Published online Dec 20, 2024. doi: 10.5662/wjm.v14.i4.91387
Revised: May 27, 2024
Accepted: June 11, 2024
Published online: December 20, 2024
Processing time: 209 Days and 21.2 Hours
The importance and utility of biobanks has increased exponentially since their inception and creation. Initially used as part of translational research, they now contribute over 40% of data for all cancer research papers in the United States of America and play a crucial role in all aspects of healthcare. Multiple classification systems exist but a simplified approach is to either classify as population-based or disease-oriented entities. Whilst historically publicly funded institutions, there has been a significant increase in industry funded entities across the world which has changed the dynamic of biobanks offering new possibilities but also new challenges. Biobanks face legal questions over data sharing and intellectual property as well as ethical and sustainability questions particularly as the world attempts to move to a low-carbon economy. International collaboration is required to address some of these challenges but this in itself is fraught with complexity and difficulty. This review will examine the current utility of biobanks in the modern healthcare setting as well as the current and future challenges these vital institutions face.
Core Tip: Biomarkers and the biobanks used to help discover them are growing in number, scope and importance. Our article reviews the different models of biobanks that exist globally as well as some of the biomarkers that have been discovered from these institutions. We review the challenges biobanks face and their future utility in biomedical research and personalised medicine.
- Citation: Colwill M, Baillie S, Pollok R, Poullis A. Biobanks and biomarkers: Their current and future role in biomedical research. World J Methodol 2024; 14(4): 91387
- URL: https://www.wjgnet.com/2222-0682/full/v14/i4/91387.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i4.91387
Biomarkers are defined as a specific characteristic that is used as an indicator of a normal or pathological biological process or a response to a therapeutic intervention[1]. Their use has grown in recent decades and they are now crucial tools in healthcare and an area of heavy investment and research.
Alongside the development of biomarkers has been the growth of biobanks with the first description of their use in the literature in 1996[2] although the earliest similar facilities, known as biorepositories, were established at the end of the 19th Century[3]. One of the first biorepositories was a programme set up by the United States Department of Defence (DoD) and is now part of the Joint Pathology Centre(JPC)[4]. This collected samples from soldiers and veterans as early as 1862 with the initial aim of providing a second diagnostic pathology opinion and this continues to be the primary aim of the JPC today. Technological advances now mean that samples historically collected in these biorepositories for a single research question can now be utilised for a variety of different projects. An example of this was the application of modern techniques to samples stored in the DoD serum repository, a biorepository of over 50000 serum specimens initially set up in the 1980s to investigate the human immunodeficiency virus epidemic, to identify over 200 potential biomarkers of occupational exposure[5] demonstrating the potential benefits of applying modern technology to historical bioreposi
Modern biobanks, as defined by the Organisation for Economic Co-operation and Development, are ‘a collection of biological material and associated data and information stored in an organised system, for a population or a large subset of a population[6]’. Their role in improving our understanding of health and disease as well as their size and scope has massively increased since their inception and now represent an industry projected to be worth $50 billion by 2026[7]. Biobanks are either academic, which are usually research-driven with institutional or grant funding, or industry-funded which tend to focus on end products and be more business-driven. The difference in these models can be problematic which will be discussed later in this article.
Whilst biobanks were initially focused on cancer research they, and the biomarkers stored within them, are now key facets in the development of personalized medicine through the emerging fields of metabolomics, proteomics and epigenomics and are playing a more and more important role in cutting edge medicine. This article will discuss their current and future roles in healthcare as well as the challenges facing them.
There are various classification systems for biobanks, but these can be cumbersome and confusing. A convenient and effective way to distinguish between them is whether they are population-based or disease-oriented entities.
