Published online Jun 20, 2023. doi: 10.5662/wjm.v13.i3.29
Peer-review started: December 13, 2022
First decision: February 8, 2023
Revised: March 14, 2023
Accepted: April 14, 2023
Article in press: April 14, 2023
Published online: June 20, 2023
Processing time: 189 Days and 9.2 Hours
The global incidence of oral cancer has steadily increased in recent years and is associated with high morbidity and mortality. Oral cancer is the most common cancer in the head and neck region, and is predominantly of epithelial origin (i.e. squamous cell carcinoma). Oral cancer treatment modalities mainly include surgery with or without radiotherapy and chemotherapy. Though proven effective, chemotherapy has significant adverse effects with possibilities of tumor resistance to anticancer drugs and recurrence. Thus, there is an imperative need to identify suitable anticancer therapies that are highly precise with minimal side effects and to make oral cancer treatment effective and safer. Among the available adjuvant therapies is curcumin, a plant polyphenol isolated from the rhizome of the turmeric plant Curcuma longa. Curcumin has been demonstrated to have anti-infectious, antioxidant, anti-inflammatory, and anticarcinogenic properties. Curcumin has poor bioavailability, which has been overcome by its various analogues and nanoformulations, such as nanoparticles, liposome complexes, micelles, and phospholipid complexes. Studies have shown that the anticancer effects of curcumin are mediated by its action on multiple molecular targets, including activator protein 1, protein kinase B (Akt), nuclear factor κ-light-chain-enhancer of activated B cells, mitogen-activated protein kinase, epidermal growth factor receptor (EGFR) expression, and EGFR downstream signaling pathways. These targets play important roles in oral cancer pathogenesis, thereby making curcumin a promising adjuvant treatment modality. This review aims to summarize the different novel formulations of curcumin and their role in the treatment of oral cancer.
Core Tip: Oral cancer has a high disease burden worldwide. Oral squamous cell carcinoma is the most predominant subtype of oral cancer. The majority of oral cancers present at an advanced stage and are associated with a poor prognosis. Timely diagnosis and early treatment are critical to achieve a superior outcome. Surgery is the recommended treatment for oral cancer; other treatment modalities are radiotherapy with or without chemotherapy. Curcumin, a plant derivative, is one among the available adjuvant therapies that has been studied for its anticarcinogenic potential in the setting of various cancers. Curcumin has been proven to modulate intracellular signaling pathways that control cancer cell growth, inflammation, invasion, apoptosis, and cell death, with evidence supporting its use in cancer therapy. This review aims to summarize the molecular pathways involved in oral carcinoma pathogenesis, to explore different therapeutic interactions of curcumin, and to highlight the role of novel curcumin formulations in oral cancer treatment.
- Citation: Mukherjee D, Krishnan A. Therapeutic potential of curcumin and its nanoformulations for treating oral cancer. World J Methodol 2023; 13(3): 29-45
- URL: https://www.wjgnet.com/2222-0682/full/v13/i3/29.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i3.29
Oral cancer, a disease of predominant epithelial origin, is the most common subtype of cancer arising in the head and neck[1]. In 2020, more than 300000 new oral cancer cases were recorded globally[2]. Ninety percent of oral cancer cases are histologically diagnosed as oral squamous cell carcinomas (OSCCs)[3]. Oral cancer is widely prevalent in developing countries of South Central Asia (e.g., India, Sri Lanka, Pakistan) and Melanesia, with a lesser disease burden in developed countries[4]. Oral cancer is the leading cause of death due to cancer in the Indian male population[5]. High incidence rates of oral cancer have been linked to alcohol consumption, tobacco smoking, betel nut chewing, and human papillomavirus (HPV) infection[6]. Despite diagnostic and therapeutic advances, the global 5-year survival rate remains less than 50%[7].
The primary treatment of oral cancer is based on the cancer stage. Surgery is the mainstay of multimodal therapy, which also includes radiotherapy and systemic treatment (chemotherapy and/or targeted agents)[8]. Chemotherapy has proven to increase treatment efficacy and improve overall survival, but has significant adverse effects and the potential for development of drug resistance[9]. As such, combination of these therapies with an adjuvant treatment to bolster their efficacy is urgently needed.
Among adjuvant therapies is curcumin, a phytochemical isolated from the turmeric plant Curcuma longa. Curcumin has been reported in a plethora of studies to have anti-infectious, antioxidant, anti-inflammatory, hepatoprotective, cardioprotective, thrombo-suppressive, anti-arthritic, chemopreven
The hydrophobic nature of curcumin leads to poor bioavailability, and the sensitivity of soluble curcumin in physiological pH has limited its use in clinical practice[13]. However, nanotechnology-based techniques have made possible various novel formulations of curcumin such as liposomes, nanoparticles, micelles, phospholipid complexes, and analogues, to improve its tissue-level absorption and increase its pharmacological efficacy[14]. Studies have shown favorable results with the use of nano-formulated curcumin in the setting of epithelial cancers. Thus, it follows that curcumin may have a therapeutic role in oral cancer treatment as an adjuvant[15-17]. The present review aims to summarize the key properties of curcumin and its novel formulations, along with their role in oral cancer treatment.
Oral cancer comprises neoplasms affecting any region of the oral cavity. The oral cavity is divided into distinct anatomic subsites, including lip, oral tongue, floor of the mouth, buccal mucosa, upper and lower gingiva, retromolar trigone, and hard palate[18]. However, 90% of oral cancers are histologically diagnosed as OSCCs[3]. OSCCs commonly present as nonhealing ulcers or growths. Early in the disease, lesions can appear as flat, discolored areas (i.e. erythroplakia or leukoplakia)[19]. Invasion of surrounding tissues can present with neck masses, trismus, referred ear pain, or specific sensory changes[20]. In cancer of the lip, there is often an exophytic, crusted lesion invading the underlying muscle with tissue damage in the adjacent lip[21]. Oral cancers are often diagnosed late due to an asymptomatic phase with fast progression and early metastasis[22]. Furthermore, the staging of oral cancer plays a significant role in survival rate, with early-stage (I and II) and advanced-stage (III and IV) lesions having a 5-year survival rate of 80% and 50% or less, respectively[22].
There are multiple pathways involved in oral carcinogenesis leading to genetic mutation (e.g., H-ras, K-ras), gene deletions (e.g., loss of chromosome 9p21 or 3p), promoter methylation (e.g., p16, Ras association domain family member 1), amplification of oncogenes and oncoproteins (e.g., EGFR, myc, bcl-2, ras, raf, stat-3, or cyclin D1) and inactivation of tumor suppressor genes (e.g., p53)[23].
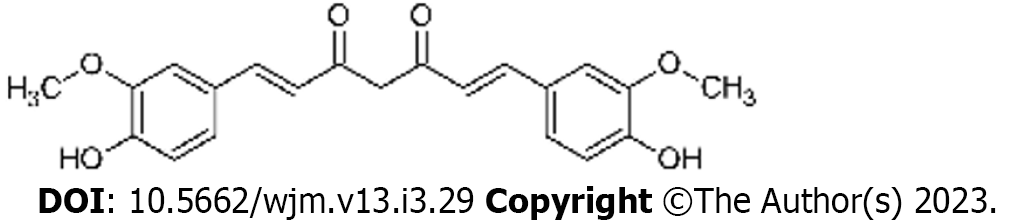
Curcumin is a yellow spice derived from the roots (rhizomes) of Curcuma longa, commonly known as turmeric[24]. Turmeric contains curcuminoids, comprising curcumin, demethoxy curcumin (DMC), and bis-demethoxycurcumin (BDMC)[25]. In 1910, the principal ingredient of curcumin was identified by Gupta et al[26] as diferuloylmethane. Curcumin is known as 1, 7-bis (4-hydroxy-3-methoxy phenyl)-1, 6-heptadiene- 3, 5-dione (1E-6E) by International Union of Pure and Applied Chemistry nomenclature. It has a molecular weight of 368.4 g/mol and a melting temperature of 183 °C[27].
Curcumin contains 2 aromatic ring systems with o-methoxy phenolic groups linked with α- and β-unsaturated β-diketone moiety (Figure 1)[28]. The absorption bands of curcumin exist in the visible spectrum (410 nm-430 nm) and the ultraviolet spectrum (250 nm–270 nm)[14]. At 488 nm, curcumin is excited by lower fluorescent yield emission in the 500 nm-530 nm range, which can be detected by flow cytometry and confocal microscopy[29]. Curcumin is insoluble in water and readily soluble in polar solvents with keto-enol tautomerism[30]. The keto-form predominates in acid or neutral solutions, with the enol-form being predominant in alkaline solutions[31].
The bioavailability of curcumin is around 1% according to various animal studies, suggesting a requirement of high doses of curcumin (3600 mg to 12000 mg) to achieve beneficial effects[32]. It is known that curcumin’s solubility in water (0.0004 mg/mL at pH 7.3) is poor, giving rise to challenges with oral administration[33]. One study has shown that curcumin has no toxic effect in patients with colorectal cancer when its oral dose is at least 3600 mg[34]. Curcumin undergoes rapid metabolism in the liver and gets excreted in the feces[35]. Curcumin is transformed into dihydrocurcumin and tetrahydrocurcumin (THC) and consequently converted into glucuronide conjugates[36,37]. In intestinal mucosa, kidney, and liver, the conjugative enzyme activity for glucuronidation and sulfation of curcumin has been discovered[37,38]. Another study demonstrated that a considerable portion of orally administered curcumin was conjugated to glucuronide in the intestine; later, the conjugated compound entered the portal vein and underwent additional conjugation to form glucuronide/sulfate metabolites of curcumin in the liver[38].
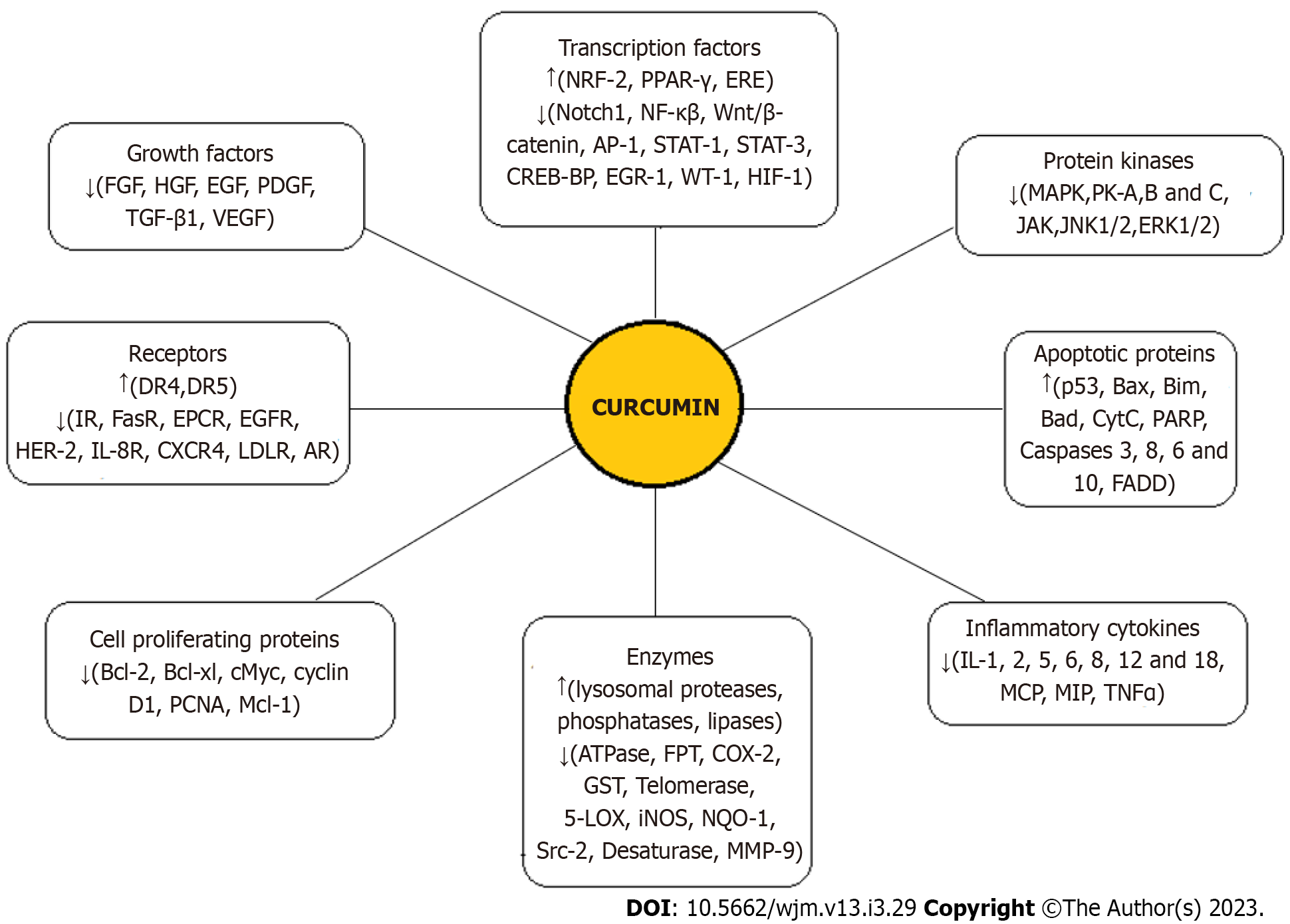
Curcumin, having a vast range of effects on various human diseases, plays an anti-tumorigenic role in different cancers by affecting multiple pathways of cancer progression[10-12]. In addition, it has been shown to have different effects on normal cells vs cancer cells, including a higher uptake by cancer cells[39]. The anticancer effects of curcumin are predominantly mediated through its regulation of various transcription factors, growth factors, inflammatory cytokines, protein kinases, and other oncogenic molecules, as summarized in Figure 2[10,11,33]. Curcumin metabolites (THC, hexahydrocurcumin, and octahydrocurcumin) also have anticancer properties[40,41]. However, the major factor restricting the use of curcumin as a novel chemotherapeutic agent is reduced bioavailability which is attributed to its poor absorption, rapid degradation, fast metabolism, and systemic elimination. Notably, as an anti-cancer drug, curcumin should be administered in a sufficiently high concentration; however, at these concentrations, patients have shown intolerance to bulk doses of the substance[33]. Using an advanced delivery system to increase the bioavailability of curcumin with satisfactory parenteral administration is the most promising solution to these challenges.
Curcumin is a potent agent that inhibits cell growth and deoxyribonucleic acid (DNA) synthesis in oral cancer cells[41]. Treatment with curcumin promotes the cell cycle’s G(2)/M phase arrest, accompanied by a decrease in cyclin B/cyclin-dependent kinase 1 and cell division cycle 25C protein levels. It induces apoptosis of oral cancer cells via reduction of Bcl-2 levels, reduction in mitochondrial membrane potential, promotion of the active forms of caspase-3, and the release of apoptosis-inducing factor (AIF) and endonuclease G from mitochondria[42]. Curcumin and curcuminoids like DMC and BDMC have shown autophagic and apoptotic activity[43,44].
Studies have shown that curcumin significantly inhibits the carcinogen-activating enzyme cytochrome P450 family 1 subfamily A member 1, which mediates benzo(a)pyrene diol bioactivation in both OSCC cells and oral mucosa[45]. Arecoline exposure is another significant risk factor for the development of oral cancer, and treatment with curcumin markedly inhibits arecoline-induced Snail expression[46]. One study showed that administration of curcumin at 100 mg/kg for 12 wk in a rat model with 4-nitroquinolone-1-oxide (4-NQO)-induced oral cancer markedly decreased the expression of proliferating cell nuclear antigen, anti-apoptosis markers (e.g., Bcl-2), suppressors of cytokine signaling 3 and 1, and STAT3. It also minimized cellular atypia and reduced expression of vimentin, E-cadherin, N-cadherin, and TWIST1, which represent epithelial-mesenchymal transition (EMT) events[47]. Combining local and systemic C3 complex (a purified mixture of curcumin, BDMC, and DMC) effectively targets cancer cell proliferation. This combination inhibits 4NQO-induced tumorigenesis via modulation of fibroblast growth factor-2/fibroblast growth factor receptor-2[48]. Moreover, curcumin inhibits the activation and expression of host transcription factors AP-1 and NF-κB, which bind to a cis-regulatory region of the HPV genome. This effect is concentration- and time-dependent, leading to the suppression of HPV16/E6 transcription and the subsequent prevention of oral carcinogenesis[49]. Curcumin triggers the activation of p38, which then interacts with binding elements in insulin-like growth factor binding protein-5, leading to the activation of the transcription factor CCAAT/enhancer binding protein α (C/EBPα). This also results in the suppression of oral carcinogenesis[50].
Additionally, curcumin reduces oral cancer cell viability and invasion by downregulating Notch 1 and NF-κB[12]. It also induces G2/M phase cell cycle arrest in a dose-dependent fashion by inhibiting the phosphorylation of EGFR and its downstream signaling molecules Akt, extracellular signal-regulated kinase (ERK1/2), and STAT3[12]. Treatment of oral cancer cells with curcumin, BDMC, and DMC leads to the production of reactive oxygen species (ROS), activation of caspase-8, -9, and -3, a decrease in the levels of matrix metalloproteinases (MMP), the release of AIF, and an alteration in the expressions of EGFR, PI3K, p-AKT, NF-κB, AMP responsive protein kinase, and MAPK[44]. In an in vivo OSCC model, curcumin has also been observed to suppress the expression of cyclo-oxygenase-2[51].
The oncogenic microRNA miR-31 is upregulated in OSCC, and curcumin downregulates the expression of this molecule in OSCC, leading to an attenuation of AKT activation and downregulation of C/EBPβ[52]. Moreover, curcumin inhibits oral cancer cell proliferation by upregulating miR-9 expression in a dose-dependent manner and suppressing Wnt/β-catenin signaling[53]. Furthermore, curcumin can also enhance the antitumor immune response by inhibiting the expression of programmed cell death ligand 1 and pSTAT3 leading to an increase in CD8+ T-cells and a decrease in T regulatory cells and myeloid-derived suppressor cells[54]. Cancer-associated fibroblasts (CAFs) are activated fibroblasts in the tumor microenvironment that play a critical role in cancer development[55]. Curcumin can reverse the phenotype of CAFs to that of peri-tumor fibroblast-like cells by downregulating the expression of α-smooth muscle actin (a unique marker for CAFs) and inhibiting the secretion of pro-carcinogenic cytokines such as transforming growth factor-β1, MMP2, and stromal cell-derived factor-1[56]. This results in decreased cancer invasion, as evidenced by a reduced release of EMT mediators in treated CAFs and reversal of EMT in treated tumor cells[57].
Hepatocyte growth factor (HGF) signaling plays an important role in EMT induction and contributes to cancer cell invasion and metastasis[58]. Curcumin inhibits HGF-induced EMT and cell motility in oral cancer cells, acting on HGF receptor c-Met and blocking the downstream activation of the pro-survival ERK pathway[59]. It also decreases proliferation in cell lines with mesenchymal characteristics and causes cell death with a dose-dependent decrease in cell–cell adhesion[60]. Curcumin treatment has been found to suppress MMP-2, MMP-9, and MMP-10, which are linked to cancer cell migration and invasion in oral cancer[61,62].
Studies have shown that curcumin can enhance the efficacy of standard platinum-based chemo
Furthermore, curcumin has a radio-sensitizer effect in OSSC and exhibits synergistic antiproliferative activity when combined with cetuximab (an anti-EGFR monoclonal antibody) in cisplatin-resistant oral cancer cells[65,66]. A study of combinations of curcumin and metformin demonstrated a reduction in tumor volume and improvement of overall survival of experimental animals, as evidenced by downregulation of cancer stem cell markers CD44 and CD133[67]. Another study showed that olaparib (a poly-ADP ribose polymerase inhibitor), when combined with curcumin in vitro and in vivo (mouse model), causes DNA damage, inhibits cell proliferation and topoisomerase activity, reduces the expression of base excision repair components, induces apoptosis, and decreases tumor volume[68].
Multiple clinical trials are ongoing or have been completed investigating the efficacy of curcumin against human diseases including oral pathologies. Kuriakose et al[69] conducted a study on oral leukoplakia, a potentially malignant oral cavity lesion with no effective treatment available. In this study, subjects with oral leukoplakia underwent a randomized, double-blinded, placebo-controlled phase IIB clinical trial with curcumin. Clinical and histological response assessments showed a significantly better outcome with curcumin treatment. Notably, the therapy was well-tolerated, and a significant and long-lasting clinical response was observed after treatment with curcumin at a dose of 3.6 g administered over 6 mo[69]. Furthermore, recent studies have shown that topical curcumin effectively treats oral mucositis[70]. Currently, a phase II randomized trial (double-blind, placebo-controlled) is ongoing to assess the therapeutic effects of curcumin in patients with stage III-IV head and neck cancer and cancer-associated anorexia-cachexia[71].
Several investigations have attempted to improve curcumin's therapeutic effectiveness and pharmacokinetic profile by developing new analogues[72,73]. The synthetic curcumin analogues that have been studied include the EF series (EF24, EF31, and UBS109), the FLLL series (FLLL11, FLLL12, FLLL31, FLLL32, and FLLL62), the GO-Y series, the 4-arylidene curcumin analogues AC17, B19 [(1E,4E)-1,5-bis(2,3-dimethoxy phenyl) penta-1,4-dien-3-one], CDF (difluorinated curcumin), and 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] CLEFMA, the diarylidenylpiperidones series, DM-1 (sodium 4-[5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2- methoxy phenolate), and dimethoxycurcumin[74]. In addition, some of these new analogues have been reported to have more potent anticancer properties than curcumin, and may have more beneficial antioxidant, antimalarial, and anti-inflammatory properties than the parent compound[75-79].
A few analogues including EF24, CDF, and FLLL12, exhibit enhanced physicochemical properties such as improved solubility and bioavailability, allowing them to overcome the limitations of curcumin[80,81]. In addition, some analogues have shown to increase the efficacy of chemotherapeutic agents and to overcome issues of resistance when combined therapy is used[82,83]. These analogues have shown promising results in breast, prostate, colon, and head-neck squamous cell cancers[77,84-87]. In a study of oral cancer cells, Chuprajob et al[88] found that curcumin analogues with the 1,4,6-trien-3-one function are more potent than the curcuminoid types. Also, structural variations in the analogues enhanced their potency; for example, the meta-oxygen function of the aromatic ring is more potent than those in the ortho and para positions, and the free phenolic hydroxy group is more potent than in the corresponding methyl analogues[88]. Furthermore, some analogues showed fewer toxic effects than curcumin when applied to normal cells[88]. In 2017, Lin et al[89] found that EF24 exhibited antitumor activity on CAL-27 oral cancer cells by deactivating the MAPK/ERK signaling pathway.
Several curcumin analogues have been developed in recent years, and most of the analogues have shown mechanisms of action similar to that of curcumin. However, some have unique mechanisms that are not associated with curcumin. For instance, the B19 analogue inhibits thioredoxin reductase 1, leading to ROS-mediated endoplasmic reticulum stress, whereas the AC17 analogue blocks proteasome function by inhibiting the deubiquitinase activity of 19S regulatory particles; neither of these mechanisms are seen with curcumin[90,91]. Further studies are required to evaluate the specific benefits of these inhibition pathways in cancer treatment.
Despite their promising potential, key parameters for the clinical development of many promising analogues remain unknown, and further attention should be given to the study of their pharmacokinetics. To reduce drug-associated toxicities and improve bioavailability, targeted drug delivery through alternative formulation has been gaining attention. Some studies have shown that curcumin analogues can be conjugated to homing moieties to direct their delivery and accumulation at specific sites. Certain homing moieties have been tested, including hyaluronic acid (HA)-targeted nanomicelles, HA dendrimers, folic acid-conjugated CDF, and EF24 conjugated to coagulation factor VIIa to target tissue factor[92-95]. These potential agents have yet to undergo clinical trials and cost-effective production strategies. Further in vivo studies can pave the way for clinical trials and future applications.
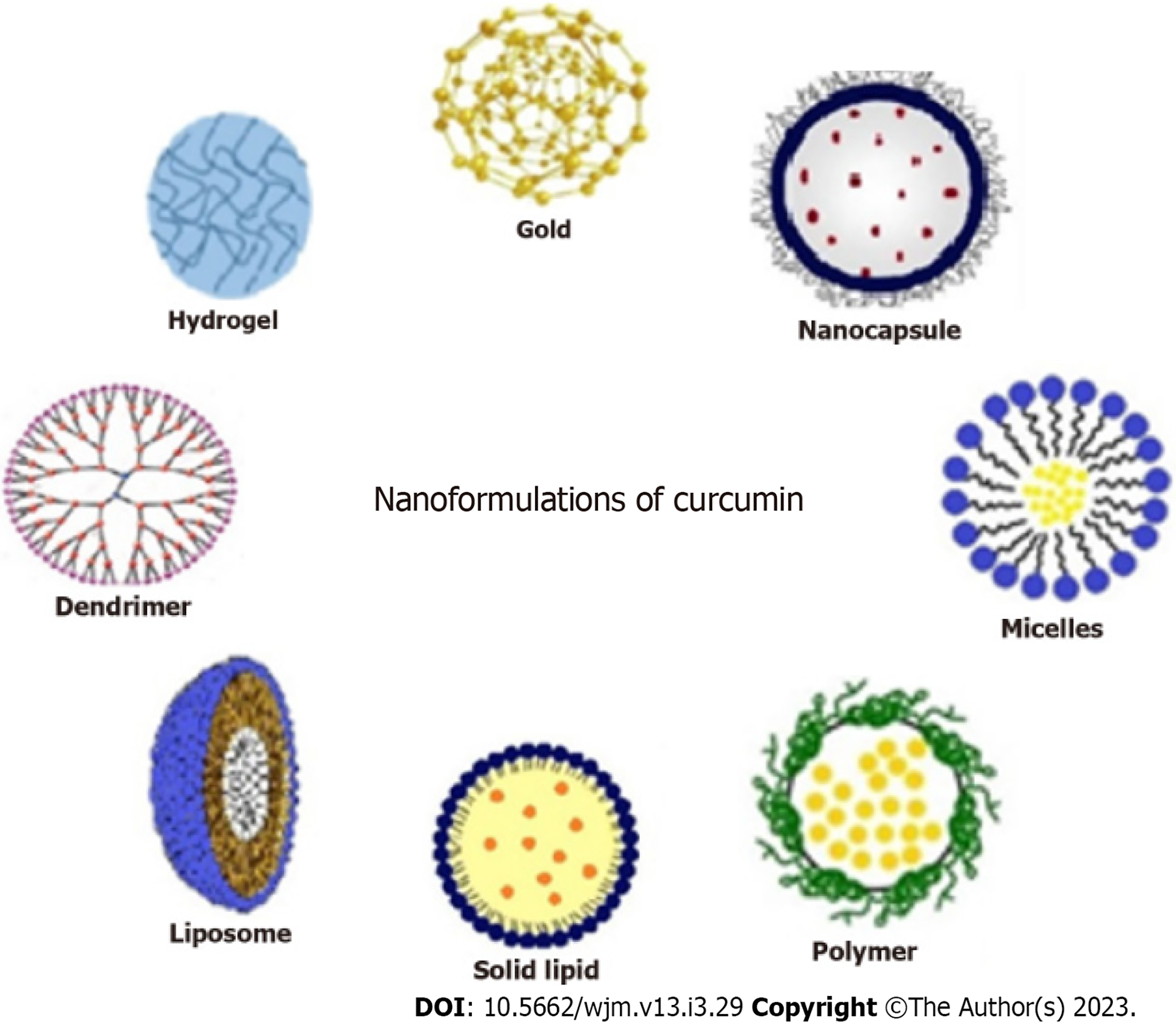
Nanotechnology has led the way in developing nanoscale drug delivery systems. Hydrophobic molecules such as curcumin can benefit from improved bioavailability as a result of the surface, small size, quantum size, and quantum tunnel effects of nanoparticles[96,97]. Several novel strategies have been developed to design curcumin nanoparticles as targeted drug-delivery systems, and these have been studied in various disease states, including cancer[33] (Figure 3).
The following is a summary of the different nanotechnology-based drug delivery modalities for potential use with curcumin.
Liposomes: These are spherical, closed phospholipid vesicles that incorporate drugs in the inner aqueous layer and have been widely used to enhance the bioavailability and efficacy of curcumin. In recent years, several liposomal curcumins with polymeric conjugates have been modified to achieve better clinical outcomes[98]. Nanoliposomes have shown properties such as sustained drug release, enhanced tumor targeting, minimized toxicity to healthy cells, and a lower dose[99].
Polymer micelles: These represent an excellent drug delivery system for curcumin, as they can overcome issues with poor solubility, low stability, and poor bioavailability. Encapsulating curcumin within cationic micelles like cetyltrimethylammonium bromide or dodecyl trimethyl ammonium bromide can enhance drug loading capacity, increase water solubility, reduce toxicity, and limit degradation[100]. Nanomicelle curcumin has been shown to prevent and treat oral mucositis caused by head and neck radiotherapy and chemotherapy[101,102].
Polymer nanoparticles: Polymer nanoparticles are another effective drug delivery system, owing to their high biocompatibility and ease of circulation in the bloodstream for longer periods. Synthetic polymer conjugates such as chitosan, d,l-lactide-co-glycolide (PLGA), polyethylene glycol (PEG), poly (n-butyl) cyanoacrylate, silk fibroin, N-isopropyl acrylamide, and hydrophobically modified starch are commonly used. Curcumin-loaded nanoparticles using PEG-5000 as a carrier stabilizer for PLGAs have shown results in mouse models of cancer, with higher cellular uptake and induction of apoptosis both in vitro and in vivo[103].
Solid lipid nanoparticles: Solid lipid nanoparticles consist of natural lipids (e.g., lecithins or triglycerides) that remain solid at 37 °C. These molecules protect labile compounds from chemical degradation and improve bioavailability. Curcumin-loaded solid lipid nanoparticles have shown enhanced cellular uptake and are promising anticancer agents against breast cancer cells in vitro[104].
Inclusion complexes: Inclusion complexes are formed of cyclodextrins and cyclic oligosaccharides composed of 6 to 8 glycosyl monomeric units (α-1,4 linked). They are widely used to improve stability, enhance water solubility, increase bioavailability, and reduce bitterness. β-Cyclodextrin is commonly used to form an inclusion complex with curcumin by solvent evaporation or pH shift techniques[105].
Solid dispersions: Solid dispersions have improved curcumin's physicochemical and pharmacokinetic activities. Wet-melting and subsequent freeze-drying are common strategies for preparing crystal and amorphous forms in which curcumin is dispersed in an inert carrier at a solid-state[106].
Magnetic nanoparticles: Drug-loaded magnetic nanoparticles can be directed to cancer-affected tissues under external magnetic fields to offer a targeted drug delivery option. Entrapping curcumin in an Fe3O4-curcumin conjugate with oleic acid or chitosan in the outer shell results in the formation of nano-sized, fluorescent-magnetic, water-dispersible nanoparticles with increased cellular uptake and enhanced bioavailability[107].
Microspheres and microcapsules: This approach encapsulates drugs or molecules like curcumin within polymeric particles to improve efficacy and organ-targeted bioavailability. Particles that have been used include camptothecin, rutin, zedoary oil, andrographolide, and eudragit S-100[108].
Emulsions: Emulsions refer to small-droplet dispersions comprising of oil and water mixtures that are stabilized by surfactant molecules to form interfacial films. These represent a lipid-based drug delivery system possessing numerous advantages, including thermodynamic stability, improved drug dissolution, and increased solubility[109]. High-speed and high-pressure homogenization procedures using triacylglycerol and Tween-20 as emulsifiers produce tiny microemulsion droplets. Incorporating curcuminoids into nanoemulsions has been shown to increase oral bioavailability[110].
Nanogels: Nanogels are 3-dimensional polymer networks with high drug loading capacity, high dispersion stability, targeted drug delivery efficiency, fast drug releasing properties, and increased drug delivery across cellular barriers. Moreover, they are easy to modify chemically. Curcumin has been used as a nanogel for targeted therapy[111].
Nanoparticle curcumin: Nanoparticle curcumin is a pure form of curcumin that is processed into nanoparticles of approximately 200 nm in size without carrier conjugates. In vitro and in vivo studies have shown that these nanoparticles of curcumin exhibit increased cellular uptake and enhanced anticancer effects due to their size, surface charge, and surface area[112-114].
Niosomes: Niosome nanocapsules are drug carriers composed of non-ionic surfactants that form a bilayered structure with hydrophobic and hydrophilic parts in an aqueous medium. Niosomes offer numerous advantages, including improved pharmacokinetics, drug stability, therapeutic effects, and reduced side effects of the administered drug[115].
Using nanoformulation-based combination therapy has gained popularity as a potent drug delivery system, often overcoming the limitations of conventional therapeutic agents. This delivery system has shown to improve intracellular drug concentrations and enhance the synergistic activity for cancer therapy[116-118]. Specific curcumin novel formulations have been studied for their efficacy in treating oral cancer, with encouraging findings (Table 1)[119,120].
| Curcumin formulations | Study type | Results | Ref. |
| Liposomes | In vitro | Size of vesicle attributed to enhanced release of curcumin and cytotoxicity in the SCC9 cells | Gosangari et al[119], 2012 |
| Cur microemulsion | In vitro | Damaged and ruptured OSCC 25 cells, cell death enhanced by ultrasound | Lin et al[121], 2012 |
| PLGA Cur- NP | In vitro | Increased ROS production, upregulated caspase-3/caspase-9, cytochrome c, Apaf- 1, AIF, Bax, downregulated Bcl-2 | Chang et al[103], 2013 |
| Cur-SiNP | In vitro | Cytotoxicity by inhibition of NF-κB activity, suppression of MMP-9, angiogenesis (VEGF), and inflammation (TNF-α) in the dark as well as on exposure to light | Singh et al[128], 2014 |
| Trienone analogues of curcuminoids | In vitro | 1,4,6-trien-3-one analogue has more potent cytotoxicity than the curcuminoid type function in oral cancer cells | Chuprajob et al[88], 2014 |
| Cur-loaded chitosan-coated PCL nanoparticle | In vitro | Mucoadhesive properties decreased SCC9 cell viability by inducing apoptosis | Mazzarino et al[129], 2015 |
| Cur analogue EF24 | In vitro | Anticancer activity on CAL-27 cancer cells via deactivation of the MAPK/ERK signaling pathway | Lin et al[89], 2017 |
| Gold nanorod-drug conjugates (Au NR@Curcumin) | In vitro | Cancer cell cycle S phase arrest, the photothermal killing of the cancer cells | Zhu et al[132], 2018 |
| NP Cur | In vitro | Chemoprotective nature of Cur towards 5-FU induced cell toxicity, antioxidant effect, altered expression of apoptotic proteins Bcl2 and Bax | Srivastava et al[112], 2018 |
| In vitro | Chemo-adjuvant property of NP Cur with Cetuximab | Mukherjee et al[136], 2022 | |
| In vitro | Cytotoxicity via apoptosis, luminescence property of NP Cur acting as a theranostic agent | Essawy et al[135], 2022 | |
| PGA- Gef/Cur NP | In vitro | NPs internalized into SAS cells, decreased cell viability, and induced apoptotic cell death via | Lai et al[134], 2019 |
| caspase-3,9 and mitochondria-dependent pathway | |||
| In vivo | Suppressed tumor size compared to the free Gef/Cur-treated group | ||
| Mucoadhesive nanostructured Cur | In vitro, Ex vivo | Improved cytotoxicity, enhanced Cur release, and permeation while selectively targeting cancer cells | Ferreira et al[137], 2019 |
| DNA Cur complex | In vitro | Enhanced cellular delivery of Cur increased cancer cell cytotoxicity in combination with FdU nucleotides | Ghosh et al[133], 2020 |
| Nano micelle | In vitro | Improved controlled-release of Cur, enhanced cellular uptake, apoptotic cell death by changing the mitochondrial membrane potential | Kumbar et al[120], 2022 |
| Cur | |||
| Cur-loaded noisome | In vitro | Significant cytotoxicity compared to free curcumin after 24 h | Fazli et al[138], 2022 |
| In vivo | Injection use (systemic) was shown to be more effective than the use of mouthwash (topical) |
In 2012, Lin et al[121] conducted a study to assess the effects of curcumin microemulsion on oral cancer cell lines. They found that exposure to curcumin-containing microemulsions for a brief period produced cytotoxic effects in the cancer cells. However, adding ultrasound enhanced these effects in OSCC-25 cells[121]. This observation is likely attributable to enhanced curcumin delivery to the cell by the fusion of microemulsion droplets with cell membranes or by overcoming transport limitations via ultrasound-induced mixing and/or heating. These ingestible microemulsions can be therapeutic in concentration-adjusted doses and have tissue-targeting properties when combined with ultrasound. Studies have shown that curcumin nanoparticles (Cur-NPs) possess significantly greater bioavailability and water solubility than free curcumin[122,123]. In a 2013 study by Chang et al[103] investigating CAL27-cisplatin-resistant human oral cancer cells (CAR cells), water-soluble PLGA Cur-NPs enhanced the drug effect. Cur-NPs increased ROS production, upregulated the expression levels of cleaved caspase-3/caspase-9, cytochrome c, apoptotic protease activating factor-1, AIF, and Bax, and downregulated the expression of Bcl-2. Cur-NPs also triggered the intrinsic apoptotic pathway by regulating the function of multiple drug resistance proteins 1 (MDR1) and the production of ROS in CAR cells[103]. Previous studies have also reported that MDR1 (a cell surface permeability glycoprotein) is a significant target of Cur-NPs[124,125]. In this study, treatment with Cur-NPs decreased MDR1 mRNA and protein levels in CAR cells, indicating the induction of CAR cell apoptosis, representing a potential treatment for cisplatin-resistant oral cancer.
Curcumin is phototoxic in the presence of oxygen[126,127]. Singh et al[128] demonstrated the use of organically-modified silica nanoparticles (SiNps) as a vehicle for the delivery of curcumin in human oral cancer cells. The results showed improved uptake of curcumin and phototoxicity in cancer cells. Incubation time-dependent cytotoxicity, inhibition of NF-κB activity, suppression of NF-κB-regulated proteins involved in invasion (MMP-9), angiogenesis (via vascular endothelial growth factor), and inflammation (tumor necrosis factor α) were observed with curcumin-SiNp. These results suggest that the curcumin–SiNp formulation has significantly improved anti-cancer effects over free curcumin in the dark and upon exposure to light[128]. These findings are likely the result of increased oxidative stress induced in the cancer cells upon visible light exposure in the presence of oxygen. The curcumin–SiNp formulation also enhances the stability of curcumin at physiological pH and increases its aqueous solubility.
In 2015, Mazzarino et al[129] conducted a study on the effect of mucoadhesive polycaprolactone (PCL) nanoparticles coated with chitosan and loaded with curcumin as a treatment for oral cancer. This study used the nanoprecipitation method to prepare the chitosan-coated PCL nanoparticles with curcumin loading[130]. The nanoparticles showed mucoadhesive properties, as evidenced by interaction with the glycoprotein mucin through electrostatic forces. In vitro studies showed that these novel curcumin nanoparticles significantly decreased the viability of SCC-9 human oral cancer cells by inducing apoptosis[129]. The study also suggested that drug retention in the mucosa after treatment with chitosan-coated curcumin-loaded nanoparticles could be helpful for local therapy in numerous diseases.
Gold nanorods (GNRs) are known for their photothermal activity and inherent tumor-targeting properties[131]. In 2018, Zhu et al[132] developed a novel system for combined plasmonic photothermal therapy and chemotherapy using the tumor microenvironment and near-infrared responsive gold nanorod-drug conjugates (Au NR@Curcumin). This study tested the antitumor effects of Au NR@Curcumin on human lung, liver, and oral carcinoma cells and found that it showed more potent cytotoxicity than the free drug. Additionally, oral cancer cells demonstrated cell cycle S phase arrest. The study suggested that Au NR@Curcumin could be effective at inducing instant photothermal killing of the cancer cells, even at a low irradiation power density[132]. In 2020, Ghosh et al[133] developed a multimodal nanoconjugate by functionalizing the GNR surface with a cytotoxic nucleoside [5-fluoro-2′-deoxyuridine (FdU)]-containing DNA hairpin followed by hydrophobic complexation of curcumin. This study showed that curcumin could be noncovalently complexed into small DNA hairpins for enhanced cellular delivery. This system caused increased cytotoxicity in SCC 131 oral cancer cells when administered in combination with FdU nucleotides, demonstrating its potential for advanced cancer therapy[133].
Several studies have investigated the potential of curcumin nanoparticles in combating oral cancer. Srivastava et al[112] found that Nano-CU, a curcumin nanoparticle, exhibited chemoprotective properties against 5-fluorouracil-induced toxicity in oral cancer cells. Nano-CU was found to have an antioxidant effect, and altered the expression of apoptotic proteins Bcl-2 and Bax in treated cells[112]. Another study by Lai et al[134] explained the anticancer properties of gefitinib (Gef) and curcumin-loaded NPs in human oral cancer SAS cells in vitro and SAS cell xenografted tumors in vivo. The results indicated that γ-polyglutamic acid-coated (PGA)-Gef/Cur NPs could be internalized into SAS cells and significantly decrease the total cell viability. Both free Gef/Cur and γ-PGA-Gef/Cur NPs induced apoptotic cell death via caspase-3, caspase-9, and mitochondria-dependent pathways. In vivo studies showed that γ-PGA-Gef/Cur NPs significantly suppressed tumor size compared to the free Gef/Cur-treated group[134]. In 2022, Essawy et al[135] developed nanoparticle curcumin using a more straightforward and cost-effective solvent-antisolvent precipitation technique and studied its effect on oral cancer cells. This study found promising cytotoxic results via apoptosis in contrast to the necrotic effect observed using doxorubicin in the cell lines. The authors also reported the observed luminescence of the nanoparticle curcumin, qualifying it as a double theranostic agent[135]. In another study, co-treating oral cancer cells with nanoparticle curcumin (approximately 200 nm size) and cetuximab showed higher cytotoxicity than cetuximab alone[136]. The above mentioned studies highlight the potential chemo-adjuvant role of curcumin nanoparticles in combating oral cancer.
In 2019, Ferreira et al[137] aimed to develop nanostructured gel formulations containing curcumin for oral cancer therapy. The authors showed that the use of this novel curcumin led to rapid incorporation and localization in the hydrophobic portion of nanometer-sized polymeric micelles, resulting in increased retention after application in the oral cavity. Cytotoxicity testing showed that the formulation selectively targeted cancer cells moreso than healthy cells. Therefore, these systems may improve the physicochemical characteristics of curcumin by increasing its release and permeation and enhancing its cancer cell-targeting properties[137]. Recently in 2022, Fazli et al[138] found that curcumin-loaded niosomes significantly inhibited the growth and necrosis of oral cancer cells compared to free curcumin. Histopathological specimens from rats with induced oral cancer showed that niosome curcumin treatment effectively inhibited cancer growth. The authors also highlighted that the injectable curcumin-loaded niosome (i.e. for systemic use) was more effective than the mouthwash form of application (i.e. for topical use)[138]. In light of these promising findings, future studies should be designed to explore the outcomes of novel curcumin formulations in preclinical and clinical trials.
Oral cancer is a highly malignant disease with a poor 5-year survival rate and limited treatment options, underscoring the importance of adjuvant therapy. Curcumin, known for its pleiotropic effects and potential therapeutic benefits, has shown promise as a treatment choice for patients with cancer. This molecule has shown improvement in the efficacy of current cancer therapeutics, including overcoming the resistance of cancer cells to chemo-radio therapy. However, several clinical and practical challenges need to be addressed before curcumin can be incorporated into regular clinical practice. The purity of the curcumin compound significantly affects its activity, and is of primary importance when used in studies or trials[139]. In addition, body tissue distribution and uptake of curcumin, which account for its biological activity, need better understanding[31]. Clinical trials with curcumin have faced various challenges, such as high metabolic instability, poor aqueous solubility, inadequate focalization, complex pharmacokinetic profile, and poor patient adherence[140].
Nanotechnology-based formulations and analogues have shown potential in overcoming the poor bioavailability issue of curcumin by improving its stability, increasing its cellular uptake, and offering controlled release. However, these formulations often lack tissue specificity. Although the various novel nano-formulations of curcumin show remarkable anti-neoplastic, theranostic, and chemo-adjuvant properties, there are technical challenges in drug development, particularly the need to regulate the size of curcumin nanoparticles for drug delivery applications. In addition, these processes are expensive and have yet to be commercialized. The effects of newer delivery systems, such as polymer nanoparticles and liposomes, on the therapeutic efficacy of curcumin need to be further investigated; while these have been shown to enhance curcumin bioavailability, the possibility of off-target toxicity has not been thoroughly studied[141]. Curcumin has shown cytotoxic and cytoprotective effects at different doses and concentrations in various cancer studies[112,135,142,143]. These findings need consideration in preclinical and clinical trials investigating newer curcumin formulations. Furthermore, the wide range of research variability in human cancer studies using these novel curcumin formulations, such as differences in study design, drug design, sample size, and route of administration, also make it difficult to conclude which formulation has the best overall pharmacokinetic properties[140].
In conclusion, encouraging findings from various studies using novel curcumin formulations indicate the need for extensive preclinical and clinical research to shed light on their pharmacokinetics, biocompatibility, toxicity, and dose regimens in normal and disease conditions in order to incorporate these agents in cancer treatment strategies. Systematic efforts must focus on identifying a potential curcumin formulation suitable for use in clinical trials. Collaboration between clinicians, translational scientists, medicinal chemists, and pharmacologists is necessary to advance these agents toward clinical use as oral cancer therapeutics.
| 1. | Balakittnen J, Weeramange CE, Wallace DF, Duijf PHG, Cristino AS, Kenny L, Vasani S, Punyadeera C. Noncoding RNAs in oral cancer. Wiley Interdiscip Rev RNA. 2022;e1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68603] [Article Influence: 13720.6] [Reference Citation Analysis (201)] |
| 3. | Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 306] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56668] [Article Influence: 7083.5] [Reference Citation Analysis (135)] |
| 5. | Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sens Int. 2020;1:100046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 6. | Irani S. New Insights into Oral Cancer-Risk Factors and Prevention: A Review of Literature. Int J Prev Med. 2020;11:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Omar E. Current concepts and future of noninvasive procedures for diagnosing oral squamous cell carcinoma--a systematic review. Head Face Med. 2015;11:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Chan AK, Huang SH, Le LW, Yu E, Dawson LA, Kim JJ, Cho BC, Bayley AJ, Ringash J, Goldstein D, Chan K, Waldron J, O'Sullivan B, Cummings B, Hope AJ. Postoperative intensity-modulated radiotherapy following surgery for oral cavity squamous cell carcinoma: patterns of failure. Oral Oncol. 2013;49:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull. 2017;7:339-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 1276] [Article Influence: 141.8] [Reference Citation Analysis (3)] |
| 10. | Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015;20:2728-2769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 11. | Giordano A, Tommonaro G. Curcumin and Cancer. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 649] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 12. | Zhen L, Fan D, Yi X, Cao X, Chen D, Wang L. Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EGFR signaling pathways. Int J Clin Exp Pathol. 2014;7:6438-6446. [PubMed] |
| 13. | Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 504] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Shome S, Talukdar AD, Choudhury MD, Bhattacharya MK, Upadhyaya H. Curcumin as potential therapeutic natural product: a nanobiotechnological perspective. J Pharm Pharmacol. 2016;68:1481-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Dash TK, Konkimalla VSB. Selection and optimization of nano-formulation of P-glycoprotein inhibitor for reversal of doxorubicin resistance in COLO205 cells. J Pharm Pharmacol. 2017;69:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Le UM, Hartman A, Pillai G. Enhanced selective cellular uptake and cytotoxicity of epidermal growth factor-conjugated liposomes containing curcumin on EGFR-overexpressed pancreatic cancer cells. J Drug Target. 2018;26:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (35)] |
| 17. | Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24:491-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 420] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 19. | Ganly I, Goldstein D, Carlson DL, Patel SG, O'Sullivan B, Lee N, Gullane P, Shah JP. Long-term regional control and survival in patients with "low-risk," early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Zhang H, Dziegielewski PT, Biron VL, Szudek J, Al-Qahatani KH, O'Connell DA, Harris JR, Seikaly H. Survival outcomes of patients with advanced oral cavity squamous cell carcinoma treated with multimodal therapy: a multi-institutional analysis. J Otolaryngol Head Neck Surg. 2013;42:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Lima AM, Meira IA, Soares MS, Bonan PR, Mélo CB, Piagge CS. Delay in diagnosis of oral cancer: a systematic review. Med Oral Patol Oral Cir Bucal. 2021;26:e815-e824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Sasahira T, Kirita T, Kuniyasu H. Update of molecular pathobiology in oral cancer: a review. Int J Clin Oncol. 2014;19:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Das T, Sa G, Saha B, Das K. Multifocal signal modulation therapy of cancer: ancient weapon, modern targets. Mol Cell Biochem. 2010;336:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and Health. Molecules. 2016;21:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 26. | Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 553] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 27. | Gera M, Sharma N, Ghosh M, Huynh DL, Lee SJ, Min T, Kwon T, Jeong DK. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget. 2017;8:66680-66698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 28. | Karthikeyan A, Senthil N, Min T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front Pharmacol. 2020;11:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 29. | Sala de Oyanguren FJ, Rainey NE, Moustapha A, Saric A, Sureau F, O'Connor JE, Petit PX. Highlighting Curcumin-Induced Crosstalk between Autophagy and Apoptosis as Supported by Its Specific Subcellular Localization. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19:20091-20112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 659] [Cited by in RCA: 861] [Article Influence: 71.8] [Reference Citation Analysis (1)] |
| 31. | Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3258] [Cited by in RCA: 3778] [Article Influence: 198.8] [Reference Citation Analysis (0)] |
| 32. | Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 425] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 33. | Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G. New insights into therapeutic activity and anticancer properties of curcumin. J Exp Pharmacol. 2017;9:31-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 34. | Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847-6854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 928] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 35. | Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors. 2013;39:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486-494. [PubMed] |
| 37. | Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105-111. [PubMed] |
| 38. | Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta. 2008;1780:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Pandey A, Chaturvedi M, Mishra S, Kumar P, Somvanshi P, Chaturvedi R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon. 2020;6:e05469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Pal A, Sung B, Bhanu Prasad BA, Schuber PT Jr, Prasad S, Aggarwal BB, Bornmann WG. Curcumin glucuronides: assessing the proliferative activity against human cell lines. Bioorg Med Chem. 2014;22:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Elattar TM, Virji AS. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res. 2000;20:1733-1738. [PubMed] |
| 42. | Ip SW, Wu SY, Yu CC, Kuo CL, Yu CS, Yang JS, Lin ZP, Chiou SM, Chung HK, Ho HC, Chung JG. Induction of apoptotic death by curcumin in human tongue squamous cell carcinoma SCC-4 cells is mediated through endoplasmic reticulum stress and mitochondria-dependent pathways. Cell Biochem Funct. 2011;29:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH, Park HR. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol. 2012;57:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Hsiao YT, Kuo CL, Chueh FS, Liu KC, Bau DT, Chung JG. Curcuminoids Induce Reactive Oxygen Species and Autophagy to Enhance Apoptosis in Human Oral Cancer Cells. Am J Chin Med. 2018;46:1145-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Rinaldi AL, Morse MA, Fields HW, Rothas DA, Pei P, Rodrigo KA, Renner RJ, Mallery SR. Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits (-)-benzo(a)pyrene-7R-trans-7,8-dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Res. 2002;62:5451-5456. [PubMed] |
| 46. | Lee SS, Tsai CH, Yu CC, Chang YC. Elevated snail expression mediates tumor progression in areca quid chewing-associated oral squamous cell carcinoma via reactive oxygen species. PLoS One. 2013;8:e67985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Gonçalves Vde P, Ortega AA, Guimarães MR, Curylofo FA, Rossa Junior C, Ribeiro DA, Spolidorio LC. Chemopreventive activity of systemically administered curcumin on oral cancer in the 4-nitroquinoline 1-oxide model. J Cell Biochem. 2015;116:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Khandelwal AR, Moore-Medlin T, Ekshyyan O, Gu X, Abreo F, Nathan CO. Local and systemic Curcumin C3 complex inhibits 4NQO-induced oral tumorigenesis via modulating FGF-2/FGFR-2 activation. Am J Cancer Res. 2018;8:2538-2547. [PubMed] |
| 49. | Mishra A, Kumar R, Tyagi A, Kohaar I, Hedau S, Bharti AC, Sarker S, Dey D, Saluja D, Das B. Curcumin modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience. 2015;9:525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Chang KW, Hung PS, Lin IY, Hou CP, Chen LK, Tsai YM, Lin SC. Curcumin upregulates insulin-like growth factor binding protein-5 (IGFBP-5) and C/EBPalpha during oral cancer suppression. Int J Cancer. 2010;127:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Maulina T, Hadikrishna I, Hardianto A, Sjamsudin E, Pontjo B, Yusuf HY. The therapeutic activity of curcumin through its anti-cancer potential on oral squamous cell carcinoma: A study on Sprague Dawley rat. SAGE Open Med. 2019;7:2050312119875982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Lu WC, Kao SY, Yang CC, Tu HF, Wu CH, Chang KW, Lin SC. EGF up-regulates miR-31 through the C/EBPβ signal cascade in oral carcinoma. PLoS One. 2014;9:e108049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Xiao C, Wang L, Zhu L, Zhang C, Zhou J. Curcumin inhibits oral squamous cell carcinoma SCC-9 cells proliferation by regulating miR-9 expression. Biochem Biophys Res Commun. 2014;454:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Liao F, Liu L, Luo E, Hu J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol. 2018;92:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 55. | Datar UV, Kale AD, Angadi PV, Hallikerimath S, Deepa M, Desai KM. Role of cancer-associated fibroblasts in oral squamous cell carcinomas, surgical margins, and verrucous carcinomas: An immunohistochemical study. J Clin Transl Res. 2022;8:80-85. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Ba P, Xu M, Yu M, Li L, Duan X, Lv S, Fu G, Yang J, Yang P, Yang C, Sun Q. Curcumin suppresses the proliferation and tumorigenicity of Cal27 by modulating cancer-associated fibroblasts of TSCC. Oral Dis. 2020;26:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Dudás J, Fullár A, Romani A, Pritz C, Kovalszky I, Hans Schartinger V, Mathias Sprinzl G, Riechelmann H. Curcumin targets fibroblast-tumor cell interactions in oral squamous cell carcinoma. Exp Cell Res. 2013;319:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Owusu BY, Galemmo R, Janetka J, Klampfer L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Ohnishi Y, Sakamoto T, Zhengguang L, Yasui H, Hamada H, Kubo H, Nakajima M. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-Met blockade. Oncol Lett. 2020;19:4177-4182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | de Campos PS, Matte BF, Diel LF, Jesus LH, Bernardi L, Alves AM, Rados PV, Lamers ML. Low Doses of Curcuma longa Modulates Cell Migration and Cell-Cell Adhesion. Phytother Res. 2017;31:1433-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Tsang RK, Tang WW, Gao W, Ho WK, Chan JY, Wei WI, Wong TS. Curcumin inhibits tongue carcinoma cells migration and invasion through downregulation of matrix metallopeptidase 10. Cancer Invest. 2012;30:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Lee AY, Fan CC, Chen YA, Cheng CW, Sung YJ, Hsu CP, Kao TY. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr Cancer Ther. 2015;14:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, Faull KF, Srivatsan ES, Wang MB. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the NFκB pathway. Mol Cancer Ther. 2010;9:2665-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Sivanantham B, Sethuraman S, Krishnan UM. Combinatorial Effects of Curcumin with an Anti-Neoplastic Agent on Head and Neck Squamous Cell Carcinoma Through the Regulation of EGFR-ERK1/2 and Apoptotic Signaling Pathways. ACS Comb Sci. 2016;18:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Chiang IT, Liu YC, Hsu FT, Chien YC, Kao CH, Lin WJ, Chung JG, Hwang JJ. Curcumin synergistically enhances the radiosensitivity of human oral squamous cell carcinoma via suppression of radiation-induced NF-κB activity. Oncol Rep. 2014;31:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Chen CF, Lu CC, Chiang JH, Chiu HY, Yang JS, Lee CY, Way TD, Huang HJ. Synergistic inhibitory effects of cetuximab and curcumin on human cisplatin-resistant oral cancer CAR cells through intrinsic apoptotic process. Oncol Lett. 2018;16:6323-6330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Siddappa G, Kulsum S, Ravindra DR, Kumar VV, Raju N, Raghavan N, Sudheendra HV, Sharma A, Sunny SP, Jacob T, Kuruvilla BT, Benny M, Antony B, Seshadri M, Lakshminarayan P, Hicks W Jr, Suresh A, Kuriakose MA. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol Carcinog. 2017;56:2446-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Molla S, Hembram KC, Chatterjee S, Nayak D, Sethy C, Pradhan R, Kundu CN. PARP inhibitor Olaparib Enhances the Apoptotic Potentiality of Curcumin by Increasing the DNA Damage in Oral Cancer Cells through Inhibition of BER Cascade. Pathol Oncol Res. 2020;26:2091-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Kuriakose MA, Ramdas K, Dey B, Iyer S, Rajan G, Elango KK, Suresh A, Ravindran D, Kumar RR, R P, Ramachandran S, Kumar NA, Thomas G, Somanathan T, Ravindran HK, Ranganathan K, Katakam SB, Parashuram S, Jayaprakash V, Pillai MR. A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev Res (Phila). 2016;9:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 70. | Normando AGC, de Menêses AG, de Toledo IP, Borges GÁ, de Lima CL, Dos Reis PED, Guerra ENS. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytother Res. 2019;33:1318-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | The Effect of Curcumin for Treatment of Cancer Anorexia-Cachexia Syndrome in Patients With Stage III-IV of Head and Neck Cancer. In: ClinicalTrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04208334 ClinicalTrials.gov Identifier: NCT04208334. |
| 72. | Mahal A, Wu P, Jiang ZH, Wei X. Synthesis and Cytotoxic Activity of Novel Tetrahydrocurcumin Derivatives Bearing Pyrazole Moiety. Nat Prod Bioprospect. 2017;7:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Nardo L, Andreoni A, Masson M, Haukvik T, Tønnesen HH. Studies on curcumin and curcuminoids. XXXIX. Photophysical properties of bisdemethoxycurcumin. J Fluoresc. 2011;21:627-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Adeluola A, Zulfiker AHM, Brazeau D, Amin ARMR. Perspectives for synthetic curcumins in chemoprevention and treatment of cancer: An update with promising analogues. Eur J Pharmacol. 2021;906:174266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Shen H, Shen J, Pan H, Xu L, Sheng H, Liu B, Yao M. Curcumin analog B14 has high bioavailability and enhances the effect of anti-breast cancer cells in vitro and in vivo. Cancer Sci. 2021;112:815-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Momtazi AA, Sahebkar A. Difluorinated Curcumin: A Promising Curcumin Analogue with Improved Anti-Tumor Activity and Pharmacokinetic Profile. Curr Pharm Des. 2016;22:4386-4397. [PubMed] [DOI] [Full Text] |
| 77. | Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl ester: a curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: potential for prostate cancer management. Neoplasia. 2003;5:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 79. | Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 80. | Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Reid JM, Buhrow SA, Gilbert JA, Jia L, Shoji M, Snyder JP, Ames MM. Mouse pharmacokinetics and metabolism of the curcumin analog, 4-piperidinone,3,5-bis[(2-fluorophenyl)methylene]-acetate(3E,5E) (EF-24; NSC 716993). Cancer Chemother Pharmacol. 2014;73:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Bertazza L, Sensi F, Cavedon E, Watutantrige-Fernando S, Censi S, Manso J, Vianello F, Casal Ide E, Iacobone M, Pezzani R, Mian C, Barollo S. EF24 (a Curcumin Analog) and ZSTK474 Emphasize the Effect of Cabozantinib in Medullary Thyroid Cancer. Endocrinology. 2018;159:2348-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Wu X, Tang W, Marquez RT, Li K, Highfill CA, He F, Lian J, Lin J, Fuchs JR, Ji M, Li L, Xu L. Overcoming chemo/radio-resistance of pancreatic cancer by inhibiting STAT3 signaling. Oncotarget. 2016;7:11708-11723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 84. | Somers-Edgar TJ, Taurin S, Larsen L, Chandramouli A, Nelson MA, Rosengren RJ. Mechanisms for the activity of heterocyclic cyclohexanone curcumin derivatives in estrogen receptor negative human breast cancer cell lines. Invest New Drugs. 2011;29:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Zhang J, Feng Z, Wang C, Zhou H, Liu W, Kanchana K, Dai X, Zou P, Gu J, Cai L, Liang G. Curcumin derivative WZ35 efficiently suppresses colon cancer progression through inducing ROS production and ER stress-dependent apoptosis. Am J Cancer Res. 2017;7:275-288. [PubMed] |
| 86. | Basak SK, Zinabadi A, Wu AW, Venkatesan N, Duarte VM, Kang JJ, Dalgard CL, Srivastava M, Sarkar FH, Wang MB, Srivatsan ES. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget. 2015;6:18504-18517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Anisuzzaman AS, Haque A, Rahman MA, Wang D, Fuchs JR, Hurwitz S, Liu Y, Sica G, Khuri FR, Chen ZG, Shin DM, Amin AR. Preclinical In Vitro, In Vivo, and Pharmacokinetic Evaluations of FLLL12 for the Prevention and Treatment of Head and Neck Cancers. Cancer Prev Res (Phila). 2016;9:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Chuprajob T, Changtam C, Chokchaisiri R, Chunglok W, Sornkaew N, Suksamrarn A. Synthesis, cytotoxicity against human oral cancer KB cells and structure-activity relationship studies of trienone analogues of curcuminoids. Bioorg Med Chem Lett. 2014;24:2839-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Lin C, Tu C, Ma Y, Ye P, Shao X, Yang Z, Fang Y. Curcumin analog EF24 induces apoptosis and downregulates the mitogen activated protein kinase/extracellular signal-regulated signaling pathway in oral squamous cell carcinoma. Mol Med Rep. 2017;16:4927-4933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Chen W, Zou P, Zhao Z, Weng Q, Chen X, Ying S, Ye Q, Wang Z, Ji J, Liang G. Selective killing of gastric cancer cells by a small molecule via targeting TrxR1 and ROS-mediated ER stress activation. Oncotarget. 2016;7:16593-16609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Zhou B, Zuo Y, Li B, Wang H, Liu H, Wang X, Qiu X, Hu Y, Wen S, Du J, Bu X. Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analog AC17 causes NF-κB inhibition and p53 reactivation in human lung cancer cells. Mol Cancer Ther. 2013;12:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Wang Z, Sau S, Alsaab HO, Iyer AK. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomedicine. 2018;14:1441-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Kesharwani P, Xie L, Banerjee S, Mao G, Padhye S, Sarkar FH, Iyer AK. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf B Biointerfaces. 2015;136:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 94. | Luong D, Kesharwani P, Killinger BA, Moszczynska A, Sarkar FH, Padhye S, Rishi AK, Iyer AK. Solubility enhancement and targeted delivery of a potent anticancer flavonoid analogue to cancer cells using ligand decorated dendrimer nano-architectures. J Colloid Interface Sci. 2016;484:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Shoji M, Sun A, Kisiel W, Lu YJ, Shim H, McCarey BE, Nichols C, Parker ET, Pohl J, Mosley CA, Alizadeh AR, Liotta DC, Snyder JP. Targeting tissue factor-expressing tumor angiogenesis and tumors with EF24 conjugated to factor VIIa. J Drug Target. 2008;16:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Moselhy S, Razvi S, Hasan N, Balamash K, Abualnaja K, Yaghmoor S, Youssri A, Kumosani A, Al-Malki L. Multifaceted Role of a Marvel Golden Molecule, Curcumin: a Review. Indian J Pharm Sci. 2018;80:400-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Wang S, Tan M, Zhong Z, Chen M, Wang Y. Nanotechnologies for Curcumin: An Ancient Puzzler Meets Modern Solutions. J Nanomater. 2011;. [DOI] [Full Text] |
| 98. | Feng T, Wei Y, Lee RJ, Zhao L. Liposomal curcumin and its application in cancer. Int J Nanomedicine. 2017;12:6027-6044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 99. | Mozafari MR. Nanoliposomes: preparation and analysis. Methods Mol Biol. 2010;605:29-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 100. | Liu M, Du H, Zhai G. The Design of Amphiphilic Polymeric Micelles of Curcumin for Cancer Management. Curr Med Chem. 2015;22:4398-4411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Kia SJ, Basirat M, Saedi HS, Arab SA. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: a randomized clinical trial. BMC Complement Med Ther. 2021;21:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Delavarian Z, Pakfetrat A, Ghazi A, Jaafari MR, Homaei Shandiz F, Dalirsani Z, Mohammadpour AH, Rahimi HR. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec Care Dentist. 2019;39:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 103. | Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC, Shieh TM, Wu TS, Tu MG, Chen MY, Yang JS. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int J Oncol. 2013;43:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 104. | Wang W, Chen T, Xu H, Ren B, Cheng X, Qi R, Liu H, Wang Y, Yan L, Chen S, Yang Q, Chen C. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Zhang L, Man S, Qiu H, Liu Z, Zhang M, Ma L, Gao W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ Toxicol Pharmacol. 2016;48:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 106. | Hu L, Shi Y, Li JH, Gao N, Ji J, Niu F, Chen Q, Yang X, Wang S. Enhancement of Oral Bioavailability of Curcumin by a Novel Solid Dispersion System. AAPS PharmSciTech. 2015;16:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 107. | Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, Kumar D, Jaggi M, Chauhan SC. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine. 2012;7:1761-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Madhavi M, Madhavi K, Jithan AV. Preparation and in vitro/in vivo characterization of curcumin microspheres intended to treat colon cancer. J Pharm Bioallied Sci. 2012;4:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Chirio D, Peira E, Dianzani C, Muntoni E, Gigliotti CL, Ferrara B, Sapino S, Chindamo G, Gallarate M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials (Basel). 2019;9:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Lu PS, Inbaraj BS, Chen BH. Determination of oral bioavailability of curcuminoid dispersions and nanoemulsions prepared from Curcuma longa Linnaeus. J Sci Food Agric. 2018;98:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Mangalathillam S, Rejinold NS, Nair A, Lakshmanan VK, Nair SV, Jayakumar R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale. 2012;4:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 112. | Srivastava S, Mohammad S, Gupta S, Mahdi AA, Dixit RK, Singh V, Samadi FM. Chemoprotective effect of nanocurcumin on 5-fluorouracil-induced-toxicity toward oral cancer treatment. Natl J Maxillofac Surg. 2018;9:160-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Tousif S, Singh DK, Mukherjee S, Ahmad S, Arya R, Nanda R, Ranganathan A, Bhattacharyya M, Van Kaer L, Kar SK, Das G. Nanoparticle-Formulated Curcumin Prevents Posttherapeutic Disease Reactivation and Reinfection with Mycobacterium tuberculosis following Isoniazid Therapy. Front Immunol. 2017;8:739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Nehra S, Bhardwaj V, Bansal A, Chattopadhyay P, Saraswat D. Nanocurcumin-pyrroloquinoline formulation prevents hypertrophy-induced pathological damage by relieving mitochondrial stress in cardiomyocytes under hypoxic conditions. Exp Mol Med. 2017;49:e404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Ge X, Wei M, He S, Yuan WE. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 315] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 116. | Duan X, Chan C, Lin W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew Chem Int Ed Engl. 2019;58:670-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 724] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 117. | Desai P, Ann D, Wang J, Prabhu S. Pancreatic Cancer: Recent Advances in Nanoformulation-Based Therapies. Crit Rev Ther Drug Carrier Syst. 2019;36:59-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Yallapu MM, Nagesh PK, Jaggi M, Chauhan SC. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015;17:1341-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 119. | Gosangari SL, Watkin KL. Effect of preparation techniques on the properties of curcumin liposomes: characterization of size, release and cytotoxicity on a squamous oral carcinoma cell line. Pharm Dev Technol. 2012;17:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Kumbar VM, Muddapur U, Bin Muhsinah A, Alshehri SA, Alshahrani MM, Almazni IA, Kugaji MS, Bhat K, Peram MR, Mahnashi MH, Nadaf SJ, Rooge SB, Khan AA, Shaikh IA. Curcumin-Encapsulated Nanomicelles Improve Cellular Uptake and Cytotoxicity in Cisplatin-Resistant Human Oral Cancer Cells. J Funct Biomater. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 121. | Lin HY, Thomas JL, Chen HW, Shen CM, Yang WJ, Lee MH. In vitro suppression of oral squamous cell carcinoma growth by ultrasound-mediated delivery of curcumin microemulsions. Int J Nanomedicine. 2012;7:941-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Luz PP, Magalhães LG, Pereira AC, Cunha WR, Rodrigues V, Andrade E Silva ML. Curcumin-loaded into PLGA nanoparticles: preparation and in vitro schistosomicidal activity. Parasitol Res. 2012;110:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 123. | Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 124. | Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6:928-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 125. | Punfa W, Yodkeeree S, Pitchakarn P, Ampasavate C, Limtrakul P. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin. 2012;33:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 126. | Dahl TA, Bilski P, Reszka KJ, Chignell CF. Photocytotoxicity of curcumin. Photochem Photobiol. 1994;59:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Banaspati A, Ramu V, Raza MK, Goswami TK. Copper(ii) curcumin complexes for endoplasmic reticulum targeted photocytotoxicity. RSC Adv. 2022;12:30722-30733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 128. | Singh SP, Sharma M, Gupta PK. Enhancement of phototoxicity of curcumin in human oral cancer cells using silica nanoparticles as delivery vehicle. Lasers Med Sci. 2014;29:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | Mazzarino L, Loch-Neckel G, Bubniak Ldos S, Mazzucco S, Santos-Silva MC, Borsali R, Lemos-Senna E. Curcumin-Loaded Chitosan-Coated Nanoparticles as a New Approach for the Local Treatment of Oral Cavity Cancer. J Nanosci Nanotechnol. 2015;15:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 130. | Mazzarino L, Travelet C, Ortega-Murillo S, Otsuka I, Pignot-Paintrand I, Lemos-Senna E, Borsali R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J Colloid Interface Sci. 2012;370:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 131. | Tong X, Wang Z, Sun X, Song J, Jacobson O, Niu G, Kiesewetter DO, Chen X. Size Dependent Kinetics of Gold Nanorods in EPR Mediated Tumor Delivery. Theranostics. 2016;6:2039-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 132. | Zhu F, Tan G, Jiang Y, Yu Z, Ren F. Rational design of multi-stimuli-responsive gold nanorod-curcumin conjugates for chemo-photothermal synergistic cancer therapy. Biomater Sci. 2018;6:2905-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 133. | Ghosh S, Mallick S, Das U, Verma A, Pal U, Chatterjee S, Nandy A, Saha KD, Maiti NC, Baishya B, Suresh Kumar G, Gmeiner WH. Curcumin stably interacts with DNA hairpin through minor groove binding and demonstrates enhanced cytotoxicity in combination with FdU nucleotides. Biochim Biophys Acta Gen Subj. 2018;1862:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Lai KC, Chueh FS, Hsiao YT, Cheng ZY, Lien JC, Liu KC, Peng SF, Chung JG. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol Appl Pharmacol. 2019;382:114734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 135. | Essawy MM, Mohamed MM, Raslan HS, Rafik ST, Awaad AK, Ramadan OR. The theranostic potentialities of bioavailable nanocurcumin in oral cancer management. BMC Complement Med Ther. 2022;22:309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 136. | Mukherjee D, Dash P, Ramadass B, Mangaraj M. Nanocurcumin in Oral Squamous Cancer Cells and Its Efficacy as a Chemo-Adjuvant. Cureus. 2022;14:e24678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 137. | Ferreira SBS, Braga G, Oliveira ÉL, da Silva JB, Rosseto HC, de Castro Hoshino LV, Baesso ML, Caetano W, Murdoch C, Colley HE, Bruschi ML. Design of a nanostructured mucoadhesive system containing curcumin for buccal application: from physicochemical to biological aspects. Beilstein J Nanotechnol. 2019;10:2304-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Fazli B, Irani S, Bardania H, Moosavi MS, Rohani B. Prophylactic effect of topical (slow-release) and systemic curcumin nano-niosome antioxidant on oral cancer in rat. BMC Complement Med Ther. 2022;22:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 139. | Pan Y, Ju R, Cao X, Pei H, Zheng T, Wang W. Optimization extraction and purification of biological activity curcumin from Curcuma longa L by high-performance counter-current chromatography. J Sep Sci. 2020;43:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Hassanzadeh K, Buccarello L, Dragotto J, Mohammadi A, Corbo M, Feligioni M. Obstacles against the Marketing of Curcumin as a Drug. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 141. | Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The Essential Medicinal Chemistry of Curcumin. J Med Chem. 2017;60:1620-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 934] [Cited by in RCA: 1293] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 142. | Atia MM, Abdel-Tawab HS, Mostafa AM, Mobarak SA. Nanocurcumin and curcumin prevent N, N'-methylenebisacrylamide-induced liver damage and promotion of hepatic cancer cell growth. Sci Rep. 2022;12:8319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 143. | Tu ZS, Wang Q, Sun DD, Dai F, Zhou B. Design, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative death. Eur J Med Chem. 2017;134:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Noncommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Liu J, China S-Editor: Liu XF L-Editor: Filipodia P-Editor: Ju JL