Published online Dec 20, 2023. doi: 10.5662/wjm.v13.i5.399
Peer-review started: September 22, 2023
First decision: September 29, 2023
Revised: October 11, 2023
Accepted: October 25, 2023
Article in press: October 25, 2023
Published online: December 20, 2023
Processing time: 88 Days and 18.2 Hours
Crohn’s Disease (CD) is an Inflammatory Bowel Disease and is characterized by an immune-mediated nature. Its etiology results from the interaction between genetic, enviromental and microbial factors. Regarding pathophysiology, it involves high levels of interleukin (IL)-12, IL-17, and Th1 profile, along with loss of tolerance mechanisms, an increase in pro-inflammatory interleukins, beyond the possibility to affect any part of the gastrointestinal tract. Its symptoms include abdominal pain, chronic diarrhea, weight loss, anorexia, and fatigue, as well as blood in the stool or rectum. Additionally, conditions comprising musculoskeletal, cutaneous, ocular, hepatic, and hematological alterations may be associated with this scenario and extra-intestinal presentation, such as erythema nodosum, anterior uveitis, osteoporosis, and arthritis can also occur. Today, clinical history, exams as fecal calprotectin, ileocolonocopy, and capsule endoscopy can be performed in the diagnosis investigation, along with treatments to induce and maintain remission. In this sense, anti-inflammatory drugs, such as corticosteroids, immunomodulators, and biological agents, as well as surgery and non-pharmacological interventions plays a role in its therapy. The aim of this review is to bring more current evidence to clinical management of CD, as well as to briefly discuss aspects of its pathophysiology, surveillance, and associated disorders.
Core Tip: Today, the clinical management of Crohn's disease (CD) involves both non-pharmacological and pharmacological therapies with the primary objective of inducing and maintaining remission. In this context, anti-inflammatory drugs, including corticosteroids, immunomodulators, and biological agents, can be employed either as monotherapy or in combination. Surgical treatment, while considered palliative, is not curative. Therefore, this review aims to provide an overview of current evidence regarding interventions for CD.
- Citation: da Silva Júnior RT, Apolonio JS, de Souza Nascimento JO, da Costa BT, Malheiro LH, Silva Luz M, de Carvalho LS, da Silva Santos C, Freire de Melo F. Crohn's disease and clinical management today: How it does? World J Methodol 2023; 13(5): 399-413
- URL: https://www.wjgnet.com/2222-0682/full/v13/i5/399.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i5.399
Inflammatory bowel disease (IBD) is a chronic condition characterized by inflammation in the gastrointestinal tract (GI)[1-3]. It encompasses a variety of diseases, with Crohn's disease (CD) and ulcerative colitis (UC) being the primary recognized types[2,3]. These diseases are persistent, debilitating, recurrent, and immune-mediated, affecting the digestive system[4,5]. The etiology of CD is multifactorial and results from the interaction between genetic and environmental factors and microbial exposure[6]. Several genes have been associated with CD, with the most evident link being related to the NOD2/CARD15 gene, which is associated with an earlier onset of the disease and a family history of CD[7]. Environmental risk factors include smoking, infections (especially Clostridium difficile, linked to disease relapses), use of medications (for example, antibiotics, mainly in the first year of life, aspirin, non-steroidal anti-inflammatory drugs, and oral contraceptives), a low-fiber diet, and stress[8]. Currently, the incidence of IBD is notably increasing, especially in developing countries and recently industrialized nations[9]. The disease, previously considered predominantly an affliction of young adults, is now diagnosed across all age groups, with about 25% of patients identified before the age of 20. The peak occurrence in childhood is during adolescence, but approximately 20% of children develop it before the age of 10, and about 5% before the age of 5[10]. Epidemiological studies in Japan also indicate an increase in IBD incidence, especially in males[11]. In Brazil, it is considered to have low rates of IBD. However, there are indications of an increase in its occurrence, even in the absence of detailed information on new cases[12]. Regarding age, research indicates a notable prevalence in individuals between 20 and 50 years old. Age groups between 20 and 60 years exhibited the highest rates of disease, with women registering a higher incidence in both CD and UC[13]. In the context of IBD, inflammation of the intestinal mucosa triggers symptoms such as abdominal pain, diarrhea, presence of blood in stools, weight loss, and the infiltration of immune cells like neutrophils and macrophages that release inflammatory substances, enzymes, and free radicals, contributing to lesions and ulcerations[2]. CD causes segmental inflammation that can affect any part of the digestive system, from the mouth to the anus. It often results in deeper ulcers that traverse all intestinal layers, potentially leading to the formation of fistulas and associated complications[5]. Although it most commonly affects the gut, this inflammatory condition can impact multiple organs[14,15]. The frequency of extra-intestinal manifestations ranges from 6% to 47%[16] and includes joint, mucocutaneous, hepatopancreatobiliary, and ocular manifestations[17,18]. The impact of IBD on patients' quality of life is significant, affecting physical and mental health as well as work performance. Moreover, it imposes a substantial burden on healthcare systems due to its chronic and recurrent nature. If inflammation is not properly controlled, serious complications can arise, such as abdominal abscesses, strictures, and intestinal obstructions, increasing the risk of developing tumors in the GI tract[5]. Therefore, this review aims to describe the current clinical management of CD to assist healthcare professionals in providing adequate care for these patients.
For this review, the authors surveyed relevant articles in the United States National Library of Medicine (PubMed). The descriptors used, along with Boolean descriptors AND/OR, were: CD; pathophysiology; immunology; diagnosis; clinical management; pharmacological; non-pharmacological; aminosalicylates; corticosteroids; immunomodulators; infliximab; adalimumab; vedolizumab; ustekinumab; surgery. The eligibility criteria for this review were based on the discussion of clinical management, covering topics from the diagnosis of CD to the exploration of new therapies. Articles published within the last 10 years and available in English, Portuguese, or Spanish were considered. A total of 24638 articles were initially identified in the database, of which 124 met the inclusion criteria. Articles that did not address the topics mentioned in the title/abstract or upon full-text examination were excluded. Additionally, a manual search of the references in the included articles was conducted, leading to the inclusion of 18 more articles. In total, 142 articles were included in this review. The summary of the articles selection process is shown in Figure 1.
Both immunological mechanisms of innate and adaptive immunity are interconnected on pathophysiology of CD. There is a dysregulation of the inflammatory and anti-inflammatory processes, as well as excessive release of cytokines[19] .
Histologically, the mucosa of the GI tract comprises three types of cells: Goblet cells, Paneth cells, and immune system cells[20]. These cells are crucial for maintaining the structural integrity and defense of the intestines and play an active role in homeostasis[21,22], causing any intestinal injury to potentially disrupt physiological functioning mechanisms, resulting in harmful responses to the body concerning food absorption and digestion[23]. Additionally, when there is tissue damage in the intestine, alterations in the microbiota can lead to mucosal inflammation, recruiting natural killer (NK) cells and monocytes to combat the injury or pathogen[23].
Studies in mice infected with microorganisms have demonstrated the crucial role of innate lymphoid cells (ILC3) in maintaining the integrity of intestinal mucosal tissues. The absence of these cells, along with IL-22, has been linked to the development of IBD[24]. In addition, Th2 profile response is observed in patients with UC while a Th1 profile response in patients with CD[24]. The high levels of interleukin (IL)-12 in the serum stimulate the maturation of immature T lymphocytes to Th1 profile[25]. Subsequently, cytokines released into the intestinal lumen contribute to the chemotaxis of Th1 cells to the mucosa of this organ. Lymphocytes synthesize chemokines like Interferon (IFN)-γ, IL-2, and tumor necrosis factor-alpha (TNF-α), which are related to the migration of neutrophils and macrophages to the site of inflammation, causing further damage to the mucosal epithelium[19,26].
Within this context, other cytokines such as IL-1, IL-6, IL-23, and transforming growth factor-beta (TGF-β) contribute to the differentiation of a Th17 profile during the antigen presentation process among a subset of immature lymphocytes[27]. These cells are responsible for synthesizing IL-17, which stimulates the migration and activity of neutrophils, thereby promoting a more inflammatory environment, along with T regulatory (T-reg) cells[27,28]. Furthermore, the high levels of IL-17 present in the tissues affected by CD can act on the intestinal epithelium, triggering the release of chemokines that further enhance the chemotaxis of inflammatory agents[28,29]. Macrophages and antigen-presenting cells (APC) also contribute to the synthesis of IL-12, IL-6, TGF-β, and IL-23. Therefore, the inflammatory process is capable of creating a feedback loop, continually stimulating the expression of substrates with Th1 and Th17 profiles[26].
Physiologically, the microbiota assist in the immune tolerance process, which occurs when the body's own antigens are captured by APCs, presented to naïve T lymphocytes, and, due to the heightened expression of immunomodulators such as IL-10 and retinoic acid, the lymphocytes are activated into T-reg cells. Studies have demonstrated that in individuals with CD, there is a loss of this tolerance mechanism and an increase in proinflammatory interleukins[30]. Supporting the theory of immune dysregulation between pro and anti-inflammatory processes, studies have shown that even in the presence of high levels of TGF-β and IL-10, which act as regulators of the immune response, local inflammation persists[27]. Furthermore, a diminished action of IL-10, responsible for regulating IL-23 levels, allows for the elevation of the latter, contributing to the stimulation of the Th17 profile[31].
The continuous and exaggerated inflammation of the intestinal mucosa, along with interactions between substrates and cytokines such as IL-13, IL-17, and TGF-β, can stimulate increased synthesis and deposition of the extracellular matrix, potentially leading to the strictures of the inflamed tissue. Furthermore, the inflammatory process could induce hyperplasia and hypertrophy of the intestinal muscle, contributing to a stenosis process in the affected region[32].
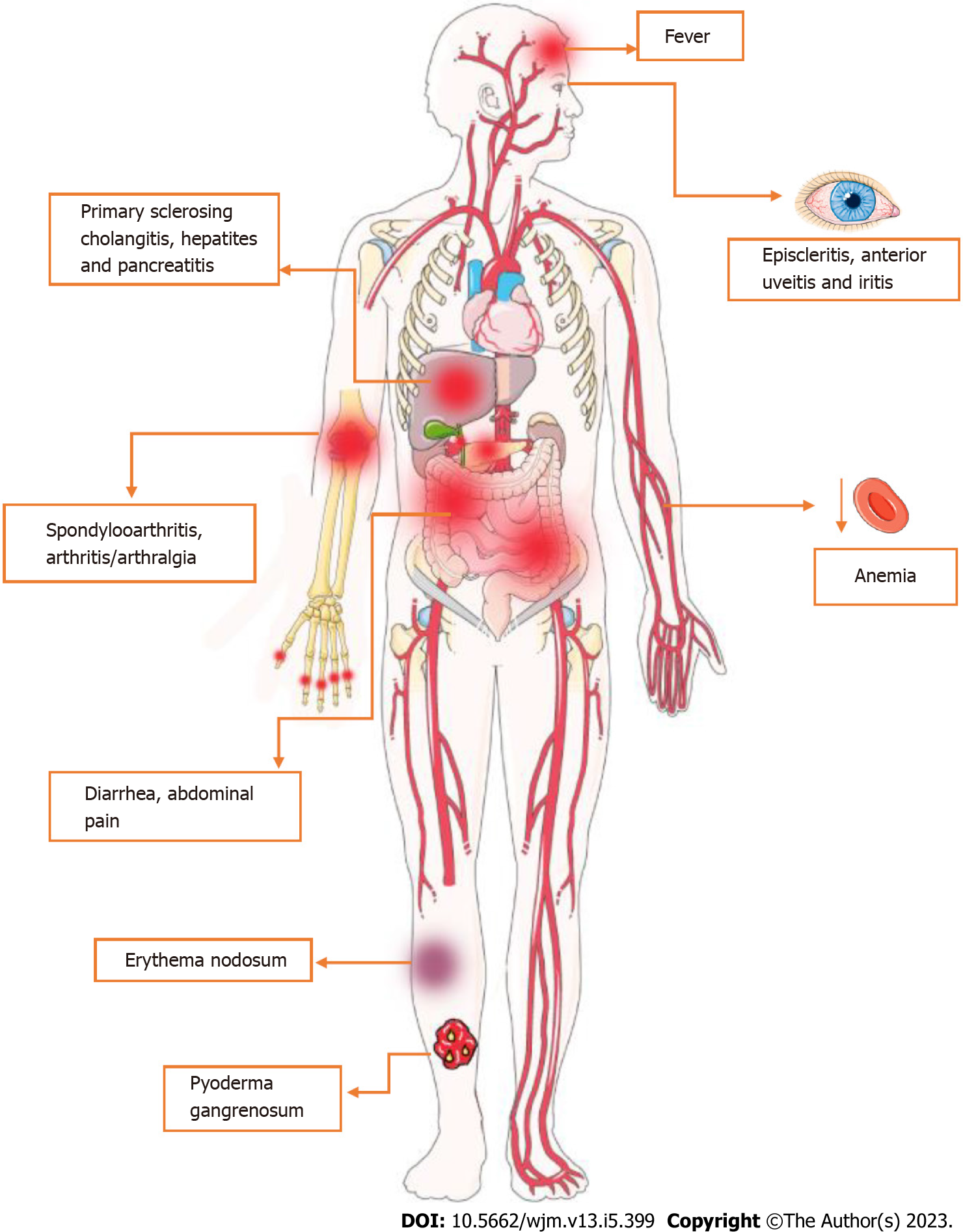
CD is regarded as a systemic illness due to its potential for complications and the presence of various extraintestinal symptoms[33]. Accordingly, the most widespread disorders associated with this condition comprise musculoskeletal, cutaneous, ocular, hepatic and hematological alterations[34]. Firstly, CD-related arthropathy occurs, apparently, due to genetic predisposition and immune system dysregulation[14]. In this sense, the human leukocyte antigen gene (HLA)-B27 is considered a major factor to the development of arthropathies, regarding genetic susceptibility[35-37]. On the other hand, increasing evidence suggests that it could also be linked to an immune-driven inflammatory reaction, especially related to IL-23, which supports IL-17 production and, consequently, neutrophil recruitment and maintenance of the inflammatory status[38,39].
Regarding cutaneous manifestations, erythema nodosum (ED) and pyoderma gangrenosum (PG) are the most prevalent disorders associated with CD[40]. Apparently, both ED and PG are more frequent in women and patients with other extraintestinal manifestations[41]. Also, a study suggests that the susceptibility to cutaneous manifestations is associated with the TRAF3IP2 gene, which plays a role in IL-17-mediated cellular immune responses[42]. The third most prevalent extraintestinal manifestation of CD involves the eye, e.g., episcleritis, scleritis, and uveitis, with approximately 3%-4% of CD patients affected[43-45]. Overall, the onset and maintenance of the inflammatory process in these structures, as mentioned above, seems to be also related to Th17 cells[46]. As for disease susceptibility, several studies suggests a relationship between HLA-DRB1*0103 and extraintestinal manifestations, including uveitis[47].
In terms of the hepatopancreatobiliary system, Primary Sclerosing Cholangitis (PSC) is considered the most common CD-related disorder[48]. While the pathogenesis of PSC is not yet fully understood, there is growing interest in the role of genetic factors, including certain HLA alleles, in its development. To date, HLA-B8, HLA-DRB1*0301, HLA-DRB3*0101 and HLA-DRB1*0401 are associated with PSC susceptibility[48-50].
The diagnosis of CD is complex and is made on the basis of symptomatic findings, physical examination, laboratory and imaging tests.
In terms of clinical aspects, CD presents diverse manifestations that are related to the intensity of transmural inflammation and its location, which, although it has a higher prevalence in the ileocolic segments, can affect any part of the gastrointestinal tract[51]. Symptoms are usually insidious, but can also develop acutely[52]. In most cases, they occur in young patients and include abdominal pain in the right iliac fossa, chronic diarrhea, weight loss, anorexia and fatigue. In cases that colonic inflammation is present, blood may appear in the stool or rectum[52,53]. In this sense, continuous blood loss and reduced absorption of iron, vitamin B12 and folic acid lead to anemia in 6.2%-73.7% of CD patients. Among these, iron deficiency anemia is the most prevalent[54,55]. It is also important to note the occurrence of extra-intestinal symptoms such as erythema nodosum, anterior uveitis, episcleritis, sclerosing cholangitis, osteoporosis, cholelithiasis, venous thromboembolism, nephrolithiasis, as well as arthritis of large joints or axial arthropathies[52-56]. These manifestations are shown in Figure 2.
When investigating the patient's clinical history, risk factors such as prior familiar cases of inflammatory bowel disease, diet low in fruit fiber, appendectomy, as well as a lifestyle with poor sleep quality, high stress and little physical activity should be taken into account[53,56]. Among the drugs with potential involvement are antibiotics, oral contraceptives and non-steroidal anti-inflammatory drugs[52,56]. Genetics also seems to be related to CD, among which it can be mentioned that the NOD2 gene in homozygosity increases the risk between 20 and 40 times[52]. In addition, one study suggests that exposure to cigarette smoke, together with chronic obstructive pulmonary disease, leads to systemic and intestinal ischemia, with epithelial dysfunction occurring in the latter and a greater risk of developing more severe forms of CD[57,58]. The physical examination should include an assessment of the hemodynamic state and look for signs of toxemia, malnutrition, dehydration and anemia. The abdomen may show distension or masses. When examining the pelvic and perianal region, lesions on skin or in the anal canal, as well as fistulas with or without abscesses should be investigated[53,57].
Laboratory tests should be guided by a search for anemia, thrombocytosis, folate and 25-hydroxyvitamin D deficiency and increased acute phase proteins. Fecal Calprotectin (FC), a neutrophil-derived factor for CD in adults with sensitivity 83%-100% and specificity 60%-100%, can be useful for assessing disease activity and monitoring after diagnosis[53,57]. Other potential biomarkers include serum IgA antibodies against Saccharomyces cerevisiae[53], antineutrophil cytoplasmic antibodies, antibodies directed against CBir1 and OmpC, elafin for predicting intestinal stenosis in CD[59], as well as microRNA expression screening for intestinal dysbiosis[60,61].
Endoscopic procedures are the first line of examination following an assessment of the clinical and laboratory aspects of patients with a non-toxic presentation[52]. Ileocolonoscopy is considered the preferred examination for assessing luminal disease[62], while esophagogastroduodenoscopy is the choice for cases suspected of upper gastrointestinal tract involvement[57,62]. Typical findings may include friability, erosion, segmental inflammation, or aphthoid, longitudinal and serpiginous ulceration. In more advanced cases, it is possible to find fistulas, stenosis, mucosal cobblestoning and wall stiffness[56,63]. Additionally, this technique allows for the collection of biopsy samples, characterized by epithelioid granulomas, preservation of globose cells, and transmural inflammatory infiltrate[63]. It is worth noting that endoscopy is also useful for determining the prognosis of CD, as well as screening for cancer associated with colitis and its characteristic lesions[64,65].
One of the limitations of traditional endoscopic methods is the difficulty of accessing the small bowel[66]. Thus, in cases of negative endoscopy, but significant symptomatology and suspicion of small bowel involvement, small bowel capsule endoscopy can be performed, which has a high negative predictive value[53]. The advantages of this method include no radiation exposure, no need for sedation and no pain. However, it is not possible to obtain a sample for biopsy or perform therapeutic interventions[57].
Radiographic techniques are considered for CD affecting the small intestine[53]. Even plain abdominal X-rays can be useful in visualizing dilation, obstruction, perforation or thickening of the intestinal wall. However, they are gradually being replaced by computed tomography (CT), especially CT-enterography, which, although it requires a high volume of intravenous contrast, has become the preferred exam for investigating the wall thickness and the relationships between the intestinal loops[52,56].
Although Magnetic Resonance Imaging (MRI) is a less available and more expensive exam, MR-enterography is an alternative to CT that allows the assessment of mesenteric vascularization and the presence of penetrating disease without the risk of exposure to ionizing radiation[62]. Another important point is that pelvic MRI is the preferred examination for investigating perianal fistulas or adjacent abscesses[53].
Another possibility is ultrasound (US), which, despite being operator-dependent, is highly available, well tolerated and has a sensitivity and specificity close to that of CT and MRI. Also, the accuracy and quality of US for visualizing the intestine can be increased with the use of oral or intravenous contrast[62]. However, it is of limited use in cases where gas is present, present less sensitive for colonic segments, and unable to assess the retroperitoneum and some areas of the GI tract[62,67].
Therefore, it is possible to classify the CD phenotype according to Montreal classification, based on age to diagnosis, localization of inflammatory lesions, and clinical behavior of the disease[65].This classification is shown in Figure 3.
That said, and despite all the complexity of diagnosing CD, professionals should also be aware of differential diagnoses that include ulcerative colitis, intestinal tuberculosis, eosinophilic gastroenteritis, diverticulitis, inflammation of Meckel's diverticulum, intestinal ischemia, chemotherapy-induced enteritis, the presence of a foreign body, neoplasms and others[66,67].
Although IBD has no known cure to date, early medical intervention in the diagnosis of CD can improve clinical response to treatment, reduce inflammatory biomarkers and increase endoscopic remission rates[68]. In addition, patients treated in the early stages of the disease have fewer complications and need for hospitalizations[69]. The goal of conservative clinical management is to induce and maintain remission in patients with active disease, undergoing non-pharmacological interventions and medications, such as aminosalicylates, corticosteroids, immunomodulators, and immunobiologicals[70,71]. Surgical treatment depends on the presentation of the disease[5].
The management of psychological comorbidities, such as anxiety and depression, can improve the disease status, treatment adherence and the need for high-cost care. Cognitive Behavioral Therapy can reduce rates of these comorbidities and improve the quality of life, both individually and in groups, in adults and adolescent patients[72]. A study showed reduction from 35.7% and 25.0% at baseline to 10.4% and 4.2% after therapy in rates of anxiety and depression, respectively[73]. Meditation and relaxation techniques can improve quality of life and possibly decrease inflammatory activity in IBD[74].
Dietetic interventions can be a viable option due to their low cost, availability, and few adverse effects. Diet can influence the immunological system and inflammatory response, although its interaction with intestinal mucosal defense and inflammatory cells is complex[75]. This approach is particularly considered for patients to avoid the use of steroid-based medications, especially children, in whom steroids may impact growth trajectory[76]. Data has indicated an elevated risk for IBD development in individuals with diets high in fat and meat, in contrast to a lower risk associated with high-fiber foods, fruits, and vegetables. Among the dietary options, Exclusive Enteral Nutrition has demonstrated efficacy in inducing CD remission and is considered the first-line therapy, especially in the pediatric population. However, adherence to this therapy can be a challenge in adults[23,77]. Additionally, the use of probiotics showed no significant effect on the induction or maintenance of CD remission[74].
Exercise has a positive impact on various clinical aspects of IBD, including disease activity, the immune system, quality of life, fatigue, and psychological factors[78]. The anti-inflammatory effects of physical exercise are based on myokines, exercise-specific cytokines that are released by myocytes during muscle contractions[79]. Resistance training is a viable and safe option; however, special attention should be given to patients with active disease, as exercise capacity may be limited during this period[78]. Smoking cessation should be strongly considered for smokers with CD, as it assists in modifying the course of the disease, reducing exacerbations, the need for surgical procedures, and improving the response to immunomodulatory therapy[80,81].
Anti-inflammatory medications are often the first choice in the treatment of IBD, such as corticosteroids and aminosalicylates[82]. Mesalazine, Balsalazide, and Olsalazine are medications derived from 5-aminosalicylic acid (5-ASA). Most guidelines do not recommend 5-ASA for the induction or maintenance period in CD[83,84]. However, 5-ASA is still used by many patients, possibly due to its safety profile, especially in the elderly[85].
Corticosteroids are the first-line treatment at the time of diagnosis and may be indicated regardless of the localization of the inflammatory lesion in moderate or severe disease. Systemic drugs such as Prednisone, Methylprednisolone, Hydrocortisone, and Budesonide can be used, with Budesonide being limited to mild or moderate disease in the ileocecal region[65]. Corticosteroid treatment should be administered for up to 4 wk, followed by a gradual dose reduction until complete cessation of use, typically concluding within 12 wk[86]. According to the British Society of Gastroenterology consensus guidelines, systemic steroids are effective for remission induction but are not suitable for the maintenance phase due to their toxicity and lack of efficacy[87]. For cases resistant to these drugs, immunomodulators or biological therapy may be considered[65].
CD relapse is common after discontinuation of corticosteroid therapy, and immunomodulators such as Azathioprine, Mercaptopurine, or Methotrexate are effective in maintaining remission. Early initiation of these medications is recommended in several cases[87,88]. Methotrexate is not recommended as monotherapy for induction, but it can be used for patients refractory to corticosteroids, with a preference for subcutaneous administration[89].
Immunobiologicals have revolutionized the management of IBD by targeting the inflammatory processes associated with the disease, either by reducing pro-inflammatory cytokines or increasing regulatory cytokines[90,91]. British Society of Gastroenterology consensus guidelines recommend choosing a biological agent based on clinical factors, cost, safety, availability for use, as well as patient adherence and preference[92]. These drugs include monoclonal antibodies (mAbs) targeting TNFα, such as Infliximab (IFX), Adalimumab (ADA), and Golimumab; mAbs targeting integrins α4β7, such as Vedolizumab; and mAbs targeting the p40 subunit of IL-12 and IL-23, such as Ustekinumab (UST)[82,91].
TNF-α is a critical pro-inflammatory cytokine in intestinal inflammation[92]. Clinical evidence has shown that anti-TNF therapy, such as IFX, leads to better clinical success, early remission, higher rates of mucosal healing, and improved quality of life in approximately 60% of IBD patients[93]. Adalimumab, administered subcutaneously, has also demonstrated efficacy in treating active CD and in patients with a loss of response to or intolerance of IFX[94,95]. However, approximately 40% of patients do not respond to anti-TNF treatment[93].The concomitant use of immunomodulators like Azathioprine or Methotrexate may prevent immunogenicity, reducing the development of anti-drug antibodies and systemic inflammatory status[96].
Various methods are employed to evaluate the response and therapeutic efficacy of these therapies, including biomarkers like FC, C-reactive protein (CRP), serum levels of the anti-TNF agent, and anti-drug antibodies[93]. Reduced levels of FC may precede disease remission and mucosal healing and are correlated with endoscopic scores[97,98]. Also, elevated levels of FC at the onset of treatment may indicate a probable non-response to anti-TNF therapy and lower rates of clinical remission[99,100], and elevated CRP rates are associated with low response in severe CD[101]. Regarding serum levels of mAbs anti-TNF, higher chances of remission and mucosal healing are associated with levels above >2 µg/mL, a minimal concentration for IFX, for example, as well as for ADA[102,103]. Anti-drug antibodies are associated with remission when their levels are below 3.15 U/mL in IFX treatment, while elevated levels may neutralize clinical efficacy, lead to a loss of response, and negatively impact quality of life and disease complications[104,105]. Novel biomarkers, such as proteomic profiles and microRNAs (miRNAs), are still under investigation for clinical practice[93]. Thus, despite the wide availability of biomarkers, most of them are not specific to CD and reflect the body's inflammatory status, necessitating further clinical studies for IBD specifically.
Anti-integrin agents modulate inflammation by targeting integrins, preventing the migration of lymphocytes to the gastrointestinal mucosa. This serves as an alternative to anti-TNF therapy and may be used in patients with a loss of response, inadequate response, and/or anti-TNF intolerance[106]. Vedolizumab, an IgG1 humanized mAbs targeting integrin α4β7 without affecting α4β1, has demonstrated efficacy in inducing remission in CD, with a better safety profile than Natalizumab, another mAbs targeting integrin α4, which is associated with the development of Progressive Multifocal Leukoencephalopathy[106,107].
Ustekinumab targets the p40 subunit of IL-12 and IL-23. Studies have shown its efficacy and safety in the treatment of CD, with approximately half of the patients achieving long-term maintenance of response without loss of response, surgery, or intolerance[108,109]. However, some individuals may experience a loss of response to UST and shorter maintenance times due to factors such as infection, elevated inflammation levels, and inadequate medication concentrations[110,111]. In such cases, optimization strategies can be employed, such as shortening the treatment interval and intravenous reinduction. UNITI studies concluded that the treatment for 12 and 8 wk safely maintained clinical response and remission in patients with CD[112,113].
Approximately 15% to 47% of patients undergoing treatment may require surgical intervention[114]. While most patients initially present with the inflammatory phenotype, about 10% exhibit the stenosing phenotype at the time of diagnosis. According to the Montreal classification, within 10 years, the disease progresses to stenosing CD in approximately 15% of cases. An anti-TNF strategy may be considered in cases of strictures without complications, but surgical therapy is indicated for refractory disease[115,116]. In cases of acute small intestinal obstruction, hospitalization and immediate evaluation are necessary to rule out complications such as perforation, abscess, fistulizing disease, and signs of underlying malignancy[115]. Surgical options include segmental resection and stenoplasty[117].
Surgery can provide palliative relief but is not curative. Many patients may experience postoperative recurrence, with risk factors such as active smoking, age younger than 30 years, and previous surgeries for penetrating disease being associated with recurrence[118]. High-risk patients may receive anti-TNF or thiopurine prophylaxis for 8 wk post-surgery, with routine endoscopy, while low-risk patients may undergo endoscopic surveillance 6-12 mo after surgery[119,120]. On the other hand, early resection in CD with ileocecal stricture is associated with prolonged clinical remission and reduced exposure to corticosteroids and biological therapies[121]. Therefore, the decision to undergo surgery should be made on a case-by-case basis, considering disease phenotype and available treatment options.
Toll-like receptor 4 (TLR4) is overexpressed in both CD and UC, and the binding between the receptor-ligand pattern recognition of DAMPs or PAMPs triggers a cascade of signaling and recruitment of inflammatory cells and cytokines, such as IL-6 and TNFα[122]. Evidence suggests a potential role for TLR4 antagonists in inflammation treatment, making it a promising alternative for future innovative treatments for IBD[123]. Therefore, more studies are necessary. Ad
The vaccination of individuals with IBD varies according to the recommendations of each country, as well as by the clinical judgment of the patient's healthcare provider[124]. It is recommended that these patients be vaccinated before starting immunosuppressive therapy, and if they have already started, vaccines with live viruses should be avoided[125,126]. Some authors recommend mandatory immunizations for these patients, including vaccines against influenza, chickenpox, hepatitis B, and triple viral. Other authors add to this list the vaccines for hepatitis A, tetanus, diphtheria, whooping cough, herpes zoster, and human papillomavirus (HPV)[127,128]. For hepatitis A and B vaccinations, it is recommended to perform serological tests to evaluate the presence of antibodies and immunity. If the test results are negative or show very low antibody levels, these patients should restart the vaccination schedule with three doses. Additionally, individuals over 50 years old should be immunized against the herpes zoster virus, and persons aged 9 to 45 years should be vaccinated for HPV, preferably covering the four main strains (6, 11, 16, and 18)[126-128]. The pneumococcal vaccine should be administered to all patients, following the recommendations of national immunization programs for chronic diseases[125]. Influenza immunization should be done annually[126]. Furthermore, in the current context, coronavirus disease 2019 (COVID-19) vaccination is essential. Although some studies have demonstrated that seroconversion rates in individuals with CD are similar to those without the disease, others have shown that the level of these antibodies and the duration of this immunity are shorter[129,130]. Therefore, some authors recommend administering one additional dose of the vaccine as a booster approximately 6 mo after completing the vaccination schedule[131].
In general, CD can predispose individuals to various intestinal neoplasms, including colorectal, small intestinal, and anal cancers. Colorectal cancer is the most strongly associated with CD, affecting mainly individuals between 40 and 50 years old who have a history of early onset symptoms[132]. The chronic inflammatory process in the rectal and colonic mucosa, excessive production of free radicals contributing to deoxyribonucleic acid damage, inactivation of tumor suppressor genes, and individual factors are all related to dysplastic mutations and the development of colorectal carcinoma[133]. Additionally, the ongoing inflammation can create an environment favorable to colonization by pathogens like Enterotoxigenic Bacteroides fragilis, which has been linked to the development of colorectal neoplasms[134]. Even the use of some immunosuppressive medications, such as TNF inhibitors, has been associated with an increased risk of cancer[135]. Similarly, the inflammatory process in the small intestine, coupled with mutations in genes like TP53 (a tumor suppressor) and IDH1 (which assists in the control of oxidative processes), has also been linked to a higher risk of small bowel adenocarcinoma[136]. Although rare, the inflammatory process of an anal fistula, a complication of CD, can increase the risk of anal cancer[137].
Recommendations for screening for colorectal cancer vary depending on the guidelines of different scientific organizations and local guidelines. Generally, it is recommended to undergo at least one colonoscopy within 8 years of the onset of symptoms to evaluate the extent and degree of mucosal damage[52,138]. TThe American Gastroenterological Association recommends annual screening for high-risk patients, evaluation every 1 to 2 years for individuals with extensive colitis or inflammation located on the left side of the colon, or every 1 to 3 years if two consecutive exams are negative. The management can be individualized based on medical criteria and the extent of mucosal damage[138]. On the other hand, the European Crohn’s disease and Colitis Organization recommends annual screening for high-risk patients, evaluation every 2 to 3 years for individuals with moderate-risk factors, and, for low-risk patients, colonoscopy every 5 years[52].
Beyond the considerable physical impact of CD, the disease process can affect various aspects of mental well-being and interpersonal relationships[139]. This disease directly interferes with family planning for couples because, while the pathogenesis of CD is not related to infertility, studies have shown that women with this disease often choose not to become mothers due to conditions such as excessive pain, dyspareunia, anemia, depression, low libido, and malnutrition[140]. Additionally, concerns about the use of medications during pregnancy and their potential side effects, as well as the possibility of active disease during labor, which increases the risk of complications, contribute to reducing discussions about family planning[141]. Other complications such as miscarriages, placental abruption, eclampsia, and pre-eclampsia can be associated with pregnancy and CD[142]. Finally, men using medications or experiencing clinical conditions associated with pain have also been related to voluntary avoidance of having children[140,141].
This review provides an overview of the immunologic aspects of CD and discusses the clinical management of these patients. Recognizing gastrointestinal symptoms such as diarrhea, abdominal pain, anorexia, and fatigue can assisti in diagnosis. These symptoms may also be accompanied by extraintestinal manifestations and associated disorders, such as arthropathy, erythema nodosum, episcleritis, and anterior uveitis. Additionally, laboratory tests, such as Fecal Calprotectin, and imaging exams play a crucial role in the evaluation process, with endoscopic procedures being the first-line approach. Both ileocolonoscopy and capsule endoscopy are important tools in this diagnostic scenario. Non-pharmacological and pharmacological treatments form the cornerstone of disease management, with surgical therapy being considered in some cases. This includes the use of anti-inflammatory medications, such as corticosteroids and immunomodulators, as well as biological agents. Therefore, psychological interventions should be more widely prescribed, as they have the potential to improve treatment responses and, consequently, disease outcomes. Finally, therapies targeting the immune system are increasingly being studied, and more research is needed to elucidate the best approach to patients with CD, taking into account their specific phenotypes. Our work summarizes the current evidence available to date.
| 1. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 709] [Article Influence: 101.3] [Reference Citation Analysis (1)] |
| 2. | Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (113)] |
| 3. | Singh N, Bernstein CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Cohen NA, Rubin DT. New targets in inflammatory bowel disease therapy: 2021. Curr Opin Gastroenterol. 2021;37:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | M'Koma AE. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Akbulut S. An assessment of serum vitamin B12 and folate in patients with Crohn's disease. Medicine (Baltimore). 2022;101:e31892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 7. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 8. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1304] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 9. | Agrawal M, Jess T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United European Gastroenterol J. 2022;10:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 10. | Borowitz SM. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front Pediatr. 2022;10:1103713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 11. | Aniwan S, Santiago P, Loftus EV Jr, Park SH. The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United European Gastroenterol J. 2022;10:1063-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Gasparini RG, Sassaki LY, Saad-Hossne R. Inflammatory bowel disease epidemiology in São Paulo State, Brazil. Clin Exp Gastroenterol. 2018;11:423-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Cassol OS, Zabot GP, Saad-Hossne R, Padoin A. Epidemiology of inflammatory bowel diseases in the state of Rio Grande do Sul, Brazil. World J Gastroenterol. 2022;28:4174-4181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 14. | Greuter T, Vavricka SR. Extraintestinal manifestations in inflammatory bowel disease - epidemiology, genetics, and pathogenesis. Expert Rev Gastroenterol Hepatol. 2019;13:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Yu YR, Rodriguez JR. Clinical presentation of Crohn's, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 16. | Vavricka SR, Rogler G, Gantenbein C, Spoerri M, PrinzVavricka M, Navarini AA, French LE, Safroneeva E, Fournier N, Straumann A, Froehlich F, Fried M, Michetti P, Seibold F, Lakatos PL, Peyrin-Biroulet L, Schoepfer AM. Chronological Order of Appearance of Extraintestinal Manifestations Relative to the Time of IBD Diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Bowel Dis. 2015;21:1794-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (1)] |
| 18. | Guillo L, D'Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J Crohns Colitis. 2021;15:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Petagna L, Antonelli A, Ganini C, Bellato V, Campanelli M, Divizia A, Efrati C, Franceschilli M, Guida AM, Ingallinella S, Montagnese F, Sensi B, Siragusa L, Sica GS. Pathophysiology of Crohn's disease inflammation and recurrence. Biol Direct. 2020;15:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 326] [Reference Citation Analysis (0)] |
| 21. | Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 625] [Article Influence: 104.2] [Reference Citation Analysis (1)] |
| 22. | Alfredsson J, Wick MJ. Mechanism of fibrosis and stricture formation in Crohn's disease. Scand J Immunol. 2020;92:e12990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Wark G, Samocha-Bonet D, Ghaly S, Danta M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Li N, Shi RH. Updated review on immune factors in pathogenesis of Crohn's disease. World J Gastroenterol. 2018;24:15-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (7)] |
| 25. | Gomez-Bris R, Saez A, Herrero-Fernandez B, Rius C, Sanchez-Martinez H, Gonzalez-Granado JM. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 26. | Iliopoulou L, Kollias G. Harnessing murine models of Crohn's disease ileitis to advance concepts of pathophysiology and treatment. Mucosal Immunol. 2022;15:10-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, Grimm MC. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021;42:1603990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 28. | Khouri A, Moreno C, Niland B. New-Onset Crohn's Disease following Initiation of Secukinumab: A Case Report and Review of the Role of IL-17 in the Pathogenesis of Crohn's Disease. Case Rep Gastrointest Med. 2023;2023:1769290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Berg DR, Colombel JF, Ungaro R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Burge K, Gunasekaran A, Eckert J, Chaaban H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 31. | Korta A, Kula J, Gomułka K. The Role of IL-23 in the Pathogenesis and Therapy of Inflammatory Bowel Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 32. | Lee B, Dane B, Katz S. Current and Emerging Approaches to the Diagnosis and Treatment of Crohn's Disease Strictures. Gastroenterol Hepatol (N Y). 2022;18:186-195. [PubMed] |
| 33. | Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 34. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 501] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 35. | Brewerton DA, Caffrey M, Nicholls A, Walters D, James DC. HL-A 27 and arthropathies associated with ulcerative colitis and psoriasis. Lancet. 1974;1:956-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Steer S, Jones H, Hibbert J, Kondeatis E, Vaughan R, Sanderson J, Gibson T. Low back pain, sacroiliitis, and the relationship with HLA-B27 in Crohn's disease. J Rheumatol. 2003;30:518-522. [PubMed] |
| 37. | Mallas EG, Mackintosh P, Asquith P, Cooke WT. Histocompatibility antigens in inflammatory bowel disease. Their clinical significance and their association with arthropathy with special reference to HLA-B27 (W27). Gut. 1976;17:906-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Kumar A, Lukin D, Battat R, Schwartzman M, Mandl LA, Scherl E, Longman RS. Defining the phenotype, pathogenesis and treatment of Crohn's disease associated spondyloarthritis. J Gastroenterol. 2020;55:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1241] [Cited by in RCA: 1298] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 40. | TavarelaVeloso F. Review article: skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (3)] |
| 41. | Ampuero J, Rojas-Feria M, Castro-Fernández M, Cano C, Romero-Gómez M. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Ciccacci C, Biancone L, Di Fusco D, Ranieri M, Condino G, Giardina E, Onali S, Lepre T, Pallone F, Novelli G, Borgiani P. TRAF3IP2 gene is associated with cutaneous extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2013;7:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Licona Vera E, Betancur Vasquez C, Peinado Acevedo JS, Rivera Bustamante T, Martinez Redondo JM. Ocular Manifestations of Inflammatory Bowel Disease. Cureus. 2023;15:e40299. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 212] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Repiso A, Alcántara M, Muñoz-Rosas C, Rodríguez-Merlo R, Pérez-Grueso MJ, Carrobles JM, Martínez-Potenciano JL. Extraintestinal manifestations of Crohn's disease: prevalence and related factors. Rev Esp Enferm Dig. 2006;98:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 686] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 47. | Roussomoustakaki M, Satsangi J, Welsh K, Louis E, Fanning G, Targan S, Landers C, Jewell DP. Genetic markers may predict disease behavior in patients with ulcerative colitis. Gastroenterology. 1997;112:1845-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819-4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 49. | Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1598-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Farrant JM, Doherty DG, Donaldson PT, Vaughan RW, Hayllar KM, Welsh KI, Eddleston AL, Williams R. Amino acid substitutions at position 38 of the DR beta polypeptide confer susceptibility to and protection from primary sclerosing cholangitis. Hepatology. 1992;16:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Cicero G, Mazziotti S. Crohn's disease at radiological imaging: focus on techniques and intestinal tract. Intest Res. 2021;19:365-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Veauthier B, Hornecker JR. Crohn's Disease: Diagnosis and Management. Am Fam Physician. 2018;98:661-669. [PubMed] |
| 53. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1973] [Article Influence: 219.2] [Reference Citation Analysis (113)] |
| 54. | Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1507-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Ott C, Liebold A, Takses A, Strauch UG, Obermeier F. High prevalence but insufficient treatment of iron-deficiency anemia in patients with inflammatory bowel disease: results of a population-based cohort. Gastroenterol Res Pract. 2012;2012:595970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1508] [Article Influence: 167.6] [Reference Citation Analysis (0)] |
| 57. | Cheifetz AS. Management of active Crohn disease. JAMA. 2013;309:2150-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 58. | Fricker M, Goggins BJ, Mateer S, Jones B, Kim RY, Gellatly SL, Jarnicki AG, Powell N, Oliver BG, Radford-Smith G, Talley NJ, Walker MM, Keely S, Hansbro PM. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Wang J, Ortiz C, Fontenot L, Xie Y, Ho W, Mattai SA, Shih DQ, Koon HW. High circulating elafin levels are associated with Crohn's disease-associated intestinal strictures. PLoS One. 2020;15:e0231796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Oliveira ECS, Quaglio AEV, Magro DO, Di Stasi LC, Sassaki LY. Intestinal Microbiota and miRNA in IBD: A Narrative Review about Discoveries and Perspectives for the Future. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 61. | Yarani R, Shojaeian A, Palasca O, Doncheva NT, Jensen LJ, Gorodkin J, Pociot F. Differentially Expressed miRNAs in Ulcerative Colitis and Crohn's Disease. Front Immunol. 2022;13:865777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 62. | Manetta R, Capretti I, Belleggia N, Marsecano C, Viscido A, Bruno F, Arrigoni F, Ma L, Guglielmi G, Splendiani A, Di Cesare E, Masciocchi C, Barile A. Magnetic resonance enterography (MRE) and ultrasonography (US) in the study of the small bowel in Crohn's disease: state of the art and review of the literature. Acta Biomed. 2019;90:38-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 63. | Kővári B, Pai RK. Upper Gastrointestinal Tract Involvement in Inflammatory Bowel Diseases: Histologic Clues and Pitfalls. Adv Anat Pathol. 2022;29:2-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Sivanathan V, Tontini GE, Möhler M, Galle PR, Neumann H. Advanced endoscopic imaging for diagnosis of inflammatory bowel diseases: Present and future perspectives. Dig Endosc. 2018;30:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Łodyga M, Eder P, Gawron-Kiszka M, Dobrowolska A, Gonciarz M, Hartleb M, Kłopocka M, Małecka-Wojciesko E, Radwan P, Reguła J, Zagórowicz E, Rydzewska G. Guidelines for the management of patients with Crohn's disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Prz Gastroenterol. 2021;16:257-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Marín-Díez E, Crespo Del Pozo J. Diagnostic approach to small-bowel wall thickening: Beyond Crohn's disease and cancer. Radiologia (Engl Ed). 2021;63:519-530. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Kedia S, Das P, Madhusudhan KS, Dattagupta S, Sharma R, Sahni P, Makharia G, Ahuja V. Differentiating Crohn's disease from intestinal tuberculosis. World J Gastroenterol. 2019;25:418-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (6)] |