Population banks obtain samples primarily from volunteers without having an inclusion or exclusion criteria. Their goal is to use vast numbers of samples to give an accurate representation of either an entire population or specific sub-population and identify specific biomarkers of genetic susceptibility that, along with external factors, contribute to disease. An example of this is the United Kingdom Biobank, a stand-alone research entity, which has collected genetic and health information from over half a million individuals since 2006. This data is anonymised and made freely available to researchers around the globe and has been used to investigate biomarkers thought to be important in sepsis[8] and, through blood and urine sample analysis, to identify biomarkers for the risk of stroke[9]. These biomarkers have since been used to develop polygenic risk scores for certain diseases including chronic kidney disease, type 2 diabetes mellitus and gout[9].
The Danish National Biobank (DNB), whilst also academic in nature and funding, uses a different model through close integration into the Danish healthcare system and incorporates various projects, including the Danish Blood Donor Biobank, Danish Cancer Biobank and the Danish National Genome Centre, which allows a wider-array of biological samples from a greater donor pool to be collected. It also incorporates a large-scale sample collection programme from new-borns, via umbilical vein blood, as well as over 5 million adult individuals. Use of DNB data has led to the development of biomarkers for dementia with the identification of micro-RNA targets involved in vasculogenesis, lipoprotein transport and amyloid precursor protein which could offer new therapeutic options for Alzheimer’s[10]. Further work using DNB data has also identified biomarkers in rheumatic disease[11], which can be used to facilitate earlier diagnosis, improve prognostication and monitor the efficacy of treatments, and the PREDICT study which aims to understand the underlying biological mechanisms in inflammatory bowel disease (IBD)[12].
As well as academic population-based biobanks, industry funded institutions are also growing in number and scope. An example is the Shanghai Zhangjiang biobank which is wholly owned and operated by Shanghai Outdo Biotech Co. Ltd and aims to collect 10 million human samples, including from the faecal microbiome, tissue and blood. Output from this biobank has included an at-home-test for liver cancer using micro-RNA biomarkers developed by Roche diagnostics[13]. The complexity of grant funding and financial pressures on academic biobanks means that these industry-funded projects are likely to become more important in the future and provide a greater share of research output.
Whilst population-based biobanks have definite advantages, the range of research questions that they can address can be limited such as when investigating a specific sub-group of patients of a particular disease. This is where disease-oriented biobanks, which collect samples focused on either a specific tissue-type or disease, can be useful and they have certainly contributed significantly to healthcare in the past decades.
Earlier disease-oriented biobanks were primarily targeting cancer with the aim of identifying novel biomarkers to aid early detection as well as developing and testing novel therapeutics such as the impact of Programmed Cell Death-1 inhibitors[14] in immunotherapy for cancer. Biobanks continue to be crucial to cancer research across the entire spectrum of the disease. A more recent example is the VIVO biobank in the United Kingdom which has a focus on childhood cancer[15]. Research utilising this resource has been able to advance treatment of acute lymphoblastic leukaemia through genomic analysis and biomarker identification[16]. Genomic analysis using VIVO biobank studies has also been used to identify biomarkers which can help risk-stratify patients with medulloblastoma[17] and a whole host of other research projects are continuing to use this valuable resource.
The number of biobanks, both academic and industry-funded, investigating cancer continues to increase but disease-oriented biobanks are also playing a more substantial role in non-oncological disease. The National Institute for Health Research (NIHR) IBD Bioresource in the United Kingdom[18] was established to provide a genetic and clinical resource that would be available to researchers and allow the development of better treatments for patients with IBD. Work using this biobank to identify epigenetic biomarkers is ongoing[19] and, with the discovery of the IBD-hi and IBD-lo[20] biomarkers predictive of more aggressive disease, the NIHR IBD Bioresource will continue to produce important research well into the future with a large number of projects ongoing.
In Europe and the United States there has also been the establishment of disease-oriented biobank networks, such as the Brain Net Europe[21] or the NIH NeuroBioBank[22], which aim to co-ordinate collection of post-mortem brain tissue for study. This has yielded results in the form of, amongst others, the discovery of biomarkers for susceptibility to Alzheimer’s disease[23] and identification of a differential regulation of tau exon 2 and exon 10 that could be useful biomarkers or possible therapeutic targets for Huntington’s disease[24]. Given the financial pressures on biobanks and relative scarcity of some pathological specimens, it is likely that the importance of biobank networks will increase in the future.
It should also be noted that these disease-oriented biobanks, whilst more focused than their population-based equivalents, can also contribute to broader research questions such as the NIHR IBD Bioresource contributing it’s stored genetic data to the 100000 genomes project using whole genome sequencing to increase the diagnostic yield of various rare diseases[25].
As can be seen, biobanks and biomarkers currently represent a key part of healthcare research and with the advent of artificial intelligence and an ever-growing understanding of metabolomics, proteomics and epigenomics it is likely their importance will only increase in the future.
The biobank industry has been growing in size, scope and complexity since it’s creation and the pace of this growth has been increasing[7]. This is representative of the move, at least in terms of research even if it has not yet translated fully into clinical practice, from generalised to personalised and precision medicine. Whilst the reality of an individual and tailored treatment for each patient based upon their genetic and metabolic profile is still some way off, it is clear that biobanks and the biomarkers contained within them will be crucial to achieving this holy grail of medicine.
Personalised medicine aims to provide the right treatment to the right patient at the right time. An area where this has been successful is through the use of oncological biobanks to predict an individual’s response to radiotherapy and chemotherapy in a wide variety of cancers. Biobank data has recently been instrumental in discovering patient-derived organoids[26] which could be used to developed targeted and personalised treatments for gastrointestinal cancers. As well as targeting treatments, biomarkers have also been used to understand a patient’s risk profile thereby facilitating earlier diagnosis and treatment, such as in Alzheimer’s[27], which can drastically impact upon prognosis. These examples highlight the potential uses of biomarkers and biobanks in personalised medicine, a field which will continue to grow into the future as technology continues to develop.
The advent of technologies such as artificial intelligence (AI) offers to bring a promising data analysis capability to the discovery and development of biomarkers. AI is able to analyze the vast volumes of data–be it proteomic, metabolomic or genomic–held in biobanks and help to identify novel biomarkers. Several have already been discovered across a variety of diseases using AI[28,29] and there have been cases where these AI-supported discoveries have outperformed current processes, such as Niu et al[30] who used plasma and liver proteomic datasets to identify circulating biomarkers that were able to diagnose the degree of fibrosis in alcohol related liver disease patients more accurately than current conventional biomarkers. As well as discovery, AI also has a role in translating these biomarkers into clinically useful tests and overcoming the logistical challenges this can pose[29] which should help to bring these discoveries from the research realm into real-world clinical use much more quickly than is currently possible.
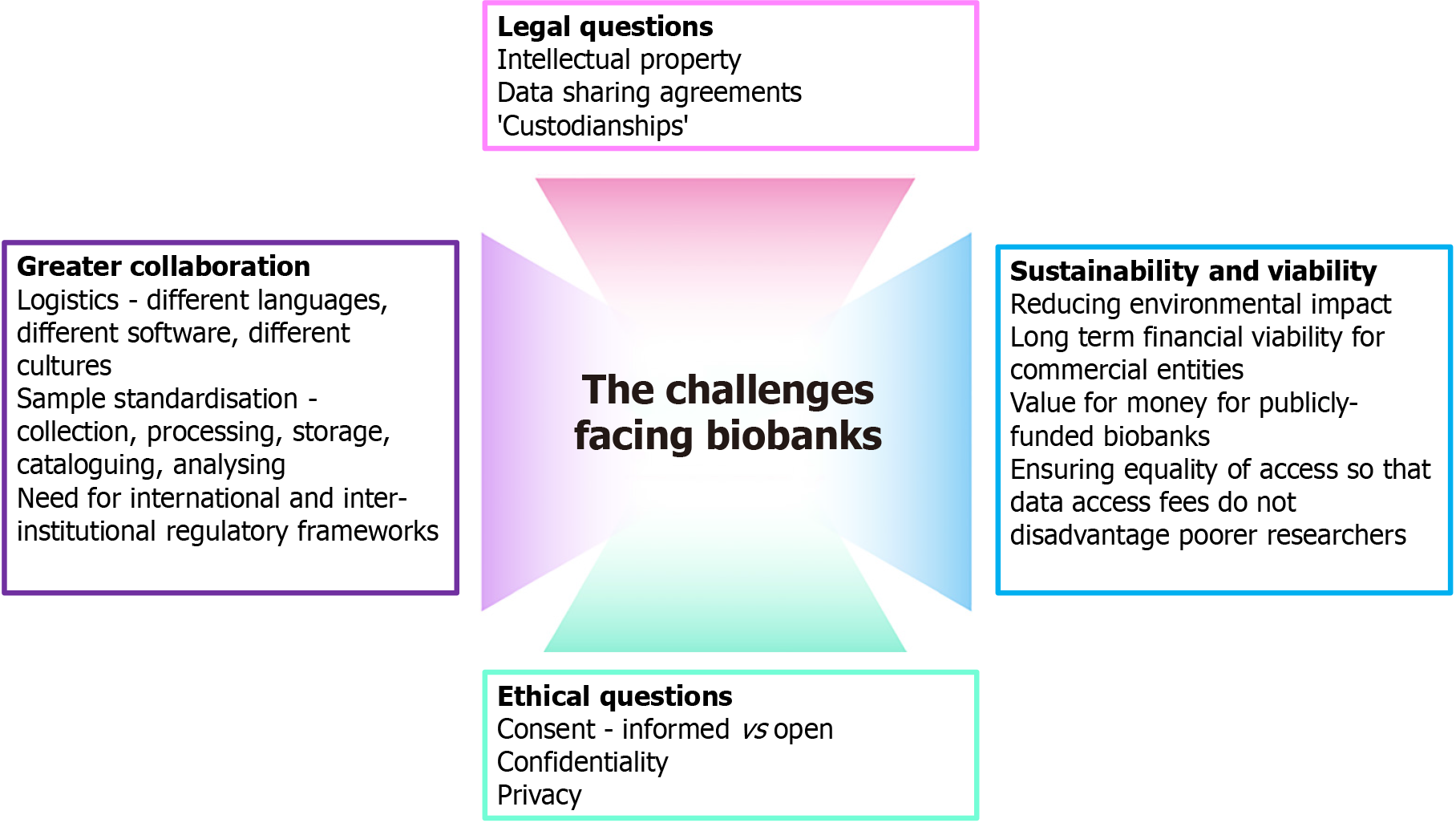
As discussed, the use of biomarkers and importance of biobanks will only increase in the coming decades and become a crucial part of cutting-edge healthcare. However, with this will come significant challenges, summarized in Figure 1, which will need to be overcome if we are to exploit their full potential.
Since the first biobanks appeared, the number of both academic and industry-funded biobanks has ballooned but has done so in a relatively hap-hazard manner often without national, let alone international, co-ordination. As a result, many biobanks are performing similar roles without pooling data sets which impacts upon the quantity and quality of output and wastes resources. Addressing this requires greater co-ordination between different institutions but this also comes with challenges, specifically around the logistics of data sharing and ethical considerations concerning data sharing and ownership. In response to these challenges international organizations have been established such as the International Society for Biological and Environmental Repositories (ISBER) and the Biobanking and Biomolecular Resource Research Infrastructure European Research Infrastructure Consortium (BBMRI-ERIC). These organizations aim to foster co-operation and data sharing between different biobanks to improve the output from biomedical research.
However, implementing this co-operation will be a challenge. Logistical barriers such as different languages spoken and computer software can be relatively easily overcome and there are multiple examples of international organisations which function well in spite of this such as the European Organisation for Nuclear Research[31]. However, a more significant challenge is sample standardisation. The protocols and standards for sample collection, cataloguing and storage can vary significantly between different biobanks even if collecting similar data. When attempting to combine data from multiple facilities, or even within the same facility but from different time periods or projects, this heterogeneity can impact upon the reproducibility and validity of the study in question. In order to foster collaborative work which produces scientifically valid results, developing and implementing standardised protocols for sample collection and storage is essential and organisations like the BBMRI-ERIC will play a crucial role.
Whilst greater collaboration will help to overcome some difficulties, it will also exacerbate some current legal conundrums. Intellectual property (IP) laws differ from country to country and prior to engaging in collaborative working there would need to be agreement amongst stakeholders about ownership of biobank facilities, the samples within them, any scientific or commercial output as well as access mechanisms to this IP for future research purposes. International agreements regarding the collective enforcement of IP legislation are needed to provide protection and security to biobanks although we do recognize this has not been possible in many other industries so may not be forthcoming in the immediate future. This will require collaboration amongst biobanks and with international organisations such as the World Trade Organisation.
One possible solution to the IP question is relinquishing the term ‘ownership’ entirely. The nature of biobanks and their various stakeholders–funding agencies, philanthropic individuals, participants, researchers–has led to the development of complex arrangements with regards to control and ownership. This negatively impacts on the access to the biobank data and research output. An alternative proposal has been to create biobanks as ‘custodianships’[32] where all those involved have a caretaking obligation from collection to distribution of any findings. It has been suggested these custodianships would then be able to endorse legal and ethical practices and share accountability amongst stakeholders[33] although this remains an area of debate.
Together with these legal challenges are ongoing ethical questions such as confidentiality, privacy and consent. The collection of large volumes of personal data and tissue requires stringent protocols and guidelines defining who has access to the data and what it is used for. Poor or inadequate implementation of these practices can result in breaking confidentiality and the invasion of participant’s privacy. The ISBER publishes guidance on best practices[34] with regards to these areas but there is no framework to ensure these are followed and this needs addressing at an international level.
Along with confidentiality and privacy, consent continues to be a challenging area and one of ongoing debate. There is variability in the consent process between different biobanks and different research projects with some obtaining fully informed consent related to one specific project. This can present challenges if investigators want to apply new tests or analyses to samples but do not have express consent and the process of re-contacting participants to obtain this consent may be considered too invasive. Other institutions use a looser or broad consent process but this has ethical implications. There is no ‘one-fits-all’ approach and this has led to debate over the optimal form of consent and whether so-called ‘open-consent’ is more appropriate for biobanks[35]. However, there is as of yet no international consensus on this and it will continue to be an area of debate in the near future.
A further challenge is with regards to the sustainability of these repositories. The collection and storage of biological samples is a costly undertaking, both financially and in terms of their environmental impact. Different funding models, as discussed earlier, create challenges such as value for money for publicly funded institutions or providing sufficient income to make privately funded institutions economically viable in the longer term. This can lead to publicly funding entities, such as public hospitals or the NIHR, not being willing to pay high maintenance costs and researchers not being able to afford high access fees charged by commercial facilities. Both of these outcomes impact upon the utility of biobanks and negatively affect the volume and quality of scientific output. These financial challenges will continue into the future[36] and co-ordination on research topics, sharing of expertise and pooling of resources will be key to maintaining a financially viable system. The environmental cost, given high energy and other resource requirements, is becoming more of a frontline issue and there has been work to try and reduce the impact of these facilities through renewable energy and minimising waste generation but there still remain significant challenges in adopting a zero-carbon and environmentally sustainable model[37].
With recent genuine advances towards that elusive goal of personalised and precision medicine, the importance of biomarkers has never been greater and the same is true of the biobanks that are essential to their development. The research output and practical, real-world applications are increasing at an almost exponential rate and with this comes questions about how to ensure the highest quality output is achieved without compromising on efficiency or legal and ethical standards. Improved international collaboration will be essential for the coming years and decades to achieve this and help us move towards truly personalised medicine.
| 1. | Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1599] [Cited by in RCA: 1260] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 2. | Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med (Berl). 1996;74:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 606] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Uruburu F. History and services of culture collections. Int Microbiol. 2003;6:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | The Joint Pathology Center. US Federal Government Pathology Reference Center. [cited 4 June 2024]. Available from: https://jpc.capmed.mil/. |
| 5. | Woeller CF, Thatcher TH, Van Twisk D, Pollock SJ, Croasdell A, Kim N, Hopke PK, Xia X, Thakar J, Mallon CT, Utell MJ, Phipps RP. Detection of Serum microRNAs From Department of Defense Serum Repository: Correlation With Cotinine, Cytokine, and Polycyclic Aromatic Hydrocarbon Levels. J Occup Environ Med. 2016;58:S62-S71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | OECD. Stat includes data and metadata for OECD countries and selected non-member economies. [cited 4 June 2024]. Available from: https://stats.oecd.org/. |
| 7. | Slušná ĽK, Balog M, Baláž V, Nemcová E, Filčák R, Jeck T, Antošová M. Rise of Biobanking in the EU: Evidence from the Framework Programmes. WSEAS Trans Bus Econ. 2021;18:1304. [DOI] [Full Text] |
| 8. | Biobank. Cholesterol and blood poisoning - the story unfolds. [cited 4 June 2024]. Available from: https://www.ukbiobank.ac.uk/Learn-more-about-uk-biobank/our-impact/cholesterol-and-blood-poisoning-the-story-unfolds. |
| 9. | Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, Venkataraman GR, Wainberg M, Ollila HM, Kiiskinen T, Havulinna AS, Pirruccello JP, Qian J, Shcherbina A; FinnGen, Rodriguez F, Assimes TL, Agarwala V, Tibshirani R, Hastie T, Ripatti S, Pritchard JK, Daly MJ, Rivas MA. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet. 2021;53:185-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 522] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 10. | Mengel-From J, Rønne ME, Carlsen AL, Skogstrand K, Larsen LA, Tan Q, Christiansen L, Christensen K, Heegaard NHH. Circulating, Cell-Free Micro-RNA Profiles Reflect Discordant Development of Dementia in Monozygotic Twins. J Alzheimers Dis. 2018;63:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kringelbach TM, Glintborg B, Hogdall EV, Johansen JS, Hetland ML; Biomarker Protocol Study Group. Identification of new biomarkers to promote personalised treatment of patients with inflammatory rheumatic disease: protocol for an open cohort study. BMJ Open. 2018;8:e019325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Center for Molecular Prediction of Inflammatory Bowel Disease (PREDICT). Aalb Univ n.d. [cited 4 June 2024]. Available from: https://www.predictibd.dk/. |
| 13. | Government online offline Shanghai. Roche Diagnostics: High-quality diagnostic solutions boost early detection of liver cancer. [cited 4 June 2024]. Available from: https://www.shanghai.gov.cn/nw48081/20231101/fcf7d38a5f654702b35efcfddcc64558.html. |
| 14. | Wang DR, Wu XL, Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther. 2022;7:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 341] [Article Influence: 85.3] [Reference Citation Analysis (1)] |
| 15. | VIVO Biobank. Home n.d. [cited 4 June 2024] Available from: https://vivobiobank.org/. |
| 16. | Schwab C, Cranston RE, Ryan SL, Butler E, Winterman E, Hawking Z, Bashton M, Enshaei A, Russell LJ, Kingsbury Z, Peden JF, Barretta E, Murray J, Gibson J, Hinchliffe AC, Bain R, Vora A, Bentley DR, Ross MT, Moorman AV, Harrison CJ. Integrative genomic analysis of childhood acute lymphoblastic leukaemia lacking a genetic biomarker in the UKALL2003 clinical trial. Leukemia. 2023;37:529-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (75)] |
| 17. | Goddard J, Castle J, Southworth E, Fletcher A, Crosier S, Martin-Guerrero I, García-Ariza M, Navajas A, Masliah-Planchon J, Bourdeaut F, Dufour C, Ayrault O, Goschzik T, Pietsch T, Sill M, Pfister SM, Rutkowski S, Richardson S, Hill RM, Williamson D, Bailey S, Schwalbe EC, Clifford SC, Hicks D. Molecular characterisation defines clinically-actionable heterogeneity within Group 4 medulloblastoma and improves disease risk-stratification. Acta Neuropathol. 2023;145:651-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (76)] |
| 18. | NIHR BioResource. The Inflammatory Bowel Disease (IBD) BioResource is a national platform designed to expedite research into Crohn’s and colitis and help develop new and better therapies. [cited 4 June 2024] Available from: https://bioresource.nihr.ac.uk/centres-programmes/ibd-bioresource/. |
| 19. | NIHR BioResource. Defining epigenetic biomarkers in whole blood DNA samples to predict response to biological therapies in inflammatory bowel disease. [cited 4 June 2024] Available from: https://bioresource.nihr.ac.uk/studies/nbr200/. |
| 20. | Lee JC, Lyons PA, McKinney EF, Sowerby JM, Carr EJ, Bredin F, Rickman HM, Ratlamwala H, Hatton A, Rayner TF, Parkes M, Smith KG. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011;121:4170-4179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Bell JE, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Budka H, Dexter DT, Falkai P, Ferrer I, Gelpi E, Gentleman SM, Giaccone G, Huitinga I, Ironside JW, Klioueva N, Kovacs GG, Meyronet D, Palkovits M, Parchi P, Patsouris E, Reynolds R, Riederer P, Roggendorf W, Seilhean D, Schmitt A, Schmitz P, Streichenberger N, Schwalber A, Kretzschmar H. Management of a twenty-first century brain bank: experience in the BrainNet Europe consortium. Acta Neuropathol. 2008;115:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | NIH NeuroBioBank. Since 2013, the NIH NeuroBioBank has catalyzed scientific discovery through the centralization of resources aimed at the collection and distribution of human post-mortem brain tissue. [cited 4 June 2024] Available from: https://neurobiobank.nih.gov/. |
| 23. | Otero-Garcia M, Mahajani SU, Wakhloo D, Tang W, Xue YQ, Morabito S, Pan J, Oberhauser J, Madira AE, Shakouri T, Deng Y, Allison T, He Z, Lowry WE, Kawaguchi R, Swarup V, Cobos I. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer's disease. Neuron. 2022;110:2929-2948.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 24. | Petry S, Nateghi B, Keraudren R, Sergeant N, Planel E, Hébert SS, St-Amour I. Differential Regulation of Tau Exon 2 and 10 Isoforms in Huntington's Disease Brain. Neuroscience. 2023;518:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 25. | 100000 Genomes Project Pilot Investigators, Smedley D, Smith KR, Martin A, Thomas EA, McDonagh EM, Cipriani V, Ellingford JM, Arno G, Tucci A, Vandrovcova J, Chan G, Williams HJ, Ratnaike T, Wei W, Stirrups K, Ibanez K, Moutsianas L, Wielscher M, Need A, Barnes MR, Vestito L, Buchanan J, Wordsworth S, Ashford S, Rehmström K, Li E, Fuller G, Twiss P, Spasic-Boskovic O, Halsall S, Floto RA, Poole K, Wagner A, Mehta SG, Gurnell M, Burrows N, James R, Penkett C, Dewhurst E, Gräf S, Mapeta R, Kasanicki M, Haworth A, Savage H, Babcock M, Reese MG, Bale M, Baple E, Boustred C, Brittain H, de Burca A, Bleda M, Devereau A, Halai D, Haraldsdottir E, Hyder Z, Kasperaviciute D, Patch C, Polychronopoulos D, Matchan A, Sultana R, Ryten M, Tavares ALT, Tregidgo C, Turnbull C, Welland M, Wood S, Snow C, Williams E, Leigh S, Foulger RE, Daugherty LC, Niblock O, Leong IUS, Wright CF, Davies J, Crichton C, Welch J, Woods K, Abulhoul L, Aurora P, Bockenhauer D, Broomfield A, Cleary MA, Lam T, Dattani M, Footitt E, Ganesan V, Grunewald S, Compeyrot-Lacassagne S, Muntoni F, Pilkington C, Quinlivan R, Thapar N, Wallis C, Wedderburn LR, Worth A, Bueser T, Compton C, Deshpande C, Fassihi H, Haque E, Izatt L, Josifova D, Mohammed S, Robert L, Rose S, Ruddy D, Sarkany R, Say G, Shaw AC, Wolejko A, Habib B, Burns G, Hunter S, Grocock RJ, Humphray SJ, Robinson PN, Haendel M, Simpson MA, Banka S, Clayton-Smith J, Douzgou S, Hall G, Thomas HB, O'Keefe RT, Michaelides M, Moore AT, Malka S, Pontikos N, Browning AC, Straub V, Gorman GS, Horvath R, Quinton R, Schaefer AM, Yu-Wai-Man P, Turnbull DM, McFarland R, Taylor RW, O'Connor E, Yip J, Newland K, Morris HR, Polke J, Wood NW, Campbell C, Camps C, Gibson K, Koelling N, Lester T, Németh AH, Palles C, Patel S, Roy NBA, Sen A, Taylor J, Cacheiro P, Jacobsen JO, Seaby EG, Davison V, Chitty L, Douglas A, Naresh K, McMullan D, Ellard S, Temple IK, Mumford AD, Wilson G, Beales P, Bitner-Glindzicz M, Black G, Bradley JR, Brennan P, Burn J, Chinnery PF, Elliott P, Flinter F, Houlden H, Irving M, Newman W, Rahman S, Sayer JA, Taylor JC, Webster AR, Wilkie AOM, Ouwehand WH, Raymond FL, Chisholm J, Hill S, Bentley D, Scott RH, Fowler T, Rendon A, Caulfield M. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care - Preliminary Report. N Engl J Med. 2021;385:1868-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 590] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (1)] |
| 26. | Yu YY, Zhu YJ, Xiao ZZ, Chen YD, Chang XS, Liu YH, Tang Q, Zhang HB. The pivotal application of patient-derived organoid biobanks for personalized treatment of gastrointestinal cancers. Biomark Res. 2022;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (77)] |
| 27. | Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. 2018;284:643-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 651] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 28. | Hou Q, Bing ZT, Hu C, Li MY, Yang KH, Mo Z, Xie XW, Liao JL, Lu Y, Horie S, Lou MW. RankProd Combined with Genetic Algorithm Optimized Artificial Neural Network Establishes a Diagnostic and Prognostic Prediction Model that Revealed C1QTNF3 as a Biomarker for Prostate Cancer. EBioMedicine. 2018;32:234-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Mann M, Kumar C, Zeng WF, Strauss MT. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021;12:759-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 30. | Niu L, Thiele M, Geyer PE, Rasmussen DN, Webel HE, Santos A, Gupta R, Meier F, Strauss M, Kjaergaard M, Lindvig K, Jacobsen S, Rasmussen S, Hansen T, Krag A, Mann M. A paired liver biopsy and plasma proteomics study reveals circulating biomarkers for alcohol-related liver disease. 2020 Preprint. Available from: bioRxiv:337592. [DOI] [Full Text] |
| 31. | CERN. Accelerating science. [cited 4 June 2024] Available from: https://home.cern/. |
| 32. | Vaz M, Vaz M, Srinivasan K. Ethical challenges in biobanking: moving the agenda forward in India. Indian J Med Ethics. 2014;11:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Yassin R, Lockhart N, González del Riego M, Pitt K, Thomas JW, Weiss L, Compton C. Custodianship as an ethical framework for biospecimen-based research. Cancer Epidemiol Biomarkers Prev. 2010;19:1012-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | ISBER. About ISBER. [cited 4 June 2024] Available from: https://isber.org. |
| 35. | Lunshof JE, Chadwick R, Vorhaus DB, Church GM. From genetic privacy to open consent. Nat Rev Genet. 2008;9:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Rush A, Catchpoole DR, Ling R, Searles A, Watson PH, Byrne JA. Improving Academic Biobank Value and Sustainability Through an Outputs Focus. Value Health. 2020;23:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Samuel G, Hardcastle F, Lucassen A. Environmental sustainability and biobanking: A pilot study of the field. New Genet Soc. 2022;41:157-175. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/