adult patients with refractory functional dyspepsia: value of routine duodenal biopsy. World J Gastroenterol. 2008;14:6948-6953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Keshavarz AA, Bashiri H, Ahmadi A, Bazargan-Hejazi S. The Prevalence of Occult Celiac Disease among Patients with Functional Dyspepsia: A Study from the Western Region of Iran. Gastroenterol Res Pract. 2010;2010:170702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Heikkinen M, Pikkarainen P, Takala J, Räsänen H, Julkunen R. Etiology of dyspepsia: four hundred unselected consecutive patients in general practice. Scand J Gastroenterol. 1995;30:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Leong RW, Nguyen NQ, Meredith CG, Al-Sohaily S, Kukic D, Delaney PM, Murr ER, Yong J, Merrett ND, Biankin AV. In vivo confocal endomicroscopy in the diagnosis and evaluation of celiac disease. Gastroenterology. 2008;135:1870-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Günther U, Daum S, Heller F, Schumann M, Loddenkemper C, Grünbaum M, Zeitz M, Bojarski C. Diagnostic value of confocal endomicroscopy in celiac disease. Endoscopy. 2010;42:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Cammarota G, Cazzato A, Genovese O, Pantanella A, Ianiro G, Giorgio V, Montalto M, Vecchio FM, Larocca LM, Gasbarrini G. Water-immersion technique during standard upper endoscopy may be useful to drive the biopsy sampling of duodenal mucosa in children with celiac disease. J Pediatr Gastroenterol Nutr. 2009;49:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 15. | Ford AC, Ching E, Moayyedi P. Meta-analysis: yield of diagnostic tests for coeliac disease in dyspepsia. Aliment Pharmacol Ther. 2009;30:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1225] [Article Influence: 45.4] [Reference Citation Analysis (1)] |
| 17. | Nenna R, Tiberti C, Petrarca L, Lucantoni F, Mennini M, Luparia RP, Panimolle F, Mastrogiorgio G, Pietropaoli N, Magliocca FM. The celiac iceberg: characterization of the disease in primary schoolchildren. J Pediatr Gastroenterol Nutr. 2013;56:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, Murray L, Metzger MH, Gasparin M, Bravi E. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 531] [Article Influence: 33.2] [Reference Citation Analysis (1)] |
| 19. | Bonamico M, Ferri M, Mariani P, Nenna R, Thanasi E, Luparia RP, Picarelli A, Magliocca FM, Mora B, Bardella MT. Serologic and genetic markers of celiac disease: a sequential study in the screening of first degree relatives. J Pediatr Gastroenterol Nutr. 2006;42:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Greco L, Romino R, Coto I, Di Cosmo N, Percopo S, Maglio M, Paparo F, Gasperi V, Limongelli MG, Cotichini R. The first large population based twin study of coeliac disease. Gut. 2002;50:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 252] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 21. | Nisticò L, Fagnani C, Coto I, Percopo S, Cotichini R, Limongelli MG, Paparo F, D’Alfonso S, Giordano M, Sferlazzas C. Concordance, disease progression, and heritability of coeliac disease in Italian twins. Gut. 2006;55:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Heap GA, van Heel DA. Genetics and pathogenesis of coeliac disease. Semin Immunol. 2009;21:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Price P, Witt C, Allcock R, Sayer D, Garlepp M, Kok CC, French M, Mallal S, Christiansen F. The genetic basis for the association of the 8.1 ancestral haplotype (A1, B8, DR3) with multiple immunopathological diseases. Immunol Rev. 1999;167:257-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 399] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 670] [Cited by in RCA: 682] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 418] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Bonamico M, Mariani P, Danesi HM, Crisogianni M, Failla P, Gemme G, Quartino AR, Giannotti A, Castro M, Balli F. Prevalence and clinical picture of celiac disease in italian down syndrome patients: a multicenter study. J Pediatr Gastroenterol Nutr. 2001;33:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (1)] |
| 28. | Molberg O, Mcadam SN, Körner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Norén O, Roepstorff P. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 847] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 29. | van de Wal Y, Kooy Y, van Veelen P, Peña S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585-1588. [PubMed] |
| 30. | Fleckenstein B, Molberg Ø, Qiao SW, Schmid DG, von der Mülbe F, Elgstøen K, Jung G, Sollid LM. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J Biol Chem. 2002;277:34109-34116. [PubMed] |
| 31. | Fleckenstein B, Qiao SW, Larsen MR, Jung G, Roepstorff P, Sollid LM. Molecular characterization of covalent complexes between tissue transglutaminase and gliadin peptides. J Biol Chem. 2004;279:17607-17616. [PubMed] |
| 32. | Molberg O, Kett K, Scott H, Thorsby E, Sollid LM, Lundin KE. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol. 1997;46:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Besterman HS, Bloom SR, Sarson DL, Blackburn AM, Johnston DI, Patel HR, Stewart JS, Modigliani R, Guerin S, Mallinson CN. Gut-hormone profile in coeliac disease. Lancet. 1978;1:785-788. [PubMed] |
| 34. | Urgesi R, Cianci R, Bizzotto A, Costamagna G, Riccioni ME. Evaluation of gastric and small bowel transit times in coeliac disease with the small bowel PillCam®: a single centre study in a non gluten-free diet adult Italian population with coeliac disease. Eur Rev Med Pharmacol Sci. 2013;17:1167-1173. [PubMed] |
| 35. | Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Implementation of a polling protocol for predicting celiac disease in videocapsule analysis. World J Gastrointest Endosc. 2013;5:313-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 36. | AGA Institute. AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Vitoria JC, Arrieta A, Arranz C, Ayesta A, Sojo A, Maruri N, García-Masdevall MD. Antibodies to gliadin, endomysium, and tissue transglutaminase for the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr. 1999;29:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Piacentini M, Colizzi V. Tissue transglutaminase: apoptosis versus autoimmunity. Immunol Today. 1999;20:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Li M, Yu L, Tiberti C, Bonamico M, Taki I, Miao D, Murray JA, Rewers MJ, Hoffenberg EJ, Agardh D. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. Am J Gastroenterol. 2009;104:154-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Bonamico M, Nenna R, Montuori M, Luparia RP, Turchetti A, Mennini M, Lucantoni F, Masotti D, Magliocca FM, Culasso F. First salivary screening of celiac disease by detection of anti-transglutaminase autoantibody radioimmunoassay in 5000 Italian primary schoolchildren. J Pediatr Gastroenterol Nutr. 2011;52:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Nenna R, Tiberti C, Petrarca L, Mennini M, Mastrogiorgio G, Lucantoni F, Panimolle F, Pontone S, Bavastrelli M, Magliocca FM. Anti-transglutaminase immunoreactivity and histological lesions of the duodenum in coeliac patients. Int Immunol. 2013;25:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Prause C, Ritter M, Probst C, Daehnrich C, Schlumberger W, Komorowski L, Lieske R, Richter T, Hauer AC, Stern M. Antibodies against deamidated gliadin as new and accurate biomarkers of childhood coeliac disease. J Pediatr Gastroenterol Nutr. 2009;49:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Dickey W, Hughes D. Prevalence of celiac disease and its endoscopic markers among patients having routine upper gastrointestinal endoscopy. Am J Gastroenterol. 1999;94:2182-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Lecleire S, Di Fiore F, Antonietti M, Savoye G, Lemoine F, Le Pessot F, Lerebours E, Ducrotté P. Endoscopic markers of villous atrophy are not useful for the detection of celiac disease in patients with dyspeptic symptoms. Endoscopy. 2006;38:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Ianiro G, Gasbarrini A, Cammarota G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol. 2013;19:8562-8570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 46. | Singh R, Lee SY, Vijay N, Sharma P, Uedo N. Update on narrow band imaging in disorders of the upper gastrointestinal tract. Dig Endosc. 2014;26:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | De Luca L, Ricciardiello L, Rocchi MB, Fabi MT, Bianchi ML, de Leone A, Fiori S, Baroncini D. Narrow band imaging with magnification endoscopy for celiac disease: results from a prospective, single-center study. Diagn Ther Endosc. 2013;2013:580526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Cammarota G, Ianiro G, Sparano L, La Mura R, Ricci R, Larocca LM, Landolfi R, Gasbarrini A. Image-enhanced endoscopy with I-scan technology for the evaluation of duodenal villous patterns. Dig Dis Sci. 2013;58:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 50. | Corazza GR, Villanacci V. Coeliac disease. J Clin Pathol. 2005;58:573-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Pontone S, Nenna R, Mastrogiorgio G, at al. Gastric pathology in celiac children and adults: the role of Helicobacter pylori. Prevent Res. 2012;2:281-287. [DOI] [Full Text] |
| 52. | Pastore L, Carroccio A, Compilato D, Panzarella V, Serpico R, Lo Muzio L. Oral manifestations of celiac disease. J Clin Gastroenterol. 2008;42:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Casella G, Villanacci V, Di-Bella C, de-Marco E, Pagni F, Drera E, Ortenzi R, Baldini V, Bassotti G. Colonoscopic findings in coeliac disease on a gluten-free diet. Rev Esp Enferm Dig. 2010;102:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Oderda G, Forni M, Morra I, Tavassoli K, Pellegrino P, Ansaldi N. Endoscopic and histologic findings in the upper gastrointestinal tract of children with coeliac disease. J Pediatr Gastroenterol Nutr. 1993;16:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Nenna R, Magliocca FM, Tiberti C, Mastrogiorgio G, Petrarca L, Mennini M, Lucantoni F, Luparia RP, Bonamico M. Endoscopic and histological gastric lesions in children with celiac disease: mucosal involvement is not only confined to the duodenum. J Pediatr Gastroenterol Nutr. 2012;55:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Stazi AV, Trecca A, Trinti B. Osteoporosis in celiac disease and in endocrine and reproductive disorders. World J Gastroenterol. 2008;14:498-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Ansaldi N, Palmas T, Corrias A, Barbato M, D’Altiglia MR, Campanozzi A, Baldassarre M, Rea F, Pluvio R, Bonamico M. Autoimmune thyroid disease and celiac disease in children. J Pediatr Gastroenterol Nutr. 2003;37:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Salardi S, Volta U, Zucchini S, Fiorini E, Maltoni G, Vaira B, Cicognani A. Prevalence of celiac disease in children with type 1 diabetes mellitus increased in the mid-1990 s: an 18-year longitudinal study based on anti-endomysial antibodies. J Pediatr Gastroenterol Nutr. 2008;46:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 60. | Di Sabatino A, Giuffrida P, Corazza GR. Still waiting for a definition of nonceliac gluten sensitivity. J Clin Gastroenterol. 2013;47:567-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657-666; quiz 667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 63. | Gear MW, Barnes RJ. Endoscopic studies of dyspepsia in a general practice. Br Med J. 1980;280:1136-1137. [PubMed] |
| 64. | Thomson AB, Barkun AN, Armstrong D, Chiba N, White RJ, Daniels S, Escobedo S, Chakraborty B, Sinclair P, Van Zanten SJ. The prevalence of clinically significant endoscopic findings in primary care patients with uninvestigated dyspepsia: the Canadian Adult Dyspepsia Empiric Treatment - Prompt Endoscopy (CADET-PE) study. Aliment Pharmacol Ther. 2003;17:1481-1491. [PubMed] |
P- Reviewer: Brogna A, Koulaouzidis A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL