Published online Sep 20, 2022. doi: 10.5662/wjm.v12.i5.381
Peer-review started: March 6, 2022
First decision: April 12, 2022
Revised: April 22, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: September 20, 2022
Processing time: 194 Days and 6 Hours
This review provides an update on the epidemiology, pathophysiology, sym
Core Tip: There are reviews in the literature regarding neuroendocrine tumors in the gastrointestinal tract specifically in the small bowel. Nevertheless, this is a first mini review to synthesize the latest data related to epidemiology, pathophysiology, clinical manifestations, diagnosis and treatment of small bowel neuroendocrine tumors.
- Citation: Gonzáles-Yovera JG, Roseboom PJ, Concepción-Zavaleta M, Gutiérrez-Córdova I, Plasencia-Dueñas E, Quispe-Flores M, Ramos-Yataco A, Alcalde-Loyola C, Massucco-Revoredo F, Paz-Ibarra J, Concepción-Urteaga L. Diagnosis and management of small bowel neuroendocrine tumors: A state-of-the-art. World J Methodol 2022; 12(5): 381-391
- URL: https://www.wjgnet.com/2222-0682/full/v12/i5/381.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i5.381
Langhans was the first person to describe small bowel (SB) neuroendocrine neoplasms (NENs) in 1867, as a polypoid tumor of the small intestine[1]. Nowadays, NENs are described as a heterogeneous group of neoplasms derived from neuroendocrine cells. The term NENs encompasses well-differentiated NENs and poorly differentiated neuroendocrine carcinomas (NECs)[2]. NENs commonly arise from the gastrointestinal tract[3,4,40].
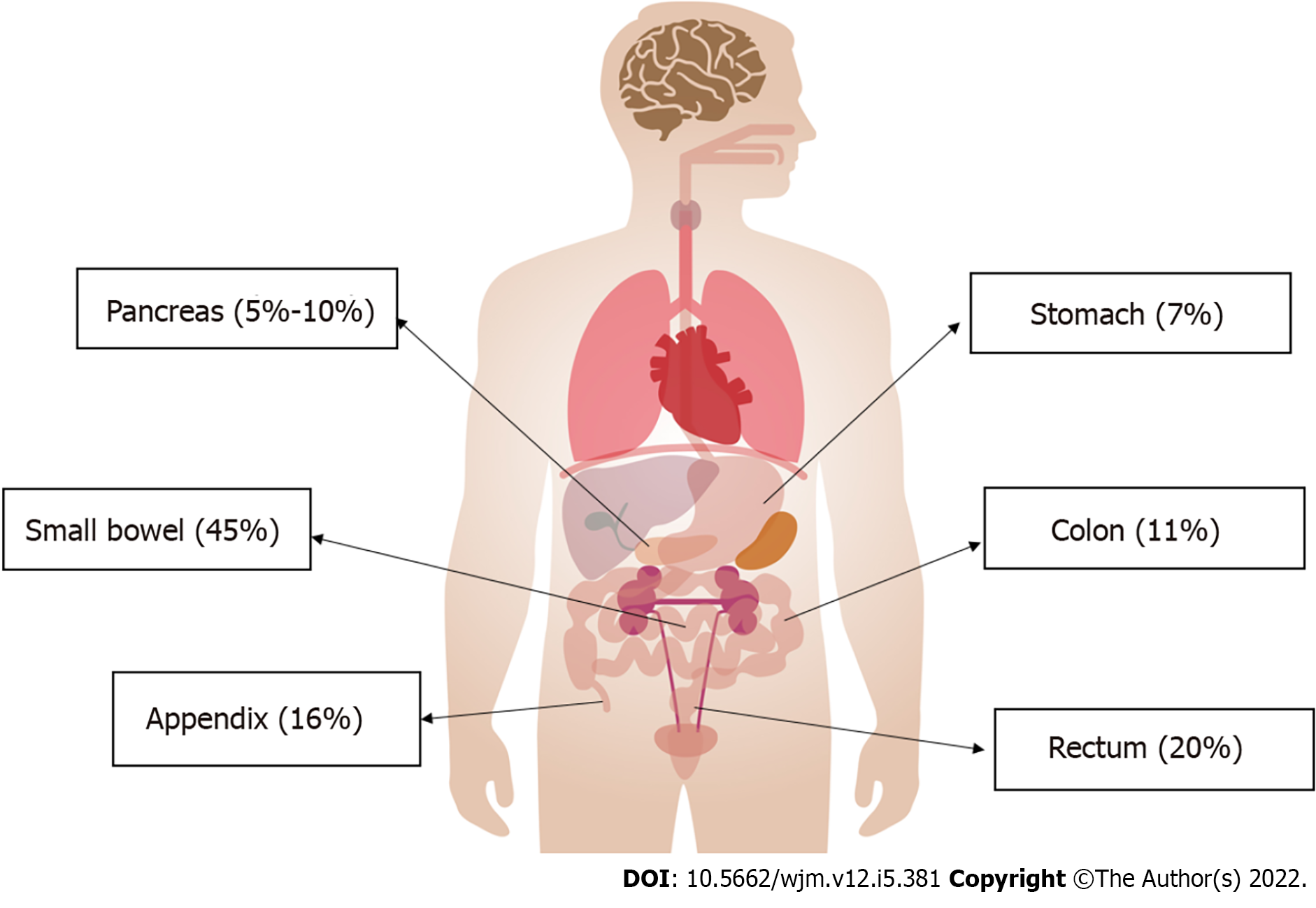
NENs can progress throughout the gastrointestinal tract, but are specifically seen in the small intestine (45%), rectum (20%), appendix (16%), colon (11%), pancreas (5%-10%) and stomach (7%)[5] (Figure 1).
NENs account for 1.0%-1.5% of all gastroenteropancreatic neoplasms[6]. SB NENs continue to increase in incidence and are today the most frequent primary malignancies of the SB[2]. This growing phenomenon seen since the 1970s is possibly due to the detection of early-stage disease[7,8].
The aim of this manuscript is to carry out not only an updated narrative review on the diagnosis and treatment but also to synthesize the data related to epidemiology, pathophysiology and clinical manifestation of SB neuroendocrine tumors.
We conducted a bibliographic review using articles indexed in PubMed/Medline, Scopus, Embase and Scielo, published between 2000 and 2022. The Medical Subject Headings used were: "Neuroendocrine Tumors”, and "Small Bowel” or “Small Intestine”. The research was limited to human-related articles. The type of articles included were: Clinical trials, prospective cohort studies, retrospective and cross-sectional studies, as well as systematic reviews and meta-analyses.
The quality of our narrative review was assessed using the SANRA scale[9], which covered the following topics: Description of the literature search, statement of the review aims, referencing, explanation of the review’s importance, presentation of relevant and appropriate endpoint data and scientific reasoning.
Small bowel cancers represent only 0.6% of all cancers and less than 5% of gastrointestinal (GI) cancers according to figures from the United States[10]. However, its incidence is increasing, reaching a growth of over a 100% in the last 40 years[10].
The two main types of SB neoplasms are adenocarcinoma of the small intestine, and NENs. Although in the 1980s, adenocarcinoma was predominant with 42%, in 2005 it had decreased to 33%, while NEN at the same time increased from 28% to 44%, positioning itself as the most frequent type of primary tumor[11]. Yao et al[6] also reported an increase in NENs of 6.4-fold from 1.31/100000 to 6.98/100000 over the timeline 1973 to 2016. In 2020, the incidence of NENs of the small intestine was estimated to be 1.2 cases per 100000 population in the United States[2].
It is thought that the rise in cases is the result of the development of better diagnostic methods, as often they are detected incidentally in endoscopic or imaging studies[11,12]. In addition, the increase in NENs compared to adenocarcinomas may be explained by the increased survival of patients with small intestine cancers, as NENs usually have a better prognosis[11].
Unlike adenocarcinomas, which are more frequent in the duodenum, small intestine NENs are more frequent in the ileum[11]. Some genetic mutations predispose to the development of NENs. The most common predisposing condition is multiple endocrine neoplasia type 1 and represents around 5 to 10% of these tumors[11].
Studies reporting the duration of symptoms preceding diagnosis varies widely, from a median of 4.3 mo up to 9.2 years[1]. Liver metastasis is seen in as many as 61%-91% at the time of diagnosis[11]. Among the risk factors associated with metastatic disease are the location in the jejunum or an unspecified site, the histology of neuroendocrine carcinoma and being a patient from a rural area[13].
The median overall survival (OS) of SB NENs is 14 years, while localized and well differentiated tumors showed a better survival. In multivariate analyses, factors that had a significant correlation were race, age, stage and site. In contrast to pancreas NENs, patients with bowel NENs are 1.5 times more likely to survive[11].
There is no objective way to define the prognosis of these patients; however, tools have been created such as the Modlin Score Nomogram that addresses 15 parameters whose objective is to determine the prognosis and guide treatment[14]. The use of this tool in tertiary referral hospitals made it possible to identify patients accurately with low and high risk of death, although Kelly and co-authors in their 2019 study indicated that it was not applicable to all patients[15].
The Epidemiology and End Results (SEER) database included 73782 patients diagnosed with NENs between 1973 and 2014 in a surveillance analysis. SB NENs were found to be the second tumor with the best prognosis, after rectum NENs[16]. Summing up the localized, regional and metastatic forms of the disease, despite the heterogeneity of these tumors, the decrease in mortality rates of all forms is well-known, regardless of an increasing incidence. In addition, although comparisons between studies is difficult due to different patient classifications, cohorts and methodology, the observations in diagnostic and therapeutic advances made, are usually similar[13].
Neuroendocrine cells release hormones by stimulation of the nervous system. They are found throughout the body, such as in the skin, lungs, gonads, pancreas, the GI tract, pituitary gland and adrenal glands. NENs are neoplasms that originate from these cells. Depending on their location, their clinical behavior is very heterogeneous[2] (Figure 2).
SB NENs are often small, multifocal, difficult to locate pre-surgically, and may not be found during surgical exploration[17]. They represent 30% of neoplasms found in the SB[18]. By definition, SB NENs are located between the ligament of Treitz and the ileocecal valve. Although duodenal NENs are sometimes included with jejunal and ileal NENs under the umbrella term "SB NEN", these tumors are clinically and biologically different and should not be considered as representatives of the same entity[1].
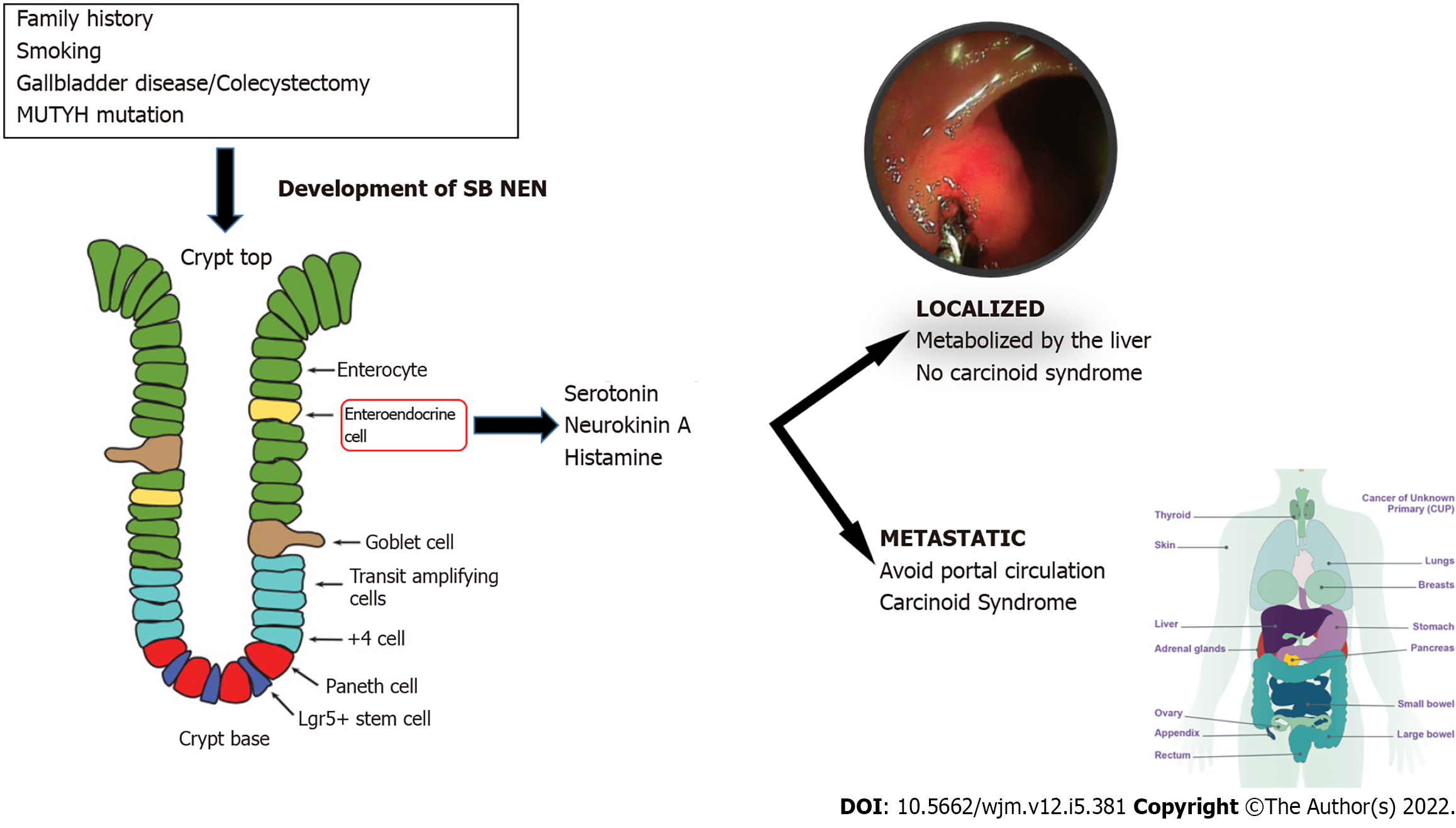
After the lung, the small intestine is the next most common location for NENs[1]. The risk factors that increase the incidence of SB NENs, have to be considered and include: A habit of smoking[19], a possible family history of cancer and the antecedent of gallbladder disease and cholecystectomy. All of them are associated with a 1.5-fold higher risk of developing SB NEN[20].
Mutation of the MutY human homologue (MYH) gene is associated with SB neuroendocrine tumors and is the main genetic background described in DNA base repair by excision[21-23] which fail in a hereditary form of SB NEN. Clinically, hereditary forms tend to be isolated endocrinopathies; however, further research is necessary.
SB NENs present in many forms, depending on the stage of the disease and the tumor burden at diagnosis. Approximately 30% of patients with SB NENs will have metastasis at the time of diagnosis, and another 40% will have regional lymph node involvement[5]. Primary tumors, in spite of being characteristically small, may cause an extensive fibrotic reaction in the SB and mesentery, resulting in narrowing or twisting of the intestine and potentiate mesenteric ischemia[1].
Metastasis of SB NENs is most commonly from the frequently seen primary site of both the small intestine as well as the pancreas. Some patients with SB NENs have synchronous or metachronous pancreatic NENs (PNENs), and it is frequently unclear whether these are separate primary tumors or metastasis. In a case series, in almost two-third of the evaluated patients, the pancreatic tumor was a metastasis of the SB NEN primary tumor, while in the remaining third of patients it represented a separate primary tumor. Determining the origin of these tumors can guide the choice of systemic therapy and surgical management[24].
The clinical manifestations are caused by the location of the primary NEN and its functionality. Most of them are non-functional, which usually have no or very few symptoms in the early stages of the disease; late symptoms are due to its mass effect or liver metastasis[25-28].
In general, the most common symptom of intestinal NENs is nonspecific abdominal pain that leads to Computing Imaging studies. Intestinal NENs can present with GI bleeding and anemia. Occasionally, NENs grow large enough to obstruct the extrahepatic bile duct or GI tract, causing jaundice or intestinal obstruction, respectively. Rarely, an intra-abdominal mass is palpable on physical examination, prompting further diagnostic studies[26]. In addition, around 15%-20% of SB NENs are symptomless and are detected incidentally, which is more frequent in patients with localized disease[1].
In patients with metastatic disease, about 10% develop carcinoid syndrome (CS), with predominance in liver metastasis. Of the wide variety of manifestations, the main manifestations are: Facial flushing (94%), diarrhea (78%), abdominal cramps (50%), heart valve disease (50%), telangiectasia (25%), wheezing (15%) and edema (19%)[29]. Almost all SB NENs produce a wide variety of biologically active peptides, including serotonin, neurokinin A, and histamine, which are responsible for CS. However, for tumors limited to the SB and its regional lymph nodes, these components are inactivated by the liver and hormonal symptoms are rare[1].
With the development of distant metastasis, the hormones secreted by SB NENs are able to bypass the portal circulation, leading to the development of CS. This syndrome was first described by Thorson in 1954. Carcinoid symptoms may be spontaneous or caused by stress, exercise, or ingestion of ethanol and amine-rich food such as chocolate or cheese[5]. The flushing associated with CS is typically transient and affects the face, neck and the upper part of the trunk[1].
The cardiac manifestations of CS, called "carcinoid heart disease", primarily affect the right side of the heart, causing valvular fibrosis. Cardiac involvement is seen in at least 20% of patients. The cause is believed to be related to high levels of serotonin that induce a fibrotic reaction in the right heart. However, the incidence is declining, possibly due to the widespread use of somatostatin analogs (SSAs). The presence of carcinoid heart disease predicts a worse prognosis[1]. This variety in presentation, combined with the relative rarity of the tumors and the nonspecific nature of the symptoms, makes diagnosis of these tumors difficult. Although the median duration of symptoms before diagnosis is 4 to 5 mo, misdiagnosis is common and a delay in diagnosis of up to 10 years has been described in the literature[1].
Published series from tertiary referral centers, mention the proportion of patients with distant metastasis of around 60% to 80%. One possible explanation for this is perhaps because early-stage lesions are removed in emergency surgeries for intestinal obstructions in less complex hospitals, while the more advanced stage of the disease is referred to these larger hospitals[30,31].
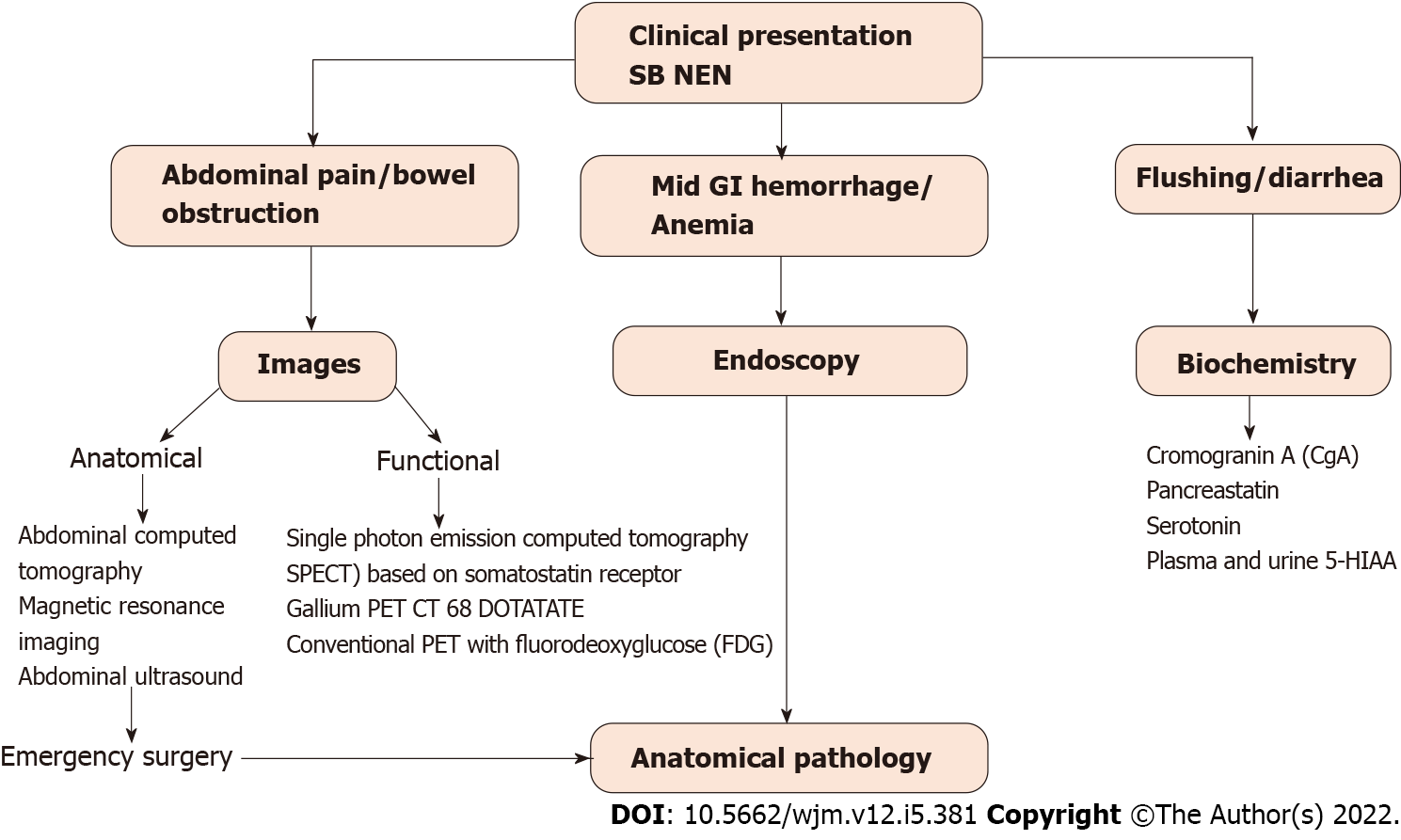
For the diagnosis of NENs, there are currently various methods available. The initial methods can be both imaging and laboratory tests; the order in which they are requested will depend on the form of clinical presentation and the initial diagnostic hypothesis. Confirmation will be histological, requiring a biopsy by endoscopy. Octreotide scan, video capsule endoscopy (VCE) and double-balloon enteroscopy (DBE) are the auxiliary exam options for diagnosis. Series reports catalog them as having a diagnostic yield of 85%, 10% and 83%, respectively. In occult SB NENs, capsule endoscopy appears to be superior to enteroscopy but may underestimate tumor burden[17].
For most patients, biochemical testing and anatomic or functional imaging will have preceded definitive diagnosis of SB NEN made by an immunohistochemical study of the tumor[6]. In addition to the hormones and neuroamines responsible for CS such as 5-hydroxyindoleacetic acid (5-HIAA) in plasma or urine[32], SB NENs secrete chromogranin A (CgA), pancreastatin, and serotonin which can be used as biomarkers for diagnosis and surveillance[32]. CgA is an acidic glycoprotein secreted by NENs, and has been extensively studied. CgA is sensitive and specific for the diagnosis of NEN, correlates with disease burden, and can predict survival. Nevertheless, renal failure, severe hypertension, vitamin B12 deficiency and proton pump inhibitor therapy can cause false CgA elevations. Serial pancreastatin measurements are useful in predicting and monitoring response to therapy[33]. A 24-h urine sample monitoring 5-HIAA, indicates serotonin breakdown. This test is highly specific for the diagnosis of SB NEN, but patients should be advised to avoid various serotonin-rich foods during collection[2].
Biochemical tests are widely used both for the diagnosis of SB NEN and for monitoring the course of the disease, but there is no agreement on how often they should be measured or how their measurement should influence treatment decisions[33].
The endoscopic technique of VCE and DBE are the most helpful exams in jejunal and ileal NENs. They allow location of the primary NEN in metastatic disease, where a basic study has been negative, to identify multifocal disease. This might change the management and prognosis. In addition, other studies have reported that multifocality does not seem to have an impact on survival or recurrence[18].
Those patients who present with hot flashes and diarrhea will probably undergo biochemical tests first, while those whose main symptom is abdominal pain or obstructive symptoms will require anatomical imaging such as computed tomography (CT) or may even be diagnosed only after an emergency surgical intervention[33].
For anatomical studies of SB NEN, CT, magnetic resonance imaging, and ultrasound are performed, while for functional studies positron emission tomography (PET) with Gallium and somatostatin receptor-based single photon emission computed tomography are carried out. Functional imaging using PET is essential for detecting small lymph node metastasis, tiny primary tumors in the SB, initial bone and bone marrow metastasis and more accurate assessment of occult liver metastasis[3]. Anatomical images provide the location of the tumors for surgical planning, while functional images have higher sensitivity and indicate the occult presence of metastasis or mistaken evidence of recurrence[2,33].
NENs of the SB are rarely visualized on CT. They are usually just millimeters in size. However, mesenteric lymph node metastasis might well appear as spiculated masses on contrast-enhanced CT, sometimes including calcifications and the regional presence of fibrosis due to its desmoplastic reaction. Additionally, as many as 30% can be multifocal[2]. CT angiography can provide details of valvular involvement. Despite this, morphological images generally significantly understate the disease[2].
For tumor classification, the Ki67 index or the number of mitoses per 10 high power fields (HPF) is used. NENs are subclassified into NENs and NECs. Grade 1 NENs have < 2 mitoses per 10 HPF or a Ki67 of < 3%. Grade 2 NENs show a Ki67 index from 3 to 20%, or 2 - 20 mitoses per 10 HPF. Grade 3 NENs give a Ki67 index of > 20%, or > 20 mitoses per 10 HPF. Further classification into G3 NENs and G3 NECs is based on their differentiation. G3 NECs are poorly differentiated but Grade 3 NENs are well differentiated[2].
Figure 3 summarizes the initial approach sequence of the patient with a suspected SB NEN and which tests should be requested depending on the form of clinical presentation.
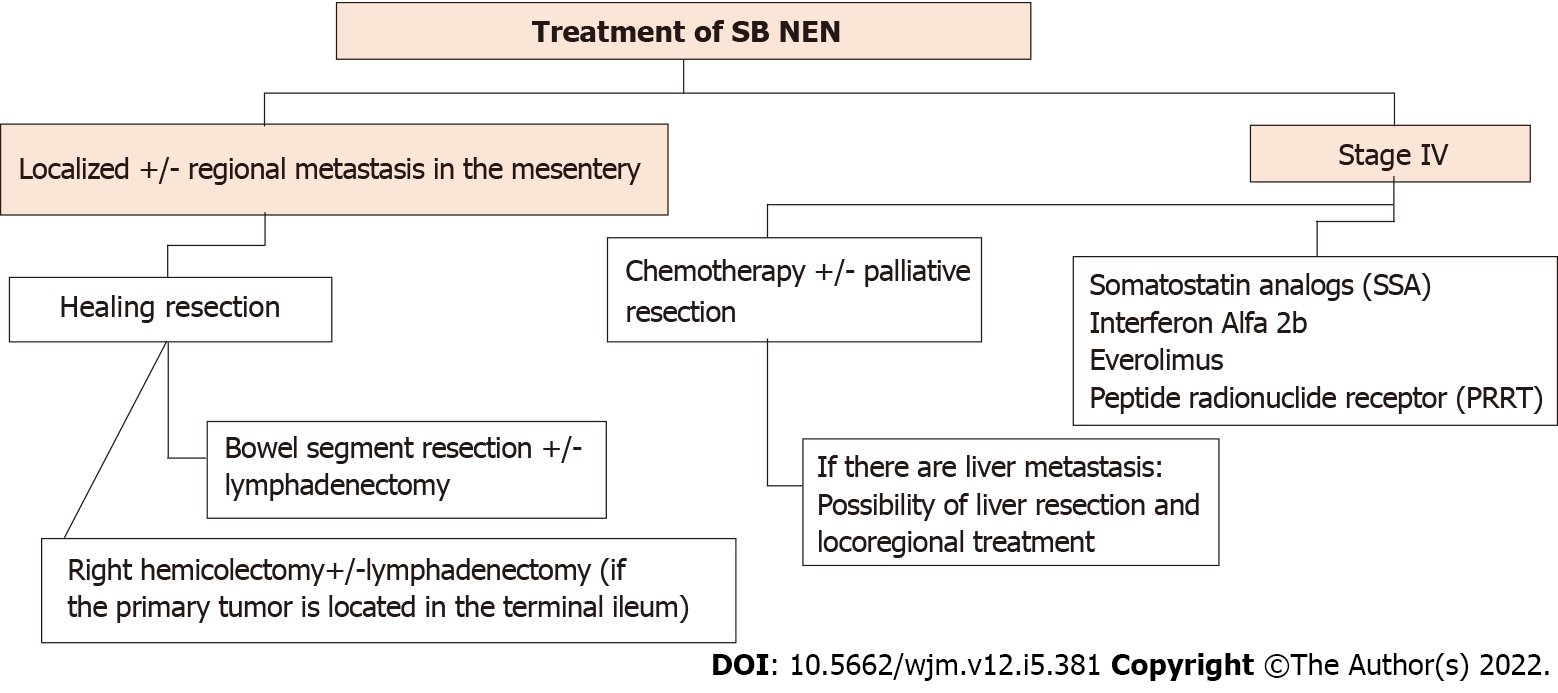
Treatment of a SB NEN depends on the staging of the disease, and whether it is locoregional or metastatic (Figure 4).
Management strategies for SB NEN include not only possible treatment of all stage tumors or metastasis, and if present, carcinoid heart disease or tumor-related symptoms and syndromes[2].
The management of these lesions is complex due to the difficulty in diagnosis, hormone secretion and more frequently, its presentation as an advanced disease. Even patients with advanced disease can have a long survival time. There are different aspects that make it difficult to determine the optimal management[34].
All patients with localized SB NEN with or without regional mesentery metastasis should be considered for curative resection. Therefore, multimodal treatment is required[2]. Although surgery is curative in most cases, recurrence rates of 42% in liver NEN have been published[1,35].
In the surgical area, meticulous exploration of the abdomen with palpation of the SB is recommended intraoperatively; this is superior to reference imaging for the detection of SB NENs, as up to 70% of these tumors are overlooked by imaging. Additionally, between 30%-54% of SB NENs are multifocal and just millimeters in size, which are very difficult to see on imaging. Therefore, a laparoscopic study is not recommended[2]. The abdomen should also be carefully examined for evidence of liver and peritoneal metastasis, reported in 20% and 60% of cases, respectively, undergoing SB NENs surgery[1].
In SB NENs, total resection it is not necessary, only the primary tumor and selective resection of the mesenteric nodes is required, taking into account the preservation of bowel function. The length of bowel resected is independent of the number of lymph nodes removed. In up to two-thirds of patients, metastasis outside the "expected" lymph node region is found, and to prevent unresectable locoregional recurrence, an extensive lymphadenectomy is required[2]. A series of reports of surgeries for SB NENs where the resection included 12 or more nodes, was related to better OS outcomes, in patients without distant metastasis[2].
If the primary tumor is located in the terminal ileum, a right hemicolectomy with or without lymphadenectomy is indicated[36]. In cases with stage IV asymptomatic SB NEN, early locoregional surgery as a prophylactic measure is controversial, as there are no convincing data associated with favorable survival outcomes, compared with locoregional surgery later in its development. SB NEN can be associated with peritoneal carcinomatosis (PC) in up to 30% of cases. As PC can cause fatal intestinal obstruction with a registered mortality of 40%, resection of peritoneal tumors should be part of the locoregional surgery[2].
Resection of the primary tumor in the setting of unresectable SB NEN liver metastasis may prevent ileus, intestinal obstruction, and desmoplastic reactions, and is registered in a retrospective study to prolong survival, independent of the tumor grade. However, such studies are biased toward an aggressive approach in patients with better baseline status, so it is unclear whether this intervention is beneficial versus the underlying characteristics of the cases[2].
Patients with metastatic NENs to the SB have a favorable prognosis, compared with other GI malignancies. An OS of 103 mo for cases with well-differentiated tumors was reported in some series between 2000 and 2012[6].
The first-line treatment of NENs consistes of SSAs, which is also the case in functional and non-functional metastatic NENs of the SB, to control CS symptoms and due to their antiproliferative effects[37,38]. The treatment consists of injections of octreotide LAR or lanreotide, which are long-acting SSAs, every four weeks. Short-acting octreotide injections are given in cases to improve symptomatic control or as a rescue therapy[1]. Octeotride LAR plus interferon alpha have shown beneficial effects by inhibiting hormone secretion and proliferation in NENs in the past decades[39].
Everolimus has been studied in advanced stages of NENs. It is a rapamycin inhibitor, used to treat CS (RADIANT-2 trial) and advanced non-functional NENs (RADIANT-4 trial). In the RADIANT-2 trial, better OS was observed after treatment with everolimus and octreotide LAR versus treatment with octreotide LAR only; however, the difference was not statistically significant[33]. Results from the RADIANT-4 trial did show a statistically significant improvement in median progression-free survival when everolimus monotherapy was compared with a placebo (11.0 vs 3.9 mo). Based on these findings, everolimus is only approved for use in progressive non-functional NENs, but is often used in patients with progressive disease regardless of tumor functionality[1,38].
Since 1992, peptide receptor radionuclide therapy (PRRT) has been used for the treatment of NENs. In PRRT, radionuclides such as Yttrium-90 (90Y) and Lutetium-177 (177Lu) are directly delivered to the tumor by radiolabeled SSA8. In the 229 patient NETTER-1 trial, all patients had well-differentiated, metastatic NENs. It was found that the PRRT treatment group had a significantly better median OS and a better response rate compared with the placebo group (18% vs 3%)[1,40].
Cytotoxic chemotherapy is also used in the treatment of PNENs, and has been shown to have an inferior role in well-differentiated SB NENs[1]. Due to easy oral administration, and their low adverse effect profile, capecitabine and temozolomide remain good practice second- or third-line choices in patients with progressive SB NENs[1].
Small intestine NECs are extremely rare. Regardless of the primary site, cisplatin or carboplatin and etoposide are used as first-line treatment, and due to the poor prognosis of NEC, they are generally not recommended for surgical intervention and treatment[41,42]. NECs with an Ki-67 index between 20% and 55% have shown low response rates to platinum-based chemotherapy, and there is no standard treatment regimen for these patients[1].
Patients with metastatic NENs of the SB are not excluded from surgery. Several studies have shown an improvement in OS together with control of symptoms following resection of metastatic lymph nodes and liver metastasis. However, these procedures are seldom curative and the recurrence rates at 5 and 10 years are 95% and 99%, respectively[1].
Finally, at the time of surgery for metastatic NENs of the SB, a cholecystectomy should be included due to the high presence of gallstones in patients receiving SSAs[1]. In addition, minimally invasive resection techniques should be performed in younger patients less prone to obstruction, without metastasis, or with small tumors. However, these techniques have limitations that will require surveillance[17].
Neuroendocrine tumors are neoplasms that can be found in any part of the body. This review is focused on those with a location or origin in the digestive tract at the level of the small intestine due to its variable form of presentation and difficult diagnosis, as well as the treatment approach, emphasizing a multidisciplinary effort. We observed that reports of current series place them in several cases as one of the most frequent tumors in the small intestine. As their incidence is increasing, the importance of understanding their behavior and how to approach them correctly increases. The presence of small bowel NENs results in variable gastrointestinal symptoms, which are frequently a cause of the delay from symptom onset to diagnosis. In addition, a suspected SB NEN must be confirmed by biochemical tests, anatomical and functional images and an anatomopathological study of tissue, the latter pre
The research team appreciates all the contributions and suggestions received from different colleagues prior to publication of this manuscript.
| 1. | Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol. 2004;35:1440-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Scott AT, Howe JR. Management of Small Bowel Neuroendocrine Tumors. J Oncol Pract. 2018;14:471-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Clift AK, Kidd M, Bodei L, Toumpanakis C, Baum RP, Oberg K, Modlin IM, Frilling A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology. 2020;110:444-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 794] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 5. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (20)] |
| 6. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3321] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 7. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2666] [Article Influence: 296.2] [Reference Citation Analysis (5)] |
| 8. | Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21:R153-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 1179] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 10. | National Cancer Institute: Surveillance E and ERP. SEER*Stat Databases: November 2015 Submission. |
| 11. | Barsouk A, Rawla P, Barsouk A, Thandra KC. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med Sci (Basel). 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Larouche V, Akirov A, Alshehri S, Ezzat S. Management of Small Bowel Neuroendocrine Tumors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Wyld D, Moore J, Tran N, Youl P. Incidence, survival and stage at diagnosis of small intestinal neuroendocrine tumours in Queensland, Australia, 2001-2015. Asia Pac J Clin Oncol. 2021;17:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Modlin IM, Gustafsson BI, Pavel M, Svejda B, Lawrence B, Kidd M. A nomogram to assess small-intestinal neuroendocrine tumor ('carcinoid') survival. Neuroendocrinology. 2010;92:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Kelly S, Aalberg J, Agathis A, Phillips K, Haile S, Haines K, Kang Kim M, Divino CM. Predicting Survival of Small Intestine Neuroendocrine Tumors: Experience From a Major Referral Center. Pancreas. 2019;48:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Man D, Wu J, Shen Z, Zhu X. Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Manag Res. 2018;10:5629-5638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 17. | Ethun CG, Postlewait LM, Baptiste GG, McInnis MR, Cardona K, Russell MC, Kooby DA, Staley CA, Maithel SK. Small bowel neuroendocrine tumors: A critical analysis of diagnostic work-up and operative approach. J Surg Oncol. 2016;114:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Gangi A, Siegel E, Barmparas G, Lo S, Jamil LH, Hendifar A, Nissen NN, Wolin EM, Amersi F. Multifocality in Small Bowel Neuroendocrine Tumors. J Gastrointest Surg. 2018;22:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Rinzivillo M, Capurso G, Campana D, Fazio N, Panzuto F, Spada F, Cicchese N, Partelli S, Tomassetti P, Falconi M, Delle Fave G. Risk and Protective Factors for Small Intestine Neuroendocrine Tumors: A Prospective Case-Control Study. Neuroendocrinology. 2016;103:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Nogueira L, Freedman ND, Engels EA, Warren JL, Castro F, Koshiol J. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol. 2014;179:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Järhult J, Landerholm K, Falkmer S, Nordenskjöld M, Sundler F, Wierup N. First report on metastasizing small bowel carcinoids in first-degree relatives in three generations. Neuroendocrinology. 2010;91:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Sei Y, Zhao X, Forbes J, Szymczak S, Li Q, Trivedi A, Voellinger M, Joy G, Feng J, Whatley M, Jones MS, Harper UL, Marx SJ, Venkatesan AM, Chandrasekharappa SC, Raffeld M, Quezado MM, Louie A, Chen CC, Lim RM, Agarwala R, Schäffer AA, Hughes MS, Bailey-Wilson JE, Wank SA. A Hereditary Form of Small Intestinal Carcinoid Associated With a Germline Mutation in Inositol Polyphosphate Multikinase. Gastroenterology. 2015;149:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Dumanski JP, Rasi C, Björklund P, Davies H, Ali AS, Grönberg M, Welin S, Sorbye H, Grønbæk H, Cunningham JL, Forsberg LA, Lind L, Ingelsson E, Stålberg P, Hellman P, Tiensuu Janson E. A MUTYH germline mutation is associated with small intestinal neuroendocrine tumors. Endocr Relat Cancer. 2017;24:427-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Scott AT, Pelletier D, Maxwell JE, Sherman SK, Keck KJ, Li G, Dillon JS, O'Dorisio TM, Bellizzi AM, Howe JR. The Pancreas as a Site of Metastasis or Second Primary in Patients with Small Bowel Neuroendocrine Tumors. Ann Surg Oncol. 2019;26:2525-2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Zhang M, Zhao P, Shi X, Zhao A, Zhang L, Zhou L. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: a large, retrospective single-centre study. BMC Endocr Disord. 2017;17:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Bonds M, Rocha FG. Neuroendocrine Tumors of the Pancreatobiliary and Gastrointestinal Tracts. Surg Clin North Am. 2020;100:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 417] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 528] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 29. | Aluri V, Dillon JS. Biochemical Testing in Neuroendocrine Tumors. Endocrinol Metab Clin North Am. 2017;46:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Dahdaleh FS, Calva-Cerqueira D, Carr JC, Liao J, Mezhir JJ, O'Dorisio TM, Howe JR. Comparison of clinicopathologic factors in 122 patients with resected pancreatic and ileal neuroendocrine tumors from a single institution. Ann Surg Oncol. 2012;19:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Keck KJ, Maxwell JE, Menda Y, Bellizzi A, Dillon J, O'Dorisio TM, Howe JR. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. 2017;161:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Canakis A, Lee LS. Current updates and future directions in diagnosis and management of gastroenteropancreatic neuroendocrine neoplasms. World J Gastrointest Endosc. 2022;14:267-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Tran CG, Sherman SK, Howe JR. Small Bowel Neuroendocrine Tumors. Curr Probl Surg. 2020;57:100823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Öberg K, Van Cutsem E, Yao JC; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 769] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 35. | Le Roux C, Lombard-Bohas C, Delmas C, Dominguez-Tinajero S, Ruszniewski P, Samalin E, Raoul JL, Renard P, Baudin E, Robaskiewicz M, Mitry E, Cadiot G; Groupe d'étude des Tumeurs Endocrines (GTE). Relapse factors for ileal neuroendocrine tumours after curative surgery: a retrospective French multicentre study. Dig Liver Dis. 2011;43:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Selberherr A, Niederle MB, Niederle B. Surgical Treatment of Small Intestinal Neuroendocrine Tumors G1/G2. Visc Med. 2017;33:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Singh S, Asa SL, Dey C, Kennecke H, Laidley D, Law C, Asmis T, Chan D, Ezzat S, Goodwin R, Mete O, Pasieka J, Rivera J, Wong R, Segelov E, Rayson D. Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence-based Canadian consensus. Cancer Treat Rev. 2016;47:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, Kunz PL, O'Dorisio TM, Salem R, Segelov E, Howe JR, Pommier RF, Brendtro K, Bashir MA, Singh S, Soulen MC, Tang L, Zacks JS, Yao JC, Bergsland EK. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas. 2017;46:707-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 39. | Dai M, Mullins CS, Lu L, Alsfasser G, Linnebacher M. Recent advances in diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. World J Gastrointest Surg. 2022;14:383-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (3)] |
| 40. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2446] [Article Influence: 271.8] [Reference Citation Analysis (0)] |
| 41. | Garcia-Carbonero R, Rinke A, Valle JW, Fazio N, Caplin M, Gorbounova V, O Connor J, Eriksson B, Sorbye H, Kulke M, Chen J, Falkerby J, Costa F, de Herder W, Lombard-Bohas C, Pavel M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology. 2017;105:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjær A, Knigge U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel). 2015;5:119-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Peru
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pausawasdi N; Tang D, China S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH