Published online Mar 20, 2025. doi: 10.5662/wjm.v15.i1.95857
Revised: June 23, 2024
Accepted: August 2, 2024
Published online: March 20, 2025
Processing time: 161 Days and 21.5 Hours
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with multifaceted origins. In recent studies, neuroinflammation and immune dysregulation have come to the forefront in its pathogenesis. There are studies suggesting that stem cell therapy may be effective in the treatment of ASD.
To evolve the landscape of ASD treatment, focusing on the potential benefits and safety of stem cell transplantation.
A detailed case report is presented, displaying the positive outcomes observed in a child who underwent intra
The study demonstrates a significant improvement in the child’s functional outcomes (Childhood Autism Rating Scale, Denver 2 Developmental Screening Test), especially in language and gross motor skills. No serious side effects were encountered during the 2-year follow-up.
The findings support the safety and effectiveness of WJ-MSC transplantation in managing ASD.
Core Tip: According to data of the World Health Organization, autism spectrum disorder (ASD) is observed in approximately 1 in every 100 children. Recent studies have revealed that immune factors and inflammation are effective in the development of ASD. In this study, we applied six doses of Wharton’s jelly-derived mesenchymal stem cell therapy to a 4-year-old patient diagnosed with autism spectrum disorder. After the applications, we observed the patient for 2 years. We did not encounter any serious side effects. According to the Childhood Autism Rating Scale values and Denver II Developmental Screening Test values, we detected significant improvements.
- Citation: Kabatas S, Civelek E, Savrunlu EC, Karaaslan U, Yıldız Ö, Karaöz E. Advances in the treatment of autism spectrum disorder: Wharton jelly mesenchymal stem cell transplantation. World J Methodol 2025; 15(1): 95857
- URL: https://www.wjgnet.com/2222-0682/full/v15/i1/95857.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i1.95857
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition that manifests in various degrees of impairment in social interaction, communication abilities, and repetitive behaviors. Despite its wide-ranging impact, ASD's etiology remains elusive, with research pointing towards a multifaceted interplay between genetic predisposition and environmental factors[1].
Recent investigations have shed light on the intricate pathogenesis of ASD, highlighting the role of neuroinflammation, immune dysregulation, and altered cytokine levels within the central nervous system (CNS)[1,2]. These underlying mechanisms contribute to the neurobiological alterations observed in individuals with ASD, providing insights into potential therapeutic targets.
In the quest for innovative treatment modalities, stem cell therapy (SCT) has emerged as a promising avenue for addressing the core pathophysiology of ASD. Mesenchymal stem cells (MSCs), in particular, have garnered attention for their immunomodulatory and regenerative properties, offering potential benefits in mitigating neuroinflammatory pro
Among the diverse types of MSCs investigated for ASD therapy, Wharton’s jelly-derived MSCs (WJ-MSCs) have gained traction due to their abundance, accessibility, and favorable biological characteristics[6]. Derived from the Whar
The application of WJ-MSCs in ASD therapy represents a paradigm shift in treatment strategies, offering a novel approach to addressing the underlying neurobiological abnormalities. By targeting neuroinflammation and promoting neuronal repair and regeneration, WJ-MSCs hold the potential to alleviate ASD symptoms and improve overall func
In this article, we present a comprehensive case report on the utilization of WJ-MSC transplantation in a child diagnosed with ASD. The study aims to elucidate the safety, efficacy, and therapeutic outcomes associated with WJ-MSC therapy, providing valuable insights into its clinical application and potential benefits for individuals with ASD.
This article describes a compelling study conducted at the University of Health Sciences, Gaziosmanpaşa Training and Research Hospital (Istanbul, Turkey). The study involved a 4-year-old patient diagnosed with ASD who underwent a comprehensive treatment protocol consisting of intrathecal (i.t.) and intravenous (i.v.) allogeneic WJ-MSC transplan
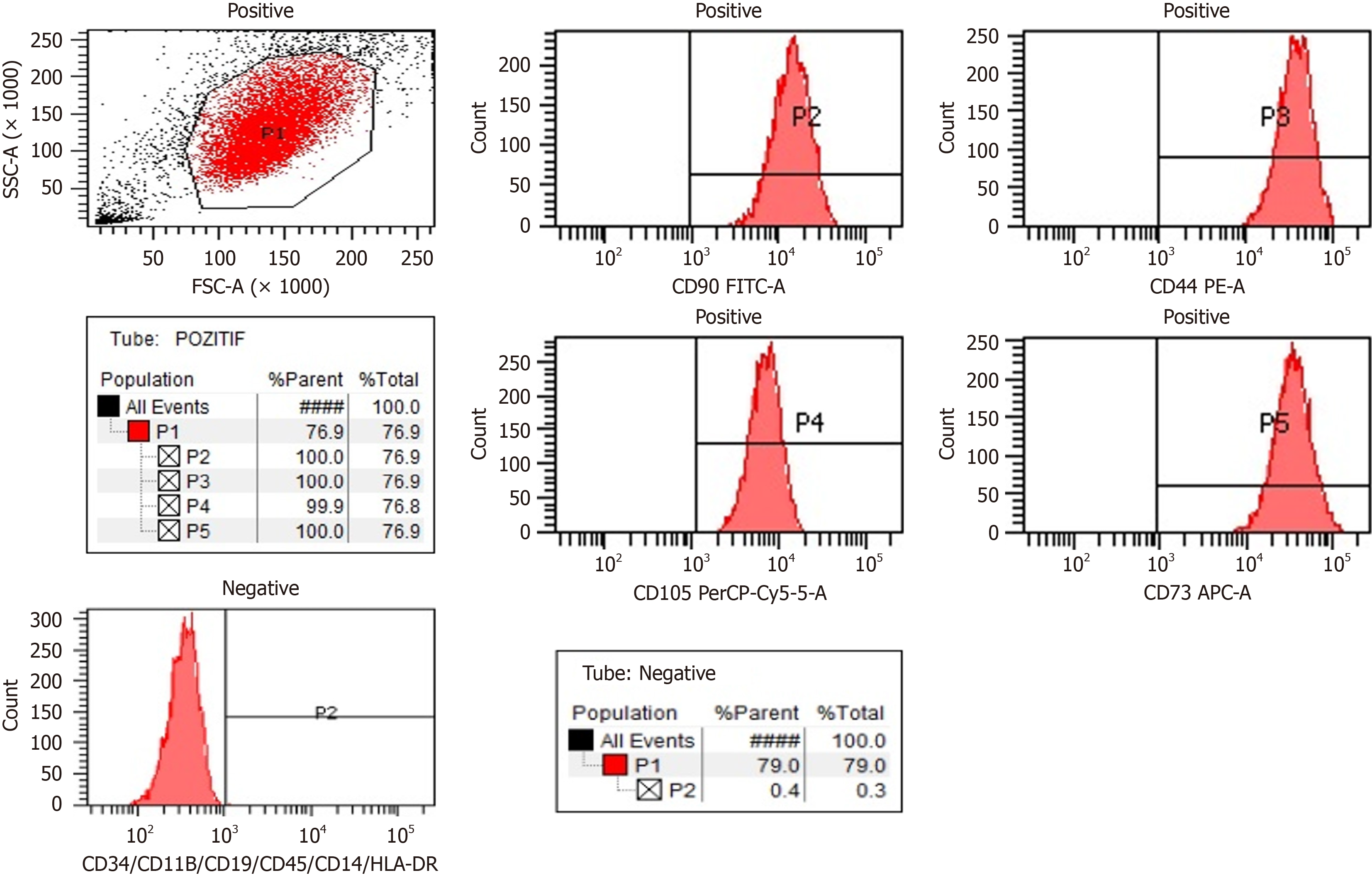
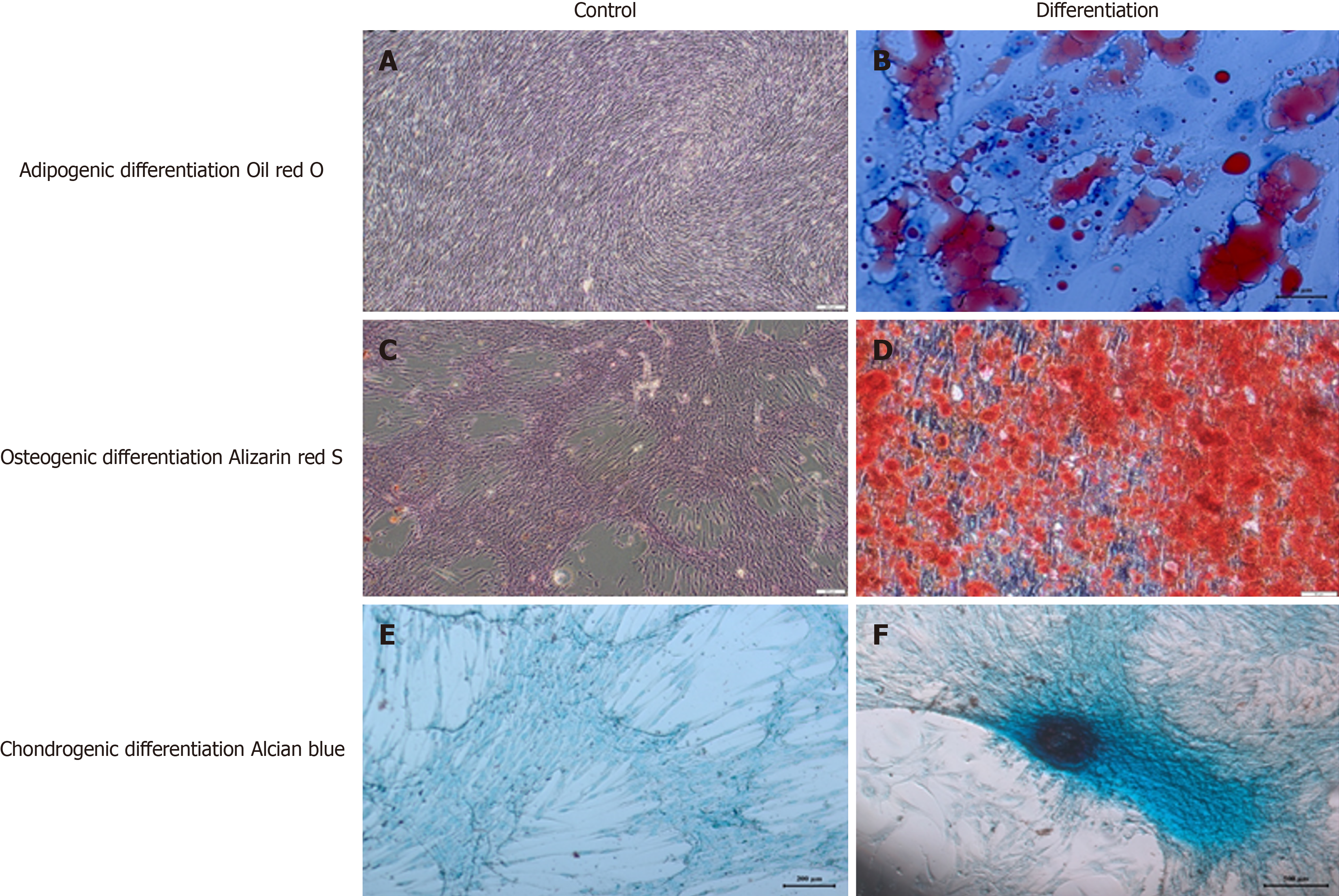
The WJ-MSCs utilized in this study were produced at Liv Hospital Ulus Stem Cell and Regenerative Medicine Center (Beşiktaş, Türkiye), a facility licensed under Good Manufacturing Practices (GMP) and approved by the Turkish Ministry of Health. The umbilical cord tissues employed were sourced from maternal donors who provided written informed consent for donation. Donors were confirmed to be free of communicable diseases through comprehensive testing conducted by the certified donor screening laboratory of Liv Hospital, in accordance with relevant regulations (EMEA/CHMP/BWP/398498/2005, 21 CFR 1271.75, 1271.80, and 1271.85).
Procedure for obtaining and processing umbilical cord tissue: Collection of cord tissue: After delivery of the placenta and umbilical cord, a sterile piece of approximately 20 cm of continuous cord tissue was obtained from placentas of infants delivered via elective cesarean section.
Transport and storage: The collected cord tissue was placed in a sterile container filled with tissue transport solution supplemented with antibiotics to maintain sterility during transport.
Transfer to GMP laboratory: Cord tissue samples were then transferred to the GMP laboratory under controlled con
Initial processing: Upon arrival in clean rooms, the cord tissue underwent sterility testing before proceeding with pro
Tissue preparation: Within a biological safety cabinet, the cord lining, umbilical arteries, and vein were meticulously removed from the cord tissue.
Enzymatic digestion: Wharton’s jelly was dissected into small pieces, minced, and digested using GMP-grade colla
Cell culture: The resulting cell suspension was cultured and allowed to grow to confluence over 7-14 days to establish the primary culture (P0).
Subsequent passages: Cells from P0 were passaged (P1, P2, and P3) under controlled conditions to expand and obtain the desired quantity of WJ-MSCs for the intended application.
Viability and cell count were assessed at each stage of cell culture. Rigorous quality control assessments included sterility testing, flow cytometry for characterization, evaluation of pyrogenicity, determination of cell count and viability, telome
Final product preparation: The WJ-MSCs were suspended in an isotonic solution and sterilely filled into vials ready for injection. As we previously published and described the procedure in detail, the production of the WJ-MSC product involved stringent adherence to GMP guidelines and meticulous quality control measures to ensure safety, efficacy, and consistency of the SCT intended for the treatment of ASD in the study setting. These detailed processes underscore the importance of standardized protocols and robust quality assurance in the development of advanced cell-based therapies[8].
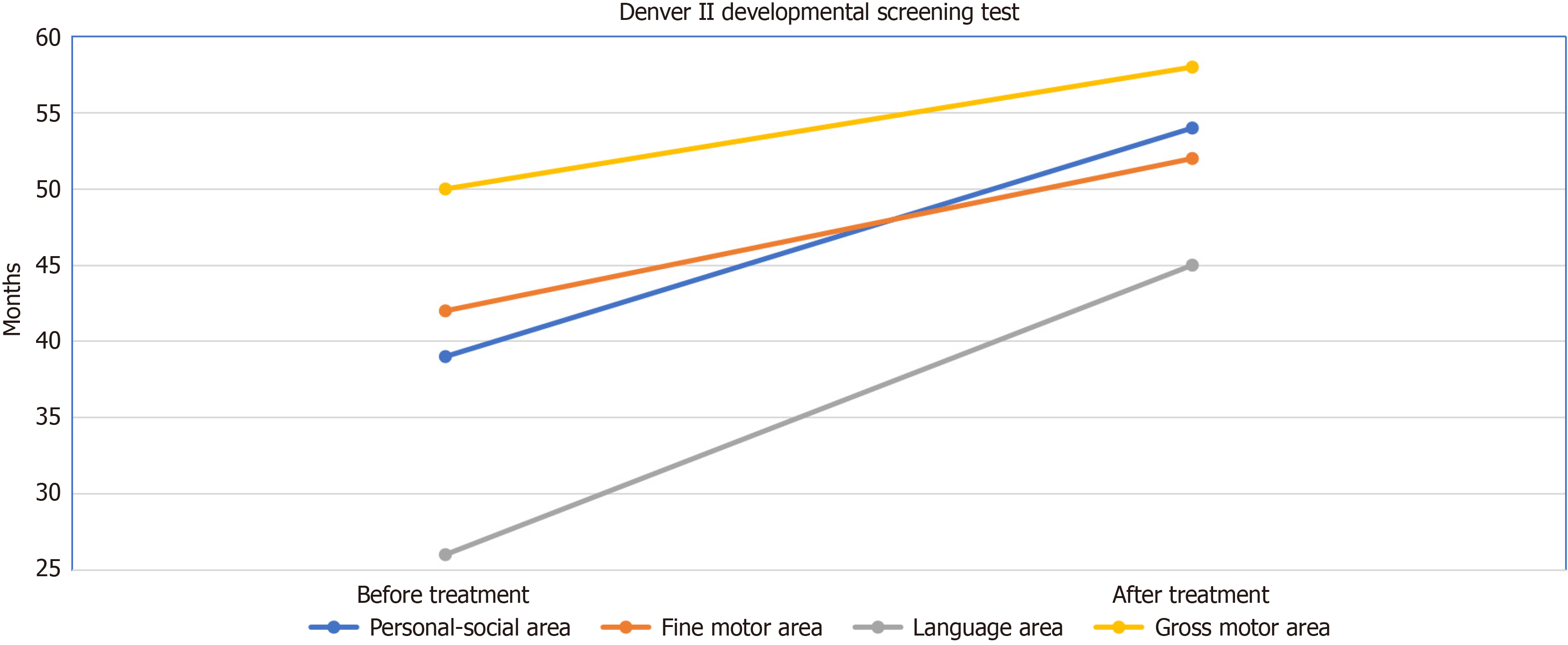
A 4-year-old child suffered from inability to make eye contact, speech delay incompatible with age, crying spells, difficulties with social interaction. The patient, who was evaluated by a child psychiatrist 6 months ago with her current complaints, was diagnosed with ASD. The patient received special training and neurorehabilitation treatments for approximately 6 months. Patient had no history of chronic disease in the patient's medical history and no significant family history. The patient's childhood autism rating scale (CARS) score was found to be 37. Denver II Developmental Screening Test results were Personal-Social Area: 3 years 3 months; Fine Motor Area: 3 years 6 months; Language area: 2 years 2 months; and Gross Motor Area: 4 years 2 months. In the patient's laboratory tests, anemia, leukocytosis, thrombocytopenia/thrombocytosis, renal failure, liver failure, and electrolyte imbalance were not observed. Acute phase reactants were negative. Additionally, hepatitis B/C and human immunodeficiency virus markers were negative. There were no mass lesions, plaques, or similar abnormal findings detected in the patient's cranial magnetic resonance imaging exa
The treatment protocol included six sessions of i.t. and i.v. WJ-MSCs were administered under sedo-anesthesia. In every instance, the cellular dose was modified to 1 × 106 WJ-MSCs/kg of the patient for each of the two application routes. To harness the anti-neuroinflammatory properties and neuro-regenerative capabilities of the MSC product, a dosage of 1 × 106 WJ-MSCs/kg was administered i.t. using a spinal needle. Prior to the injection, an equivalent amount of cerebrospinal fluid was extracted to prevent any elevation in intracranial pressure. In addition, a dosage of 1 × 106 WJ-MSCs/kg was administered i.v. by a blood infusion set, with the intention of achieving a systemic immune modulation and promoting tissue regeneration.
Remarkably, the treatment demonstrated both safety and efficacy, with no severe adverse events reported throughout the 2-year follow-up period (3rd, 6th, 12th and 24th month). This case study underscores the promising potential of WJ-MSCs transplantation as a therapeutic intervention for ASD and highlights the importance of further research in this area to validate and expand upon these initial findings.
The patient, who initially exhibited a CARS score of 37, experienced a notable improvement, with scores decreasing to 31 after the 6th application. While the Denver II Developmental Screening Test results were compatible with Personal-Social Area: 3 years 3 months, Fine Motor Area: 3 years 6 months, Language area: 2 years 2 months, Gross Motor Area: 4 years 2 months before the applications; after the 6th application, it was evaluated as compatible with Personal-Social Area: 4 years 6 months, Fine Motor Area: 4 years 4 months, Language area: 3 years 9 months, and Gross Motor Area: 4 years 10 months (Figure 3). Positive outcomes were particularly prominent in language and gross motor functions (Video 1 - before treatment, Video 2 - after round 6). No major complication occurred, and minor complications improved with symptomatic treatment (Table 1).
| Complications | Administration | ||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | ||
| Early | Infection | - | - | - | - | - | - |
| Fever | - | + | + | - | - | - | |
| Pain | + | - | - | - | - | - | |
| Headache | - | - | - | - | + | - | |
| Increased C-reactive protein levels | - | - | - | - | - | - | |
| Leukocytosis | - | - | - | - | - | - | |
| Allergic reaction or shock | - | - | - | - | - | - | |
| Perioperative complications | - | - | - | - | - | - | |
| Late | Secondary infections | - | - | - | - | - | - |
| Urinary tract infections | - | - | - | - | - | - | |
| Deterioration of neurological status | - | - | - | - | - | - | |
| Neuropathic pain | - | - | - | - | - | - | |
| Carcinogenesis | - | - | - | - | - | - | |
The intricate interplay between genetic predisposition and environmental factors in ASD is underscored by recent advancements in single-cell brain organoid screening. Li et al[9] identified developmental defects associated with 36 high-risk ASD genes, shedding light on their effects on cell fate determination, particularly in dorsal intermediate progenitors, ventral progenitors, and upper-layer excitatory neurons. This insight emphasizes the relevance of transcriptional regu
Exploring neuroinflammation in ASD, recent research by Villarreal-Martínez et al[10] aligns with the well-documented inflammatory processes within the CNS. Their findings corroborate dysregulation of proinflammatory processes invol
One of the most important reasons why we chose WJ-MSCs in our study was that there were many studies in the literature indicating that these cells could be used safely in clinical studies. It is well tolerated by the immune system and thus the risk of rejection is reduced. Additionally, WJCs release dopamine-loaded exosomes that protect against neuro
Our case report aligns with the evolving field of stem cell research and its application in neurodevelopmental disorders. Santos et al[12] and Wang et al[13] provide additional support by emphasizing the use of induced pluripotent stem cell-derived brain organoids to model ASD. This approach complements our utilization of WJ-MSCs trans
Pertinent to the clinical outcomes observed in our case, Pavinato et al[14] describe an autosomal dominant disorder associated with loss-of-function variants in the CAPRIN1 gene. Their findings underscore the importance of under
The advancements in stem cell research and organoid modeling discussed by Li et al[9], Santos et al[12], and Wang et al[13] underscore the need for ongoing research to define optimal stem cell doses and transplantation frequencies. Addi
In conclusion, the integration of diverse literature strengthens the evidence supporting the safety and effectiveness of WJ-MSCs transplantation in managing ASD. The combination with neurorehabilitation aligns with emerging paradigms in ASD treatment. Promising outcomes in language and gross motor functions suggest the potential of this multifaceted therapeutic strategy to alleviate autism severity. However, the intricate nature of ASD, involving both genetic and envi
| 1. | Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 2. | Szachta P, Skonieczna-Żydecka K, Adler G, Karakua-Juchnowicz H, Madlani H, Ignyś I. Immune related factors in pathogenesis of autism spectrum disorders. Eur Rev Med Pharmacol Sci. 2016;20:3060-3072. [PubMed] |
| 3. | Lv YT, Zhang Y, Liu M, Qiuwaxi JN, Ashwood P, Cho SC, Huan Y, Ge RC, Chen XW, Wang ZJ, Kim BJ, Hu X. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Maric DM, Papic V, Radomir M, Stanojevic I, Sokolovac I, Milosavljevic K, Maric DL, Abazovic D. Autism treatment with stem cells: a case report. Eur Rev Med Pharmacol Sci. 2020;24:8075-8080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Kabatas S, Civelek E, Savrunlu EC, Kaplan N, Boyalı O, Diren F, Can H, Genç A, Akkoç T, Karaöz E. Feasibility of allogeneic mesenchymal stem cells in pediatric hypoxic-ischemic encephalopathy: Phase I study. World J Stem Cells. 2021;13:470-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 437] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 8. | Boyalı O, Kabatas S, Civelek E, Ozdemir O, Bahar-Ozdemir Y, Kaplan N, Savrunlu EC, Karaöz E. Allogeneic mesenchymal stem cells may be a viable treatment modality in cerebral palsy. World J Clin Cases. 2024;12:1585-1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Li C, Fleck JS, Martins-Costa C, Burkard TR, Themann J, Stuempflen M, Peer AM, Vertesy Á, Littleboy JB, Esk C, Elling U, Kasprian G, Corsini NS, Treutlein B, Knoblich JA. Single-cell brain organoid screening identifies developmental defects in autism. Nature. 2023;621:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 10. | Villarreal-Martínez L, González-Martínez G, Sáenz-Flores M, Bautista-Gómez AJ, González-Martínez A, Ortiz-Castillo M, Robles-Sáenz DA, Garza-López E. Stem Cell Therapy in the Treatment of Patients With Autism Spectrum Disorder: a Systematic Review and Meta-analysis. Stem Cell Rev Rep. 2022;18:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (70)] |
| 11. | Nabetani M, Mukai T, Taguchi A. Cell therapies for autism spectrum disorder based on new pathophysiology: A review. Cell Transplant. 2023;32:9636897231163217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Santos JLS, Araújo CA, Rocha CAG, Costa-Ferro ZSM, Souza BSF. Modeling Autism Spectrum Disorders with Induced Pluripotent Stem Cell-Derived Brain Organoids. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Wang L, Owusu-Hammond C, Sievert D, Gleeson JG. Stem Cell-Based Organoid Models of Neurodevelopmental Disorders. Biol Psychiatry. 2023;93:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Pavinato L, Delle Vedove A, Carli D, Ferrero M, Carestiato S, Howe JL, Agolini E, Coviello DA, van de Laar I, Au PYB, Di Gregorio E, Fabbiani A, Croci S, Mencarelli MA, Bruno LP, Renieri A, Veltra D, Sofocleous C, Faivre L, Mazel B, Safraou H, Denommé-Pichon AS, van Slegtenhorst MA, Giesbertz N, van Jaarsveld RH, Childers A, Rogers RC, Novelli A, De Rubeis S, Buxbaum JD, Scherer SW, Ferrero GB, Wirth B, Brusco A. CAPRIN1 haploinsufficiency causes a neurodevelopmental disorder with language impairment, ADHD and ASD. Brain. 2023;146:534-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Liu Z, Tan S, Zhou L, Chen L, Liu M, Wang W, Tang Y, Yang Q, Chi S, Jiang P, Zhang Y, Cui Y, Qin J, Hu X, Li S, Liu Q, Chen L, Li S, Burstein E, Li W, Zhang X, Mo X, Jia D. SCGN deficiency is a risk factor for autism spectrum disorder. Signal Transduct Target Ther. 2023;8:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 16. | Marinho LSR, Chiarantin GMD, Ikebara JM, Cardoso DS, de Lima-Vasconcellos TH, Higa GSV, Ferraz MSA, De Pasquale R, Takada SH, Papes F, Muotri AR, Kihara AH. The impact of antidepressants on human neurodevelopment: Brain organoids as experimental tools. Semin Cell Dev Biol. 2023;144:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Li J, Xu X, Liu J, Zhang S, Tan X, Li Z, Zhang J, Wang Z. Decoding microRNAs in autism spectrum disorder. Mol Ther Nucleic Acids. 2022;30:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/