Published online Mar 26, 2014. doi: 10.5662/wjm.v4.i1.11
Revised: January 2, 2014
Accepted: January 15, 2014
Published online: March 26, 2014
Processing time: 118 Days and 13.2 Hours
Human leukocyte antigen-G (HLA-G) is a non-classical HLA class I molecule that differs from classical HLA class I molecules by low polymorphism and tissue distribution. HLA-G is a tolerogenic molecule with an immune-modulatory and anti-inflammatory function on both innate and adaptative immunity. This peculiar characteristic of HLA-G has led to investigations of its role in pathological conditions in order to define possible uses in diagnosis, prevention and treatment. In recent years, HLA-G has been shown to have an important implication in different inflammatory and autoimmune diseases, pregnancy complications, tumor development and aggressiveness, and susceptibility to viral infections. In fact, HLA-G molecules have been reported to alternate at both genetic and protein level in different disease situations, supporting its crucial role in pathological conditions. Specific pathologies show altered levels of soluble (s)HLA-G and different HLA-G gene polymorphisms seem to correlate with disease. This review aims to update scientific knowledge on the contribution of HLA-G in managing pathological conditions.
Core tip: Human leukocyte antigen-G (HLA-G) is a tolerogenic molecule. HLA-G has been shown to have important implications in different pathological conditions where it is reported to alternate at both protein and genetic level. The peculiar immunoregulatory function of HLA-G and its dysregulation in different diseases have led to investigation of its role in pathological conditions in order to define possible uses in diagnosis, prevention and treatment. This review aims to update scientific knowledge on the contribution of HLA-G in managing pathological conditions.
- Citation: Bortolotti D, Gentili V, Rotola A, Cassai E, Rizzo R, Luca DD. Impact of HLA-G analysis in prevention, diagnosis and treatment of pathological conditions. World J Methodol 2014; 4(1): 11-25
- URL: https://www.wjgnet.com/2222-0682/full/v4/i1/11.htm
- DOI: https://dx.doi.org/10.5662/wjm.v4.i1.11
Diagnosis and prevention of diseases is mainly based on the identification of specific biological markers and drug targets. In view of this, the possibility of easy and fast identification of molecules, for example in biological fluids, seems to be even more necessary.
In recent years, different studies have demonstrated that human leukocyte antigen-G (HLA-G), a non-classical class I molecule, could fulfil this necessity[1-3]. In fact, HLA-G expression and levels in biological fluids, cells and tissues in different pathological conditions have been shown. Several authors reported that the level of soluble HLA-G and gene polymorphisms correlate with disease outcome and the therapeutic success of treatment[4-6].
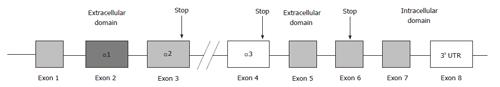
HLA-G is a major histocompatibility complex class I antigen encoded by a gene on chromosome 6p21. It differs from classical HLA class I molecules by its restricted tissue distribution and limited polymorphism in the coding region. To date, 50 alleles (IMGT HLA database, August 2013) and 16 proteins are known. The gene structure of HLA-G is homologous to other HLA class I (Ia) genes consisting of 7 introns and 8 exons coding the heavy chain of the molecule. Exon 1 encodes the peptide signal, while exons 2, 3 and 4 encode the extracellular α1, α2 and α3 domains, respectively. Exons 5 and 6 encode the transmembrane and cytoplasmic domains of the heavy chain. Exon 7 is always absent from mature mRNA due to the stop codon in exon 6; exon 8 is not translated (Figure 1). Seven HLA-G isoforms exist due to mRNA alternative splicing and differential association with β2-microglobulin; two of these are found on the cell surface and in biological fluids: Membrane-bound G1 and soluble G5, which lacks the trans-membrane and intracellular domains of membrane-bound G1 (Figure 1)[7]. HLA-G possesses an unpaired cysteine residue at position 42 on an external loop of the peptide binding groove that enables the dimerisation[8,9]. HLA-G monomers are recognized by the inhibitory receptors LILRB1 and LILRB2 and by KIR2DL4[10]. LILR receptors have a greater affinity for the dimeric form that increases the signaling transduction, especially in natural killer (NK) cells[11,12]. The interaction of HLA-G molecules with inhibitory receptors induces apoptosis of activated Crohn’s disease (CD8+) T cells[11], modulates the activity of NK cells[13,14] and of dendritic cells (DC)[15,16], blocks allo-cytotoxic T lymphocyte response[17] and induces expansion of suppressor T cell populations, such as CD4+CD25+FoxP3+ regulatory T (Treg) cells[18,19]. Moreover, HLA-G is expressed at high levels on DC-10 cells, human DCs with tolerogenic activity and an outstanding ability to produce interleukin (IL)-10[16]. Interestingly, the expression of membrane-bound HLA-G1 and that of its receptors is up-regulated by IL-10 on DC-10 and the expression of high levels of membrane-bound HLA-G1, ILT4 and IL-10 by DC-10 is critical to the generation of allergen-specific Tr1 cells by DC-10[16].
The HLA-G production is controlled by several polymorphisms, both in the promoter and in the 3’ untranslated region (3’ UTR), modifying the affinity of gene targeted sequences for transcriptional or post-transcriptional factors, respectively[20] .
Twenty-nine single nucleotide polymorphisms (SNPs) have been identified in the HLA-G promoter region which may be involved in the regulation of HLA-G expression, considering that many of these polymorphisms are within or close to known or putative regulatory elements. The HLA-G 5’ upstream regulatory region (URR) is unique among the HLA genes[21] and is unresponsive to NF-κB[22] and interferon (IFN)-γ[23] due to the presence of a modified enhancer A and a deleted interferon-stimulated response element (ISRE). A locus control region located -1.2 kb from exon 1 exhibits a binding site for CREB1 factor, which also binds to two additional cAMP response elements at -934 and -770 positions from the ATG. In addition, a binding site ISRE for interferon response factor-1 is located at the -744 base pair (bp) position[24] and is involved in HLA-G transactivation following IFN-β treatment[24]. The HLA-G promoter also contains a heat shock element at the -459/-454 position that binds heat shock factor-1[25] and a progesterone receptor binding site at -37 bp from ATG[26]. Several promoter region polymorphisms coincide with or are close to known or putative regulatory elements and thus may affect the binding of HLA-G regulatory factors[27]. The -725 C > G/T SNP is very close to ISRE, in which the -725 G allele is associated with a significantly higher expression level compared with the others[28]. The polymorphic sites at the 5’ URR are frequently in linkage disequilibrium with the polymorphic sites identified at the 3’ UTR, some of them influencing alternative splicing and mRNA stability.
A 14 bp insertion/deletion (INS/DEL) polymorphism (rs66554220) in exon 8 involves mRNA stability and expression[29,30]. In particular, the DEL allele stabilizes the mRNA with a consequent higher HLA-G expression[30,31]. The presence of an adenine at position +3187 modifies an AU-rich motif in the HLA-G mRNA and decreases its stability[32]. One SNP C > G at the +3142 bp position (rs1063320) affects the expression of the HLA-G locus by increasing the affinity of this region for the microRNAs (miR)-148a, miR-148b and miR-152, therefore decreasing the mRNA availability by mRNA degradation and translation suppression[33]. The influence of the +3142G allele has been demonstrated by a functional study in which HLA-G high-expressing JEG-3 choriocarcinoma-derived cells have been transfected with miR-148a, decreasing soluble HLA-G levels. The discordant results obtained by Manaster et al[34], who have reported the lack of +3142 C > G effect on the miRNA control of membrane HLA-G expression, prompt further considerations on the relationship between this polymorphism and membrane HLA-G expression. Other SNPs are identified as implicated in miRNA interaction. In particular, +3003, +3010, +3027 and +3035 SNPs are targets for miR-513a-5p, miR-518c*, miR-1262 and miR-92a-1*, miR-92a-2*, miR-661, miR-1224-5p and miR-433 miRNAs[35]. The miR-2110, miR-93, miR-508-5p, miR-331-5p, miR-616, miR-513b, and miR-589* miRNAs target the 14bp INS/DEL fragment region and miR-148a, miR-19a*, miR-152, mir-148b, and miR-218-2 target the +3142 C/G polymorphism.
HLA-G is a stress-inducible gene; heat shock, hypoxia and arsenite increase different HLA-G alternative transcripts[25,36,37]. The indoleamine 2,3-dioxygenase, an enzyme which metabolizes tryptophan, induces HLA-G expression during monocyte differentiation into DCs[38]. The anti-inflammatory and immunosuppressive IL-10 has been correlated with concomitant HLA-G expression[30,39]. Transactivation of HLA-G transcription has also been demonstrated by leukemia inhibitory factor[40], progesterone[26] and methotrexate[41] cell exposure. Furthermore, IFN-α, -β and -γ enhance HLA-G cell-surface expression by tumors or monocytes[42,43]. HLA-G expression could be acquired by trogocytosis, where a “donor” cell that expresses membrane HLA-G exchanges membrane parts containing HLA-G with a “recipient” cell that is not expressing HLA-G molecules. In this particular situation, “recipient” cells will acquire and make use of membrane HLA-G molecules from a “donor” HLA-G positive cell without the activation of HLA-G gene. Trogocytosis of antigen presenting cell HLA-G1 by T cells in humans makes T cells unresponsive[44]. It has been shown that HLA-G1 can be acquired by NK cells from tumor cells. NK cells that acquire HLA-G1 stop proliferating, are no longer cytotoxic and behave like suppressor cells capable of inhibiting other NK-cell functions[14].
HLA-G’s role in immune-tolerance was discovered studying its expression in trophoblast cells at the fetus-maternal interface[45]. The importance of HLA-G production by placental trophoblasts is evident in pre-eclampsia and unexplained recurrent spontaneous abortion (RSA). Several studies have found an aberrant or reduced expression of both HLA-G mRNA and protein in pathological compared with control placentas[46-48], with a possible implication in fetal protection and vascular events.
HLA-G expression has been documented in a few tissues during physiological conditions, such as cornea, thymus, erythroid and endothelial precursors[49-51], and in a variable percentage of serum/plasma samples from healthy subjects[52] where the main producers are activated CD14 positive monocytes[53]. A modified expression of HLA-G molecules has been observed during “no-physiological” conditions, such as viral infection[54-57], cancer[58,59], transplantation[60-64], inflammatory and autoimmune diseases[65,66].
Thus, a growing body of evidence has indicated HLA-G as a suitable key factor in different pathologies. In fact, the immune-modulation by HLA-G may exhibit two distinct effects in pathological conditions: It could be protective in inflammatory and autoimmune diseases[2,65-67], or on the other hand it could be dangerous, for example in tumors or infectious diseases[54-56,58,59]. Based on this evidence, the role of HLA-G in inflammatory and autoimmune diseases has gained considerable clinical interest for the possibility of exploiting it as a molecular biomarker and a therapeutic target.
Given the immunomodulatory nature of HLA-G molecule, it could be considered a good reference parameter for prevention, diagnosis and treatment in autoimmune and inflammatory diseases.
HLA-G has been analyzed in different pathologies. In this review, we focus on the importance of HLA-G analysis in common and debilitating pathologies characterized by a dysregulation in host immune system in which HLA-G plays a central role.
Rheumatic disease is a general term used to describe numerous conditions that affect the joints [rheumatoid arthritis (RA)], connective tissues [scleroderma, systemic lupus erythematosus (SLE)] and vessels (vasculitis). Rheumatic diseases are inflammatory and autoimmune diseases, the second most common cause of disability after musculoskeletal injuries. RA (OMIM, #180300) is caused by the immune system attacking synovial cells and treatments include disease modifying anti-rheumatic drugs (DMARDs) and, more recently, biological agents. An important goal of RA therapy has shifted to initiate treatment early and aggressively to achieve remission or low disease activity as quickly as possible. This “treat-to-target” concept has been shown to maximize long-term healthy life[68,69].
Interestingly, RA patients present with an abnormal regulatory network in the immune response, which includes HLA-G gene[70]. Serum sHLA-G protein concentration is significantly lower in RA[71] patients than in controls. The decreased sHLA-G concentrations may lead to a chronic activation of inflammatory cells and contribute to the development of the disease. The evaluation of sHLA-G molecules at the specific inflammation site of the synovia reported higher levels of sHLA-G in RA[72] patients. The release of HLA-G in the inflamed synovium may be related to the recruitment of activated HLA-G positive immune cells and the local production by activated synovial fibroblasts[73] that could interact with immune inhibitory receptors and maintain a chronic inflammatory response. These data suggest that there is a different production of HLA-G molecules on the basis of the local and systemic environments, characterized by different molecular factors and cell types. Interestingly, a recent work confirmed the role of HLA-G molecules in RA. The authors used an intracutaneous treatment of HLA-G monomer or dimer molecules in collagen-induced arthritis model mice. These molecules produced excellent anti-inflammatory effects with a single, local administration[74]. Notably, the dimer exhibited higher immunosuppressive effects than the monomer due to the higher dimer affinity for PIR-B, the mouse homolog of the LILRBs. The HLA-G 14 bp INS/DEL polymorphism has been evaluated as a pharmacogenetic marker of MTX therapy[41]. The authors showed an increase of the 14 bp DEL/DEL genotype in the responder group, characterized by a reduction in disease activity score (DAS28) measured before and after six months of treatment with MTX. In contrast to this study, there are two researches with negative results: (1) 130 RA patients responsive to MTX did not show a significant difference in 14 bp DEL/INS allelic and genotypic distribution (DAS28 < 3.2)[75]; and (2) 186 RA patients, previously untreated with MTX, were prospectively followed up and considered as responders with a DAS28 of up to 2.4 after six months of treatment[76]. No significant association between HLA-G 14 bp INS/DEL and MTX efficacy was observed. Comparing these studies, the discordant results may reflect population differences in gene expression that could influence the power of association studies and lead to different levels of association. In addition, the different doses of MTX and the different cut-off used for RA therapy response definition could affect the results obtained.
Rizzo et al[2] evaluated the possible role of HLA-G molecules as biomarkers for RA treatment in a follow-up study. Twenty-three early RA (ERA) patients were analyzed during a 12 mo follow-up disease treatment for sHLA-G levels in plasma samples, mHLA-G and ILT2 expression on peripheral blood CD14 positive cells, and typed HLA-G 14 bp DEL/INS polymorphism. Interestingly, the authors observed that ERA patients with low sHLA-G and membrane HLA-G expression suffered a more severe disease. In fact, sHLA-G levels inversely correlated with DAS28 and ultrasonographic power Doppler scores, used to define the severity and progression of the disease. Interestingly, sHLA-G up-modulation is evident after 3 mo of DMARDs therapy, while a significant reduction in tumor necrosis factor-α levels is evident after 9 mo therapy when a clear amelioration of the disease is evident, with a high specificity for HLA-G detection in EA condition. Moreover, the implication of the HLA-G 14 bp INS/DEL polymorphism is confirmed as the presence of the DEL allele characterizes the patients with a significant improvement in disease status.
SLE (OMIM, #601744) is a systemic autoimmune disease of the connective tissue that can affect any part of the body. Rosado et al[77] and Chen et al[78] showed higher sHLA-G and IL-10 levels in SLE patients in comparison with healthy controls, while Rizzo et al[66] observed lower sHLA-G concentrations in SLE patients. The differences in sHLA-G levels in these two papers could be due to the difference in the analyzed samples (serum or plasma) since it is known that the highest sHLA-G levels are recovered from plasma samples compared with serum collected from the same subjects because of a trapping phenomenon during clot formation that could subtract sHLA-G from the serum[79]. As a proof, Monsiváis-Urenda et al[80] evidenced a diminished expression of HLA-G in monocytes and in mature CD83 positive DCs from SLE patients compared with healthy controls. In addition, monocytes from SLE patients showed a decreased induction of HLA-G expression in response to IL-10. Finally, lymphocytes from SLE patients displayed a lower acquisition of HLA-G (by trogocytosis) from autologous monocytes compared to controls. Interestingly, ILT-2 receptor expression is increased on lymphocytes from SLE patients, in particular, in CD3 positive cells, CD19 positive cells, CD56 positive cells and related to IL-10 and anti-DNA antibodies[78]. These results confirm the presence of a HLA-G impaired expression in patients with SLE and a possible role in the pathogenesis. Using a SNP mapping approach, HLA-G gene is reported to be a novel independent locus with SLE interaction[81]. In particular, HLA-G 14 bp INS/DEL polymorphism and HLA-G +3142 C > G SNP were analyzed in a SLE population. SLE patients showed a higher frequency of 14 bp INS allele and 14 bp INS/INS genotype[66]. Moreover, 14 bp INS/INS patients presented the highest disease activity[82]. On the contrary, the evaluation of HLA-G 14 bp INS/DEL polymorphism in a SLE Brazilian population failed to present an association[83], while the +3142 G allele was found to be associated with SLE susceptibility[84]. The +3142 G allele and the +3142 GG genotype frequencies are increased among SLE patients compared with controls[85]. These data support the role of HLA-G molecules in the control of the SLE condition and in particular several results sustain the lower HLA-G expression as a risk factor for SLE development.
Multiple sclerosis (MS) (OMIM, #126200) is a chronic inflammatory demyelinating and neurodegenerative disease of the central nervous system (CNS) with unknown etiology that is widely considered to be autoimmune in nature[86]. The presence in CSF of detectable sHLA-G levels in relapsing-remitting MS (RRMS) patients and, occasionally, in other inflammatory neurological disorders and non-inflammatory neurological disorders was reported for the first time by Fainardi and coauthors[87]. In addition, sHLA-G levels in CSF are higher in RRMS than in controls and increased, in association with IL-10 values, in RRMS patients without than in those with magnetic resonance imaging (MRI) evidence of disease activity[88]. The importance of sHLA-G level evaluation as a biomarker for MS is confirmed[89]. Of note, in RRMS patients, CSF concentrations of sHLA-G and IL-10 are positively correlated with inactive MRI disease and CSF IL-10 titers are more elevated in patients with than in those without CSF measurable levels of sHLA-G. These data suggest that CSF sHLA-G levels may modulate MS disease activity acting as anti-inflammatory molecules under the control of IL-10 CSF levels which may enhance sHLA-G production together with the influence due to HLA-G polymorphisms[67]. The existence of high CSF concentrations of sHLA-G in MS patients and their association with clinical and MRI stable disease have been repeatedly confirmed in subsequent investigations in which: (1) An intrathecal production of sHLA-G is more frequent in MS than in inflammatory and non-inflammatory controls and predominated in clinically and MRI inactive compared to clinically and MRI active MS[88]; (2) sHLA-G concentrations reciprocally fluctuate in CSF and serum of MS patients because they are decreased in the serum of clinically stable MS and increased in CSF of MRI inactive MS[65]; (3) CSF levels of HLA-G5 and not those of sHLA-G1 isoforms are increased in MS compared to controls and in MS patients without MRI appearance of disease activity than in those with MRI Gd-enhancing lesions[90]; and (4) CSF values of sHLA-G and antiapoptotic sFas molecules are inversely correlated in MS patients with no evidence of MRI disease activity since CSF concentrations of sFas are lower in MS than in controls and in MRI inactive than in MRI active MS[90]. Interestingly, HLA-G and its inhibitory receptors (ILT-2 and ILT-4) are strongly up-regulated within and around MS lesions where microglia, macrophages and endothelial cells are recognized as the cellular sources[91]. Furthermore, protein HLA-G expression is higher on cultured human MS microglial cells after activation with Th1 proinflammatory cytokines and a novel subpopulation of naturally occurring CD4 positive and CD8 positive Treg cells expressing HLA-G (HLA-Gpos Treg) has been recently described in peripheral blood of MS patients with relapse[92].
Further studies demonstrated that IL-10 contributes to mediating the suppressive activity of CD4 positive HLA-Gpos Treg[93] which are highly represented in CSF and inflammatory brain lesions of MS patients as activated central memory T cells capable of migrating from the periphery to intrathecal compartment due to the expression of CCR5[94]. These results strengthen the assumption of an association between HLA-G antigens and MS.
Collectively, these observations provide evidence that HLA-G antigens are likely to be involved in the resolution of MS autoimmunity acting as anti-inflammatory molecules and suggest that HLA-G positive Treg could play a role in the development of a CNS immunosuppressive microenvironment at the sites of inflammation in MS.
HLA-G proves to also be an important biological marker in other pathologies, for example, gastrointestinal and allergic diseases and diabetes.
Inflammatory bowel disease (OMIM, #266600) is the general term for CD and ulcerative colitis (UC), two chronic inflammatory disorders of the intestine which have different clinical, morphological and immunological characteristics.
Torres et al[95] studied intestinal samples of UC and CD patients and, by using an immunohistochemistry technique, demonstrated that while UC intestinal cells presented with HLA-G on their surface, CD intestinal biopsies did not. This result combined with high levels of IL-10 found in the lamina propria of the colon of UC patients suggested that HLA-G can regulate the mucosal immune responses in UC. The distribution of the 14 bp INS/DEL polymorphism in UC and CD was investigated by Glas et al[96]. They observed an increase of both 14 bp DEL/INS and 14 bp INS/INS genotypes and a consequent decrease of the high producer genotype (14 bp DEL/DEL) in UC subjects in comparison with CD patients. Also, Rizzo et al[97] found a different HLA-G expression in UC and CD patients. Non activated peripheral blood mononuclear cells from CD patients spontaneously secrete sHLA-G, while those from UC patients and healthy donors do not. Furthermore, after stimulation with LPS, both cells from CD and healthy subjects show sHLA-G production, while this does not happen in UC patients. This defective production in UC patients seems to be due to an altered secretion of IL-10 in response to inflammation. The different HLA-G expression profiles in UC and CD patients sustain the different etiopathogenesis at the origin of these two diseases. This hypothesis is sustained by the different modulation of HLA-G observed in the two pathologies after therapy[98]. On the basis of this evidence, it is possible to propose sHLA-G and IL-10 levels as diagnostic parameters to facilitate the diagnosis of UC and CD patients.
Asthma (OMIM, #600807) is a chronic disease affecting approximately 300 million people worldwide, with 180000 deaths resulting annually from severe asthma attacks. Asthma is characterized by chronic inflammation in the airway, which consequently narrows more easily in response to a variety of triggers than the airway of a healthy individual. Nicolae et al[99] suggested the role of HLA-G as a potential asthma and bronchial hyperresponsiveness (BHR) susceptibility gene. In particular, susceptibility varies depending on whether the mother has asthma or BHR. A G/G genotype at SNP -964G/A in the promoter region was associated with asthma in the offspring of mothers with either asthma or BHR, whereas the A/A genotype was associated with asthma in the offspring of asthma- and BHR-free mothers. Tan et al[33] discovered an association between +3142 C > G (rs1063320) and asthma. HLA-G5 is expressed by airway epithelium and is present in the bronchoalveolar lavage fluid from asthmatic patients[100,101]. In addition to the local presence in airways, sHLA-G may also be found in asthmatic subjects outside the lung. The plasma sHLA-G levels are higher in atopic asthmatic children than in both non-atopic, asthmatic and non-atopic, non-asthmatic children[101]. The 14 bp INS/DEL polymorphism has no impact on plasma sHLA-G levels in the atopic, asthmatic children. Thus, circulating HLA-G may be important as a biomarker and could potentially modulate immune function more broadly, while the local abundance in airways may have a more direct relationship with immune modulation in the mucosa. There is also in vitro evidence that the presence of HLA-G may be different in an asthma condition in comparison with physiological status. sHLA-G expression by peripheral blood mononuclear cells is reduced in asthmatic patients[102] while it is increased in asthma induced by isocyanates[103]. This different behavior may represent differences in biological roles in different disease contexts. A loss of HLA-G could reduce immunosuppression and perpetuate inflammation, whereas increased HLA-G in asthma could be an attempt to reassert immunosuppression. Interestingly, HLA-G is differentially expressed during the lung development[104], suggesting a potential role in lung inflammation induction and chronicization.
Allergic rhinitis (AR) (OMIM, #607154) is characterized by a Th2 polarized immune response. sHLA-G molecules are increased in sera of patients with pollen-induced AR studied outside the pollen season[105], during the pollen season[106] and in perennial AR patients[107]. Interestingly, sublingual immunotherapy (SLIT) for AR is able to reduce sHLA-G serum levels in pollen allergic patients[108,109], suggesting a clinical implication as a biomarker of response to SLIT. Interestingly, children with AR have significantly higher levels of sHLA-G molecules than normal controls or children with allergic asthma[110].
During human pregnancy, the maternal immune system recognizes and eliminates alloantigens derived from bacteria or virus, but it tolerates genetically different fetal cells, especially extravillous trophoblast cells invading the maternal decidua or entering the spiral arteries. The expression of HLA-G antigens by trophoblasts is of major importance in protecting the fetus from the semiallogeneic response of the mother[111].
The lack of an established immunological tolerance in pregnancy results in an immune response against paternal antigens expressed by the fetus at the placenta, causing severe health problems for both the fetus and the mother. Complications during pregnancy may affect the woman, the fetus, or both. Miscarriage, RSA and pre-eclampsia account for the most frequent pregnancy complications[112] and the dysregulation of the immunological control at the fetal-maternal interface seems to play a role in these pregnancy complications.
Interestingly, there is a reduced expression of both HLA-G mRNA and protein in pathological compared with control placentas[46-48,113]. In pregnant women, there is a peak of sHLA-G levels in plasma samples in the first trimester that is not evidenced in complicated pregnancies[114,115]. In particular, pregnant women with low sHLA-G plasma levels are characterized by a relative risk of 7.12 of developing placental abruption[116].
The lower secretion of HLA-G by maternal immune cells seems to be in part influenced by HLA-G gene polymorphisms, affecting mRNA stability. In particular, the HLA-G 14 bp ins allele decreases mRNA stability[29,117] and protein production[30,39,118-120]. The HLA-G 14 bp INS/DEL polymorphism seems to affect the fetal HLA-G expression as independent studies have reported fetuses carrying the homozygous genotype for the 14 bp INS allele with a significantly increased risk of pre-eclampsia[121-124]. In addition, the 5’ URR seems to be implicated in pathological pregnancies[125]. The confirmed role of HLA-G molecules during pregnancy suggests a potential use in clinical practice. Most pregnancy complications are controversial in terms of diagnosis and treatment. As an example, pre-eclampsia can mimic and be confused with many other diseases and none of the signs are specific. The lower levels of sHLA-G detected in maternal plasma and the HLA-G polymorphism association could assist clinicians in an accurate and reliable diagnosis. Moreover, the HLA-G genetic background of the mother could be an a priori sign of an increased risk of complication during pregnancy. These women could be identified and proposed for a stricter follow-up. It is noteworthy that with an appropriate and timely treatment, the success rate is approximately 80%. Therefore, the use of HLA-G as a biological and genetic marker could improve the management of pregnant women. Moreover, the ability to control HLA-G expression in pathological pregnancies and in women with a high risk of pregnancy complications and infertility could be a tool to cure and prevent these conditions with a deep impact, not only for the individual but also for society.
Until now, more than 15000 embryo culture supernatants have been evaluated for sHLA-G expression, with a positive correlation with embryo implantation rate and pregnancy outcome[126]. However further research is needed to investigate HLA-G in assisted reproductive technologies, but recent studies suggest that sHLA-G is a good candidate as a valuable non-invasive embryo marker to improve pregnancy outcome[127]. Three aspects should be taken into consideration: (1) The recognition of a common sHLA-G detection protocol; (2) The necessity to identify a standardized range for positivity; and (3) The comprehension of the factors involved in the differential expression of sHLA-G between equal stage embryos originating from the same woman.
A high frequency of HLA-G surface expression and increased sHLA-G serum levels has been detected in both hematological and solid tumors. HLA-G and sHLA-G expression correlates with a poor clinical outcome in tumor patients, suggesting a role in the immune escape mechanism of tumors. The frequency of HLA-G expression varies between different types of cancer and even between different studies in the same type of tumor, probably due to the criteria of patient selection and the methodology used. In hematological malignancies, HLA-G expression was documented with a higher frequency in acute myeloid leukemia cases[128], B and T acute lymphoid leukemia and chronic B lymphocyte leukemia[1,129].
HLA-G expression is frequent in choriocarcinoma[45,130,131], breast[132-135], endometrial[136], and ovarian cancers[137]. In digestive tumors, HLA-G expression was described in esophageal squamous cell carcinoma[138], colorectal cancer[139,140], gastric cancer[19], and liver cancer[141]. In relation to increased membrane HLA-G expression in cancer, higher circulating sHLA-G concentrations were described in patients suffering from different types of cancer[142,143].
These data suggest that HLA-G levels might be used as a diagnostic tool to distinguish between malignant and benign tumors and during disease follow-up. Moreover, HLA-G might serve as a possible marker for tumor sensitivity to chemotherapy and as a prognostic marker for advanced disease stage and clinical outcome. HLA-G assay, either in biological fluids or in biopsies, may have a clinical value in diagnosis, staging, or prognosis of cancer, but prospective validation studies should be conducted in order to use it as a biomarker.
Indeed, it would be important to suppress its immune-suppressive expression in cancer. HLA-G blockade in those tumors that express it remains an attractive therapeutic strategy against cancer. Targeting HLA-G-expressing cancer cells would be also important for maximize the efficacy of anticancer therapies. An experimental approach to target HLA-G-expressing cells in a renal cell carcinoma model was the use of HLA-G- derived peptides based on the binding motif to the HLA-A24[144]. HLA-G peptides induced a cytotoxic attack against HLA-G-expressing HLA-A24 tumor cells, suggesting that HLA-G-mediated suppression can be overcome using peptide-derived immunotherapy.
Host immune defence mechanisms are efficient at eliminating most viral infections. However, some viruses have developed multiple strategies for subverting host immune defences, thus facilitating their spread in the host[145]. Virus-infected cells are protected against attack by NK cells by HLA-G, providing a long-term immunosuppression function. It may be, therefore, that the diminished immune function induced by HLA-G in the host sometimes leads to an advantage for virus progression by helping viruses subvert the host’s antiviral defences[146].
Human immunodeficiency virus type 1 (HIV-1) infection is associated with severe and progressive loss of the immune function in infected persons. It is known that HIV-1 protects infected cells from T lymphocytes and NK cell recognition and lyses by classical HLA-A and B down-regulation and non-classical HLA-G molecule up-regulation, respectively. Since the immunoregulatory ability of HLA-G has become known, the involvement of this molecule in the progression of HIV-1 infection has been widely examined. Studies have focused on the expression of HLA-G in monocytes, which are relevant as reservoirs of HIV-1, and in lymphocytes, which are more susceptible to be infected by HIV-1. Monocytes obtained from HIV-1 seropositive patients expressed HLA-G, although only a small proportion of healthy individuals express this molecule[147]. This might be a consequence of highly active antiretroviral therapy (HAART) since a greater proportion of monocytes expressing HLA-G was observed in patients undergoing HAART compared to untreated[148]. T cells obtained from HIV-1 seropositive individuals were found to express HLA-G at a higher proportion[149] and behave as HLA-G+ Treg.
Human cytomegalovirus (HCMV) is a herpes virus causing widespread, persistent human infection in a delicate balance between the progression of the virus and the defences of the host[150]. HCMV has evolved a number of independent strategies to evade the immune system. HLA-G is produced during viral reactivation in macrophages and astrocytoma cells[56] and the percentage of HLA-G-positive monocytes and sHLA-G levels in patients with active HCMV infection were both dramatically higher than in healthy individuals[151]. The up-regulation observed in HLA-G is probably related to a virus-encoded homologue of human IL-10 (cmvIL-10)[151], which prevents NK cell recognition of infected cells.
Evidence also supports a role of HLA-G in human papilloma virus (HPV) infections. In fact, HLA-G may play a role in mediating HPV infection risk[152] and facilitate cervical cancer development[153].
The ability of specific neurotropic viruses to induce the formation of HLA-G in infected neurons, thus conferring protection against NK cells, was demonstrated. For example, herpes simplex virus-1 and Rhabdovirus[154], trigger the expression and up-regulation of membrane and soluble HLA-G molecules in actively infected neurons.
There is also some evidence that HCV and HBV viruses use HLA-G as a strategy to evade the immune response[155-158].
In summary, one of the main mechanisms of virus evasion is the induction of changes in levels of the classical HLA-G proteins. This enables the virus to prevent infected cells from being recognized and attacked by CTL and NK cells. The main challenge would be to block HLA-G up-modulation by viral infection in order to allow the recognition by immune cells.
This review has underlined the importance of HLA-G molecules in pathological conditions.
The literature data suggest that HLA-G could be implicated in both risk and disease chronicization where this antigen is characterized by an impaired expression depending on the different disease environment.
In fact, HLA-G proteins seem to be involved in the regulation of the immune system during autoimmune and allergic conditions, such as gastrointestinal, skin, neurological, rheumatic diseases, in pathological pregnancies and in the immune escape mechanisms during viral infections and tumor transformation. In particular, in these disorders, HLA-G proteins could directly interact with immune cells or control the balance between Th1 and Th2 cytokines. In fact, a disequilibrium in this setting would maintain an inflammatory and immune-deregulated condition.
The comprehension of the specific role and mechanisms of action of HLA-G antigens in the development and progression of inflammatory and autoimmune disorders could justify the use of HLA-G molecules as a marker of inflammation and drug treatment and open up new therapeutic perspectives. Moreover, the definition of the role of HLA-G genetic polymorphisms as risk and pharmacogenetic markers could sustain the clinical relevance of HLA-G typing in the laboratory routine. In particular, the possibility to use simple, non-invasive and standardized tools for HLA-G analysis makes it quickly transferable to the health care system practice. These could help in pathology outcome prediction and support treatment decisions.
As reported in Table 1, there are still contrasting results that need to be taken into consideration. The present challenge is to confirm whether HLA-G molecules have a potential role in prevention and diagnosis of pathological conditions. The perspective to identify pharmacological strategies to control the HLA-G production would represent a concrete possibility to improve the control of inflammation and to guide the therapeutic approach. In fact, the possible use of HLA-G as a therapeutic target is of extreme interest.
| Topics | HLA-G genetics and polymorphism | Protein | Ref. |
| Autoimmune and inflammatory pathologies | |||
| Rheumatoid arthritis | Lower plasma sHLA-G levels than in controls | 71 | |
| Higher sHLA-G levels in the synovia | 73 | ||
| Plasma level of sHLA-G correlates with disease activity parameters | 2 | ||
| Increase in 14 bp DEL/DEL genotype frequency in responsive patients to MTX treatment | 41 | ||
| Increase in 14 bp DEL allele frequency in patients with improved disease status | 2 | ||
| Systemic lupus erythematosus | Higher level of sHLA-G and IL-10 in plasma that in controls | 77,78 | |
| Higher frequency of 14 bp INS allele and 14 bp INS/INS genotype than in controls | Lower concentration of sHLA-G in serum than in controls | 66 | |
| Decrease in HLA-G expression in monocytes and DCs | 80 | ||
| 14 bp INS/INS genotype is associated to the highest disease activity | 82 | ||
| +3142 G allele and +3142 GG genotype are more frequent in SLE and associated to SLE susceptibility | 84,85 | ||
| Multiple sclerosis | sHLA-G levels in MS CSF are higher than in controls | 87,88 | |
| sHLA-G levels in MS could be influenced by HLA-G 14 bp and +3142 C < G polymorphisms | 67 | ||
| sHLA-G level are increased in serum of CFS of MRI inactive MS | 65,90 | ||
| HLA-G expression in monocytes is lower than in controls | 91 | ||
| Presence of HLA-Gpos Treg cells in peripherial blood | 92 | ||
| Inflammatory bowel disease Crohn’s disease and ulcerative colitis | HLA-G is present on UC intestinal cells but not in CD biopsies | 95 | |
| 14 bp INS/DEL and 14 bp INS/INS are increased in UC in comparison with CD patients | 96 | ||
| PBMCs from CD patients secrete spontaneously sHLA-G | 97 | ||
| Different modulation of HLA-G by therapy in UC and CD | 98 | ||
| Asthma | -964 G < A and +3142 C < G SNPs are associated with asthma | Expression of HLA-G in airway epithelium and airway system | 99,100 |
| sHLA-G plasma levels are higher in atopic asthmatic children | 101 | ||
| sHLA-G secretion is increased in asthma induced by isocyanates | 103 | ||
| Allergic rhinitis | Higher sHLA-G serum levels than controls | 106-110 | |
| Pathological pregnancy | Decreased HLA-G expression in placenta than uncomplicated pregnancies | 46-48,112,113 | |
| Pre-eclampsia | Increased 14 bp INS/INS genotype frequency than uncomplicated pregnancies | 120-125 | |
| Tumors | Increased HLA-G expression in tumor cells | 1,45,128-143 | |
| Higher sHLA-G serum levels than controls | 142 | ||
| Viral infection | Increased HLA-G expression in viral infected cells | 145 | |
| HIV-1 | Increased HLA-G expression in infected monocytes and T cells | 147 | |
| HCMV | Increased HLA-G expression in infected monocytes | 56 | |
| Increased sHLA-G serum levels than controls | 151 | ||
The ability to modulate HLA-G molecules on the cell surface and to administer HLA-G molecules[74] seems to be at the basis of these cell therapies, suggesting the importance of further studies on HLA-G role in pathological conditions and the possibility of having a controlled modification of the HLA-G level according to disease status and pregnancy complications.
We thank Linda Marie Sartor for revision of the English language.
| 1. | Alkhouly N, Shehata I, Ahmed MB, Shehata H, Hassan S, Ibrahim T. HLA-G expression in acute lymphoblastic leukemia: a significant prognostic tumor biomarker. Med Oncol. 2013;30:460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Rizzo R, Farina I, Bortolotti D, Galuppi E, Rotola A, Melchiorri L, Ciancio G, Di Luca D, Govoni M. HLA-G may predict the disease course in patients with early rheumatoid arthritis. Hum Immunol. 2013;74:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Dardano A, Rizzo R, Polini A, Stignani M, Tognini S, Pasqualetti G, Ursino S, Colato C, Ferdeghini M, Baricordi OR. Soluble human leukocyte antigen-g and its insertion/deletion polymorphism in papillary thyroid carcinoma: novel potential biomarkers of disease? J Clin Endocrinol Metab. 2012;97:4080-4086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Jin HL, Li CR, Xiao L, Shi BY, Cai M, Li ZL, Wang S, Chen LP, Zhan SL, Li PC. Clinical relevance of sHLA-G-mediated with better graft acceptance in early posttransplantation. Transplant Proc. 2012;44:1259-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Lau DT, Norris MD, Marshall GM, Haber M, Ashton LJ. HLA-G polymorphisms, genetic susceptibility, and clinical outcome in childhood neuroblastoma. Tissue Antigens. 2011;78:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Waterhouse M, Duque-Afonso J, Wäsch R, Bertz H, Finke J. Soluble HLA-G molecules and HLA-G 14-base pair polymorphism after allogeneic hematopoietic cell transplantation. Transplant Proc. 2013;45:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci USA. 1992;89:3947-3951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 393] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Favier B, HoWangYin KY, Wu J, Caumartin J, Daouya M, Horuzsko A, Carosella ED, LeMaoult J. Tolerogenic function of dimeric forms of HLA-G recombinant proteins: a comparative study in vivo. PLoS One. 2011;6:e21011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856-8861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 438] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Gonen-Gross T, Achdout H, Gazit R, Hanna J, Mizrahi S, Markel G, Goldman-Wohl D, Yagel S, Horejsí V, Levy O. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171:1343-1351. [PubMed] |
| 12. | Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, Kurimoto E, Kato K, Kohda D, Maenaka K. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc Natl Acad Sci USA. 2006;103:16412-16417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Marchal-Bras-Goncalves R, Rouas-Freiss N, Connan F, Choppin J, Dausset J, Carosella ED, Kirszenbaum M, Guillet J. A soluble HLA-G protein that inhibits natural killer cell-mediated cytotoxicity. Transplant Proc. 2001;33:2355-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6--STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105:8357-8362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 451] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 17. | Kapasi K, Albert SE, Yie S, Zavazava N, Librach CL. HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology. 2000;101:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Naji A, Le Rond S, Durrbach A, Krawice-Radanne I, Creput C, Daouya M, Caumartin J, LeMaoult J, Carosella ED, Rouas-Freiss N. CD3+CD4low and CD3+CD8low are induced by HLA-G: novel human peripheral blood suppressor T-cell subsets involved in transplant acceptance. Blood. 2007;110:3936-3948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Du L, Xiao X, Wang C, Zhang X, Zheng N, Wang L, Zhang X, Li W, Wang S, Dong Z. Human leukocyte antigen-G is closely associated with tumor immune escape in gastric cancer by increasing local regulatory T cells. Cancer Sci. 2011;102:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Donadi EA, Castelli EC, Arnaiz-Villena A, Roger M, Rey D, Moreau P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol Life Sci. 2011;68:369-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Solier C, Mallet V, Lenfant F, Bertrand A, Huchenq A, Le Bouteiller P. HLA-G unique promoter region: functional implications. Immunogenetics. 2001;53:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Gobin SJ, Keijsers V, Cheong C, van Zutphen M, Van den Elsen PJ. Transcriptional regulation of HLA-G. Transplant Proc. 1999;31:1857-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ. Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J Immunol. 1999;163:1428-1434. [PubMed] |
| 24. | Lefebvre S, Berrih-Aknin S, Adrian F, Moreau P, Poea S, Gourand L, Dausset J, Carosella ED, Paul P. A specific interferon (IFN)-stimulated response element of the distal HLA-G promoter binds IFN-regulatory factor 1 and mediates enhancement of this nonclassical class I gene by IFN-beta. J Biol Chem. 2001;276:6133-6139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Ibrahim EC, Morange M, Dausset J, Carosella ED, Paul P. Heat shock and arsenite induce expression of the nonclassical class I histocompatibility HLA-G gene in tumor cell lines. Cell Stress Chaperones. 2000;5:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Yie SM, Xiao R, Librach CL. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum Reprod. 2006;21:2538-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Castelli EC, Mendes-Junior CT, Veiga-Castelli LC, Roger M, Moreau P, Donadi EA. A comprehensive study of polymorphic sites along the HLA-G gene: implication for gene regulation and evolution. Mol Biol Evol. 2011;28:3069-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Ober C, Billstrand C, Kuldanek S, Tan Z. The miscarriage-associated HLA-G -725G allele influences transcription rates in JEG-3 cells. Hum Reprod. 2006;21:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Hviid TV, Hylenius S, Rørbye C, Nielsen LG. HLA-G allelic variants are associated with differences in the HLA-G mRNA isoform profile and HLA-G mRNA levels. Immunogenetics. 2003;55:63-79. [PubMed] |
| 30. | Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics. 2004;56:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Svendsen SG, Hantash BM, Zhao L, Faber C, Bzorek M, Nissen MH, Hviid TV. The expression and functional activity of membrane-bound human leukocyte antigen-G1 are influenced by the 3’-untranslated region. Hum Immunol. 2013;74:818-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Yie SM, Li LH, Xiao R, Librach CL. A single base-pair mutation in the 3’-untranslated region of HLA-G mRNA is associated with pre-eclampsia. Mol Hum Reprod. 2008;14:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Tan Z, Randall G, Fan J, Camoretti-Mercado B, Brockman-Schneider R, Pan L, Solway J, Gern JE, Lemanske RF, Nicolae D. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am J Hum Genet. 2007;81:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 309] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 34. | Manaster I, Goldman-Wohl D, Greenfield C, Nachmani D, Tsukerman P, Hamani Y, Yagel S, Mandelboim O. MiRNA-mediated control of HLA-G expression and function. PLoS One. 2012;7:e33395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Castelli EC, Moreau P, Oya e Chiromatzo A, Mendes-Junior CT, Veiga-Castelli LC, Yaghi L, Giuliatti S, Carosella ED, Donadi EA. In silico analysis of microRNAS targeting the HLA-G 3’ untranslated region alleles and haplotypes. Hum Immunol. 2009;70:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Chang CC, Murphy SP, Ferrone S. Differential in vivo and in vitro HLA-G expression in melanoma cells: potential mechanisms. Hum Immunol. 2003;64:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Mouillot G, Marcou C, Zidi I, Guillard C, Sangrouber D, Carosella ED, Moreau P. Hypoxia modulates HLA-G gene expression in tumor cells. Hum Immunol. 2007;68:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | López AS, Alegre E, LeMaoult J, Carosella E, González A. Regulatory role of tryptophan degradation pathway in HLA-G expression by human monocyte-derived dendritic cells. Mol Immunol. 2006;43:2151-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Rizzo R, Hviid TV, Stignani M, Balboni A, Grappa MT, Melchiorri L, Baricordi OR. The HLA-G genotype is associated with IL-10 levels in activated PBMCs. Immunogenetics. 2005;57:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Bamberger AM, Jenatschke S, Schulte HM, Löning T, Bamberger MC. Leukemia inhibitory factor (LIF) stimulates the human HLA-G promoter in JEG3 choriocarcinoma cells. J Clin Endocrinol Metab. 2000;85:3932-3936. [PubMed] |
| 41. | Rizzo R, Rubini M, Govoni M, Padovan M, Melchiorri L, Stignani M, Carturan S, Ferretti S, Trotta F, Baricordi OR. HLA-G 14-bp polymorphism regulates the methotrexate response in rheumatoid arthritis. Pharmacogenet Genomics. 2006;16:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen--G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Lefebvre S, Moreau P, Guiard V, Ibrahim EC, Adrian-Cabestre F, Menier C, Dausset J, Carosella ED, Paul P. Molecular mechanisms controlling constitutive and IFN-gamma-inducible HLA-G expression in various cell types. J Reprod Immunol. 1999;43:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 45. | Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1060] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 46. | Hara N, Fujii T, Yamashita T, Kozuma S, Okai T, Taketani Y. Altered expression of human leukocyte antigen G (HLA-G) on extravillous trophoblasts in preeclampsia: immunohistological demonstration with anti-HLA-G specific antibody “87G” and anti-cytokeratin antibody “CAM5.2”. Am J Reprod Immunol. 1996;36:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Yie SM, Li LH, Li YM, Librach C. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am J Obstet Gynecol. 2004;191:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Peng B, Zhang L, Xing AY, Hu M, Liu SY. [The expression of human leukocyte antigen G and E on human first trimester placenta and its relationship with recurrent spontaneous abortion]. Sichuan Daxue Xuebao Yixueban. 2008;39:976-979. [PubMed] |
| 49. | Le Discorde M, Moreau P, Sabatier P, Legeais JM, Carosella ED. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum Immunol. 2003;64:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Mallet V, Blaschitz A, Crisa L, Schmitt C, Fournel S, King A, Loke YW, Dohr G, Le Bouteiller P. HLA-G in the human thymus: a subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int Immunol. 1999;11:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Blaschitz A, Lenfant F, Mallet V, Hartmann M, Bensussan A, Geraghty DE, Le Bouteiller P, Dohr G. Endothelial cells in chorionic fetal vessels of first trimester placenta express HLA-G. Eur J Immunol. 1997;27:3380-3388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Rizzo R, Andersen AS, Lassen MR, Sørensen HC, Bergholt T, Larsen MH, Melchiorri L, Stignani M, Baricordi OR, Hviid TV. Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy. Am J Reprod Immunol. 2009;62:320-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Rebmann V, Busemann A, Lindemann M, Grosse-Wilde H. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Derrien M, Pizzato N, Dolcini G, Menu E, Chaouat G, Lenfant F, Barré-Sinoussi F, Bouteiller PL. Human immunodeficiency virus 1 downregulates cell surface expression of the non-classical major histocompatibility class I molecule HLA-G1. J Gen Virol. 2004;85:1945-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Matte C, Lajoie J, Lacaille J, Zijenah LS, Ward BJ, Roger M. Functionally active HLA-G polymorphisms are associated with the risk of heterosexual HIV-1 infection in African women. AIDS. 2004;18:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Onno M, Pangault C, Le Friec G, Guilloux V, André P, Fauchet R. Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J Immunol. 2000;164:6426-6434. [PubMed] |
| 57. | Donaghy L, Gros F, Amiot L, Mary C, Maillard A, Guiguen C, Gangneux JP. Elevated levels of soluble non-classical major histocompatibility class I molecule human leucocyte antigen (HLA)-G in the blood of HIV-infected patients with or without visceral leishmaniasis. Clin Exp Immunol. 2007;147:236-240. [PubMed] |
| 58. | Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol. 2007;17:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Pistoia V, Morandi F, Wang X, Ferrone S. Soluble HLA-G: Are they clinically relevant? Semin Cancer Biol. 2007;17:469-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Crispim JC, Duarte RA, Soares CP, Costa R, Silva JS, Mendes-Júnior CT, Wastowski IJ, Faggioni LP, Saber LT, Donadi EA. Human leukocyte antigen-G expression after kidney transplantation is associated with a reduced incidence of rejection. Transpl Immunol. 2008;18:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Sebti Y, Le Maux A, Gros F, De Guibert S, Pangault C, Rouas-Freiss N, Bernard M, Amiot L. Expression of functional soluble human leucocyte antigen-G molecules in lymphoproliferative disorders. Br J Haematol. 2007;138:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED. Implication of HLA-G molecule in heart-graft acceptance. Lancet. 2000;355:2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Lila N, Amrein C, Guillemain R, Chevalier P, Latremouille C, Fabiani JN, Dausset J, Carosella ED, Carpentier A. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation. 2002;105:1949-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 64. | Qiu J, Terasaki PI, Miller J, Mizutani K, Cai J, Carosella ED. Soluble HLA-G expression and renal graft acceptance. Am J Transplant. 2006;6:2152-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Fainardi E, Rizzo R, Melchiorri L, Stignani M, Castellazzi M, Caniatti ML, Baldi E, Tola MR, Granieri E, Baricordi OR. Soluble HLA-G molecules are released as HLA-G5 and not as soluble HLA-G1 isoforms in CSF of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;192:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Rizzo R, Hviid TV, Govoni M, Padovan M, Rubini M, Melchiorri L, Stignani M, Carturan S, Grappa MT, Fotinidi M. HLA-G genotype and HLA-G expression in systemic lupus erythematosus: HLA-G as a putative susceptibility gene in systemic lupus erythematosus. Tissue Antigens. 2008;71:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Rizzo R, Bortolotti D, Fredj NB, Rotola A, Cura F, Castellazzi M, Tamborino C, Seraceni S, Baldi E, Melchiorri L. Role of HLA-G 14bp deletion/insertion and +3142C>G polymorphisms in the production of sHLA-G molecules in relapsing-remitting multiple sclerosis. Hum Immunol. 2012;73:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med. 2007;120:936-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 69. | Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, Combe B, Cutolo M, de Wit M, Dougados M. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1641] [Cited by in RCA: 1508] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 70. | Brenol CV, Veit TD, Chies JA, Xavier RM. The role of the HLA-G gene and molecule on the clinical expression of rheumatologic diseases. Rev Bras Reumatol. 2012;52:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Sonoda Y, Okuda T, Yokota S, Maekawa T, Shizumi Y, Nishigaki H, Misawa S, Fujii H, Abe T. Actions of human interleukin-4/B-cell stimulatory factor-1 on proliferation and differentiation of enriched hematopoietic progenitor cells in culture. Blood. 1990;75:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | LeMaoult J, Le Discorde M, Rouas-Freiss N, Moreau P, Menier C, McCluskey J, Carosella ED. Biology and functions of human leukocyte antigen-G in health and sickness. Tissue Antigens. 2003;62:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Ongaro A, Stignani M, Pellati A, Melchiorri L, Massari L, Caruso G, De Mattei M, Caruso A, Baricordi OR, Rizzo R. Human leukocyte antigen-G molecules are constitutively expressed by synovial fibroblasts and upmodulated in osteoarthritis. Hum Immunol. 2010;71:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Kuroki K, Hirose K, Okabe Y, Fukunaga Y, Takahashi A, Shiroishi M, Kajikawa M, Tabata S, Nakamura S, Takai T. The long-term immunosuppressive effects of disulfide-linked HLA-G dimer in mice with collagen-induced arthritis. Hum Immunol. 2013;74:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Stamp LK, O’Donnell JL, Chapman PT, Barclay ML, Kennedy MA, Frampton CM, Roberts RL. Lack of association between HLA-G 14 bp insertion/deletion polymorphism and response to long-term therapy with methotrexate response in rheumatoid arthritis. Ann Rheum Dis. 2009;68:154-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Kooloos WM, Wessels JA, van der Straaten T, Allaart CF, Huizinga TW, Guchelaar HJ. Functional polymorphisms and methotrexate treatment outcome in recent-onset rheumatoid arthritis. Pharmacogenomics. 2010;11:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Rosado S, Perez-Chacon G, Mellor-Pita S, Sanchez-Vegazo I, Bellas-Menendez C, Citores MJ, Losada-Fernandez I, Martin-Donaire T, Rebolleda N, Perez-Aciego P. Expression of human leukocyte antigen-G in systemic lupus erythematosus. Hum Immunol. 2008;69:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Chen J, Shen B, Jiang Y, Jun L, Zhu M, Chen B, Liu C. Analysis of immunoglobulin-like transcripts (ILTs) in lymphocytes with sHLA-G and IL10 from SLE patients. Clin Exp Med. 2013;13:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Rudstein-Svetlicky N, Loewenthal R, Horejsi V, Gazit E. HLA-G levels in serum and plasma. Tissue Antigens. 2007;69 Suppl 1:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Monsiváis-Urenda AE, Baranda L, Alvarez-Quiroga C, Abud-Mendoza C, González-Amaro R. Expression and functional role of HLA-G in immune cells from patients with systemic lupus erythematosus. J Clin Immunol. 2011;31:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Fernando MM, Freudenberg J, Lee A, Morris DL, Boteva L, Rhodes B, Gonzalez-Escribano MF, Lopez-Nevot MA, Navarra SV, Gregersen PK. Transancestral mapping of the MHC region in systemic lupus erythematosus identifies new independent and interacting loci at MSH5, HLA-DPB1 and HLA-G. Ann Rheum Dis. 2012;71:777-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Veit TD, Cordero EA, Mucenic T, Monticielo OA, Brenol JC, Xavier RM, Delgado-Cañedo A, Chies JA. Association of the HLA-G 14 bp polymorphism with systemic lupus erythematosus. Lupus. 2009;18:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Pedroza LS, Sauma MF, Vasconcelos JM, Takeshita LY, Ribeiro-Rodrigues EM, Sastre D, Barbosa CM, Chies JA, Veit TD, Lima CP. Systemic lupus erythematosus: association with KIR and SLC11A1 polymorphisms, ethnic predisposition and influence in clinical manifestations at onset revealed by ancestry genetic markers in an urban Brazilian population. Lupus. 2011;20:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Lucena-Silva N, de Souza VS, Gomes RG, Fantinatti A, Muniz YC, de Albuquerque RS, Monteiro AL, Diniz GT, Coelho MR, Mendes-Junior CT. HLA-G 3’ untranslated region polymorphisms are associated with systemic lupus erythematosus in 2 Brazilian populations. J Rheumatol. 2013;40:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Consiglio CR, Veit TD, Monticielo OA, Mucenic T, Xavier RM, Brenol JC, Chies JA. Association of the HLA-G gene +3142C>G polymorphism with systemic lupus erythematosus. Tissue Antigens. 2011;77:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1575] [Cited by in RCA: 1655] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 87. | Fainardi E, Rizzo R, Melchiorri L, Vaghi L, Castellazzi M, Marzola A, Govoni V, Paolino E, Tola MR, Granieri E. Presence of detectable levels of soluble HLA-G molecules in CSF of relapsing-remitting multiple sclerosis: relationship with CSF soluble HLA-I and IL-10 concentrations and MRI findings. J Neuroimmunol. 2003;142:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 88. | Fainardi E, Rizzo R, Melchiorri L, Castellazzi M, Paolino E, Tola MR, Granieri E, Baricordi OR. Intrathecal synthesis of soluble HLA-G and HLA-I molecules are reciprocally associated to clinical and MRI activity in patients with multiple sclerosis. Mult Scler. 2006;12:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Waschbisch A, Sandbrink R, Hartung HP, Kappos L, Schwab S, Pohl C, Wiendl H. Evaluation of soluble HLA-G as a biomarker for multiple sclerosis. Neurology. 2011;77:596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Fainardi E, Rizzo R, Melchiorri L, Stignani M, Castellazzi M, Tamborino C, Paolino E, Tola MR, Granieri E, Baricordi OR. CSF levels of soluble HLA-G and Fas molecules are inversely associated to MRI evidence of disease activity in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2008;14:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Wiendl H, Feger U, Mittelbronn M, Jack C, Schreiner B, Stadelmann C, Antel J, Brueck W, Meyermann R, Bar-Or A. Expression of the immune-tolerogenic major histocompatibility molecule HLA-G in multiple sclerosis: implications for CNS immunity. Brain. 2005;128:2689-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Feger U, Tolosa E, Huang YH, Waschbisch A, Biedermann T, Melms A, Wiendl H. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Huang YH, Zozulya AL, Weidenfeller C, Schwab N, Wiendl H. T cell suppression by naturally occurring HLA-G-expressing regulatory CD4+ T cells is IL-10-dependent and reversible. J Leukoc Biol. 2009;86:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Huang YH, Zozulya AL, Weidenfeller C, Metz I, Buck D, Toyka KV, Brück W, Wiendl H. Specific central nervous system recruitment of HLA-G(+) regulatory T cells in multiple sclerosis. Ann Neurol. 2009;66:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Torres MI, Le Discorde M, Lorite P, Ríos A, Gassull MA, Gil A, Maldonado J, Dausset J, Carosella ED. Expression of HLA-G in inflammatory bowel disease provides a potential way to distinguish between ulcerative colitis and Crohn’s disease. Int Immunol. 2004;16:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Glas J, Török HP, Tonenchi L, Wetzke M, Beynon V, Teshome MY, Cotofana S, Schiemann U, Griga T, Klein W. The 14-bp deletion polymorphism in the HLA-G gene displays significant differences between ulcerative colitis and Crohn’s disease and is associated with ileocecal resection in Crohn’s disease. Int Immunol. 2007;19:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Rizzo R, Melchiorri L, Simone L, Stignani M, Marzola A, Gullini S, Baricordi OR. Different production of soluble HLA-G antigens by peripheral blood mononuclear cells in ulcerative colitis and Crohn’s disease: a noninvasive diagnostic tool? Inflamm Bowel Dis. 2008;14:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Zelante A, Borgoni R, Galuppi C, Cifalà V, Melchiorri L, Gullini S, Baricordi O, Rizzo R. Therapy modifies HLA-G secretion differently in Crohn’s disease and ulcerative colitis patients. Inflamm Bowel Dis. 2011;17:E94-E95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 99. | Nicolae D, Cox NJ, Lester LA, Schneider D, Tan Z, Billstrand C, Kuldanek S, Donfack J, Kogut P, Patel NM. Fine mapping and positional candidate studies identify HLA-G as an asthma susceptibility gene on chromosome 6p21. Am J Hum Genet. 2005;76:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 100. | Tahan F, Patiroglu T. Plasma soluble human leukocyte antigen G levels in asthmatic children. Int Arch Allergy Immunol. 2006;141:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Zheng XQ, Li CC, Xu DP, Lin A, Bao WG, Yang GS, Yan WH. Analysis of the plasma soluble human leukocyte antigen-G and interleukin-10 levels in childhood atopic asthma. Hum Immunol. 2010;71:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | Rizzo R, Mapp CE, Melchiorri L, Maestrelli P, Visentin A, Ferretti S, Bononi I, Miotto D, Baricordi OR. Defective production of soluble HLA-G molecules by peripheral blood monocytes in patients with asthma. J Allergy Clin Immunol. 2005;115:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Mapp CE, Ferrazzoni S, Rizzo R, Miotto D, Stignani M, Boschetto P, Maestrelli P, Baricordi OR. Soluble human leucocyte antigen-G and interleukin-10 levels in isocyanate-induced asthma. Clin Exp Allergy. 2009;39:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Melén E, Kho AT, Sharma S, Gaedigk R, Leeder JS, Mariani TJ, Carey VJ, Weiss ST, Tantisira KG. Expression analysis of asthma candidate genes during human and murine lung development. Respir Res. 2011;12:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 105. | Ciprandi G, Colombo BM, Contini P, Cagnati P, Pistorio A, Puppo F, Murdaca G. Soluble HLA-G and HLA-A,-B,-C serum levels in patients with allergic rhinitis. Allergy. 2008;63:1335-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Ciprandi G, Contini P, Murdaca G, DeAmici M, Gallina AM, Puppo F. Soluble serum HLA-G and HLA-A, -B, -C molecules in patients with seasonal allergic rhinitis exposed to pollens. Int Immunopharmacol. 2009;9:1058-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Ciprandi G, Contini P, Murdaca G, Gallina AM, Puppo F. Soluble HLA-G molecule in patients with perennial allergic rhinitis. Int Arch Allergy Immunol. 2009;150:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Ciprandi G, Contini P, Pistorio A, Murdaca G, Puppo F. Sublingual immunotherapy reduces soluble HLA-G and HLA-A,-B,-C serum levels in patients with allergic rhinitis. Int Immunopharmacol. 2009;9:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Ciprandi G, De Amici M, Caimmi S, Marseglia A, Marchi A, Castellazzi AM, Marseglia G. Soluble serum HLA-G in children with allergic rhinitis and asthma. J Biol Regul Homeost Agents. 2010;24:221-224. [PubMed] |
| 110. | Ciprandi G, Continia P, Fenoglio D, Sormani MP, Negrini S, Puppo F, Indiveri F. Relationship between soluble HLA-G and HLA-A,-B,-C serum levels, and interferon-gamma production after sublingual immunotherapy in patients with allergic rhinitis. Hum Immunol. 2008;69:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology. 1986;59:595-601. [PubMed] |
| 112. | Gracia CR, Sammel MD, Chittams J, Hummel AC, Shaunik A, Barnhart KT. Risk factors for spontaneous abortion in early symptomatic first-trimester pregnancies. Obstet Gynecol. 2005;106:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol. 1997;151:1809-1818. [PubMed] |
| 114. | Alegre E, Díaz-Lagares A, Lemaoult J, López-Moratalla N, Carosella ED, González A. Maternal antigen presenting cells are a source of plasmatic HLA-G during pregnancy: longitudinal study during pregnancy. Hum Immunol. 2007;68:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000;183:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H. Placental abruption is associated with decreased maternal plasma levels of soluble HLA-G. J Clin Immunol. 2003;23:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Rousseau P, Le Discorde M, Mouillot G, Marcou C, Carosella ED, Moreau P. The 14 bp deletion-insertion polymorphism in the 3’ UT region of the HLA-G gene influences HLA-G mRNA stability. Hum Immunol. 2003;64:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 118. | Fujii T, Ishitani A, Geraghty DE. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J Immunol. 1994;153:5516-5524. [PubMed] |
| 119. | Hiby SE, King A, Sharkey A, Loke YW. Molecular studies of trophoblast HLA-G: polymorphism, isoforms, imprinting and expression in preimplantation embryo. Tissue Antigens. 1999;53:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Hviid TV, Hylenius S, Lindhard A, Christiansen OB. Association between human leukocyte antigen-G genotype and success of in vitro fertilization and pregnancy outcome. Tissue Antigens. 2004;64:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Hylenius S, Andersen AM, Melbye M, Hviid TV. Association between HLA-G genotype and risk of pre-eclampsia: a case-control study using family triads. Mol Hum Reprod. 2004;10:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Larsen MH, Hylenius S, Andersen AM, Hviid TV. The 3’-untranslated region of the HLA-G gene in relation to preeclampsia: revisited. Tissue Antigens. 2010;75:253-261. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 123. | Moreau P, Contu L, Alba F, Lai S, Simoes R, Orrù S, Carcassi C, Roger M, Rabreau M, Carosella ED. HLA-G gene polymorphism in human placentas: possible association of G*0106 allele with preeclampsia and miscarriage. Biol Reprod. 2008;79:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 124. | O’Brien M, McCarthy T, Jenkins D, Paul P, Dausset J, Carosella ED, Moreau P. Altered HLA-G transcription in pre-eclampsia is associated with allele specific inheritance: possible role of the HLA-G gene in susceptibility to the disease. Cell Mol Life Sci. 2001;58:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 125. | Ober C, Aldrich CL, Chervoneva I, Billstrand C, Rahimov F, Gray HL, Hyslop T. Variation in the HLA-G promoter region influences miscarriage rates. Am J Hum Genet. 2003;72:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 126. | Rizzo R, Vercammen M, van de Velde H, Horn PA, Rebmann V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol Life Sci. 2011;68:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 127. | Kotze D, Kruger TF, Lombard C, Padayachee T, Keskintepe L, Sher G. The effect of the biochemical marker soluble human leukocyte antigen G on pregnancy outcome in assisted reproductive technology--a multicenter study. Fertil Steril. 2013;100:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Yan WH, Lin A, Chen BG, Luo WD, Dai MZ, Chen XJ, Xu HH, Li BL. Unfavourable clinical implications for HLA-G expression in acute myeloid leukaemia. J Cell Mol Med. 2008;12:889-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | Erikci AA, Karagoz B, Ozyurt M, Ozturk A, Kilic S, Bilgi O. HLA-G expression in B chronic lymphocytic leukemia: a new prognostic marker? Hematology. 2009;14:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Kalhor N, Ramirez PT, Deavers MT, Malpica A, Silva EG. Immunohistochemical studies of trophoblastic tumors. Am J Surg Pathol. 2009;33:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Mao TL, Kurman RJ, Huang CC, Lin MC, Shih IeM. Immunohistochemistry of choriocarcinoma: an aid in differential diagnosis and in elucidating pathogenesis. Am J Surg Pathol. 2007;31:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | He X, Dong DD, Yie SM, Yang H, Cao M, Ye SR, Li K, Liu J, Chen J. HLA-G expression in human breast cancer: implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann Surg Oncol. 2010;17:1459-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 133. | de Kruijf EM, Sajet A, van Nes JG, Natanov R, Putter H, Smit VT, Liefers GJ, van den Elsen PJ, van de Velde CJ, Kuppen PJ. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185:7452-7459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 134. | Eskandari-Nasab E, Hashemi M, Hasani SS, Omrani M, Taheri M, Mashhadi MA. Association between HLA-G 3’UTR 14-bp ins/del polymorphism and susceptibility to breast cancer. Cancer Biomark. 2013;13:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 135. | Rolfsen GB, Castelli EC, Donadi EA, Duarte RA, Soares CP. HLA-G polymorphism and breast cancer. Int J Immunogenet. Int J Immunogenet. 2013;in press. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Bijen CB, Bantema-Joppe EJ, de Jong RA, Leffers N, Mourits MJ, Eggink HF, van der Zee AG, Hollema H, de Bock GH, Nijman HW. The prognostic role of classical and nonclassical MHC class I expression in endometrial cancer. Int J Cancer. 2010;126:1417-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Davidson B, Elstrand MB, McMaster MT, Berner A, Kurman RJ, Risberg B, Trope CG, Shih IeM. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol. 2005;96:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of HLA-G is associated with prognosis in esophageal squamous cell carcinoma. Am J Clin Pathol. 2007;128:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 139. | Ye SR, Yang H, Li K, Dong DD, Lin XM, Yie SM. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol. 2007;20:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 140. | Zeestraten EC, Reimers MS, Saadatmand S, Dekker JW, Liefers GJ, van den Elsen PJ, van de Velde CJ, Kuppen PJ. Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer. 2014;110:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 141. | Cai MY, Xu YF, Qiu SJ, Ju MJ, Gao Q, Li YW, Zhang BH, Zhou J, Fan J. Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection. Clin Cancer Res. 2009;15:4686-4693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 142. | Schütt P, Schütt B, Switala M, Bauer S, Stamatis G, Opalka B, Eberhardt W, Schuler M, Horn PA, Rebmann V. Prognostic relevance of soluble human leukocyte antigen-G and total human leukocyte antigen class I molecules in lung cancer patients. Hum Immunol. 2010;71:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Dong DD, Yie SM, Li K, Li F, Xu Y, Xu G, Song L, Yang H. Importance of HLA-G expression and tumor infiltrating lymphocytes in molecular subtypes of breast cancer. Hum Immunol. 2012;73:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 144. | Komohara Y, Harada M, Ishihara Y, Suekane S, Noguchi M, Yamada A, Matsuoka K, Itoh K. HLA-G as a target molecule in specific immunotherapy against renal cell carcinoma. Oncol Rep. 2007;18:1463-1468. [PubMed] |
| 145. | Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Trends Microbiol. 2000;8:410-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 146. | Tripathi P, Agrawal S. The role of human leukocyte antigen E and G in HIV infection. AIDS. 2007;21:1395-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 147. | Lozano JM, González R, Kindelán JM, Rouas-Freiss N, Caballos R, Dausset J, Carosella ED, Peña J. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS. 2002;16:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 148. | Cabello A, Rivero A, Garcia MJ, Lozano JM, Torre-Cisneros J, González R, Dueñas G, Galiani MD, Camacho A, Santamaria M. HAART induces the expression of HLA-G on peripheral monocytes in HIV-1 infected individuals. Hum Immunol. 2003;64:1045-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 149. | Li C, Toth I, Schulze Zur Wiesch J, Pereyra F, Rychert J, Rosenberg ES, van Lunzen J, Lichterfeld M, Yu XG. Functional characterization of HLA-G+ regulatory T cells in HIV-1 infection. PLoS Pathog. 2013;9:e1003140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Söderberg-Nauclér C, Nelson JY. Human cytomegalovirus latency and reactivation - a delicate balance between the virus and its host‘s immune system. Intervirology. 1999;42:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Yan WH, Lin A, Chen BG, Chen SY. Induction of both membrane-bound and soluble HLA-G expression in active human cytomegalovirus infection. J Infect Dis. 2009;200:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 152. | Ferguson R, Ramanakumar AV, Koushik A, Coutlée F, Franco E, Roger M. Human leukocyte antigen G polymorphism is associated with an increased risk of invasive cancer of the uterine cervix. Int J Cancer. 2012;131:E312-E319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 153. | Rodríguez JA, Galeano L, Palacios DM, Gómez C, Serrano ML, Bravo MM, Combita AL. Altered HLA class I and HLA-G expression is associated with IL-10 expression in patients with cervical cancer. Pathobiology. 2012;79:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 154. | Mégret F, Prehaud C, Lafage M, Moreau P, Rouas-Freiss N, Carosella ED, Lafon M. Modulation of HLA-G and HLA-E expression in human neuronal cells after rabies virus or herpes virus simplex type 1 infections. Hum Immunol. 2007;68:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 155. | Cordero EA, Veit TD, da Silva MA, Jacques SM, Silla LM, Chies JA. HLA-G polymorphism influences the susceptibility to HCV infection in sickle cell disease patients. Tissue Antigens. 2009;74:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 156. | Weng PJ, Fu YM, Ding SX, Xu DP, Lin A, Yan WH. Elevation of plasma soluble human leukocyte antigen-G in patients with chronic hepatitis C virus infection. Hum Immunol. 2011;72:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 157. | Park Y, Park Y, Lim HS, Kim YS, Hong DJ, Kim HS. Soluble human leukocyte antigen-G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue Antigens. 2012;79:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Shi WW, Lin A, Xu DP, Bao WG, Zhang JG, Chen SY, Li J, Yan WH. Plasma soluble human leukocyte antigen-G expression is a potential clinical biomarker in patients with hepatitis B virus infection. Hum Immunol. 2011;72:1068-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
P- Reviewers: Ballestri S, Bao ZJ, Liu QG S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Liu SQ