Published online Sep 20, 2023. doi: 10.5662/wjm.v13.i4.366
Peer-review started: June 14, 2023
First decision: July 7, 2023
Revised: August 4, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 20, 2023
Processing time: 97 Days and 11.6 Hours
Marginal zone lymphoma (MZL) is an indolent non-Hodgkin B cell lymphoma with various architectural pattern including perifollicular, follicular colonization, nodular, micronodular, and diffuse patterns. A sclerotic variant has not been pre
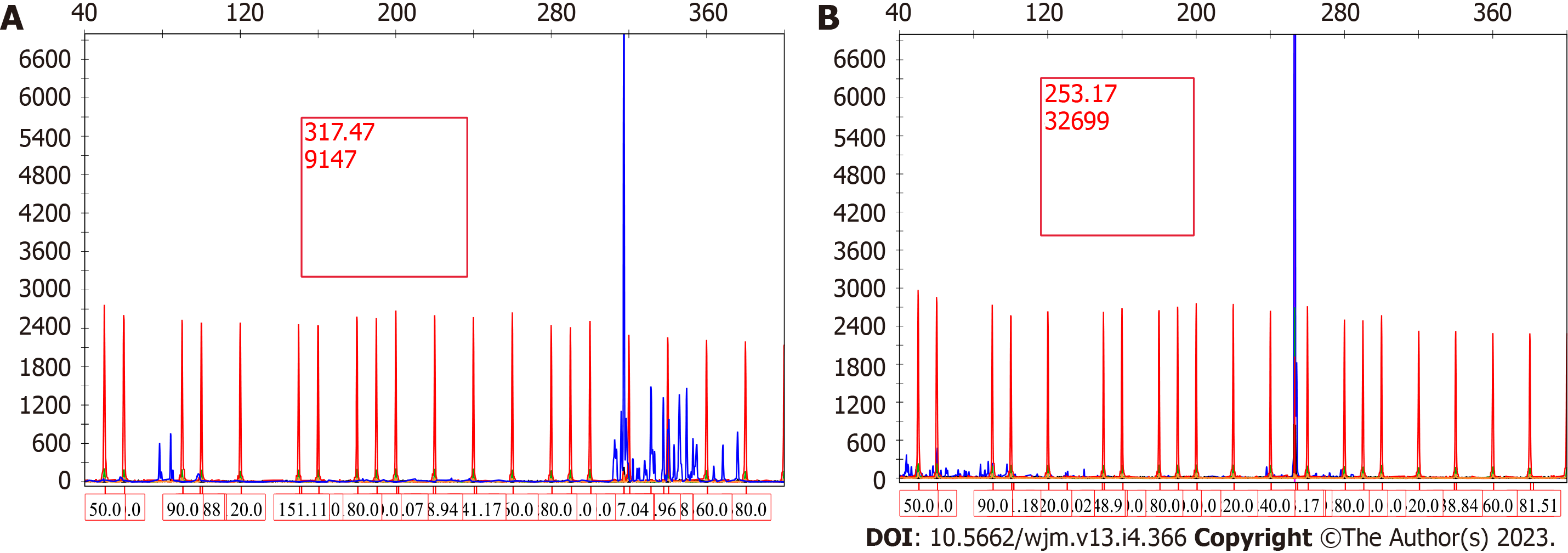
A 66-year-old male developed left upper extremity swelling. Chest computed tomography (CT) in September 2020 showed 14 cm mass in left axilla. Needle core biopsy of axillary lymph node showed sclerotic tissue with atypical B lymphoid infiltrate but was non-diagnostic. Excisional biopsy was performed for diagnosis and showed extensive fibrosis and minor component of infiltrating B cells. Flow cytometry showed a small population of CD5-, CD10-, kappa restricted B cells. Monoclonal immunoglobulin heavy chain and light chain gene rearrangement were identified. Upon being diagnosed with MZL, patient was treated with ritu
This is an important case report because by morphology this case could have easily been overlooked as non-specific fibrosis with chronic inflammation representing a significant diagnostic pitfall. Moreover, this constitutes a new archi
Core Tip: In the clinical context of suspicious lymphadenopathy, the presence of an extensive sclerosis on biopsy should not deter the clinician from a diagnosis of lymphoma, and careful evaluation and work up is needed to exclude covert lym
- Citation: Moureiden Z, Tashkandi H, Hussaini MO. Sclerotic marginal zone lymphoma: A case report. World J Methodol 2023; 13(4): 366-372
- URL: https://www.wjgnet.com/2222-0682/full/v13/i4/366.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i4.366
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma derived from marginal zone B cells within the lymphatic system[1]. The incidence of MZL, based on data from the United States SEER-18 program, is 19.6 per 1000000 person-years[2]. MZL is typically classified into extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (61%), nodal MZL (NMZL) (30%), and splenic MZL (SMZL) (9%). Other subtypes include pediatric MZL and immunoproliferative small intestinal disease[3]. 5-year relative survival rate for EMZL, NMZL, and SMZL are 88.7%, 76.5% and 79.7%, respectively with EMZL being most likely to transform to diffuse large B cell lymphoma[4,5].
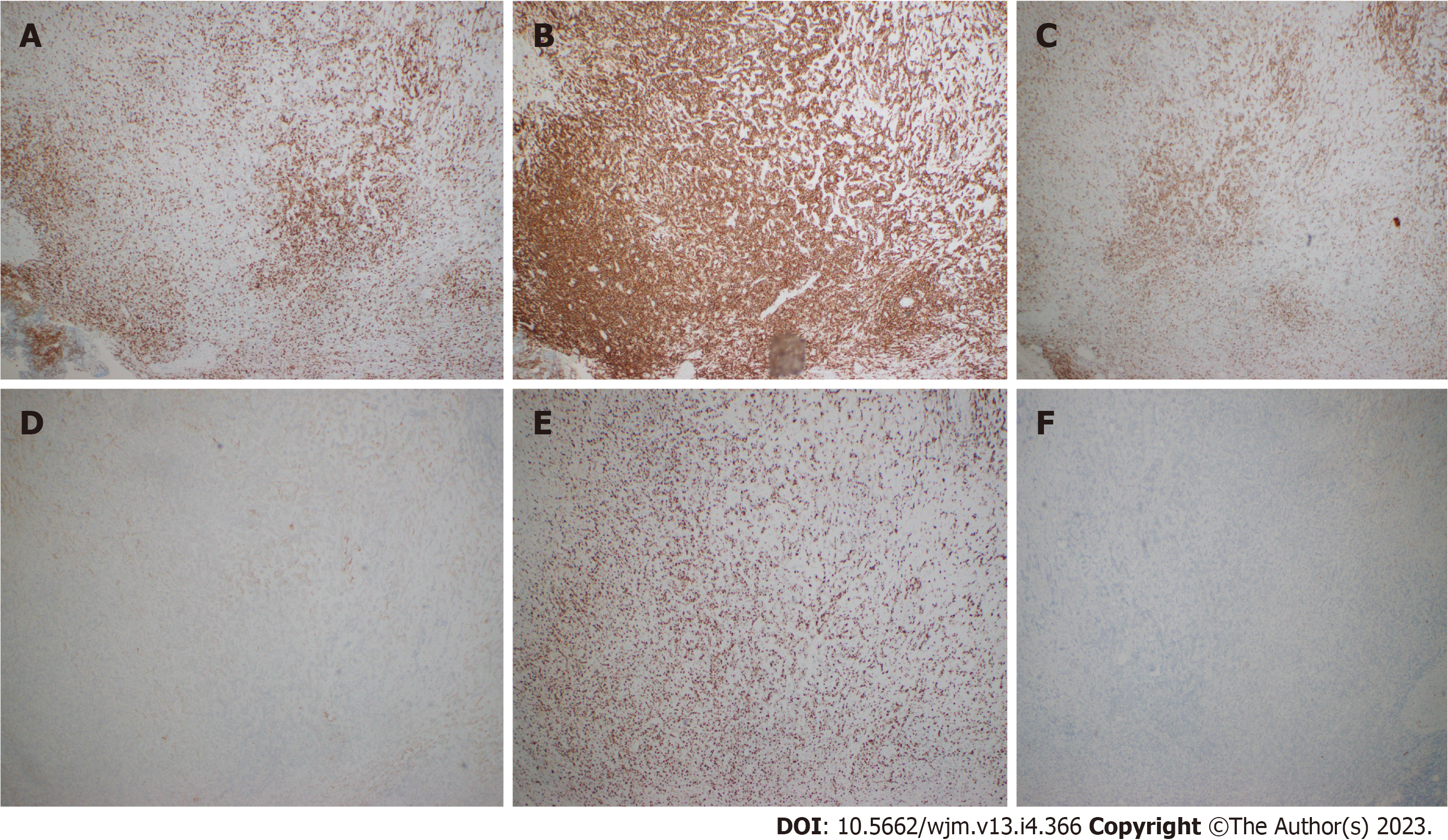
Marginal zone B cells typically display a morphology of small to medium sized lymphocytes with somewhat irregular nuclei containing mature chromatin and relatively abundant pale cytoplasm. They may classically assume a monocytoid morphology[6]. In SMZL, villous lymphocytes can be seen in the periphery. MZL typically expresses an immunohistochemistry profile positive for B cell-associated antigens (CD19, CD20, CD22, CD79a) and complement receptors (CD21 and CD35). MZLs are usually negative for CD5, CD10, CD23, BCL6, and cyclin D1. Furthermore, SMZL has a high con
We present a remarkable case of MZL masquerading in a sclerotic background as fibrosis with chronic inflammation. This constitutes the first report of this architectural pattern in MZL and represents a serious and important diagnostic pitfall in lymphoma diagnosis.
The patient is a 66-year-old male who developed left upper extremity swelling.
The patient had an episode of syncope of unclear etiology in January 2020. He started to have pain under his left arm in February that waxed and waned. Then over the course of a few weeks, he started having left upper extremity swelling. The patient had an ultrasound and mammogram that were reportedly negative. This was followed by a computed tomo
Past medical history is notable for benign prostatic hyperplasia, chronic kidney disease stage III, type 2 diabetes, hyper
The patient had a mother with history of breast cancer and colon cancer.
Temperature: 36.78 °C, heart rate: 93/min, respiratory rate: 16/min, blood pressure: 125/73 mmHg, SpO2: 100%, weight: 104.5 kg, body mass index: 31.20 kg/m2. General: Alert and oriented, no acute distress. Eye: Normal conjunctivae, anicteric. Head/ears/nose/mouth/throat: Normocephalic, no trauma, normal hearing. Neck: Supple, non-tender. Cardiovascular: Regular rate and rhythm, normal peripheral perfusion. Lung: Lungs were clear to auscultation, respirations were non-labored. Abdomen: Soft, nontender, nondistended, no splenomegaly. Hematological/lymphatics: No lymphadenopathy: Cervical, supraclavicular, axillary, inguinal. Extremities: Normal range of motion, normal strength, no deformity. Integumentary: Warm, dry, intact. Neurologic: Alert, oriented, no focal defects. Cognition and speech: Ori
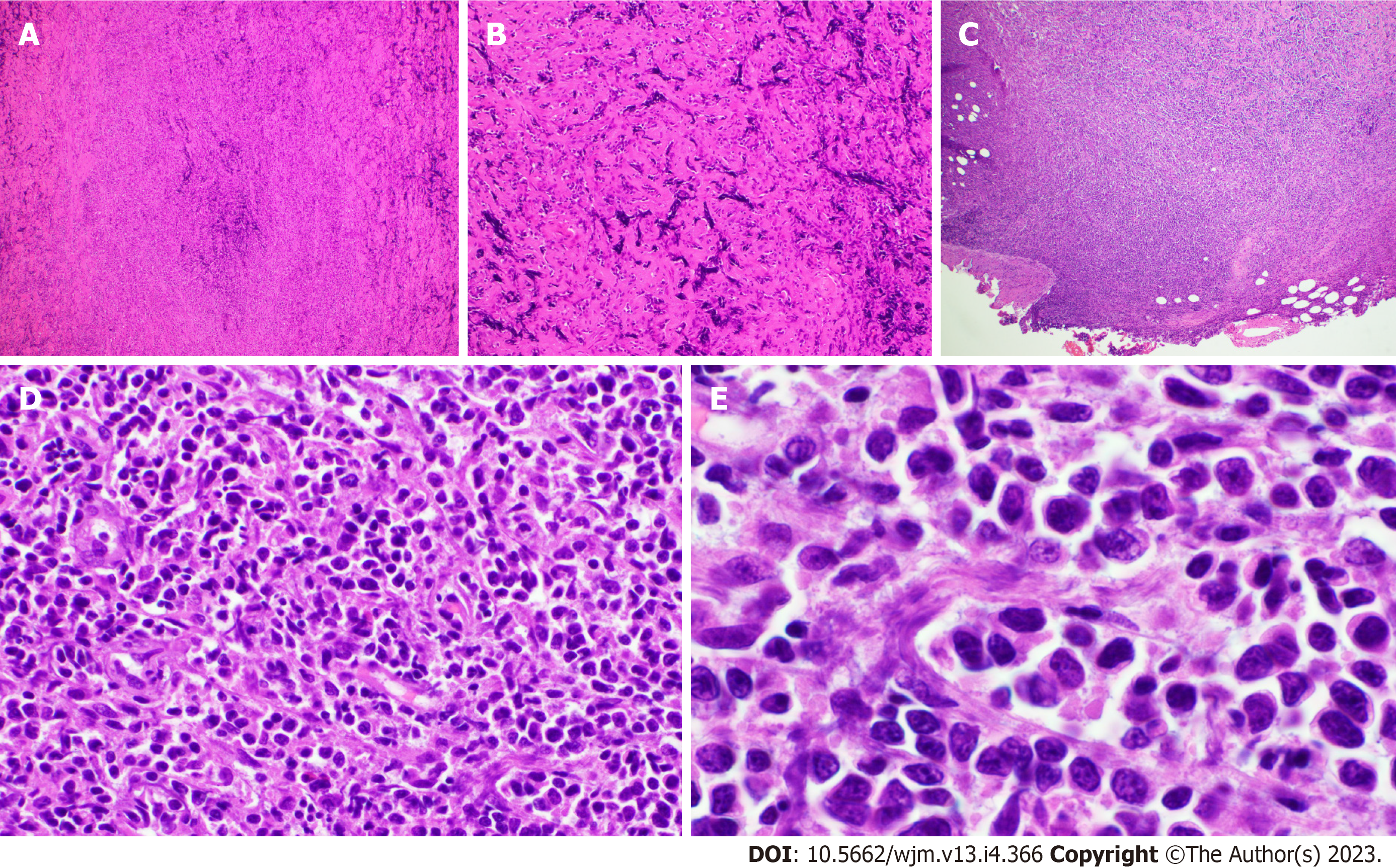
The specific examinations and results are listed in Table 1. Pathology results are provided in Figures 1-3.
| Value w/units | Normal range | |
| CBC | ||
| WBC | 5.59 k/uL | 4.00-10.90 |
| Preliminary ANC | > 1.5 k/uL | |
| RBC | 3.50 mil/uL | 4.45-5.73 |
| Hemoglobin | 11.1 g/dL | 13.4-16.9 |
| Hematocrit | 34.1% | 40.0-48.0 |
| Mean cell volume | 97.4 FL | 80.3-94.0 |
| MCH | 31.7 pg | 27.4-33.4 |
| MCHC | 32.6 g/dL | 32.0-36.8 |
| RDW | 50.6 FL | 36.8-46.1 |
| Platelet count | 113 k/uL | 143-382 |
| MPV | 10.1 FL | 7.4-11.7 |
| Differential | ||
| Neutro auto | 3.57 k/uL | 1.80-7.80 |
| Eos auto | 0.37 k/uL | 0.00-0.45 |
| Basophil auto | 0.04 k/uL | 0.00-0.20 |
| Immature Gran auto | 0.03 k/uL | 0.00-0.10 |
| Mono auto | 0.71 k/uL | 0.30-0.80 |
| Lymph auto | 0.87 k/uL | 1.10-3.50 |
| Nucleated RBC | 0.00 k/uL | 0.00-0.10 |
| Differential % | ||
| Neutro auto % | 63.9 | |
| Eos auto % | 6.6 | |
| Basophil auto % | 0.7 | |
| Immature Gran auto % | 0.5 | |
| Mono auto % | 12.7 | |
| Lymph auto % | 15.6 | |
| Nucleated RBC %/100 WBC | 0.0/100 WBC | 0.0-1.0 |
| Coagulation | ||
| Prothrombin time | 11.6 s | 10.2-12.9 |
| INR | 1.0 | 0.8-1.1 |
| APTT | 27.0 s | 25.1-36.5 |
| Reticulocyte percent | 1.78% | 0.80-1.90 |
| Reticulocyte number | 0.0623 mil/uL | 0.0360-0.1000 |
| Immature retic fraction (%) | 14.5 | 3.0-13.4 |
| Eryth. sed rate | 20 mm/hr | 0-15 |
| Metabolic panel | ||
| Sodium | 140 mmol/L | 134-145 |
| Potassium | 4.0 mmol/L | 3.4-4.5 |
| Chloride | 102 mmol/L | 96-107 |
| Total CO2 | 26 mmol/L | 22-30 |
| Glucose level | 111 mg/dL | 70-110 |
| BUN | 13 mg/dL | 6-23 |
| Creatinine | 1.5 mg/dL | 0.7-1.3 |
| Est. GFR | 47 mL/min/1.73 m2 | |
| Est. GFR (Af-Am) | 57 mL/min/1.73 m2 | |
| Uric acid | 6.9 mg/dL | 3.5-8.5 |
| Calcium | 11.3 mg/dL | 8.6-10.2 |
| Calcium corrected | 11.2 mg/dL | 8.6-10.2 |
| Phosphorus | 2.7 mg/dL | 2.5-4.5 |
| Total protein | 6.8 gm/dL | 6.6-8.7 |
| Albumin | 4.1 gm/dL | 3.5-5.2 |
| Total bilirubin | 0.80 mg/dL | 0.00-1.20 |
| Alk. phosphatase | 65 U/L | 40-130 |
| AST | 34 U/L | 10-50 |
| ALT | 25 U/L | 0-41 |
| LDH | 158 U/L | 135-225 |
| Magnesium level | 1.4 mg/dL | 1.6-2.3 |
Chest CT in September 2020 showed a 14 cm irregularly, marginated mass arising in left axilla. There was questionable invasion of the left subscapularis muscle and thickening the right pectoralis minor muscle. Positron emission tomography (PET) scan showed standardized uptake value (SUV) of 26.
The final diagnosis is MZL.
Upon being diagnosed with lymphoma, patient was treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). More specifically, he received 6 cycles of R-CHOP, and doxo/cyclophosphamide 50% dose reduction starting cycle 4 due to severe cytopenias.
The patient achieved complete remission by PET/CT (July 20, 2021). He is under surveillance post treatment and 1-year post-treatment scans show no evidence of disease.
MZL can demonstrate a wide spectrum of clinical manifestations due organ-specific variability. Genomically, there is also heterogeneity, although dysregulation of B-cell receptor, nuclear factor κB, and NOTCH signaling pathways is typical[3]. Significant variation is also seen in the architectural patterns manifested by MZL[7]. This is complicated by the fact that there is no single universal biomarker for MZL, and the diagnosis is often only arrived at after integrating phenotypic, cytogenetic, and molecular features[9].
There are a few studies that have cataloged the architectural patterns in MZL. Salama et al[10] evaluated 51 NMZL and found four major patterns, namely: Diffuse (75%), well-formed nodular/follicular (10%), interfollicular (14%) and perifollicular (2%). Interestingly, they noted compartmentalizing interstitial sclerosis in 28% of cases most commonly in the diffuse variant (12/15 cases). However, this was illustrated as relatively inconspicuous comprising of delicate tendrils of sclerosis which in no way resembles our case. Others have documented variable architectural patterns depending on the site of involvement: Spleen (nodular to diffuse), bone marrow (intrasinusoidal, interstitial, nodular, and even paratrabecular), lymph node (nodular to diffuse, liver (intrasinusoidal and portal tract lymphoid nodules), etc[9].
Rarely, lymphomas can present in a markedly fibrotic background or be clinically misdiagnosed as retroperitoneal fibrosis[11,12]. Sclerosing lymphomas are rare and typically of follicle center origin[13]. We do not know of any cases describing this architectural presentation of MZL. As such, this case could have easily been overlooked as non-specific fibrosis with chronic inflammation representing a significant diagnostic pitfall. Clues with regard to the diagnosis include the radiologic findings of a large (14 cm) mass with high SUV. Furthermore, the B cell predominance by immunohistochemistry was atypical. Flow cytometry and immunoglobulin heavy and light chain gene rearrangement studies were vital in order to arrive at the correct diagnosis. Response to R-CHOP further confirms the diagnosis clinically.
In summary, we present the first case report of sclerotic MZL which should be recognized as a rare architectural pattern in MZL and poses a diagnostic challenge, especially on limited fine-needle aspiration or needle core biopsy specimens. Integration of all clinical and pathological data is essential to arrive at the correct diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shahriari M, Iran; Tusubira D, Uganda S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6978] [Article Influence: 697.8] [Reference Citation Analysis (0)] |
| 2. | Jain MD, Zhao H, Wang X, Atkins R, Menges M, Reid K, Spitler K, Faramand R, Bachmeier C, Dean EA, Cao B, Chavez JC, Shah B, Lazaryan A, Nishihori T, Hussaini M, Gonzalez RJ, Mullinax JE, Rodriguez PC, Conejo-Garcia JR, Anasetti C, Davila ML, Locke FL. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137:2621-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 3. | Bertoni F, Rossi D, Zucca E. Marginal-Zone Lymphomas. Reply. N Engl J Med. 2022;386:1962-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Alderuccio JP, Kahl BS. Current Treatments in Marginal Zone Lymphoma. Oncology (Williston Park). 2022;36:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Florindez JA, Alderuccio JP, Reis IM, Lossos IS. Splenic marginal zone lymphoma: A US population-based survival analysis (1999-2016). Cancer. 2020;126:4706-4716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Meda BA, Buss DH, Woodruff RD, Cappellari JO, Rainer RO, Powell BL, Geisinger KR. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol. 2000;113:688-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Piris MA, Onaindía A, Mollejo M. Splenic marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Franco V, Florena AM, Iannitto E. Splenic marginal zone lymphoma. Blood. 2003;101:2464-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | van den Brand M, van Krieken JH. Recognizing nodal marginal zone lymphoma: recent advances and pitfalls. A systematic review. Haematologica. 2013;98:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Salama ME, Lossos IS, Warnke RA, Natkunam Y. Immunoarchitectural patterns in nodal marginal zone B-cell lymphoma: a study of 51 cases. Am J Clin Pathol. 2009;132:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ouchani M, Bachir H, Hamaz S, Alaoui H, Serraj K. Retroperitoneal Fibrosis: Beware of Lymphoma. Cureus. 2021;13:e17587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Dlabal PW, Mullins JD, Coltman CA Jr. An unusual manifestation of non-Hodgkin's lymphoma. Fibrosis masquerading as Ormond's disease. JAMA. 1980;243:1161-1162. [PubMed] |
| 13. | Chim CS, Liang R, Chan AC. Sclerosing malignant lymphoma mimicking idiopathic retroperitoneal fibrosis: importance of clonality study. Am J Med. 2001;111:240-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |