Published online Mar 20, 2024. doi: 10.5662/wjm.v14.i1.89709
Peer-review started: November 9, 2023
First decision: December 12, 2023
Revised: December 21, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 20, 2024
Processing time: 118 Days and 13.2 Hours
The surgeon performing a distal gastrectomy, has an arsenal of reconstruction techniques at his disposal, Billroth II among them. Braun anastomosis performed during a Billroth II procedure has shown evidence of superiority over typical Billroth II, in terms of survival, with no impact on postoperative morbidity and mortality.
To compare Billroth II vs Billroth II and Braun following distal gastrectomy, regarding their postoperative course.
Patients who underwent distal gastrectomy during 2002-2021, were separated into two groups, depending on the surgical technique used (Billroth II: 74 patients and Billroth II and Braun: 28 patients). The daily output of the nasogastric tube (NGT), the postoperative day that NGT was removed and the day the patient started per os feeding were recorded. Postoperative complications were at the same time noted. Data were then statistically analyzed.
There was difference in the mean NGT removal day and the mean start feeding day. Mean total postoperative NGT output was lower in Braun group (399.17 mL vs 1102.78 mL) and it was statistically significant (P < 0.0001). Mean daily postoperative NGT output was also statistically significantly lower in Braun group. According to the postoperative follow up 40 patient experienced bile reflux and alkaline gastritis from the Billroth II group, while 9 patients who underwent Billroth II and Braun anastomosis were presented with the same conditions (P < 0.05).
There was evidence of superiority of Billroth II and Braun vs typical Billroth II in terms of bile reflux, alkaline gastritis and NGT output.
Core Tip: This is a retrospective study to evaluate the efficacy of the addition of Braun enteroenteroanastomosis to Bilroth II reconstruction compared to Billroth II alone in terms of the postoperative outcomes of these surgical techniques, following distal gastrectomy. The addition of Braun anastomosis demonstrated superiority in terms of survival without impacting complications or mortality. The study highlights the significance of considering bile reflux and alkaline gastritis in postoperative quality of life after gastrectomy, emphasizing the role of Braun’s anastomosis in reducing bile reflux and associated complications.
- Citation: Christodoulidis G, Kouliou MN, Koumarelas KE, Argyriou K, Karali GA, Tepetes K. Billroth II anastomosis combined with brown anastomosis reduce reflux gastritis in gastric cancer patients. World J Methodol 2024; 14(1): 89709
- URL: https://www.wjgnet.com/2222-0682/full/v14/i1/89709.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i1.89709
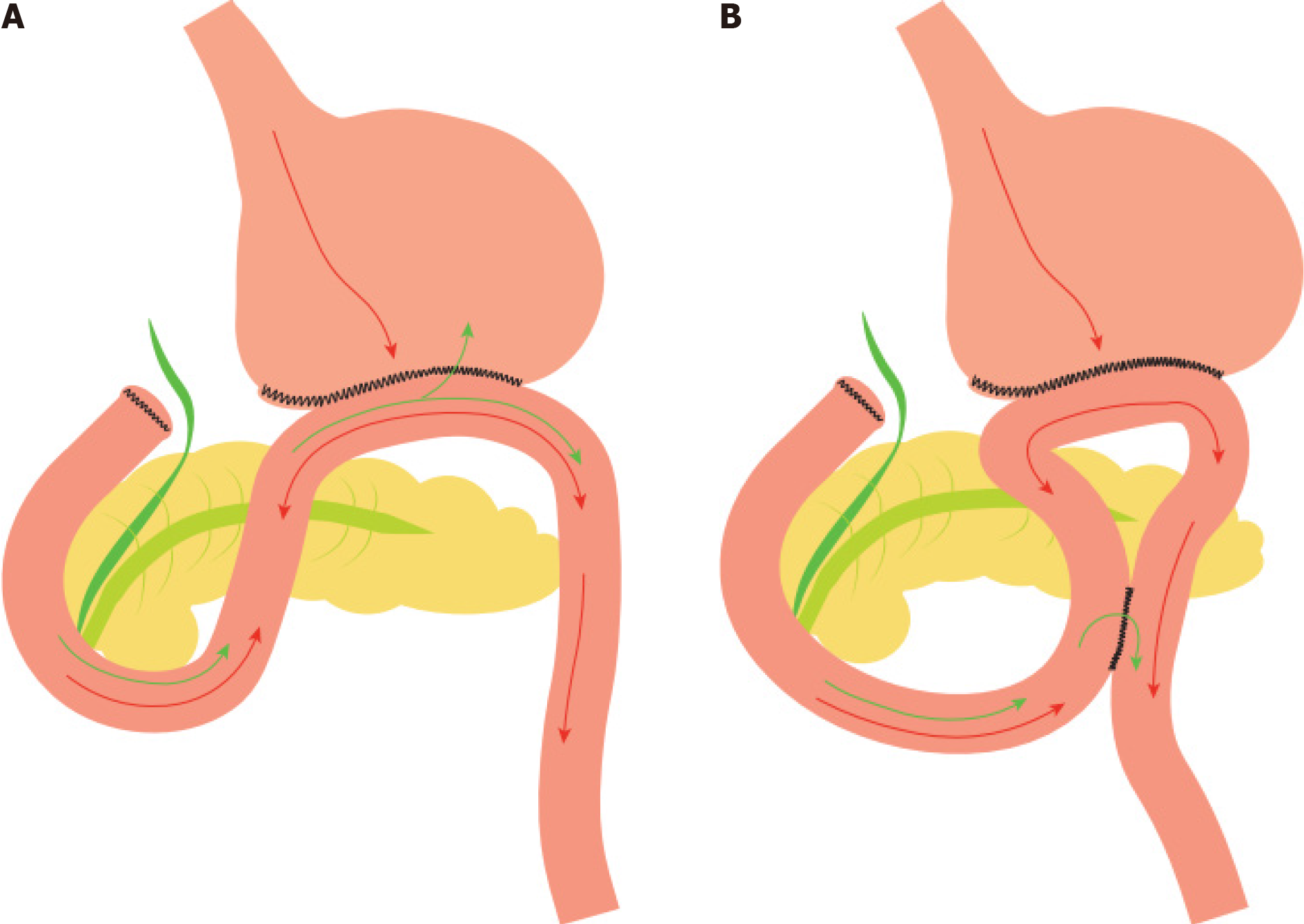
Theodor Billroth (1829-1894) was a prominent figure of surgery during the 19th century, being the first to perform a subtotal gastrectomy[1,2]. Among his heritage, Billroth II[1], an operation, in which, after a partial gastrectomy and closure of duodenum stump, a side-to-side anastomosis is performed between jejunum and the greater curvature of the stomach. Although Heinrich Braun (1862-1934), another surgical pioneer, was the first to describe the formation of an ulcer in the jejunum after a gastroenterostomy, he is widely known for the homonymous enteroenterostomy[3]. Braun enteroenterostomy[4], is defined as the anastomosis between the afferent and efferent loops of jejunum, distal to a gastroenterostomy. The purpose of a Braun anastomosis, originally introduced in 1892[5], is to reduce the reflux of bile and pancreatic secretions into the stomach[6], as well as the possibility of ileus[7] and divert oral intake from the afferent limb[8], which is crucial, given the fact that bile reflux is one of the most important factors that determine the posto
Distal gastrectomy[12-14] remains the operation of choice for distal-third gastric cancer, as followed by lower mortality and morbidity rates, higher quality of life and no significant difference as far as long-term survival rates are concerned, compared to total gastrectomy, with the efforts now leaning on maintaining the continuity of the Gastrointestinal tract[15,16]. Moreover, the surgeon performing a distal gastrectomy, has a variety of reconstruction techniques, Billroth-I, Billroth-II, and Roux-en-Y, each with its respective advantages and disadvantages, at his disposal[5,10-12,16-18]. Among those, Billroth II with or without Braun anastomosis is often preferred worldwide[19]. Braun anastomosis performed during a Billroth II procedure has shown evidence of superiority over typical Billroth II, in terms of survival, with no impact on postoperative complications and mortality[20].
Therefore, this study compared the two above mentioned surgical techniques regarding their postoperative course.
For the purpose of this retrospective study, data were collected from patients undergoing distal gastrectomy at the Department of Surgery, University Hospital of Larissa, during 2002-2021. No patients were excluded based on their underlying disease. As far as primary diagnosis is concerned, from the entire sample, 5 patients were diagnosed with gastrointestinal stromal tumor, 6 patients with gastric ulcer, and all the remaining patients suffered from gastric adenocarcinoma. They were then separated into two groups, depending on the surgical technique used (Billroth II: 74 patients, mean age: 70.75 years, 44 male, 30 female; and Billroth II and Braun: 28 patients, mean age: 70.41 years, 21 male, 7 female). As minimum and maximum age in the sample was 42 and 92 years respectively, patients were also divided into two subgroups (≤ 67 years and > 67 years). There was no categorization, on the basis of the way the anastomoses were performed, for example hand sewn or with the use of a mechanical stapler. Demographic data, including age and gender, the output of the nasogastric tube (NGT), the postoperative day (POD) that NGT was removed, the day the patient started feeding and the total postoperative hospitalization days (PHD) were recorded. Patients on their 5th POD underwent gastroscopy in order to investigate any possible development of alkaline gastritis and bile reflux. NGT output was measured on a daily basis at a fixed hour and data were collected until the 10th POD. Patients with NGT ≥ 10th POD or need for NGT reinsertion after 10th POD were excluded from the study. Before comparisons between the two surgical techniques were made, the data from each subgroup underwent Shapiro-Wilk test for normality (P < 0.05)[21-26]. Due to the fact that normality could not be proven, Mann-Whitney U test (P < 0.05) was used[27]. Aligned Rank Transform three way ANOVA was then performed (ARTool) for the effects of age, gender and surgical method on hospitalization days and NGT total output, to be examined[28]. All statistical analysis was conducted using IBM SPSS statistics v22 software.
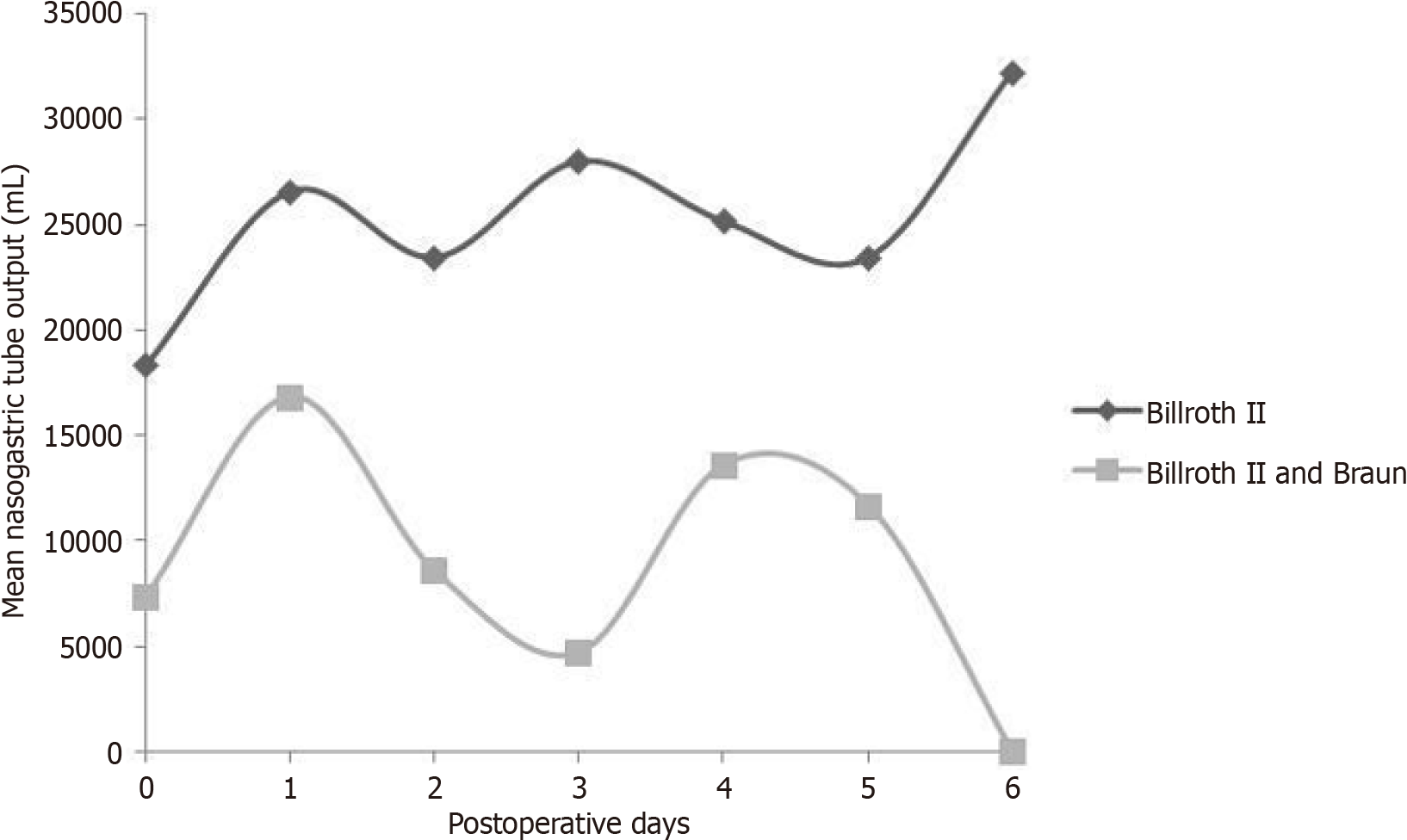
The study outcomes are displayed in Table 1. The mean PHD for Billroth II was 13.09, while for Billroth II and Braun was 10.17 (P < 0.0001). Moreover, there was statistically significant difference between the two methods as far as feeding start day and NGT removal day are concerned (P < 0.0001). Data from BII and Braun for NGT removal day follow the normal distribution, but since BII data do not, Mann Whitney was applied. NGT output mean is systematically lower for BII + B group during all POD (Figures 1 and 2). Moreover, patients from BII + B group had their NGT removed by the 6th POD due to lack of drainage, while patients from BII group had still an increased drainage volume due to reflux gastritis.
| Operation | n | Mean | SD | P value | |
| Postoperative hospitalization days | Billroth II | 74 | 13.09 | 1.41 | < 0.0001 |
| Billroth II + Braun | 28 | 10.17 | 2.01 | ||
| Feeding start day | Billroth II | 74 | 6.33 | 0.66 | < 0.0001 |
| Billroth II + Braun | 28 | 5.17 | 0.41 | ||
| NGT removal day | Billroth II | 74 | 4.31 | 0.31 | < 0.0001 |
| Billroth II + Braun | 28 | 4.00 | 0.67 | ||
| NGT output (day 0) | Billroth II | 74 | 183.56 | 56.29 | < 0.0001 |
| Billroth II + Braun | 26 | 73.64 | 25.70 | ||
| NGT output (day 1) | Billroth II | 72 | 265.80 | 50.08 | < 0.0001 |
| Billroth II + Braun | 26 | 168.18 | 76.95 | ||
| NGT output (day 2) | Billroth II | 61 | 234.59 | 44.82 | < 0.0001 |
| Billroth II + Braun | 21 | 85.56 | 43.08 | ||
| NGT output (day 3) | Billroth II | 49 | 280.00 | 57.76 | < 0.0001 |
| Billroth II + Braun | 16 | 47.14 | 17.82 | ||
| NGT output (day 4) | Billroth II | 30 | 251.67 | 70.48 | < 0.0001 |
| Billroth II + Braun | 12 | 136.00 | 93.25 | ||
| NGT output (day 5) | Billroth II | 21 | 234.62 | 65.08 | < 0.0001 |
| Billroth II + Braun | 7 | 116.67 | 92.80 | ||
| NGT output (day 6) | Billroth II | 13 | 322.50 | 76.11 | < 0.0001 |
| Billroth II + Braun | 1 | 0 | NA | ||
| NGT output (sum) | Billroth II | 74 | 1102.78 | 203.94 | < 0.0001 |
| Billroth II + Braun | 28 | 399.17 | 140.18 |
According to the postoperative complications, out of the 74 patients who underwent Billroth II, forty presented with bile reflux and alkaline gastritis (54%), and from the group of patients who underwent BII and Braun these complications were observed in only 9 patients (32%) (P < 0.05) (Table 2). These findings were mainly confirmed by the patients gastroscope report during their postoperative follow-up and the NGT output.
| Postoperative complications | Billroth II | Billroth II with Braun | P value |
| Bile reflux | 40 | 9 | 0.048 |
| Alkaline gastritis | 40 | 9 | 0.048 |
| Anastomotic bleeding | 2 | 1 | 0.820 |
| Anastomotic fistula | 1 | 1 | 0.470 |
In our study BII outweighed BII and Braun in terms of operation time, with a mean operating time of 226.4 min ± 41.6 min. for the BII group vs a duration of 255.8 min ± 66.2 min. for the BII and Braun group (P < 0.05). However, there were no statistically significant difference in blood loss during surgery (Table 3).
| Characteristics | Billroth II | Billroth II and Braun |
| Age | 70.75 | 70.41 |
| Gender | ||
| Male | 44 | 21 |
| Female | 30 | 7 |
| Operation time (min) | 226.4 ± 41.6 | 255.8 ± 66.2 |
| Blood loss (mL) | 175.4 ± 121.3 | 148.7 ± 96.8 |
An Aligned Rank Transform three-way ANOVA was conducted and examined the effect of age, gender and surgical method on hospitalization days and NGT total output (Table 4). There was a statistically significant interaction between age and gender in total NGT output (P < 0.05) or PHD (P < 0.05). Similarly, there was an interaction effect on NGT output or PHD between age and method, gender and method or age, gender, and method (P < 0.05). This means that younger and male patients had smaller values of NGT output as well as less hospitalization days. Moreover, whichever of the independent factors were combined with BII and Braun anastomosis had also a better outcome.
| NGT output (sum) | Postoperative hospitalization days | |
| Age | 0.929 | 0.339 |
| Gender | 0.325 | 0.093 |
| Method | 0.170 | 0.469 |
| Interaction age and gender | 0.861 | 0.288 |
| Interaction age and method | 0.946 | 0.635 |
| Interaction gender and method | 0.579 | 0.177 |
| Interaction age, gender, and method | 0.983 | 0.998 |
Patients on their follow-up were given questionnaires for the postoperative quality-of-life assessing data about patients recovery, in terms of physical, emotional and cognitive behavior. A short analysis of the data acquired indicated that the patients of the two groups had a similar postoperative status.
Gastric malignancies account for 930000 over 1000000 new cases and 700000 deaths annually[15,29]. Gastric cancer is considered the third deadliest, while being the fifth most commonly diagnosed[15]. Considering the progress in earlier diagnosis a more preservative attitude towards distal gastric cancer resection has been recently adopted, since there is no difference regarding long term survival rates between distal and total gastrectomy[12,15]. The extent of the portion of the stomach removed, given adequate oncologic margins (≥ 3 cm for T2 tumors or types 1 and 2 and ≥ 5 cm for types 3 and 4) does not constitute a prognostic factor, unlike perigastric lymph node clearance. Regarding lymph node clearance during distal gastrectomy, JGCA recommends D1 or D1+ for cT1N0 and D2 for cT2-T4 tumors[13]. Due to the fact that the most important factor affecting the decision between distal or total gastrectomy is the proximal resection margin, patients suffering from malignancy in the middle part of the stomach can also be submitted to distal gastrectomy, thus counting almost for 23%-70% of all cancer gastrectomies in Europe and Asia[12,30,31]. Although five-year survival rates of gastrectomy range between 33%-50%, patients can suffer from ongoing gastrointestinal symptoms for up to 6 months postoperatively[29,32].
The selection of reconstruction methods following distal gastrectomy presents a significant dilemma. Options such as Billroth I, Billroth II, and Roux-en-Y are available, with the latter gaining prominence in the 1970s and 1980s as a response to the elevated incidence of post-gastrectomy alkaline reflux gastritis[33]. Roux-en-Y exhibits superiority over Billroth II in terms of functional and endoscopic outcomes, attributed to the mitigated risks of gastroduodenal and duodenogastroesophageal reflux (DGER), identified as precipitating factors for malignancy development based on reflux gastritis and esophagitis[7,17,18]. However, Roux-en-Y anastomosis entails certain drawbacks, such as a potential occurrence of Roux stasis syndrome (observed in approximately 0-13% of patients), leading to vomiting, stomach dilation, and prolonged hospitalization[18]. Additionally, the procedure necessitates an extended operation time and is associated with increased intraoperative blood loss and greater postoperative weight loss compared to Billroth II and Braun[10,15]. Billroth II with Braun is often proposed as the primary surgical approach, with Roux-en-Y considered a secondary option in case of Braun’s failure[34].
A retrospective analysis involving 720 patients with gastric malignancy from 1997-2011 suggested that Billroth II and Braun may enhance lifespan without escalating postoperative complications and mortality rates[20]. The literature, including a Randomised Clinical Trial and a prospective randomized trial, underscores the comparable acceptability of Billroth II and Braun to Roux-en-Y gastrojejunostomy, emphasizing the significance of considering operation time and blood loss in critically ill patients during the selection of the appropriate procedure[10,15,17]. However, a prospective randomized trial exhibited statistically significant differences in favor of Roux-en-Y regarding the degree and extent of gastritis and bile reflux, though no distinctions were observed in the overall Gastrointestinal Quality of Life Index score[17,35,36].
The application of Braun anastomosis in pancreaticoduodenectomy (PD) has garnered substantial attention, with studies indicating its potential benefits. Comparative analyses between Child non-Braun and Child Braun cohorts revealed statistically significant reductions in DGER rates in the Braun group, positioning Braun enteroenterostomy as a significant independent factor in mitigating DGER[37]. These findings align with similar results in pylorus-preserving pancreatoduodenectomy, affirming the advantageous role of Braun anastomosis in minimizing postoperative DGER incidence[38]. A recent meta-analysis of ten studies comprising 1614 patients reported no significant differences in mortality, intraoperative blood loss, postoperative pancreatic fistula, bile leakage, gastrointestinal hemorrhage, intra-abdominal abscesses, wound complications, and overall hospital stay between Braun PD and typical PD. Nevertheless, the Braun group exhibited lower rates of reoperation, morbidity, clinically relevant DGER, postoperative NGT reinsertion, and vomiting[6].
Our study, albeit contributing valuable insights, is not without limitations. It bears the inherent biases of a retrospective, non-randomized trial conducted over a nineteen-year span, during which accumulated experience may have exerted an influential role. Notably, the study group lacked stratification based on anastomosis techniques (manual suturing or the use of a stapling device), surgery type (open vs laparoscopic) and the specific disease leading to gas
In conclusion, there were evidence of superiority of Billroth II and Braun against typical Billroth II, in terms of bile reflux, alkaline gastritis and NGT output. These results were statistical significant, eventhough the several study limitations. The need for randomized controlled trials is highlighted.
The study focuses on comparing two reconstruction techniques, Billroth II and Billroth II with Braun anastomosis, commonly used after distal gastrectomy, examining their impact on postoperative outcomes. The retrospective study collected data from patients undergoing distal gastrectomy, dividing them into two groups based on the reconstruction technique used. The significance of our research lies to the close follow-up in accordance with the gastroenterologists to confirm the diagnosis of alkaline reflux gastritis.
The research is motivated by the debate on the optimal reconstruction technique following distal gastrectomy for gastric cancer. The study aims to contribute valuable insights by comparing the postoperative outcomes of the two reconstruction methods, Billroth II and Billroth II with Braun anastomosis, in order to inform clinical decision-making and potentially improve patient outcomes in the treatment of distal gastric cancer.
To evaluate and compare the postoperative course of patients undergoing distal gastrectomy with either Billroth II or Billroth II with Braun anastomosis. Specific outcomes under scrutiny involve factors such as postoperative hospitalization days (PHD), feeding initiation, nasogastric tube (NGT) removal, and the occurrence of complications like bile reflux and alkaline gastritis, aiming to discern potential advantages between the two reconstruction techniques.
The study employed a retrospective design, collecting data from patients who underwent distal gastrectomy at the Department of Surgery, University Hospital of Larissa, spanning from 2002 to 2021. Patients were categorized based on the reconstruction technique used (Billroth II or Billroth II with Braun), and statistical analyses, including Mann-Whitney U test and Aligned Rank Transform three-way ANOVA, were performed to assess variables such as PHD, feeding start day, NGT removal, and complications.
The research revealed that distal gastrectomy with Billroth II and Braun anastomosis demonstrated superiority over typical Billroth II in terms of postoperative outcomes. Statistically significant differences were observed, including shorter PHD, earlier feeding initiation, quicker NGT removal, and a lower incidence of complications such as bile reflux and alkaline gastritis, highlighting potential benefits of the Billroth II and Braun anastomosis technique in the surgical management of distal gastric cancer.
In conclusion, the study suggests evidence of the superiority of Billroth II with Braun anastomosis over typical Billroth II in the context of distal gastrectomy for gastric cancer. Despite inherent limitations in the retrospective design, the findings emphasize the potential benefits of the specific reconstruction technique, such as reduced postoperative complications and improved outcomes.
Future research should focus on addressing limitations such as sample size constraints, variations in surgical techniques, and the absence of quantitative assessments for gastric reflux, aiming to provide more conclusive evidence on the optimal reconstruction method for enhanced postoperative outcomes in patients with distal gastric cancer.
| 1. | Kazi RA, Peter RE. Christian Albert Theodor Billroth: master of surgery. J Postgrad Med. 2004;50:82-83. [PubMed] |
| 2. | Santoro E. The history of gastric cancer: legends and chronicles. Gastric Cancer. 2005;8:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Robinson JO. The history of gastric surgery. Postgrad Med J. 1960;36:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Riha O. 100 Jahre Braun-anastomose. Langenbecks Arch Chir. 1993;378:106-109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 5. | Li S, Zang L. [Research advance in Billroth II with Braun anastomosis after distal gastrectomy]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:956-960. [PubMed] |
| 6. | Xu B, Zhu YH, Qian MP, Shen RR, Zheng WY, Zhang YW. Braun Enteroenterostomy Following Pancreaticoduodenectomy: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Jiao YJ, Lu TT, Liu DM, Xiang X, Wang LL, Ma SX, Wang YF, Chen YQ, Yang KH, Cai H. Comparison between laparoscopic uncut Roux-en-Y and Billroth II with Braun anastomosis after distal gastrectomy: A meta-analysis. World J Gastrointest Surg. 2022;14:594-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Vogel SB, Drane WE, Woodward ER. Clinical and radionuclide evaluation of bile diversion by Braun enteroenterostomy: prevention and treatment of alkaline reflux gastritis. An alternative to Roux-en-Y diversion. Ann Surg. 1994;219:458-65; discussion 465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Svensson JO. Duodenogastric reflux after gastric surgery. Scand J Gastroenterol. 1983;18:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Cui LH, Son SY, Shin HJ, Byun C, Hur H, Han SU, Cho YK. Billroth II with Braun Enteroenterostomy Is a Good Alternative Reconstruction to Roux-en-Y Gastrojejunostomy in Laparoscopic Distal Gastrectomy. Gastroenterol Res Pract. 2017;2017:1803851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | He L, Zhao Y. Is Roux-en-Y or Billroth-II reconstruction the preferred choice for gastric cancer patients undergoing distal gastrectomy when Billroth I reconstruction is not applicable? A meta-analysis. Medicine (Baltimore). 2019;98:e17093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Santoro R, Ettorre GM, Santoro E. Subtotal gastrectomy for gastric cancer. World J Gastroenterol. 2014;20:13667-13680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 14. | Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Shishegar A, Vahedi M, Kamani F, Kazerouni MF, Pasha MA, Fathi F. Comparison between Roux-en-Y gastrojejunostomy and Billroth-II with Braun anastomosis following partial gastrectomy: A randomized controlled trial. Ann Med Surg (Lond). 2022;76:103544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | In Choi C, Baek DH, Lee SH, Hwang SH, Kim DH, Kim KH, Jeon TY. Comparison Between Billroth-II with Braun and Roux-en-Y Reconstruction After Laparoscopic Distal Gastrectomy. J Gastrointest Surg. 2016;20:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Piessen G, Triboulet JP, Mariette C. Reconstruction after gastrectomy: which technique is best? J Visc Surg. 2010;147:e273-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Garofalo F, Abouzahr O, Atlas H, Denis R, Garneau P, Huynh H, Pescarus R. Laparoscopic revision of Billroth II with Braun anastomosis into Roux-en-Y anatomy in a patient with intestinal malrotation. Surg Endosc. 2018;32:511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Wang F, Zu HL, Jiang H, Kang Y, Dong PD, Xue YW. Clinical investigation of combined Billroth II with Braun anastomosis for patients with gastric cancer. Hepatogastroenterology. 2014;61:1812-1816. [PubMed] |
| 21. | Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310-4; discussion 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 452] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 22. | Goei TH, van Berge Henegouwen MI, Slooff MJ, van Gulik TM, Gouma DJ, Eddes EH. Pylorus-preserving pancreatoduodenectomy: influence of a Billroth I versus a Billroth II type of reconstruction on gastric emptying. Dig Surg. 2001;18:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | van Berge Henegouwen MI, van Gulik TM, DeWit LT, Allema JH, Rauws EA, Obertop H, Gouma DJ. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, Cameron JL. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218:229-37; discussion 237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 269] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Henderson AR. Testing experimental data for univariate normality. Clin Chim Acta. 2006;366:112-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Shapiro SS, Wilk MB, Chen HJ. A Comparative Study of Various Tests for Normality. J Am Stat Assoc. 1968;63:1343-1372. [RCA] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 306] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Gaddis GM, Gaddis ML. Introduction to biostatistics: Part 5, Statistical inference techniques for hypothesis testing with nonparametric data. Ann Emerg Med. 1990;19:1054-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Wobbrock JO, Findlater L, Gergle D, Higgins JJ. The aligned rank transform for nonparametric factorial analyses using only anova procedures. 2011. [cited 24 December 2023]. Available from: https://dl.acm.org/doi/10.1145/1978942.1978963. |
| 29. | Shan B, Shan L, Morris D, Golani S, Saxena A. Systematic review on quality of life outcomes after gastrectomy for gastric carcinoma. J Gastrointest Oncol. 2015;6:544-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 30. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 271] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | De Manzoni G, Verlato G, Roviello F, Di Leo A, Marrelli D, Morgagni P, Pasini F, Saragoni L, Tomezzoli A; Italian Research Group for Gastric Cancer. Subtotal versus total gastrectomy for T3 adenocarcinoma of the antrum. Gastric Cancer. 2003;6:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Dassen AE, Dikken JL, van de Velde CJ, Wouters MW, Bosscha K, Lemmens VE. Changes in treatment patterns and their influence on long-term survival in patients with stages I-III gastric cancer in The Netherlands. Int J Cancer. 2013;133:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Kennedy T, Green R. Roux diversion for bile reflux following gastric surgery. Br J Surg. 1978;65:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Almerie MQ, Darrien JH, Javed S, Kerrigan DD. Braun Procedure Is Effective in Treating Bile Reflux Following One Anastomosis Gastric Bypass: a Case Series. Obes Surg. 2021;31:3880-3882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Lindecken KD, Salm B. [The effectiveness of Braun’s anastomosis in Billroth II surgery. The role of hepatobiliary sequence scintigraphy (HBSS) in the diagnosis of bile flow following stomach resection]. Rofo. 1993;159:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Li XG, Song QY, Wu D, Li S, Zhang BL, Zhang LY, Guan D, Wang XX, Liu L. Does the addition of Braun anastomosis to Billroth II reconstruction on laparoscopic-assisted distal gastrectomy benefit patients? World J Gastrointest Oncol. 2022;14:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 37. | Xu B, Meng H, Qian M, Gu H, Zhou B, Song Z. Braun enteroenterostomy during pancreaticoduodenectomy decreases postoperative delayed gastric emptying. Am J Surg. 2015;209:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Watanabe Y, Ohtsuka T, Kimura H, Matsunaga T, Tamura K, Ideno N, Aso T, Miyasaka Y, Ueda J, Takahata S, Tanaka M. Braun enteroenterostomy reduces delayed gastric emptying after pylorus-preserving pancreatoduodenectomy: a retrospective review. Am J Surg. 2015;209:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yu WB, China S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