Published online Mar 20, 2024. doi: 10.5662/wjm.v14.i1.88519
Peer-review started: September 27, 2023
First decision: December 7, 2023
Revised: December 22, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 20, 2024
Processing time: 162 Days and 0.1 Hours
Alcohol use disorder (AUD) represents a major public health issue which affects millions of people globally and consist a chronic relapsing condition associated with substantial morbidity and mortality. The gut microbiome plays a crucial role in maintaining overall health and has emerged as a significant contributor to the pathophysiology of various psychiatric disorders. Recent evidence suggests that the gut microbiome is intimately linked to the development and progression of AUD, with alcohol consumption directly impacting its composition and function. This review article aims to explore the intricate relationship between the gut microbiome and AUD, focusing on the implications for mental health outcomes and potential therapeutic strategies. We discuss the bidirectional communication between the gut microbiome and the brain, highlighting the role of microbiota-derived metabolites in neuroinflammation, neurotransmission, and mood regulation. Furthermore, we examine the influence of AUD-related factors, such as alcohol-induced gut dysbiosis and increased intestinal permeability, on mental health outcomes. Finally, we explore emerging therapeutic avenues targeting the gut microbiome in the management of AUD, including prebiotics, probiotics, and fecal microbiota transplantation. Understanding the complex interplay between the gut microbiome and AUD holds promise for developing novel interventions that could improve mental health outcomes in individuals with AUD.
Core Tip: The emerging field of research on the gut microbiome’s role in alcohol use disorder (AUD) has revealed significant implications for health outcomes and potential therapeutic strategies. Alcohol consumption has profound effects on the gut microbiome, leading to dysbiosis and increased systemic inflammation but their association has been found bidirectional. The gut microbiome represents a promising therapeutic target for the treatment of AUD, with dietary interventions such as probiotics and prebiotics, as well as fecal transplantation showing potential in improving gut dysbiosis and reducing inflammation.
- Citation: Koutromanos I, Legaki E, Gazouli M, Vasilopoulos E, Kouzoupis A, Tzavellas E. Gut microbiome in alcohol use disorder: Implications for health outcomes and therapeutic strategies-a literature review. World J Methodol 2024; 14(1): 88519
- URL: https://www.wjgnet.com/2222-0682/full/v14/i1/88519.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i1.88519
Alcohol use disorder (AUD) represents a major public health issue which affects millions of people globally. AUD is characterized by excessive drinking and persistent alcohol-seeking behavior. It has been described as a single spectrum of problematic use and clinically significant impairment based on endorsement of at least two of a total of 12 criteria that assess behavioral and physical manifestations of heavy alcohol consumption according to the Diagnostic and Statistical Manual of Mental Disorders. The terms alcohol abuse and alcohol dependence fall under the umbrella of the general term AUD, and can be can be classified as mild (if patient meet 2 or 3 criteria), moderate (if patient meet 4 or 5 criteria), or severe AUD (if patient meet more than 6 criteria)[1].
The prevalence of AUD is increased in high/upper middle-income countries in both males and females. Some estimates show almost 6% of individuals meet the AUD criteria, leading to significant socioeconomic problems and public health losses[1,2].
The worldwide prevalence of heavy episodic drinking surpassed 18% of total population in 2016[3]. Nevertheless, alcohol use and its effects present substantial variations across different countries. The European Union is the region with the highest alcohol consumption at a global scale, with 87% of its adolescents having consumed alcohol at least once during their lifetime, even higher compared to the United States’ 70% of adolescents[4].
Alcohol abuse is responsible for approximately 3 million deaths per year (5.3% of all deaths), along with more than 5% of the disease burden globally according to the WHO[5]. AUD is attributed as a causal factor for a plethora of diseases, which include communicable diseases, such as maternal, perinatal and nutritional conditions, and non-communicable diseases, such as epilepsy, cancer, cardiovascular, digestive diseases and injuries[3]. While the physical health consequences of AUD have been well-described, the impact of alcohol on mental health is a matter of ongoing deliberation. The interplay between alcohol consumption, mental health, and physical health outcomes is complex and multifaceted. AUD syndrome is the result of cumulative effects caused by excessive alcohol consumption, a person's genetic susceptibility, and several environmental factors, as such, deep understanding of its pathophysiology could be vital for the development of an effective treatment[6].
Abundant evidence have highlighted that alcohol dependence (alcoholism) is a complex genetic disease, with a heritability estimate as high as 50%, and a large number of variants across the genome influencing the onset and development of a person's addiction to alcohol, with some of these genes being involved in alcohol metabolism[1]. Acquiring a better understanding of the way that the environment influences genetic risk which contributes to the onset of alcoholism is of major importance in deciphering the underlying mechanisms of AUDs[7].
The gut microbiome, the complex ecosystem of microorganisms residing in the gastrointestinal tract, has risen as a fascinating field of study, as it affects several physiological processes, including digestion, metabolism, and immune function. Recently, the gut microbiome is referred to hold a substantial role in the pathophysiology of mental illnesses such as schizophrenia, bipolar disorder, anxiety disorders, depression and AUD. Preclinical studies have indicated the influence of gut microbiota in the gut-brain axis (GBA) and the bidirectional interactions between the central nervous system, the enteric nervous system, and the gastrointestinal tract, potentially affecting mental health outcomes[8].
In this review, we will discuss the latest findings regarding the changes in gut microbiome associated with AUD, and how they contribute to the development and progression of the disorder. We will further discuss the potential mechanisms through which gut dysbiosis leads to implications on health outcomes. Additionally, we will delve into the potential therapeutic approaches targeting the gut microbiome for the treatment of AUD, such as probiotics, prebiotics, dietary interventions and fecal transplantation. By considering the gut microbiome when evaluating and treating individuals with AUD, clinicians may be able to improve the health outcomes of these patients and reduce the burden of disease associated with AUD.
A comprehensive literature search was conducted using the PubMed database to identify relevant articles for this review. The following search terms were used: "gut microbiome," "alcohol use disorder," "alcohol abuse," "alcohol consumption," "microbiota," and "microbiome." This review focused on articles published in the English language between 2010 and 2022, to ensure the inclusion of recent research while capturing significant developments in the field.
The initial search yielded a broad range of articles related to the gut microbiome and AUD. After careful evaluation, articles were selected based on their relevance to the topic. Studies that investigated the changes in gut microbiome composition and function in individuals with AUD, as well as those which explored the impact of alcohol on gut dysbiosis and associated health outcomes were included. Additionally, articles focusing on therapeutic strategies targeting the gut microbiome for the treatment of AUD were considered.
The selected articles were thoroughly reviewed, and the key findings, methodologies, and conclusions were extracted. Data related to the mechanisms linking alcohol consumption to gut dysbiosis, the implications of gut dysbiosis on health outcomes, and the potential therapeutic approaches targeting the gut microbiome were synthesized and organized in a coherent manner.
By analyzing the available literature and synthesizing the findings, this review intends to contribute to the growing body of knowledge on the gut microbiome's role in AUD and provide valuable insights for future research and clinical practice.
The human body is inhabited by a vast number of microorganisms that live in concordance with their host and are commonly referred to as human microbiota or microflora. The largest proportion of microbiota, approximately 70%, can be found in the gut. Intestinal microflora is involved in host’s physiology, regulating digestion, vitamin production, metabolism of xenobiotics, immunological responses and at the same time conferring protection against pathogen perturbation[9,10].
Although it was originally believed that gut contains about 1000 bacterial species, a large scale study has estimated that the collective human gut microbiota is composed of over 35000 bacterial species. Overall, the healthy gut microbiota is predominantly constituted by strict anaerobes that dominate over anaerobes from the phyla Firmicutes and Bacteroidetes. This is followed by the phyla Actinobacteria, and Proteobacteria, with minor proportions of species belonging to the phyla of Fusobacteria, Tenericutes, and Verrucomicrobia. Despite the constant appearance of this general profile remains constant, gut microbiota can exhibit temporal and spatial differences regarding distribution at the genus level and higher classification[10,11].
A microbiome that has been linked with a healthy host, requires an extent of resistance towards external (e.g. dietary, pharmaceutical) and internal (e.g. age) changes and the ability of resilience afterwards. Thus, microbial health does not reflect a static but rather a dynamic state. Any such disturbance could jeopardize the balance of the composition and/or regulation of microbial communities, a condition that is commonly described as dysbiosis. This condition is far more likely to occur due to inadequate presence of commensal microorganisms, possible alteration of regular microbial diversity, and competition between commensal and pathogenic species for a particular body region or nutrients. Additional external factors that favor the progression of a dysbiotic state involve malnutrition, as in the case of low dietary fibers or vitamins, food additives (e.g. preservatives, emulsifers), chronic alcohol consumption, drug abuse or certain medication (most often antibiotics, over-the-counter anti-inflammatories and chemotherapeutics), exposure to hazardous environmental agents (toxins, heavy metals or radiation), and increased stress levels (anxiety, depression). Current literature suggests that mental health disorders (as in the case of drug and alcohol abuse) are often correlated with dysbiosis[12].
Alcohol abuse is known to cause deleterious effects all over human body. Despite that, it remains to be proved whether alcohol drinking is the cause or the consequence of changes in the gut microbiota. As stated above, alcohol consumption can cause significant imbalances to the gut microbiome such as the promotion of potentially pathogenic bacteria in alcoholics with and without liver disease, resulting in dysbiosis. de Timary et al[13], observed no differences in alcohol intake between the dysbiotic and non-dysbiotic group, while the total concentration in bacteria and in most bacterial families, genera or species failed to recover after detoxification, suggesting that the difference in composition of the gut microbiota might not be the consequence of drinking, which raises the issue of whether the alteration in the gut microbiota could be a precursor to the development of alcohol-dependence in some subjects. The majority of data deriving from human-based studies show a link between alcohol intake and bacterial abundance that does not imply causality. Current knowledge suggests that innate gut bacteria may bey key influencers of voluntary alcohol intake in rats that have been selectively bred for their increased ethanol intake. Administration of antibiotics that are non-absorbable prior to ethanol access has been reported to inhibit approximately 70% of volunteray ethanol intake. This effect has been fully observed during the first day of access to alcohol. The available data suggest that the firewall mechanisms that typically prevent increased alcohol intake tend to be suppressed by endogenous microbiota of a rat strain that is selected for their preference towards alcohol[14]. Carbia et al[15] demonstrated that the most typical patten of alcohol misuse in adolescence is linked with alterations of the gut microbiome, even prior to the development of addiction. An animal- based study of Segovia-Rodríguez et al[16] shed light to whether intestinal microbiota could be the cause of increased alcohol intake in animals that received fecal transplantation from alcohol-dependent laboratory animals, leading to a higher alcohol consumption and to a reduced spontaneous locomotor activity compared to animals which received transplant from control animals or those treated with the buffer without feces[17]. This finding indicated that alterations that were induced by microbiota would affect the host in a more universal fashion. Authors proposed a synergistic mechanism of interaction between the new microbiota received and alcohol consumption. A plausible explanation of the bidirectional effect is that in the presence of alcohol, microbiota can promote a positive feedback mechanism that favors the abundance of bacteria that benefit from alcohol intake[17]. These alterations reflect a dysregulation in the microbiome-gut brain axis that could further trigger dysregulation and lead to an even more increased risk of psychopathology and especially when appearing during key windows across a person’s lifetime[15].
Most data about the influence of alcohol on the relative abundance of gut microbiome comes from animal-based studies; expose to alcohol does not alter the abundance of gut microbiota, but remarkably alternates its composition. In animal models, alcohol intake decreases the relative abundance of the genera Lactobacillus (or Sporolactobacillus) and promotes the relative abundance of the genera Blautia, Allobaculum[18], Ruminococcus, Coprococcus[19], Adlercreutzia and Turicibacter[20], Alistipes and Odoribacter[21], resulting in memory loss, and neuropsychiatric behaviors, like anxiety and depression-like disorders[18,20,21].
Different patterns of drinking, such as chronic drinking (chronic) or recent heavy drinking (acute) could lead to a different composition of gut microbiota[22]. Specifically, in a mouse model with acute alcohol consumption, an upregulation of the phyla Actinobacteria and Verrucomicrobia and the genera Bacteroidales and Lachnospiraceae was recorded[23], while in chronic alcohol consumption Bacteroidetes, Bacteroides genus, and Akkermansia genus were present at a higher proportion[22]. Furthermore, acute alcohol consumption decreased the levels of Lactobacillus, Escherichia-Shigella, and Turicibacter[23], while in chronic alcohol intake the relative abundance of Firmicutes phylum, Lactococcus, Pediococcus, Lactobacillus, and Leuconostoc genus was downregulated[22].
Even though alcohol consumption could be a critical factor that influences gut microbiome in terms of function and composition, alcohol metabolism itself, along with its effects on the patient/consumer can be influenced by the microbiome, establishing a bidirectional relationship. However, existing data on alcohol’s effect on gut microbiome in humans are scarce. The first studies dealing with changes in the gut microbiome of individuals with AUD concluded a reduction in the abundance of beneficial bacteria such as Bacteroidetes, Lactobacillus and Bifidobacterium, and an increase in potentially harmful bacteria, such as Enterobacteriaceae, Streptococcus and Proteobacteria[24,25].
On the other side, alcohol dependents with higher intestinal permeability seems to present a more unique profile with a sharp reduction in the abundance of Ruminoccaceae family (and in particular in Rumminococcus, Faecalibacterium, Subdoligranulum. Oscillibacter and Anaerofilum), and an increase in Lachnospiraceae and the genera Blautia and Megasphaera[25].
Dubinkina et al[26] were the first to describe the gut metagenome of patients with alcoholic disorder using shotgun (whole-genome) metagenomic sequencing. They found significant differences on the gut microbial community in gut dysbiosis, when comparing alcoholics with and without liver disease, as opposed to Mutlu et al[24], who reported similarities between the two groups. Dubinkina et al[26] reported only a slight overlap in the metagenomic signature of alcoholics with and without liver disease. Increased populations of Klebsiella and decreased Coprococcus, Faecalibacterium prausnitzii, and unclassified Clostridiales were associated with alcoholics without liver cirrhosis, whereas both groups were characterized by reduced Acidaminococcus sp, as well as increased Lactobacilli and Bifidobacterium members, however with different species in each group. Most recently, Bjørkhaug et al[27] confirmed via sequencing the higher relative abundance of Proteobacteria in alcohol overconsumers in a dose independent manner. Their results revealed reduced relative abundance of Faecalibacterium, a plethora of taxonomic groups inside the Firmicutes phylum, and specifically in the Clostridia and Actinobacteria class in the group of alcohol overconsumers, and a higher relative abundance of Sutterella, Clostridium, and Holdemania[27].
A more recent meta-analysis highlighted the causal role of alcohol in gut dysbiosis confirming that alcohol consumption could favor the proliferation of some bacterial species in the gut, such as the already mentioned Bacteroidetes and Proteobacteria and suppress other species like the probiotic Lactobacillus and Bifidobacterium and the physcobiotics Faecalibacterium prausnitzii and Akkermansia muciniphila[28].
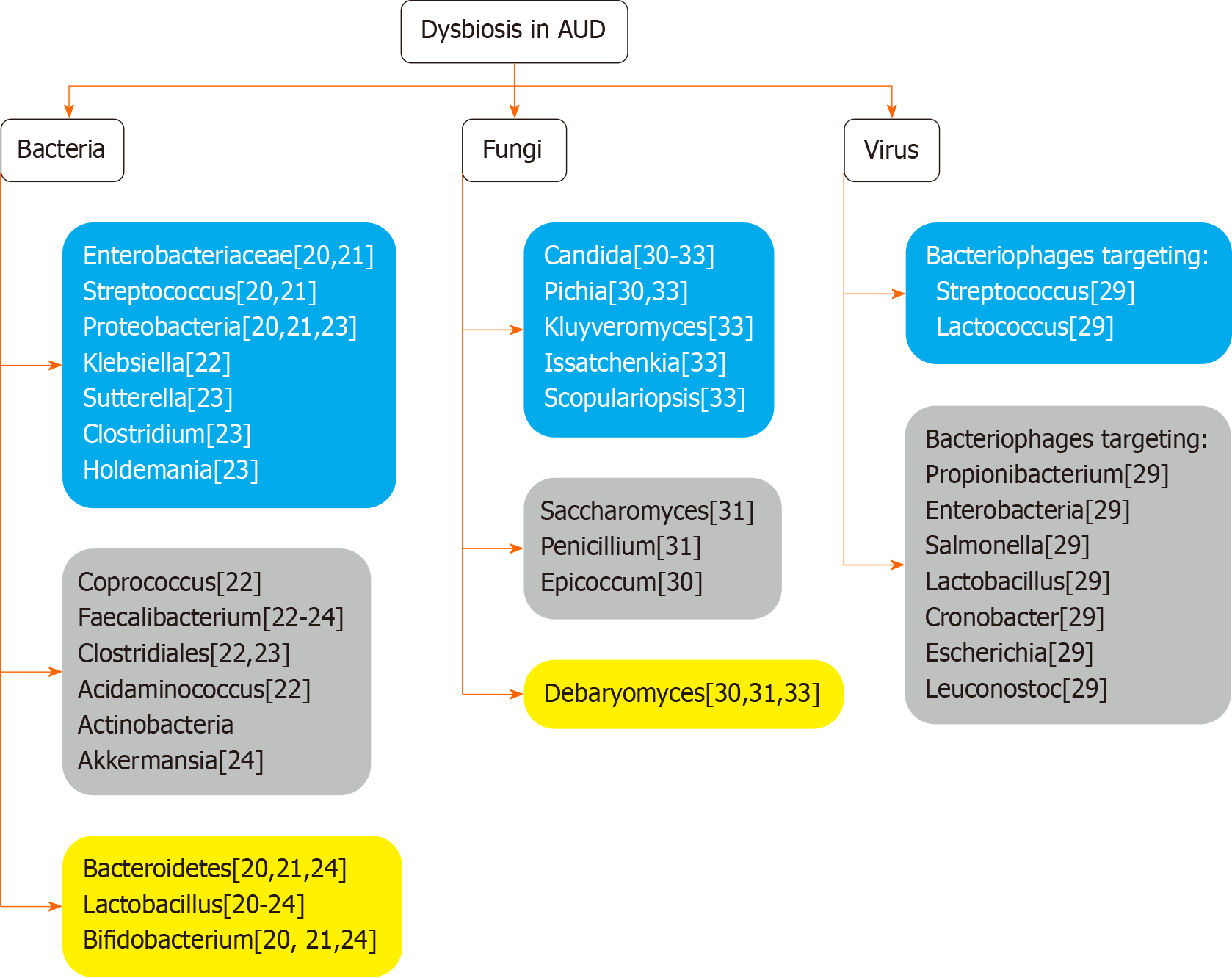
However, the gut microbiome does not consist of bacteria only, but also includes viruses and fungi. Major differences have been observed regarding the fecal viromes of AUD patients, specifically in the composition of bacteriophage species. At least 18 bacteriophages were found to be more abundant in the control subjects, including eight bacteriophages that target Propionibacterium, five that target Enterobacteria, while the remaining seem to target Salmonella, Lactobacillus, Cronobacter, Escherichia, and Leuconostoc. Concerning the bacteriophage species which were more abundant in the AUD patient population, the findings include two bacteriophages that target Streptococcus and two that target Lactococcus[29]. Concerning mycobiome, there are scarce data investigating fungal abundance differentiation in AUD patients compared to non-alcoholic individuals. An increase in the abundance of Candida[30-33], Pichia[30,33] Kluyveromyces, Issatchenkia and Scopulariopsis[33], genus level has been reported, while Saccharomyces, Penicillium[31], and Epicoccum[30], presented lower levels. Literature data are conflicting regarding Debaryomyces[30,31,33] (Figure 1).
The dysbiosis observed in the gut microbiome of individuals with AUD can have significant implications for health outcomes. One of the most notable consequences is the increased risk of developing liver disease. Chronic alcohol consumption leads to imbalanced gut microbiome, which in turn causes hepatocytes damage, leading to the development of alcoholic liver disease (ALD), ranging from steatosis to cirrhosis. Gut dysbiosis has also been linked to the development of other chronic diseases, including cardiovascular disease and type 2 diabetes. Gut microbiota may influence brain function through neural, endocrine, and immune pathways[34] related to vagus nerve signaling[35]. In order to ameliorate the health outcomes of gut dysbiosis, it is essential to understand the role of the gut microbiome in health maintenance, and how an imbalance could affect the human body.
Inflammation, a hallmark of gut dysbiosis, plays a pivotal role in the development of these diseases. Research has shown that gut dysbiosis can lead to the production of pro-inflammatory cytokines, lipopolysaccharides (LPS), and bacterial endotoxins, resulting in increased inflammation, oxidative stress, insulin resistance and endothelial dysfunction, and other widespread effects on the body, further exacerbating the detrimental health outcomes associated with AUD[36]. The intestinal mucosa can be detrimentally affected by inflammation caused by microbiome metabolites or ethanol, leading to damage and increased permeability. Although the exact mechanism has not yet been characterized, alcohol may lead to gut inflammation via alterations in the gut microbiota by promoting an increase in pro-inflammatory bacteria and/or decrease in anti-inflammatory bacteria, and affecting cytokine expression. Accordingly, in an animal model treated with human gut microbiota, the relative population of a pro-inflammatory bacterium (Clostridium cluster XIVa) increased, whereas there was a decrease in anti-inflammatory bacteria (Akkermansia muciniphila, Atopobium, Faecalibacterium prausnitzii), compared to untreated[37]. Interestingly, this kind of alcohol-induced dysbiosis was found to mediate intestinal barrier dysfunction, as well as ALD, via activation of tumor necrosis factor receptor I, in intestinal epithelial cells. Inflammation is primarily facilitated via introduction of leukocytes, as well as the presence of inflammation mediators, such as histamine, reactive oxygen species (ROS) and leukotriene. Mucin can be altered by reduced Mucin 2 (MUC-2) expression, which in turn is caused by increased matrix metalloproteinase-9 (MMP-9) expression by epithelial cells. MMP-9 can also be induced by Claudin-1 up-regulation, inhibiting goblet cell differentiation via Notch signalling, also leading to inflammation, due to the resulting reduction in MUC-2 expression[37].
The amount of episodes that involve binge drinking are associated with increased responsiveness of the stimulated cytokines [interleukin (IL)-6 and IL-8]. Moreover, blood cytokine response is stimulated (mostly increases via the Toll-like receptor 4 (TLR4) triggered cytokines IL-6, IL-8, Il-1b), as the frequency of the binge drinking events increases. Additionally, craving behavior is linked to increased levels of circulating markers of inflammation in AUD patients[15].
Release of endotoxins by dysbiotic microbiota in the gut was also found to induce production of pro-inflammatory cytokines and ROS by the stimulated hepatic Kupffer cells[38,39]. Even a sole binge drinking event can elevate serum endotoxin, most possibly due to translocation of products derived from gut bacteria and disturbance of the innate immune response, which contribute to the deleterious effects of binge drinking[15]. For instance, gut microbiota possibly affect the intestinal barrier’s integrity. The following release of cytokines could signal to the brain via activation of the vagus nerve or via signaling across the blood–brain barrier. In parallel, substances that are produced by gut microbiota are possibly absorbed reaching the brain through the bloodstream. Subsequently, the brain, can affect the gut microbiota through neuronal and endocrine mechanisms and by the adoption of health behaviors. Thus, it is obvious that a possible imbalance of gut microbiota may affect the brain and lead to dysfunctions in the form of psychiatric disorders including as emotional and cognitive alterations.
AUD patients have enhanced levels of LPS, as a result of the increased abundance of the gram-negative bacteria which produce it, and have been shown to trigger significant inflammation; high levels of LPS can cause sepsis, even septic shock, and health implications including neurodegenerative disorders such as Alzheimer’s disease, via chronic neuroinflammation[40]. Previous studies have shown that alcohol and gut dysbiosis elevate the LPS serum levels that derive from bacteria[22,41]. LPS can release inflammatory factors such as tumour necrosis factor alpha, IL-1, IL-6, IL-8, IL-10, interferon gamma (IFN-γ), and MMP-9 and induce inflammation by activating TLR4 complexes[42] and subsequently switches on the hypothalamic-pituitary-adrenal axis (HPA) axis to influence the brain. The brain can affect the intestine via the HPA axis which releases adrenocorticotropic hormones, leading to elevated intestinal permeability. In alcoholics, the higher relative abundance of gram-negative bacteria also includes Enterobacteriaceae[43] and Proteobacteria[26]; their increased population highly correlates with elevated LPS levels, and results in a more potent immunological response, compared to other phyla. These, combined with increased gut permeability, are significant contributors of AUD-related inflammation[44].
Metabolites that derive from the gut microbiome can exert diverse functions in the body with the majority of them involved in pathways of the digestive system formation and function. These molecules range from short chain fatty acids (SCFAs) and neurotransmitters to precursors of neurotransmitters, bile acids, hormones and vitamins. Each one of these metabolites has important impact on the brain function of the host. Notably, a decrease in the metabolic potential of alcohol dependent patients microbiome has been marked, characterized with an overall functional decrease in pathways related to methane metabolism, bacterial chemotaxis, and pyrimidine metabolism, and increase in metabolic pathways related to the phosphotransferase system[26].
The intestinal microbiome produces a range of small molecules representing most major metabolite classes, allowing them to ferment complex dietary polysaccharides and carbohydrates, as well as dietary fibers, producing SCFAs[45]. SCFAs (acetate, propionate, and butyrate) constitute a significant energy source for gut epithelial cells[46], and have been associated with improvement in the function and maintenance of the intestinal barrier’s integrity, for an overall protective effect against pathogens[47]. Their anti-inflammatory nature supports epithelia cell proliferation, as well as T cell differentiation in the colon, contributing to gut homeostasis[48]. The bacterial SCFA butyrate can enhance tight junction integrity by inducing the production of relevant proteins, including claudin, occludin and zonula occludes, which as a result inhibits bacterial translocation[49]. Since both the intestinal bacteria and the levels of produced metabolites have such interlinked functions with the human body, affecting barrier integrity, there should be substantial caution of the altered populations to avoid collateral harm[50]. SCFAs are part of a satiety-inducing mechanism, with propionate enhancing gluconeogenesis, and the aforementioned butyrate playing a role in increasing glucagon-like peptide-a via gut cell stimulation[51]. Importantly, through the GBA, each metabolite present a neuromodulatory function, regulating the levels of critical neurotransmitters, including enteroendocrine serotonin, noradrenaline, and dopamine,) which affect numerous functions of the nervous system, as well as range of emotions, behaviors and other central nervous system (CNS) functions. Even gamma aminobutyric acid, a predominant inhibitory neurotransmitter, has been found to be secreted by bacterial strains, such a Bifidobacterium and Lactobacillus[24]. Lower levels of isovalerate in fecal SCFAs correlated with higher alcohol consumption in AUD patients, compared to controls, and were successfully reversed post-faecal microbiota transplantation (FMT) treatment[15,52,53]. The therapeutic efficacy may be attributed to recovery in the levels of bacteria such as Alistipes, Faecalibacterium, Ruminococcus, and Ocillibacter, which have been previously associated with increased isovalerate[54].
A metagenomic analysis in AUD patients by Litwinowicz et al[55] (concluded to a significant increase in facultative anaerobes (such as the Enterobacteriaceae family), that may be a result of a simultaneous decrease in levels of butyrate-producing bacteria (Butyricicoccaceae, Lachnospiraceae, Ruminococcaceae, Oscillospiraceae), thus reducing beta-oxidation, which in turn ends up increasing oxygen levels. Most of the produced butyrate gets used up for energy, with only approximately 5% remaining in circulation, however, this small fraction appears to be capable of inducing a potent anti-inflammatory response, through various pathways, including through the G-protein-coupled receptors 41 and 43[56], inhibition of IFN-γ signaling, and induction of nuclear factor kappaB[57]. Butyrate’s functions extends to strengthening the intestinal barrier via permeability reduction, therefore lower levels of bacteria producing it (Ruminococcaceae, Lachnospiraceae), could negatively affect AUD progression[55,58].
The connection among gut dysbiosis and brain function is firm given the ability of the gut microbiome to affect the functions of the central nervous system. SCFAs and the condition of the gut have been shown to influence a plethora of psycho-neurological conditions that include depression, anxiety, stress, Autism Spectrum Disorder (ASD), schizophrenia, and Parkinson disease[59,60]. Apart from negatively affecting the progress of AUD itself, intestinal microbiota can have a detrimental effect in various factors associated with relapse, such as alcohol craving and negative emotional states, such as depression and anxiety[13]. Of note, the level of intestinal permeability was associated with behaviors, depression recovery, anxiety, and craving levels observed in individuals with reduced intestinal permeability, but not in individuals with increased intestinal permeability. This finding seems to suggest a role of the microbiome GBA in AUD. Both addiction and withdrawal are known to arise from modifications in the neuronal function. The GBA is regarded as the bidirectional communication pathway that links the brain with the gastrointestinal tract. Different pathways for communication between the gut and the brain involve neuronal signaling via the vagus nerve, endocrine actions via the HPA, and stimulation of neural inflammation, or a wide range of metabolic changes. Several studies have proved that bacteria affect the GBA and interfere in conditions and phenotypes such as ASDs, social behavior, anxiety, depression, eating habits as well as the amount of food consumed[61].
Treatment outcomes in AUD can vary across patients and different medications. Most pharmacotherapies focus on limiting alcohol craving through neuromodulation, including opioid, glutamate, gamma-aminobutyric acid, and serotonin systems. While achieving abstinence is the desirable result, its rarely achieved, by merely 16% of AUD patients. The three approved AUD medications (disulfiram, acamprosate, and naltrexone) have shown moderate therapeutic efficacy, and with AUD being a heterogenous disorder, a single medication is unlikely to be effective for every patient[62]. As it is increasingly recognized that the gut microbiome is correlated with AUDs, it has emerged as a potential therapeutic target for the treatment of AUD, aiming to reverse the dysbiotic state and reduce inflammation. Dysbiosis of microbiota can be restored through different approaches.
Dietary interventions have been investigated as a means to improve gut dysbiosis in individuals with various pathologies including AUD. A Mediterranean-style diet, rich in fruits, vegetables, whole grains, and healthy fats, is widely known for its beneficial effects on overall health, promotion of maintenance of a diverse and beneficial gut microbiota, increase in the concentration of SCFAs, and protection of the intestinal mucus layer[63]. Conversely, another study found that a diet with unsaturated fats affected the intestinal barrier, inducing inflammation and liver injury in mice exposed to chronic alcohol consumption[64].
Another approach to modulate gut microbiota are the dietary supplements in the form of either probiotics or prebiotics since it has been proposed as an emerging therapeutic target for the management of cognitive and/or behavior pathology. The definition of probiotics states that they are “living microorganisms which, when administered in adequate quantities, present an overall benefit to the host’s health”[65]. Due to probiotics’ potential benefits to the CNS and mental disorders, it has been proposed they be characterized as “psychobiotic,” expecting low side effects, and anti-inflammatory, antidepressant, and anti-anxiety effects, ameliorating mental functions in Alzheimer’s disease and ASD[66].
The administration of probiotics in health subjects has been linked with alterations in brain activity related to emotional memory and decision-making procedures as well as changes in functional connectivity.
The beneficial use of probiotics has been indicated in impaired social cognition and emotional functioning disorders, which have been linked to AUDs[67,68]. The administration of probiotics in healthy individual has been linked with alterations in brain activity related to emotional memory and decision-making procedures[69], as well as changes in functional connectivity during the performance of different emotional tasks[70,71], while stress conditions leading to rapid reaction times in an emotional recognition task and reduction of proinflammatory cytokines[72]. In autism-animal model, a probiotic administration could reverse social behavior deficits[73]. A very early study (1995) showed that consuming 100 mL of a Bacillus natto-fermented product, could reduce breath alcohol (44% reduction) and aldehyde (45% reduction) concentrations in a group of participants 1 h after drinking whisky, compared to a control group of rats[74].
Most data about the benefits of probiotics use in alcohol disorders arise from studies of alcohol liver disease with a focus on the improvement of the liver tissue. Probiotic supplementation restores the number of fecal Bifidobacteria, Lactobacilli, and Enterococci in alcoholic patients. The proposed mechanism of action is via modulation of dysbiosis and balance restoration, which in turn promotes an anti-inflammatory microenvironment allowing the reduction of the intestinal permeability and the translocation of bacterial components (LPS) to the systemic circulation. Furthermore, endotoxemia is found to be reduced while at the same time, probiotics can prevent bacterial metabolites from reaching the liver and triggering inflammatory responses[75]. By reducing systemic proinflammatory status and neuroinflammation, probiotics also offer an excellent alternative to relieve CNS damage reinforcing beneficial effects on addiction and, consequently, alcohol consumption[76].
Lim et al[77] studied the effects of 19 probiotic species on alcohol and acetaldehyde metabolism identifying four probiotic species, which present a relatively higher tolerance to alcohol, and a more effective alcohol and acetaldehyde metabolism: Lactobacillus gasseri CBT LGA1, Lactobacillus casei CBT LC5, Bifidobacterium lactis CBT BL3, and Bifidobacterium breve CBT BR3. These species also showed high mRNA expression levels of alcohol and acetaldehyde dehydrogenase (ALDH). A mixture of these four probiotics species and excipients, the ProAP4, was then administered to rats for 2 wk in advance of acute alcohol administration. The serum alcohol and acetaldehyde concentrations were significantly decreased in the treated group than in the control. Thus, the administration of these four probiotic species, rapidly reduced blood alcohol and acetaldehyde levels in an alcohol and ALDH-dependent fashion. Subsequently, another randomized placebo-cotroled crossover study examined the effect of Duolac ProAP4 supplementation on alcohol detoxification in humans, which lead to a reduction in both blood alcohol and acetaldehyde levels in ALDH2 2 hetorozygotes[78].
A number of prebiotics and probiotics alone or their combination could hold the potential of regulating brain’s neurotransmition and in turn could attenuate alcohol-related addictive processes, and the related affective and cognitive-behavioral modifications. Accordingly, synbiotic supplementation was reported by Pizarro et al[79] to induce alterations in the relative population of gut’s bacteria, tryptophan derivatives, g-aminobutyric acid and norepinephrine levels in the hippocampus and prefrontal cortex of mice, post alcohol withdrawal. Interestingly, although alcohol appeared to induce a detrimental effect in long-term memory and mobility in female mice (which was reduced by synbiotics), male mice showed no significant changes, indicating a higher alcohol tolerance in males. A double-blind, placebo-controlled trial randomized participants in four groups supplemented with placebo, prebiotics, probiotics and synbiotics, respectively. This study highlighted the increase of the number of supplement-specific bacteria after probiotic administration in healthy individuals, but no improvement in the metabolism of an acute dose of alcohol was reported[80]. Furthermore, the combination of drugs that inhibit the hyper-glutamatergic state [N-acetylcysteine (NAC) and acetylsalicylic acid (ASA)] with probiotics (Lactobacillus rhamnosus ) markedly inhibit relapse ethanol intake. Two mechanisms were induced by these treatments; NAC + ASA reduced the glutamatergic tone and antibiotic + LGG reduced the dopaminergic tone, that reduced the alcohol binge–drinking relapse effect independently and complementary[81].
Most recently, a recombinant probiotic Lactococcus lactis expressing human ADH1B (hADH1B) was constructed, to enhance alcohol degradation in the intestinal tract after oral administration. Alcohol’s metabolism includes its decomposition from the enzyme alcohol dehydrogenase the action of which is followed by the liver enzyme ALDH; thus, as expected, the administration of hADH1B-expressing probiotic reduced alcohol absorption, and extended alcohol tolerance time and a reduction of recovery time and protected the intestine and liver from damage after acute alcohol consumption in mice[82].
A number of recent studies (both preclinical and clinical) have shown that transplantation of fecal microbiota from AUD patient is capable to alter the intestinal barrier and modify brain function. In total, important alterations in the gut microbiome in time occur as a response to chronic alcohol exposure and correspond to severe intestinal barrier dysfunction and ALD development. Moreover, the altered bacterial communities of the gut may serve as a significant therapeutic target for the prevention/treatment of chronic alcohol intake induced intestinal barrier dysfunction and liver disease[83]. An animal model study showed that when sensitive to alcohol mice were fed with intestinal microbiota from resistant mice, the development of alcohol-induced liver lesions was prevented and a better gut homeostasis was observed. In total FMT treated mice indicated a similar intestinal microbiota profile to alcohol-resistant mice[84].
A phase 1 randomised clinical trial of FMT for AUD demonstrated short-term and long-term changes in AUD patients with cirrhosis using microbial manipulation. The FMT group presented a higher number of Alistipes and Roseburia, and increased production of SCFA. In contrast, there was a reduction in stool isovalerate and 2- methylbutyrate in placebo. Alcohol craving was negatively associated with Ruminococcaceae genera after FMT, and the reverse pattern was seen with Proteobacteria genera, such as Pseudomonas and with other potential pathobionts, such as Enterococcus. Ethanoligenens, which is associated with endogenous alcohol production, was also negatively linked with FMT. Potentially beneficial genera and those higher in post-FMT, such as Bilophila and Ruminococcus, were associated with lower alcohol-craving score after FMT. FMT subjects also showed reduced intestinal permeability, short-term decreases in inflammation, and decreased lipopolysaccharide binding protein, all of which supports FMT as a beneficial treatment for AUD patients, that often suffer from impaired intestinal barrier function[53].
A later study in germ-free mice, colonized with microbiota from post-FMT humans, showed a striking decrease in both initial ethanol acceptance as well as ethanol preference, compared to control group. The beneficial impact was attributed to changes in the microbial taxa (increased Lachnospiraceae and Ruminococcaceae, decreased Enterobacteriaceae), which appeared comparable to those in post-FMT humans, and was complemented with improvements in intestinal barrier function, increase in SCFAs, and lower butyrate. Notably, changes in gene expression were limited in the intestine, but not the liver or prefrontal cortex, and were associated primarily with inflammation and immune response, proliferation of epithelial cells, as well as response to oxidative stress. These results promote gut microbiota and the intestinal interface as therapeutic targets to lower alcohol intake in AUD patients[85].
The emerging field of research on the gut microbiome’s role in AUD has revealed significant implications for health outcomes and potential therapeutic strategies. Alcohol consumption has profound effects on the gut microbiome, leading to dysbiosis and increased systemic inflammation. These alterations have significant implications for health outcomes, including the development of liver disease, cardiovascular disease, and type 2 diabetes. The gut microbiome represents a promising therapeutic target for the treatment of AUD, with interventions such as probiotics, prebiotics, and dietary modifications showing potential in improving gut dysbiosis and reducing inflammation. However, further research is needed to fully understand the intricate interactions between alcohol consumption and the gut microbiome, and to develop effective interventions that can mitigate the detrimental effects of AUD on gut health.
| 1. | Deak JD, Miller AP, Gizer IR. Genetics of alcohol use disorder: a review. Curr Opin Psychol. 2019;27:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Nehring SM, Chen RJ, Freeman AM. Alcohol Use Disorder. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 3. | World Health Organization. Global status report on alcohol and health. 2018. [cited August 2023]. Available from: https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf. |
| 4. | World Health Organization. Alcohol in the European Union: consumption, harm and policy approaches: 27 March 2012, Copenhagen, Denmark: final report. 2012. [cited August 2023]. Available from: https://iris.who.int/handle/10665/107302. |
| 5. | Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 457] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 6. | Jones JD, Comer SD, Kranzler HR. The pharmacogenetics of alcohol use disorder. Alcohol Clin Exp Res. 2015;39:391-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Spanagel R, Bartsch D, Brors B, Dahmen N, Deussing J, Eils R, Ende G, Gallinat J, Gebicke-Haerter P, Heinz A, Kiefer F, Jäger W, Mann K, Matthäus F, Nöthen M, Rietschel M, Sartorius A, Schütz G, Sommer WH, Sprengel R, Walter H, Wichmann E, Wienker T, Wurst W, Zimmer A. An integrated genome research network for studying the genetics of alcohol addiction. Addict Biol. 2010;15:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Tzavellas EO, Logotheti M, Stefanis N. The Gut Microbiome in Serious Mental Illnesses. Gut Microbiome-Relat Dis Ther. 2021;1:243-263. [DOI] [Full Text] |
| 9. | Gouba N, Hien YE, Guissou ML, Fonkou MDM, Traore Y, Tarnagda Z. Digestive tract mycobiota and microbiota and the effects on the immune system. Human Microb J. 2019;12:100056. [DOI] [Full Text] |
| 10. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1969] [Article Influence: 179.0] [Reference Citation Analysis (78)] |
| 11. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2814] [Article Influence: 175.9] [Reference Citation Analysis (3)] |
| 12. | Andreou NP, Gazouli M. The Human Microbiome. Gut Microbiome-Relat Dis Ther. 2021;1:1-28. [DOI] [Full Text] |
| 13. | de Timary P, Leclercq S, Stärkel P, Delzenne N. A dysbiotic subpopulation of alcohol-dependent subjects. Gut Microbes. 2015;6:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Ezquer F, Quintanilla ME, Moya-Flores F, Morales P, Munita JM, Olivares B, Landskron G, Hermoso MA, Ezquer M, Herrera-Marschitz M, Israel Y. Innate gut microbiota predisposes to high alcohol consumption. Addict Biol. 2021;26:e13018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Carbia C, Bastiaanssen TFS, Iannone LF, García-Cabrerizo R, Boscaini S, Berding K, Strain CR, Clarke G, Stanton C, Dinan TG, Cryan JF. The Microbiome-Gut-Brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine. 2023;89:104442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 16. | Segovia-Rodríguez L, Echeverry-Alzate V, Rincón-Pérez I, Calleja-Conde J, Bühler KM, Giné E, Albert J, Hinojosa JA, Huertas E, Gómez-Gallego F, Bressa C, Rodríguez de Fonseca F, López-Moreno JA. Gut microbiota and voluntary alcohol consumption. Transl Psychiatry. 2022;12:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Zhao W, Hu Y, Li C, Li N, Zhu S, Tan X, Li M, Zhang Y, Xu Z, Ding Z, Hu L, Liu Z, Sun J. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC ε levels in mouse. Biofactors. 2020;46:38-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Xiao HW, Ge C, Feng GX, Li Y, Luo D, Dong JL, Li H, Wang H, Cui M, Fan SJ. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett. 2018;287:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Posteraro B, Paroni Sterbini F, Petito V, Rocca S, Cubeddu T, Graziani C, Arena V, Vassallo GA, Mosoni C, Lopetuso L, Lorrai I, Maccioni P, Masucci L, Martini C, Gasbarrini A, Sanguinetti M, Colombo G, Addolorato G. Liver Injury, Endotoxemia, and Their Relationship to Intestinal Microbiota Composition in Alcohol-Preferring Rats. Alcohol Clin Exp Res. 2018;42:2313-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Xu Z, Wang C, Dong X, Hu T, Wang L, Zhao W, Zhu S, Li G, Hu Y, Gao Q, Wan J, Liu Z, Sun J. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors. 2019;45:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Wang G, Liu Q, Guo L, Zeng H, Ding C, Zhang W, Xu D, Wang X, Qiu J, Dong Q, Fan Z, Zhang Q, Pan J. Gut Microbiota and Relevant Metabolites Analysis in Alcohol Dependent Mice. Front Microbiol. 2018;9:1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 658] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 23. | Ming L, Qiao X, Yi L, Siren D, He J, Hai L, Guo F, Xiao Y, Ji R. Camel milk modulates ethanol-induced changes in the gut microbiome and transcriptome in a mouse model of acute alcoholic liver disease. J Dairy Sci. 2020;103:3937-3949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 610] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 25. | Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485-E4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 734] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 26. | Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 27. | Bjørkhaug ST, Aanes H, Neupane SP, Bramness JG, Malvik S, Henriksen C, Skar V, Medhus AW, Valeur J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes. 2019;10:663-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 28. | Qamar N, Castano D, Patt C, Chu T, Cottrell J, Chang SL. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav Brain Res. 2019;376:112196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Hsu CL, Zhang X, Jiang L, Lang S, Hartmann P, Pride D, Fouts DE, Stärkel P, Schnabl B. Intestinal virome in patients with alcohol use disorder and after abstinence. Hepatol Commun. 2022;6:2058-2069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, Bluemel S, Hartmann P, Xu J, Koyama Y, Kisseleva T, Torralba MG, Moncera K, Beeri K, Chen CS, Freese K, Hellerbrand C, Lee SM, Hoffman HM, Mehal WZ, Garcia-Tsao G, Mutlu EA, Keshavarzian A, Brown GD, Ho SB, Bataller R, Stärkel P, Fouts DE, Schnabl B. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127:2829-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 31. | Lang S, Duan Y, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M, Abraldes JG, Bosques-Padilla F, Verna EC, Brown RS Jr, Vargas V, Altamirano J, Caballería J, Shawcross D, Lucey MR, Louvet A, Mathurin P, Garcia-Tsao G, Ho SB, Tu XM, Bataller R, Stärkel P, Fouts DE, Schnabl B. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology. 2020;71:522-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (2)] |
| 32. | Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, Liu J, Mogavero S, Bosques-Padilla F, Abraldes JG, Vargas V, Tu XM, Yang L, Hou X, Hube B, Stärkel P, Schnabl B. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol. 2020;72:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 33. | Hartmann P, Lang S, Zeng S, Duan Y, Zhang X, Wang Y, Bondareva M, Kruglov A, Fouts DE, Stärkel P, Schnabl B. Dynamic Changes of the Fungal Microbiome in Alcohol Use Disorder. Front Physiol. 2021;12:699253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: A review. Brain Behav Immun. 2017;66:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 35. | Dinan TG, Cryan JF. Brain-Gut-Microbiota Axis and Mental Health. Psychosom Med. 2017;79:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 36. | Meroni M, Longo M, Dongiovanni P. Alcohol or Gut Microbiota: Who Is the Guilty? Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 37. | Chen G, Shi F, Yin W, Guo Y, Liu A, Shuai J, Sun J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front Microbiol. 2022;13:916765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 38. | Meroni M, Longo M, Dongiovanni P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 39. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1456] [Article Influence: 242.7] [Reference Citation Analysis (1)] |
| 40. | Kim HS, Kim S, Shin SJ, Park YH, Nam Y, Kim CW, Lee KW, Kim SM, Jung ID, Yang HD, Park YM, Moon M. Gram-negative bacteria and their lipopolysaccharides in Alzheimer's disease: pathologic roles and therapeutic implications. Transl Neurodegener. 2021;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 41. | Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1745] [Cited by in RCA: 1728] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 42. | Leclercq S, de Timary P, Delzenne NM, Stärkel P. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl Psychiatry. 2017;7:e1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 43. | Simeonova D, Stoyanov D, Leunis JC, Carvalho AF, Kubera M, Murdjeva M, Maes M. Increased Serum Immunoglobulin Responses to Gut Commensal Gram-Negative Bacteria in Unipolar Major Depression and Bipolar Disorder Type 1, Especially When Melancholia Is Present. Neurotox Res. 2020;37:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M; DIABIMMUNE Study Group, Xavier RJ. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:842-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 857] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 45. | Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science. 2015;349:1254766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 46. | Dumas ME. The microbial-mammalian metabolic axis: beyond simple metabolism. Cell Metab. 2011;13:489-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Swidergall M, Ernst JF. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot Cell. 2014;13:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2211] [Article Influence: 170.1] [Reference Citation Analysis (3)] |
| 49. | Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, Shen Z, Günzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 50. | Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 752] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 51. | Chambers ES, Preston T, Frost G, Morrison DJ. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7:198-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 52. | Bajaj JS, Shamsaddini A, Fagan A, McGeorge S, Gavis E, Sikaroodi M, Brenner LA, Wade JB, Gillevet PM. Distinct gut microbial compositional and functional changes associated with impaired inhibitory control in patients with cirrhosis. Gut Microbes. 2021;13:1953247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M, Patel S, Davis B, Meador J, Puri P, Sikaroodi M, Gillevet PM. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology. 2021;73:1688-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 54. | Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. 2016;19:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Litwinowicz K, Gamian A. Microbiome Alterations in Alcohol Use Disorder and Alcoholic Liver Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 56. | Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312-11319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1823] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 57. | Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: A Double-Edged Sword for Health? Adv Nutr. 2018;9:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 795] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 58. | Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 59. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 2146] [Article Influence: 306.6] [Reference Citation Analysis (2)] |
| 60. | Taylor AM, Holscher HD. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr Neurosci. 2020;23:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 61. | Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018;12:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 878] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 62. | Burnette EM, Nieto SJ, Grodin EN, Meredith LR, Hurley B, Miotto K, Gillis AJ, Ray LA. Novel Agents for the Pharmacological Treatment of Alcohol Use Disorder. Drugs. 2022;82:251-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 63. | David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8117] [Cited by in RCA: 7168] [Article Influence: 597.3] [Reference Citation Analysis (1)] |
| 64. | McDevitt HO, Wraith DC, Smilek DE, Lundberg AS, Steinman L. Evolution, function, and utilization of major histocompatibility complex polymorphism in autoimmune disease. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 2:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Food and Agriculture Organization and World Health Organization Expert Consultation. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Córdoba, Argentina: Food and Agriculture Organization of the United Nations and World Health Organization; 2001. Available from: https://www.fao.org/3/a0512e/a0512e.pdf. |
| 66. | Ansari F, Pourjafar H, Tabrizi A, Homayouni A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr Pharm Biotechnol. 2020;21:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 67. | Hranilovic D, Bucan M, Wang Y. Emotional response in dopamine D2L receptor-deficient mice. Behav Brain Res. 2008;195:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 853] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 69. | Bagga D, Reichert JL, Koschutnig K, Aigner CS, Holzer P, Koskinen K, Moissl-Eichinger C, Schöpf V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 70. | Rode J, Edebol Carlman HMT, König J, Repsilber D, Hutchinson AN, Thunberg P, Andersson P, Persson J, Kiselev A, Lathrop Stern L, Salomon B, Mohammed AA, Labus JS, Brummer RJ. Probiotic Mixture Containing Lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum Affects Brain Responses Toward an Emotional Task in Healthy Subjects: A Randomized Clinical Trial. Front Nutr. 2022;9:827182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401, 1401.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 788] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 72. | Chong HX, Yusoff NAA, Hor YY, Lew LC, Jaafar MH, Choi SB, Yusoff MSB, Wahid N, Abdullah MFIL, Zakaria N, Ong KL, Park YH, Liong MT. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes. 2019;10:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 73. | Adıgüzel E, Çiçek B, Ünal G, Aydın MF, Barlak-Keti D. Probiotics and prebiotics alleviate behavioral deficits, inflammatory response, and gut dysbiosis in prenatal VPA-induced rodent model of autism. Physiol Behav. 2022;256:113961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 74. | Sumi H, Yatagai C, Wada H, Yoshida E, Maruyama M. [Effect of Bacillus natto-fermented product (BIOZYME) on blood alcohol, aldehyde concentrations after whisky drinking in human volunteers, and acute toxicity of acetaldehyde in mice]. Arukoru Kenkyuto Yakubutsu Ison. 1995;30:69-79. [PubMed] |
| 75. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 396] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 76. | Fuenzalida C, Dufeu MS, Poniachik J, Roblero JP, Valenzuela-Pérez L, Beltrán CJ. Probiotics-Based Treatment as an Integral Approach for Alcohol Use Disorder in Alcoholic Liver Disease. Front Pharmacol. 2021;12:729950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 77. | Lim TJ, Lim S, Yoon JH, Chung MJ. Effects of multi-species probiotic supplementation on alcohol metabolism in rats. J Microbiol. 2021;59:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Jung SJ, Hwang JH, Park EO, Lee SO, Chung YJ, Chung MJ, Lim S, Lim TJ, Ha Y, Park BH, Chae SW. Regulation of Alcohol and Acetaldehyde Metabolism by a Mixture of Lactobacillus and Bifidobacterium Species in Human. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Pizarro N, Kossatz E, González P, Gamero A, Veza E, Fernández C, Gabaldón T, de la Torre R, Robledo P. Sex-Specific Effects of Synbiotic Exposure in Mice on Addictive-Like Behavioral Alterations Induced by Chronic Alcohol Intake Are Associated With Changes in Specific Gut Bacterial Taxa and Brain Tryptophan Metabolism. Front Nutr. 2021;8:750333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Irwin C, Khalesi S, Cox AJ, Grant G, Davey AK, Bulmer AC, Desbrow B. Effect of 8-weeks prebiotics/probiotics supplementation on alcohol metabolism and blood biomarkers of healthy adults: a pilot study. Eur J Nutr. 2018;57:1523-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Ezquer F, Quintanilla ME, Morales P, Santapau D, Munita JM, Moya-Flores F, Ezquer M, Herrera-Marschitz M, Israel Y. A dual treatment blocks alcohol binge-drinking relapse: Microbiota as a new player. Drug Alcohol Depend. 2022;236:109466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Jiang X, Yan C, Zhang H, Chen L, Jiang R, Zheng K, Jin W, Ma H, Liu X, Dong M. Oral Probiotic Expressing Human Ethanol Dehydrogenase Attenuates Damage Caused by Acute Alcohol Consumption in Mice. Microbiol Spectr. 2023;11:21. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 83. | Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 439] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 84. | Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, Ciocan D, Rainteau D, Humbert L, Hugot C, Gaudin F, Noordine ML, Robert V, Berrebi D, Thomas M, Naveau S, Perlemuter G, Cassard AM. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 85. | Wolstenholme JT, Saunders JM, Smith M, Kang JD, Hylemon PB, González-Maeso J, Fagan A, Zhao D, Sikaroodi M, Herzog J, Shamsaddini A, Peña-Rodríguez M, Su L, Tai YL, Zheng J, Cheng PC, Sartor RB, Gillevet PM, Zhou H, Bajaj JS. Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat Commun. 2022;13:6198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu C, China; Stachowska E, Poland; Wang YD, China S-Editor: Qu XL L-Editor: A P-Editor: Zhao YQ