| 68. | Estevinho MM, Leão Moreira P, Silva I, LaranjeiraCorreia J, Santiago M, Magro F. A scoping review on early inflammatory bowel disease: definitions, pathogenesis, and impact on clinical outcomes. Therap Adv Gastroenterol. 2022;15:17562848221142673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Oh EH, Oh K, Han M, Seo H, Chang K, Lee SH, Kim GU, Song EM, Seo M, Lee HS, Hwang SW, Park SH, Yang DH, Kim KJ, Byeon JS, Myung SJ, Yang SK, Ye BD. Early anti-TNF/immunomodulator therapy is associated with better long-term clinical outcomes in Asian patients with Crohn's disease with poor prognostic factors. PLoS One. 2017;12:e0177479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Ben Ghezala I, Charkaoui M, Michiels C, Bardou M, Luu M. Small Molecule Drugs in Inflammatory Bowel Diseases. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | El Menyiy N, El Allam A, Aboulaghras S, Jaouadi I, Bakrim S, El Omari N, Shariati MA, Miftakhutdinov A, Wilairatana P, Mubarak MS, Bouyahya A. Inflammatory auto-immune diseases of the intestine and their management by natural bioactive compounds. Biomed Pharmacother. 2022;151:113158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Dubinsky MC, Dotan I, Rubin DT, Bernauer M, Patel D, Cheung R, Modesto I, Latymer M, Keefer L. Burden of comorbid anxiety and depression in patients with inflammatory bowel disease: a systematic literature review. Expert Rev Gastroenterol Hepatol. 2021;15:985-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | BennebroekEvertsz' F, Sprangers MAG, Sitnikova K, Stokkers PCF, Ponsioen CY, Bartelsman JFWM, van Bodegraven AA, Fischer S, Depla ACTM, Mallant RC, Sanderman R, Burger H, Bockting CLH. Effectiveness of cognitive-behavioral therapy on quality of life, anxiety, and depressive symptoms among patients with inflammatory bowel disease: A multicenter randomized controlled trial. J Consult Clin Psychol. 2017;85:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Torres J, Ellul P, Langhorst J, Mikocka-Walus A, Barreiro-de Acosta M, Basnayake C, Ding NJS, Gilardi D, Katsanos K, Moser G, Opheim R, Palmela C, Pellino G, Van der Marel S, Vavricka SR. European Crohn's and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:673-685e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 75. | Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017;152:398-414.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 76. | Aljebab F, Choonara I, Conroy S. Systematic Review of the Toxicity of Long-Course Oral Corticosteroids in Children. PLoS One. 2017;12:e0170259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 77. | Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, Wu GD. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015;148:1087-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 292] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 78. | Ordille AJ, Phadtare S. Intensity-specific considerations for exercise for patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2023;11:goad004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 79. | Lee JH, Jun HS. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front Physiol. 2019;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 80. | Santus P, Radovanovic D, Raiteri D, Pini S, Spagnolo G, Maconi G, Rizzi M. The effect of a multidisciplinary approach for smoking cessation in patients with Crohn's disease: Results from an observational cohort study. Tob Induc Dis. 2020;18:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn's disease. Aliment Pharmacol Ther. 2016;43:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 82. | M'Koma AE. Inflammatory Bowel Disease: Clinical Diagnosis and Pharmaceutical Management. Med Res Arch. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 979] [Article Influence: 163.2] [Reference Citation Analysis (2)] |
| 84. | Akobeng AK, Zhang D, Gordon M, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn's disease. Cochrane Database Syst Rev. 2016;9:CD003715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 85. | Burisch J, Bergemalm D, Halfvarson J, Domislovic V, Krznaric Z, Goldis A, Dahlerup JF, Oksanen P, Collin P, de Castro L, Hernandez V, Turcan S, Belousova E, D'Incà R, Sartini A, Valpiani D, Giannotta M, Misra R, Arebi N, Duricova D, Bortlik M, Gatt K, Ellul P, Pedersen N, Kjeldsen J, Andersen KW, Andersen V, Katsanos KH, Christodoulou DK, Sebastian S, Barros L, Magro F, Midjord JM, Nielsen KR, Salupere R, Kievit HA, Kiudelis G, Kupčinskas J, Fumery M, Gower-Rousseau C, Kaimakliotis IP, Schwartz D, Odes S, Lakatos L, Lakatos PL, Langholz E, Munkholm P; Epi-IBD group. The use of 5-aminosalicylate for patients with Crohn's disease in a prospective European inception cohort with 5 years follow-up - an Epi-IBD study. United European Gastroenterol J. 2020;8:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;2008:CD006792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1718] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 88. | Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;2015:CD000067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 89. | Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2014;2014:CD006884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Padoan A, Musso G, Contran N, Basso D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr Issues Mol Biol. 2023;45:5534-5557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 91. | Tallarico M, Palleria C, Ruffolo L, Spagnuolo R, Naturale MD, De Francesco AE, De Sarro C, Romeo R, Citraro R, Doldo P, Abenavoli L, Gallelli L, Luzza F, Leo A, De Sarro G. Biologics for Inflammatory Bowel Disease in Clinical Practice: A Calabria (Southern Italy) Prospective Pharmacovigilance Study. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 92. | Aardoom MA, Veereman G, de Ridder L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 93. | Cui G, Fan Q, Li Z, Goll R, Florholmen J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBio Medicine. 2021;66:103329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 94. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-33; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1212] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 95. | Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, D'Haens G, Li J, Rosenfeld MR, Kent JD, Pollack PF. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 741] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 96. | Yarur AJ, Abreu MT, Deshpande AR, Kerman DH, Sussman DA. Therapeutic drug monitoring in patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3475-3484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Liu F, Lee SA, Riordan SM, Zhang L, Zhu L. Global Studies of Using Fecal Biomarkers in Predicting Relapse in Inflammatory Bowel Disease. Front Med (Lausanne). 2020;7:580803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 98. | Kristensen V, Røseth A, Ahmad T, Skar V, Moum B. Fecal Calprotectin: A Reliable Predictor of Mucosal Healing after Treatment for Active Ulcerative Colitis. Gastroenterol Res Pract. 2017;2017:2098293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Bertani L, Blandizzi C, Mumolo MG, Ceccarelli L, Albano E, Tapete G, BaianoSvizzero G, Zanzi F, Coppini F, de Bortoli N, Bellini M, Morganti R, Marchi S, Costa F. Fecal Calprotectin Predicts Mucosal Healing in Patients With Ulcerative Colitis Treated With Biological Therapies: A Prospective Study. Clin Transl Gastroenterol. 2020;11:e00174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Mumolo MG, Bertani L, Ceccarelli L, Laino G, Di Fluri G, Albano E, Tapete G, Costa F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018;24:3681-3694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (2)] |
| 101. | Detrez I, Dreesen E, Van Stappen T, de Vries A, Brouwers E, Van Assche G, Vermeire S, Ferrante M, Gils A. Variability in Golimumab Exposure: A 'Real-Life' Observational Study in Active Ulcerative Colitis. J Crohns Colitis. 2016;10:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 102. | Moore C, Corbett G, Moss AC. Systematic Review and Meta-Analysis: Serum Infliximab Levels During Maintenance Therapy and Outcomes in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 103. | Verstockt B, Moors G, Bian S, Van Stappen T, Van AsscheG, Vermeire S, Gils A, Ferrante M. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn's disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | VandeCasteele N, Khanna R, Levesque BG, Stitt L, Zou GY, Singh S, Lockton S, Hauenstein S, Ohrmund L, Greenberg GR, Rutgeerts PJ, Gils A, Sandborn WJ, Vermeire S, Feagan BG. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut. 2015;64:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 105. | Bendtzen K. Immunogenicity of Anti-TNF-α Biotherapies: I. Individualized Medicine Based on Immunopharmacological Evidence. Front Immunol. 2015;6:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | McLean LP, Cross RK. Integrin antagonists as potential therapeutic options for the treatment of Crohn's disease. Expert Opin Investig Drugs. 2016;25:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1635] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 108. | Ren H, Kang J, Wang J, Su J, Zou L, Yin A, Li J, Zhou Q, Wang W, Tang Z, Zhang J, Lu Y, Yang Y, Qiu C, Ding Y, Dong W, An P. Efficacy of Ustekinumab Optimization by 2 Initial Intravenous Doses in Adult Patients With Severe Crohn's Disease. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Harris KA, Horst S, Gadani A, Nohl A, Annis K, Duley C, Beaulieu D, Ghazi L, Schwartz DA. Patients with Refractory Crohn's Disease Successfully Treated with Ustekinumab. Inflamm Bowel Dis. 2016;22:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 110. | Dalal RS, Njie C, Marcus J, Gupta S, Allegretti JR. Predictors ofUstekinumab Failure in Crohn's Disease After Dose Intensification. Inflamm Bowel Dis. 2021;27:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 111. | Ma C, Fedorak RN, Kaplan GG, Dieleman LA, Devlin SM, Stern N, Kroeker KI, Seow CH, Leung Y, Novak KL, Halloran BP, Huang VW, Wong K, Blustein PK, Ghosh S, Panaccione R. Long-term Maintenance of Clinical, Endoscopic, and Radiographic Response to Ustekinumab in Moderate-to-Severe Crohn's Disease: Real-world Experience from a Multicenter Cohort Study. Inflamm Bowel Dis. 2017;23:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 112. | Yao J, Peng X, Zhong Y, Su T, Bihi A, Zhao J, Liu T, Wang W, Hu P, Zhang M, Zhi M. Extra intravenous Ustekinumabreinduction is an effective optimization strategy for patients with refractory Crohn's disease. Front Med (Lausanne). 2023;10:1105981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 113. | Sandborn WJ, Rebuck R, Wang Y, Zou B, Adedokun OJ, Gasink C, Sands BE, Hanauer SB, Targan S, Ghosh S, de Villiers WJS, Colombel JF, Feagan BG, Lynch JP. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn's Disease: The IM-UNITI Trial. Clin Gastroenterol Hepatol. 2022;20:578-590.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 114. | Khatri V, Kalyanasundaram R. Therapeutic implications of inflammasome in inflammatory bowel disease. FASEB J. 2021;35:e21439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 115. | El Ouali S, Click B, Holubar SD, Rieder F. Natural history, diagnosis and treatment approach to fibrostenosing Crohn's disease. United European Gastroenterol J. 2020;8:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 116. | Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 117. | Rieder F, Latella G, Magro F, Yuksel ES, Higgins PD, Di Sabatino A, de Bruyn JR, Rimola J, Brito J, Bettenworth D, van Assche G, Bemelman W, d'Hoore A, Pellino G, Dignass AU. European Crohn's and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn's Disease. J Crohns Colitis. 2016;10:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 118. | Regueiro M, Velayos F, Greer JB, Bougatsos C, Chou R, Sultan S, Singh S. American Gastroenterological Association Institute Technical Review on the Management of Crohn's Disease After Surgical Resection. Gastroenterology. 2017;152:277-295.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 119. | Tang S, Liu W, Qi W, Yu T, Cao Q, Ge X, Zhou W. Real-World Experience with AGA Guidelines in the Management of Crohn's Disease following Ileocolonic Resection: A Retrospective Cohort Study. Gastroenterol Res Pract. 2020;2020:8618574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Nguyen GC, Loftus EV Jr, Hirano I, Falck-Ytter Y, Singh S, Sultan S; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Management of Crohn's Disease After Surgical Resection. Gastroenterology. 2017;152:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 121. | Golovics PA, Lakatos L, Nagy A, Pandur T, Szita I, Balogh M, Molnar C, Komaromi E, Lovasz BD, Mandel M, Veres G, Kiss LS, Vegh Z, Lakatos PL. Is early limited surgery associated with a more benign disease course in Crohn's disease? World J Gastroenterol. 2013;19:7701-7710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 122. | Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1579] [Cited by in RCA: 2429] [Article Influence: 202.4] [Reference Citation Analysis (0)] |
| 123. | Tam JSY, Coller JK, Hughes PA, Prestidge CA, Bowen JM. Toll-like receptor 4 (TLR4) antagonists as potential therapeutics for intestinal inflammation. Indian J Gastroenterol. 2021;40:5-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 124. | Park SB, Kim KO, Lee HS, Choi CH, Wei SC, Chen MH, Matsuoka K. Vaccination in patients with inflammatory bowel disease-Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn's and Colitis meeting. Intest Res. 2023;21:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 125. | Kochar B, Herfarth HH. Vaccinations in Adult Patients with Inflammatory Bowel Diseases in the West. Inflamm Intest Dis. 2018;3:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Shah BB, Goenka MK. A comprehensive review of vaccination in patients with inflammatory bowel diseases: An Indian perspective. Indian J Gastroenterol. 2020;39:321-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Cushing K, Higgins PDR. Management of Crohn Disease: A Review. JAMA. 2021;325:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 128. | Beaugerie L, Rahier JF, Kirchgesner J. Predicting, Preventing, and Managing Treatment-Related Complications in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1324-1335.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 129. | Jena A, James D, Singh AK, Dutta U, Sebastian S, Sharma V. Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients With IBD: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2022;20:1456-1479.e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 130. | Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, Abreu MT, Dubinsky MC; International Organization for the Study of Inflammatory Bowel Disease (IOIBD); International Organization for the Study of Inflammatory Bowel Diseases (IOIBD). SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 131. | Tepasse PR, Vollenberg R, Nowacki TM. Vaccination against SARS-CoV-2 in Patients with Inflammatory Bowel Diseases: Where Do We Stand? Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Fiorillo C, Schena CA, Quero G, Laterza V, Pugliese D, Privitera G, Rosa F, Schepis T, Salvatore L, Di Stefano B, Larosa L, Minordi LM, Natale L, Tortora G, Armuzzi A, Alfieri S. Challenges in Crohn's Disease Management after Gastrointestinal Cancer Diagnosis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 133. | Shah SC, Itzkowitz SH. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology. 2022;162:715-730.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 554] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 134. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (116)] |
| 135. | Xiao L, Sun L, Zhao K, Pan YS. Crohn's disease with infliximab treatment complicated by rapidly progressing colorectal cancer: A case report. World J Gastrointest Oncol. 2021;13:305-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | Aparicio T, Pachev A, Laurent-Puig P, Svrcek M. Epidemiology, Risk Factors and Diagnosis of Small Bowel Adenocarcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 137. | Kotsafti A, Scarpa M, Angriman I, Castagliuolo I, Caruso A. Fistula-Related Cancer in Crohn's Disease: A Systematic Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol. 2019;25:4148-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 139. | Jones JL, Nguyen GC, Benchimol EI, Bernstein CN, Bitton A, Kaplan GG, Murthy SK, Lee K, Cooke-Lauder J, Otley AR. The Impact of Inflammatory Bowel Disease in Canada 2018: Quality of Life. J Can Assoc Gastroenterol. 2019;2:S42-S48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 140. | Cao RH, Grimm MC. Pregnancy and medications in inflammatory bowel disease. Obstet Med. 2021;14:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 141. | Shannahan SE, Erlich JM, Peppercorn MA. Insights into the treatment of inflammatory bowel disease in pregnancy. Therap Adv Gastroenterol. 2019;12:1756284819852231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 142. | Picciarelli Z, Stransky OM, Leech MM, Michel HK, Schwartz M, Kim SC, Gray WM, Kazmerski TM. Exploring Reproductive Health Decision Experiences and Preferences of Women With Pediatric-Onset Inflammatory Bowel Diseases. Crohns Colitis 360. 2022;4:otab083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gica N, Romania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH