Published online Jun 20, 2024. doi: 10.5662/wjm.v14.i2.92608
Revised: March 15, 2024
Accepted: April 9, 2024
Published online: June 20, 2024
Processing time: 135 Days and 16.2 Hours
It is increasingly common to find patients affected by a combination of type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD), and studies are able to correlate their relationships with available biological and clinical evidence. The aim of the current study was to apply association rule mining (ARM) to discover whether there are consistent patterns of clinical features relevant to these diseases. ARM leverages clinical and laboratory data to the meaningful patterns for diabetic CAD by harnessing the power help of data-driven algorithms to optimise the decision-making in patient care.
To reinforce the evidence of the T2DM-CAD interplay and demonstrate the ability of ARM to provide new insights into multivariate pattern discovery.
This cross-sectional study was conducted at the Department of Biochemistry in a specialized tertiary care centre in Delhi, involving a total of 300 consented subjects categorized into three groups: CAD with diabetes, CAD without diabetes, and healthy controls, with 100 subjects in each group. The participants were enrolled from the Cardiology IPD & OPD for the sample collection. The study employed ARM technique to extract the meaningful patterns and relationships from the clinical data with its original value.
The clinical dataset comprised 35 attributes from enrolled subjects. The analysis produced rules with a maximum branching factor of 4 and a rule length of 5, necessitating a 1% probability increase for enhancement. Prominent patterns emerged, highlighting strong links between health indicators and diabetes likelihood, particularly elevated HbA1C and random blood sugar levels. The ARM technique identified individuals with a random blood sugar level > 175 and HbA1C > 6.6 are likely in the “CAD-with-diabetes” group, offering valuable insights into health indicators and influencing factors on disease outcomes.
The application of this method holds promise for healthcare practitioners to offer valuable insights for enhancing patient treatment targeting specific subtypes of CAD with diabetes. Implying artificial intelligence techniques with medical data, we have shown the potential for personalized healthcare and the development of user-friendly applications aimed at improving cardiovascular health outcomes for this high-risk population to optimise the decision-making in patient care.
Core Tip: The study is aimed to apply association rule mining (ARM) to discover the correlation between type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD). ARM leverages clinical and laboratory data to the meaningful patterns for diabetic CAD by harnessing the power help of data-driven algorithms. It will assist to optimise the decision-making in patient care by providing new insights into multivariate pattern discovery. Thus, this research facilitates targeted medication for patients to regulate uncontrolled clinical parameters in case of CAD with T2DM.
- Citation: Dabla PK, Upreti K, Shrivastav D, Mehta V, Singh D. Discovering hidden patterns: Association rules for cardiovascular diseases in type 2 diabetes mellitus. World J Methodol 2024; 14(2): 92608
- URL: https://www.wjgnet.com/2222-0682/full/v14/i2/92608.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i2.92608
Heart disease has become a global threat and considered to be a major cause of mortality and death rate in modern society[1]. The importance of detailed medical assessment cannot be denied; however; in this era of artificial intelligence, if this skillful tool can be given the added advantage of data-driven algorithms and association rule mining (ARM) techniques, it can prove to be helpful to perform this complicated task to estimate the probability of disease based on data and facts provided to the system[2]. In ARM, frequent item sets are built from symptom transactions using a minimum threshold specified by the user, and a confidence level of 0.9 is established by researchers to rank rules. For positively correlated rules, a minimum support and lift of 0.001 and 1, respectively, can be set. Low-confidence and low-support criteria can capture rare or unusual things, while low-support and high-confidence thresholds yield a small number of customer analytics rules[3]. In medical diagnosis, where many symptom combinations are rare, ARM can help identify linked patterns and rules, making symptom analysis more focused[4].
In several previous studies, the authors used ARM techniques to analyze lung cancer data from the SEER program and used hotspots[5,6]. In healthcare, the relationship between diseases is represented by an association rule among symptoms (or diseases), and in medicine it is represented as P→Q, where P and Q are distinct sets of symptoms (or diseases). P is the rule’s antecedents; Q is its consequent. Also, known as “if→then”, “if” is antecedent, and “then” is consequent. Support, confidence, and lift are used to quantify the effectiveness of generated rules, which are respectively calculated as follows:
Support (P→Q) = Number of patients having P and Q /total number of patients
Confidence (P→Q) = Number of patients having P and Q/number of patients having P
Lift (P→Q) = [(Number of Patients having both P and Q)/(number of patients having P)] (fraction of patients having Q)
Support establishes a rule’s frequency (i.e., generality) for a particular data collection. Lift controls the frequency of symptom Q when illness P occurs. Lift determines P and Q’s similarity: Independent (= 1), positive (> 1), negative (< 1)[7]. The Hotspot algorithm, as described previously[8,9], follows a straightforward approach. It starts with the input data and proceeds in a depth-first manner using a greedy strategy. At each node, it branches on the attribute that results in the most significant improvement in the target value while respecting the constraints and then repeats the process recursively for each child node. Each node corresponds to a segment of an association, and the target value can be enhanced by adjusting the target fraction or average target value. To implement the algorithm, the Hotspot program from “Waika to Environment for Knowledge Analysis” (WEKA)[10] is employed.
This program[8,9] provides an overview of disease predictions based on computational intelligence. Available data suggest that neural networks were used for lumen wall evaluation, vessel contour determination, and plaque characterization, optimizing for phase with each vessel segment analysis[11].
Recent advances in data mining techniques have provided unprecedented opportunities to uncover hidden patterns and associations in complex datasets, leading to new insights into the pathogenesis and management of cardiovascular diseases (CVD). Despite the growing interest in this area, there remains a need for more comprehensive studies that explore the intricate relationships between various clinical and genetic factors influencing the development and progression of coronary artery disease (CAD) in type 2 diabetes mellitus (T2DM) patients. By applying ARM to a large dataset of T2DM patients, this study aimed to identify novel associations between clinical and genetic factors that may help in the early detection and management of CAD in this high-risk population. Understanding these hidden patterns could lead to the development of more personalized approaches for the prevention and treatment of CAD in T2DM patients, ultimately improving their overall prognosis and quality of life.
Thus, our work focused on the application of ARM techniques to evaluate the association between angiographic coronary artery stenosis patterns and its severity in diabetic and non-diabetic CAD patients based on their glycemic control. It can prove useful to assist doctors and patients in early detection and intervention of complicated multifactorial disease like CAD.
This hospital-based cross-sectional study was performed at the Department of Biochemistry in a tertiary care referral cardiac centre in Delhi, India. We analysed the data of 200 consented, age- and sex-matched, angiographically proven patients diagnosed as having CAD with or without diabetes and 100 healthy control individuals.
Venous blood samples were collected from consented patients under proper aseptic techniques for the analysis of serum glycated haemoglobin (HbA1c), blood sugar levels, lipids, and other routine parameters. The level of HbA1c was taken as a serum biomarker for diabetes where HbA1c levels more than 6.5% were considered as diabetic and less than 6.5% as non-diabetic[12]. We enrolled patients who had undergone coronary angiography using Judkin’s technique[13] to study angiography patterns.
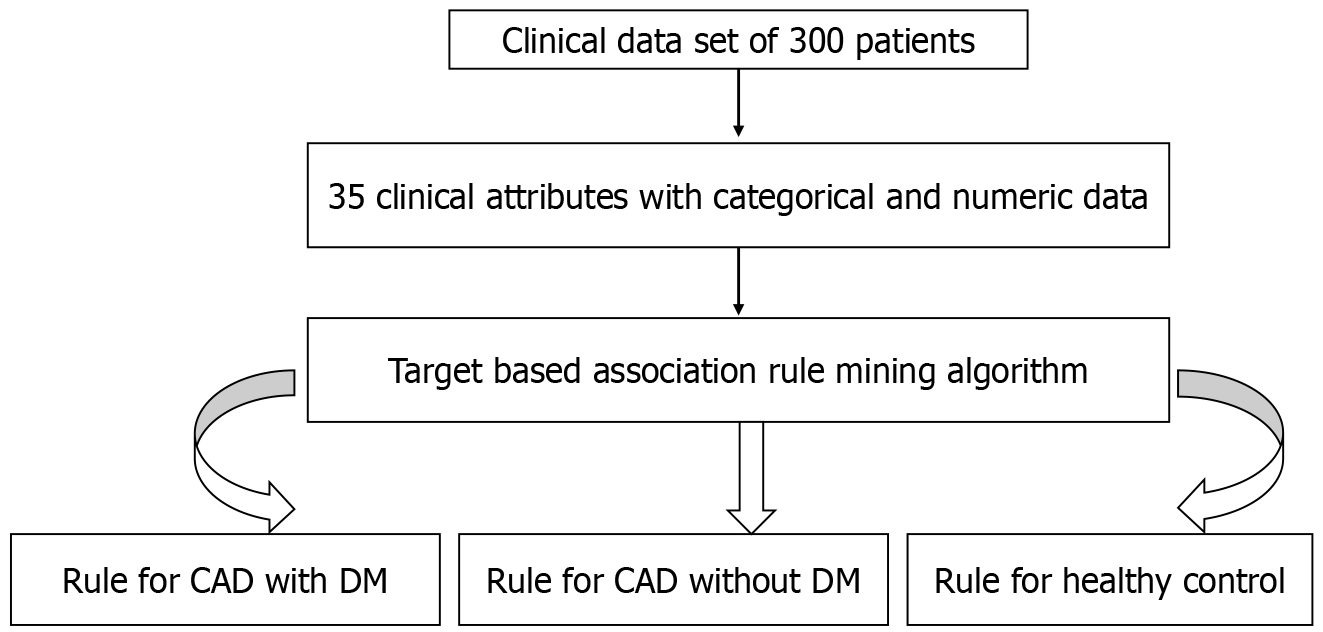
In the present investigation, the clinical dataset comprised 35 attributes from enrolled patients, with their real/original clinical values serving as input for the proposed model (Table 1). Data extraction and management are illustrated in Figure 1. Utilizing a Hotspot rule-based data mining method, the study successfully resolved the test, leading to the identification of novel and significant symptom patterns in patients with CAD and diabetes.
| S. No | Attribute name | Data type | Categorical value |
| 1 | Age | Numeric | Minimum = 23 years, maximum = 78 yr |
| 2 | Gender | Categorical | Female, male |
| 3 | Diet | Categorical | Non-veg, veg |
| 4 | Systolic_BP | Numeric | Minimum = 73, maximum = 200 |
| 5 | Diastolic_BP | Numeric | Minimum = 24, maximum = 120 |
| 6 | Smoking | Categorical | No, yes |
| 7 | Alcoholic | Categorical | No, yes |
| 8 | Tobacco | Categorical | No, yes |
| 9 | Hypertensive | Categorical | No, yes |
| 10 | Disease type | Categorical | Double-vessel, single-vessel, triple-vessel, no-disease |
| 11 | Gensini score | Numeric | Minimum = 0, maximum = 136 |
| 12 | Total cholesterol | Numeric | Minimum = 45, maximum = 381 |
| 13 | Triglycerides | Numeric | Minimum = 25.2, maximum = 924 |
| 14 | High density lipoprotein | Numeric | Minimum = 11, maximum = 132 |
| 15 | Low density lipoprotein | Numeric | Minimum = 15, maximum = 303 |
| 16 | Very low density lipoprotein | Numeric | Minimum = 5.4, maximum = 184 |
| 17 | Random blood sugar | Numeric | Minimum = 72, maximum = 683 |
| 18 | HbA1C (glycated haemoglobin) | Numeric | Minimum = 3.1, maximum = 16 |
| 19 | Sodium | Numeric | Minimum = 122, maximum = 156 |
| 20 | Potassium | Numeric | Minimum = 1.8, maximum = 6 |
| 21 | Haemoglobin | Numeric | Minimum = 1.8, maximum = 17.4 |
| 22 | WBC | Numeric | Minimum = 1300, maximum = 66000 |
| 23 | Neutrophils | Numeric | Minimum = 0, maximum = 34.6 |
| 24 | Lymphocytes | Numeric | Minimum = 0, maximum = 11 |
| 25 | Monocyte | Numeric | Minimum = 0.026, maximum = 5.26 |
| 26 | Eosinophils | Numeric | Minimum = 0, maximum = 4.6 |
| 27 | Platelet count | Numeric | Minimum = 0.67, maximum = 173 |
| 28 | Left atrium | Numeric | Minimum = 1, maximum = 4.1 |
| 29 | Aortic root | Numeric | Minimum = 1, maximum = 3.4 |
| 30 | LVIDd | Numeric | Minimum = 3.9, maximum = 5.7 |
| 31 | LVIDs | Numeric | Minimum = 1, maximum = 4.7 |
| 32 | IVSdIVDd | Numeric | Minimum = 0.6, maximum = 1.5 |
| 33 | PWTd_PWTs | Numeric | Minimum = 0.7, maximum = 1.5 |
| 34 | Ejection fraction | Numeric | Minimum = 20, maximum = 60 |
| 35 | Group | Categorical | CAD-with-diabetes, CAD-without-diabetes, healthy control |
As far as available literature is concerned, the current study is the main review to extract the most well-known manifestations of cardiac vessel blockade in cases of CAD with diabetes using straightforward yet incredible Hotspot-based mining. A Hotspot learns a bunch of rules that augment or limit an objective variable or worth of interest[6]. The Hotspot algorithm processes real/numeric clinical data, utilizing the actual values of clinical attributes as input to identify associations among attributes. It aims to discover various rules/patterns specifically for patient groups, including CAD with DM, CAD without DM, and healthy control (HC).
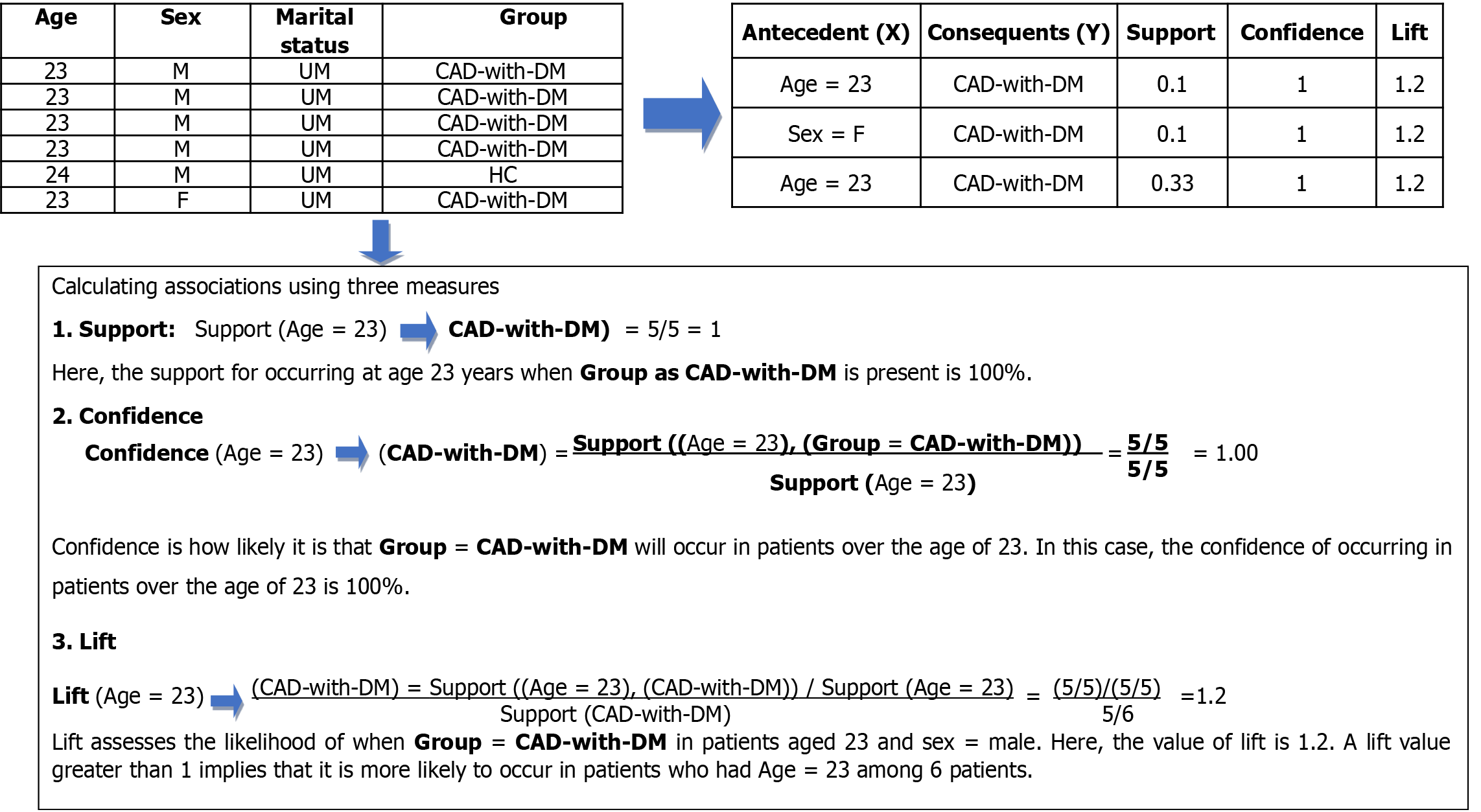
In Figure 2, when we used the clinical data of CAD with DM patients and HCs, the rule mining method produced two rules with support scores of 0.1 and 0.33, confidence of 1, and lift of 1.2 for the antecedent (X) Age = 23 and consequent (Y) “Group = CAD-with-DM” in rule 1. All patients had CAD with DM with a confidence level of 1. Similarly, lift 1.2 shows that Sex = F and Age = 23 are connected with “Group = CAD with DM”.
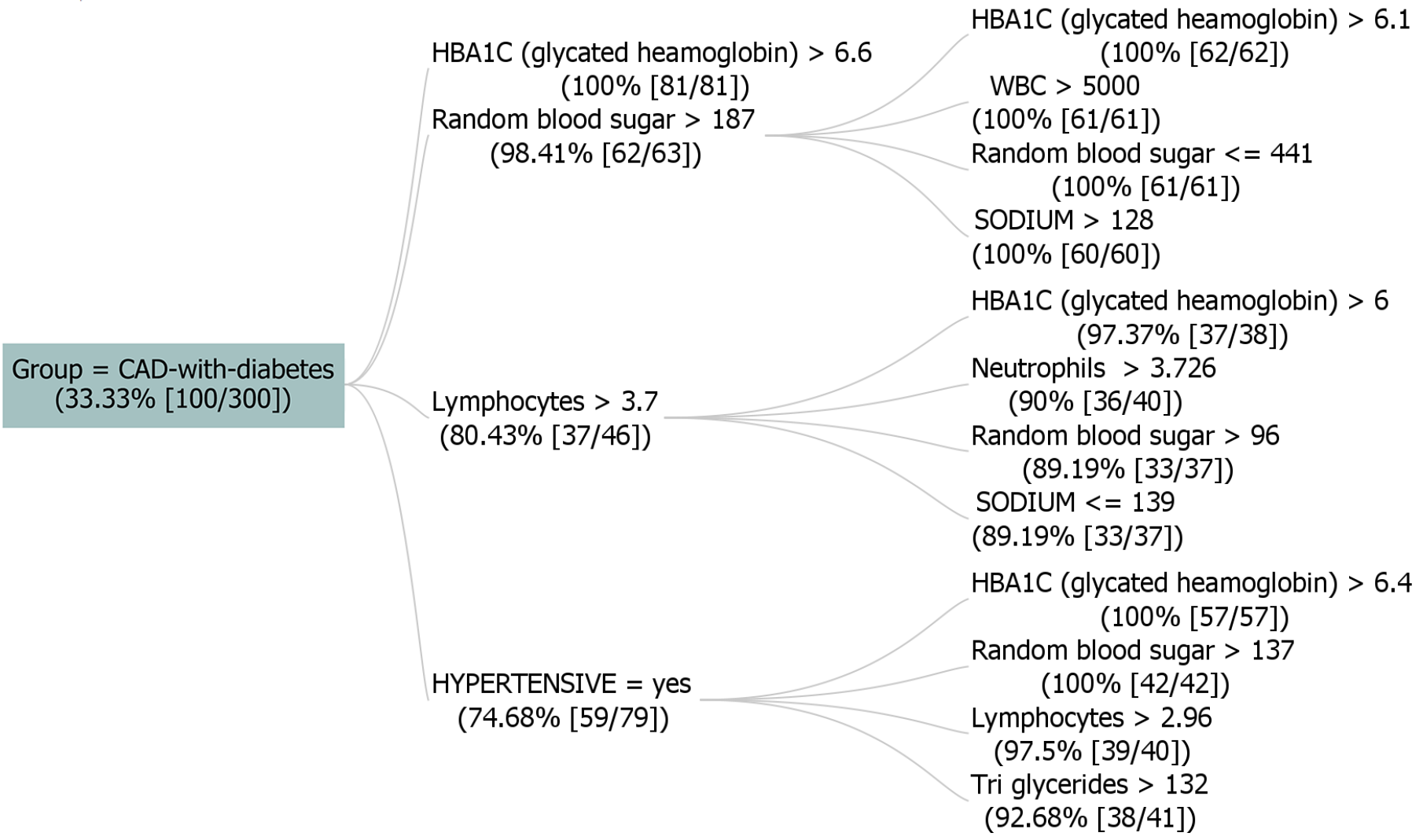
Figure 3 displays a dataset with 300 instances, where the "GROUP" attribute signifies "CAD-with-diabetes" at 33.33% support (100 instances). The figure helps doctors recommend medication for controlling clinical parameters, and pharmacists can develop customized medicines. Rules were generated in the analysis with a maximum branching factor of 4 and rule length of 5, requiring a 1% increase in probability for improvement. Notable rules were found, showing strong connections between health indicators and diabetes likelihood, including elevated HbA1C and random blood sugar levels. These rules provide insights into potential risk factors for CAD with diabetes. For instance, if random blood sugar exceeds 175 and HbA1C is above 6.6, the instance is likely classified as "CAD-with-diabetes." Overall, these rules demonstrate high confidence, lift, and conviction, showcasing their strong predictive capability.
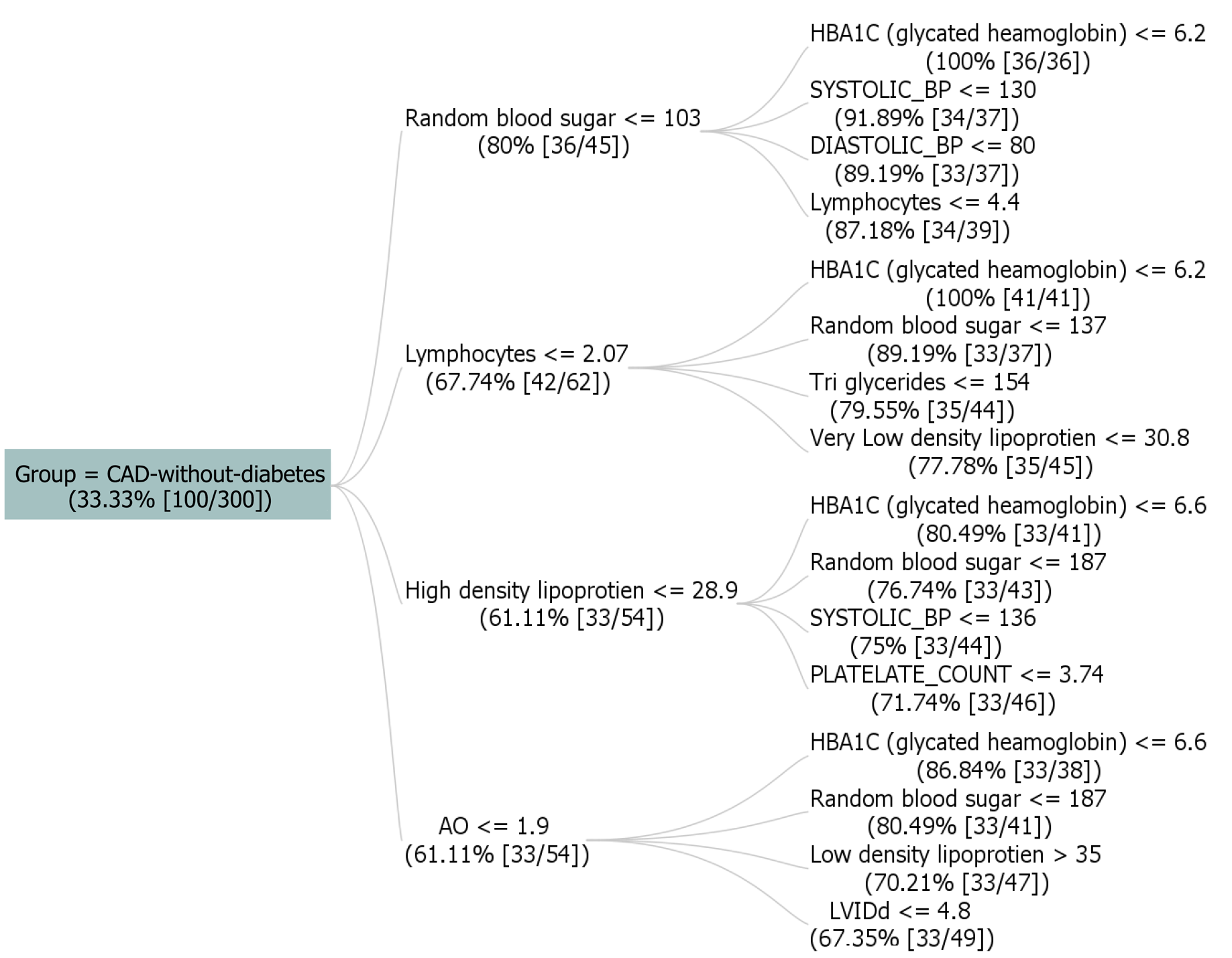
Figure 4 shows a dataset with 300 instances, with the target attribute "GROUP" indicating "CAD-without-diabetes" with support value taken as 33.33% (100 instances) of cases. Rules provided outline various conditions, including blood sugar levels, blood pressure, and other biomarkers, each contributing to the prediction of "CAD-without-diabetes" with associated confidence, lift, and conviction measures. For example, one rule specifies that if lymphocytes are less than or equal to 2.83, random blood sugar is less than or equal to 167, and HbA1C levels are less than or equal to 6.2, the predicted group is "CAD-without-diabetes." The presented rules demonstrate diverse combinations involving lymphocytes (≤ 5.05), high-density lipoprotein (≤ 96), triglycerides (≤ 559), platelet count (≤ 5.35), diastolic blood pressure (≤ 100), systolic blood pressure (≤ 150), monocyte levels (> 0.189), and sodium (> 125). These rules unveil intricate relationships, providing insights into the factors influencing the presence of CAD without diabetes, along with associated confidence, lift, leverage, and conviction metrics.
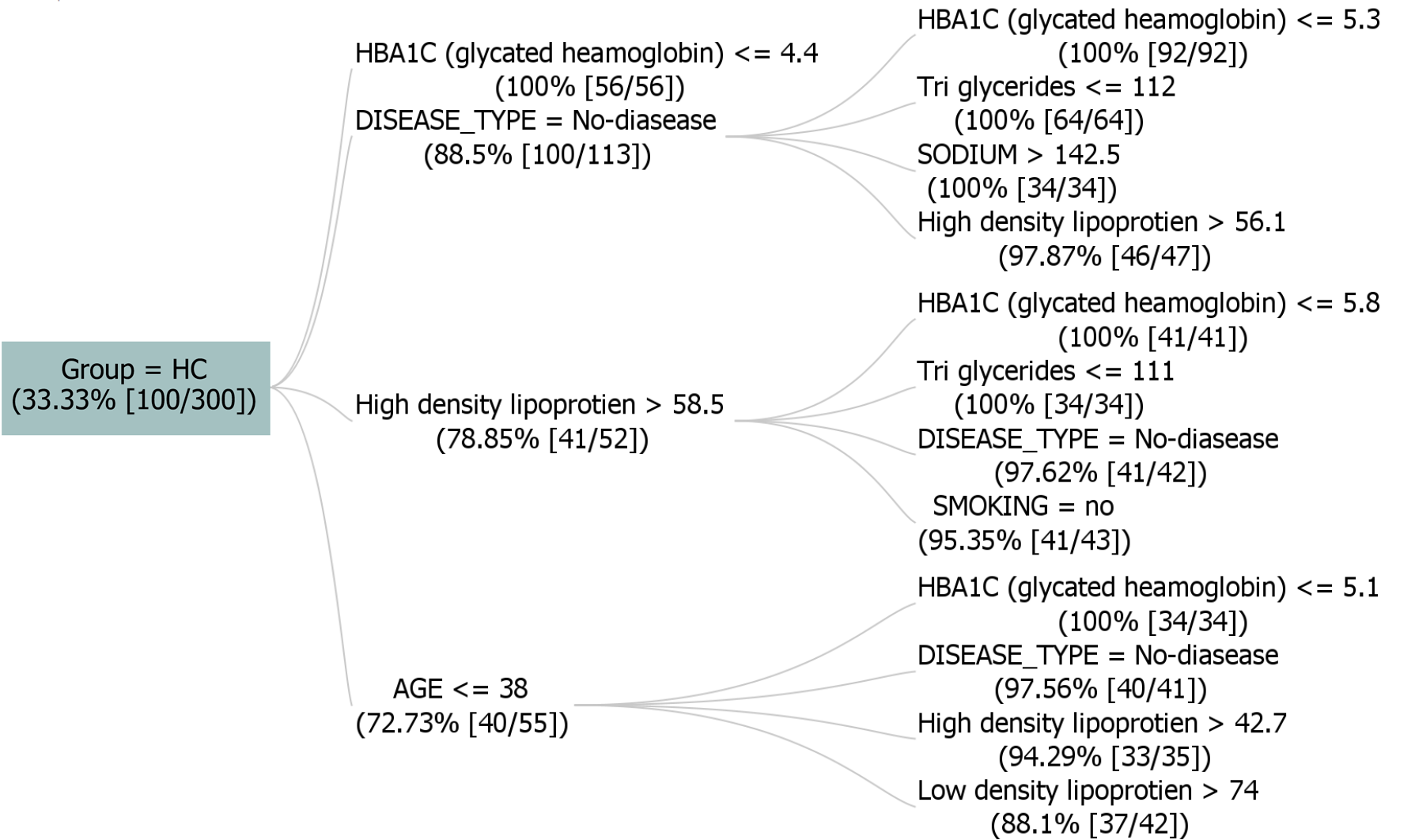
Figure 5 shows a dataset that comprises 300 instances, with the target attribute "GROUP" indicating "HC" with support value taken as 33.33%(100 instances). It depicts significant rules, including associations such as [DISEASE_TYPE = no disease, HbA1C ≤ 5.3] and [High density lipoprotein > 58.5, smoking= no], which exhibit robust relationships with the target value HC. The tree incorporates factors like HbA1C levels, lipoprotein concentrations, and lifestyle indicators. Criteria involve conditions such as low HbA1C levels (≤ 4.8), normal blood sugar levels, and specific ranges for lipoproteins. The presented rules highlight connections between various health parameters and the likelihood of disease. These rules offer insights into potential health indicators and contribute to understanding factors influencing disease outcomes within the specified parameters.
The association rules, presented as segment descriptions in Table 2, pertain to clinical feature datasets with support values ranging from 0.33 to 0.99. A confidence level approaching 1 indicates a robust association between clinical attributes and the specified target class (Consequent). Additionally, a lift value exceeding 1 signifies a strong correlation with the clinical attribute[7].
| Rules | Antecedents ≥ consequents | Support | Confidence | Lift | Segment description |
R1 | [Random blood sugar > 187, HbA1C > 6.1] ≥ [Group = CAD-with-diabetes] | 0.33 | 1 | 3 | If random blood sugar > 187 and HbA1C > 6.1, then Group = CAD-with-diabetes |
| R2 | [Random blood sugar > 175, High density lipoprotein > 11, HbA1C > 6.1] ≥ [Group = CAD-with-diabetes] | 0.66 | 1 | 3 | If random blood sugar > 175, high density lipoprotein > 11, and HbA1C > 6.1, then Group = CAD-with-diabetes |
| R3 | [Eosinophils ≤ 0.792, sodium ≤ 145, HbA1C > 6.2] ≥ [Group = CAD-with-diabetes] | 0.99 | 0.96 | 2.88 | If eosinophils ≤ 0.792, sodium ≤ 145, and HbA1C > 6.2, then Group = CAD-with-diabetes |
| R4 | [Random blood sugar ≤ 103, Systolic_BP ≤ 130, HbA1C ≤ 6.2] ≥ [Group = CAD-without-diabetes] | 0.33 | 1 | 3 | If random blood sugar ≤ 103, Systolic_BP ≤ 130, and HbA1C ≤ 6.2, then Group = CAD-without-diabetes |
R5 | [Lymphocytes ≤ 2.83, random blood sugar ≤ 167, HBA1C ≤ 6.4, Systolic_BP ≤ 150] ≥ [Group = CAD-without-diabetes] | 0.66 | 1 | 3 | If lymphocytes ≤ 2.83, random blood sugar ≤ 167, HbA1C ≤ 6.4, and Systolic_BP ≤ 150, then Group = CAD-without-diabetes |
| R6 | [Lymphocytes ≤ 5.05, high density lipoprotein ≤ 96, HBA1C ≤ 6.6] ≥ [Group = cad-without-diabetes] | 0.99 | 0.87 | 2.61 | If lymphocytes ≤ 5.05, high density lipoprotein ≤ 96, and HBA1C ≤ 6.6, then Group = CAD-without-diabetes |
| R7 | [DISEASE_TYPE = No-disease, sodium > 142.5] ≥ [Group = HC] | 0.33 | 1 | 3 | If DISEASE_TYPE = No-disease and sodium > 142.5, then [Group = HC] |
| R8 | [HbA1C ≤ 4.8, low density lipoprotein > 52, DISEASE_TYPE = No-disease] ≥ [Group = HC] | 0.66 | 1 | 3 | If HbA1C ≤ 4.8, low-density lipoprotein > 52, and there is no disease DISEASE_TYPE = No−disease, then [Group = HC] |
| R9 | [DISEASE_TYPE = No-disease, smoking = no, HbA1C ≤ 5.9, tobacco = no] ≥ [Group = HC] | 0.99 | 1 | 3 | If DISEASE_TYPE = No−disease, smoking = no, HbA1C ≤ 5.9, and tobacco = no, then [Group = HC] |
Oxidative stress and endothelial dysfunction are frequently observed in diabetic patients and may directly contribute to the development of atherosclerosis and CVD[14]. Our study focused on investigating significant differences in diabetic-specific parameters (random blood sugar and serum HbA1c levels) between diabetic and non-diabetic CAD patients. Studies suggested that fasting blood glucose is a major contributor to HbA1c levels in T2DM patients. The underlying multifactorial mechanisms of the disease still pose a challenge[15]. The Hoorn study proposed that T2DM and endothelial dysfunction may interact in a bidirectional manner in the pathogenesis of cardiovascular events[16]. The endothelial dysfunction can be due to various mechanisms, including dyslipidemia, formation of advanced glycated end products, intra-endothelial accumulation of glucose, increased oxidative stress, and low-grade inflammatory responses[17].
Recent advancements in cardiovascular research have shown promising developments. Chen et al[18] utilized self-supervised learning to transfer knowledge of cardiovascular progression between cohorts, enhancing the detection of specific cardiovascular events and improving examination strategies and prognosis. Ma et al[19] introduced a highly accurate and lightweight deep learning model for phonocardiogram classification, crucial for screening valvular heart disease via mobile health applications, demonstrating exceptional accuracy and robustness.
In another study, six machine learning algorithms demonstrated comparable diagnostic accuracy, suggesting stress-only myocardial perfusion imaging supported by machine learning models as a promising alternative in clinical settings[20]. Lee et al[21] developed a deep learning model for detecting obstructive CAD on coronary computed tomography angiography, showing high diagnostic accuracy and improved discrimination. Veroneze et al[22] applied ARM to discover consistent patterns of clinical features and differentially expressed genes relevant to T2DM, dyslipidemia, and periodontitis, offering insights into these chronic inflammatory diseases. Reti-CVD, an artificial intelligence (AI) software as a medical device utilizing retinal images for personalized CVD risk assessment, demonstrated a significant association with increased CVD risk, leading to regulatory authorization[23]. Additionally, Singh et al[24] validated the ARM method, discovering hidden patterns and associations of early-onset myocardial infarction with hypertension and diabetes mellitus.
Our findings align with previous studies regarding the relationship between T2DM, endothelial dysfunction, and adverse cardiovascular outcomes[25]. Pathak et al[26] observed a significantly higher occurrence of triple vessel disease/multivessel disease in patients with a diabetes mellitus duration > 10 years compared to those with a duration < 10 years.
In summary, our study contributes to the growing body of evidence supporting the association between T2DM, endo
A larger multicenter cohort clinical trial, spanning diverse geographic locations and ethnicities, would be beneficial. Nevertheless, our study lays the groundwork for future large-scale studies that can help healthcare providers establish plasma glucose testing goals based on specific HbA1c values, thereby improving patient care.
In conclusion, we demonstrated that ARM is a powerful data analysis technique to identify consistent patterns between the clinical profiles of patients affected by specific pathological panels. In addition, ARM is able to evidence relevant associations among important parameters of HbA1C and random blood sugar levels of the patients. These insights shed light on potential risk factors for cardiovascular disease with diabetes. A standout rule suggests that instances with random blood sugar > 175 and HBA1C > 6.6 are likely in the "CAD with diabetes" group. These findings can contribute to the valuable insights into health indicators and factors influencing disease outcomes. With the aid of this research, doctors can effortlessly prescribe medication to regulate uncontrolled clinical parameters, while pharmacists can innovate customized medicines tailored to address specific attributes with uncontrolled clinical parameters. This research facilitates targeted medication for patients. Thus, it is pertinent to widen the horizon of AI applications, while integrating with CAD research and development in terms of systems development, process modelling, and its optimization.
| 1. | Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6994] [Cited by in RCA: 7472] [Article Influence: 1245.3] [Reference Citation Analysis (0)] |
| 2. | Bohr A, Memarzadeh K. The rise of artificial intelligence in healthcare applications. Artif Intell Healthc. 2020;25-60. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 3. | Szathmary L, Valtchev P, Napoli A. Generating Rare Association Rules Using the Minimal Rare Itemsets Family. Int J Software Informat. 2010;4:219-238. |
| 4. | Kumar Y, Koul A, Singla R, Ijaz MF. Artificial intelligence in disease diagnosis: a systematic literature review, synthesizing framework and future research agenda. J Ambient Intell Humaniz Comput. 2023;14:8459-8486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 290] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 5. | Tickle KS, Imam T, Nahar J, Chen YPP. Association rule mining to detect factors which contribute to heart disease in males and females. Exp Syst Appl. 2013;40:1086-1093. [DOI] [Full Text] |
| 6. | Dabla PK, Upreti K, Singh D, Singh A, Sharma J, Dabas A, Gruson D, Gouget B, Bernardini S, Homsak E, Stankovic S. Target association rule mining to explore novel paediatric illness patterns in emergency settings. Scand J Clin Lab Invest. 2022;82:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Agrawal R, Imieliaski T, Swami A. Mining association rules between sets of items in large databases, in Proceedings of the 1993 ACM SIGMOD International Conference on Management of Data. ACM SIGMOD Record. 1993;22:207-216. [DOI] [Full Text] |
| 8. | Agrawal A, Choudhary A. Association Rule Mining Based HotSpot Analysis on SEER Lung Cancer Data. IJKDB. 2011;2:34-54. [DOI] [Full Text] |
| 9. | Agrawal A, Choudhary AN. Identifying hotspots in lung cancer data using association rule mining. In 2nd IEEE ICDM Workshop on Biological Data Mining and its Applications in Healthcare (BioDM), 2011; 995-1002. [DOI] [Full Text] |
| 10. | Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten I. The Weka data mining software: An update. SIGKDD Explorat. 2008;11:10-18. [DOI] [Full Text] |
| 11. | Jonas RA, Crabtree TR, Jennings RS, Marques H, Katz RJ, Chang HJ, Stuijfzand WJ, van Rosendael AR, Choi JH, Doh JH, Her AY, Koo BK, Nam CW, Park HB, Shin SH, Cole J, Gimelli A, Khan MA, Lu B, Gao Y, Nabi F, Nakazato R, Schoepf UJ, Driessen RS, Bom MJ, Thompson RC, Jang JJ, Ridner M, Rowan C, Avelar E, Généreux P, Knaapen P, de Waard GA, Pontone G, Andreini D, Al-Mallah MH, Guglielmo M, Bax JJ, Earls JP, Min JK, Choi AD, Villines TC. Diabetes, Atherosclerosis, and Stenosis by AI. Diabetes Care. 2023;46:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Zemlin AE, Matsha TE, Hassan MS, Erasmus RT. HbA1c of 6.5% to diagnose diabetes mellitus -- does it work for us? -- the Bellville South Africa study. PLoS One. 2011;6:e22558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Page HL Jr. The Judkin's technique. Cathet Cardiovasc Diagn. 1979;5:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | An Y, Xu BT, Wan SR, Ma XM, Long Y, Xu Y, Jiang ZZ. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc Diabetol. 2023;22:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 200] [Reference Citation Analysis (0)] |
| 15. | Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health. 2015;73:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | van Sloten TT, Henry RM, Dekker JM, Nijpels G, Unger T, Schram MT, Stehouwer CD. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn study. Hypertension. 2014;64:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Domingueti CP, Dusse LM, Carvalho Md, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 18. | Chen LC, Hung KH, Tseng YJ, Wang HY, Lu TM, Huang WC, Tsao Y. Self-Supervised Learning-Based General Laboratory Progress Pretrained Model for Cardiovascular Event Detection. IEEE J Transl Eng Health Med. 2024;12:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Ma S, Chen J, Ho JWK. An edge-device-compatible algorithm for valvular heart diseases screening using phonocardiogram signals with a lightweight convolutional neural network and self-supervised learning. Comput Methods Programs Biomed. 2024;243:107906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Wang F, Yuan H, Lv J, Han X, Zhou Z, Lu W, Lu L, Jiang L. Stress-only versus rest-stress SPECT MPI in the detection and diagnosis of myocardial ischemia and infarction by machine learning. Nucl Med Commun. 2024;45:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Lee DY, Chang CC, Ko CF, Lee YH, Tsai YL, Chou RH, Chang TY, Guo SM, Huang PH. Artificial intelligence evaluation of coronary computed tomography angiography for coronary stenosis classification and diagnosis. Eur J Clin Invest. 2024;54:e14089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Veroneze R, Cruz Tfaile Corbi S, Roque da Silva B, de S Rocha C, V Maurer-Morelli C, Perez Orrico SR, Cirelli JA, Von Zuben FJ, Mantuaneli Scarel-Caminaga R. Using association rule mining to jointly detect clinical features and differentially expressed genes related to chronic inflammatory diseases. PLoS One. 2020;15:e0240269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Lee CJ, Rim TH, Kang HG, Yi JK, Lee G, Yu M, Park SH, Hwang JT, Tham YC, Wong TY, Cheng CY, Kim DW, Kim SS, Park S. Pivotal trial of a deep-learning-based retinal biomarker (Reti-CVD) in the prediction of cardiovascular disease: data from CMERC-HI. J Am Med Inform Assoc. 2023;31:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 24. | Singh A, Singh D, Sharma S, Upreti K, Maheshwari M, Mehta V, Sharma J, Mehra P, Dabla PK. Discovering Patterns of Cardiovascular Disease and Diabetes in Myocardial Infarction Patients Using Association Rule Mining. Folia Medica Indonesiana. 2022;58:242-250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 26. | Pathak SR, Gajurel RM, Poudel CM, Shrestha H, Thapa S, Koirala P. Angiographic Severity of Coronary Artery Disease in Diabetic and Non-diabetic Acute STEMI Patients in a Tertiary Care Centre of Nepal. Kathmandu Univ Med J. 2021;19:410-414. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/