Published online Jul 20, 2022. doi: 10.5662/wjm.v12.i4.305
Peer-review started: December 6, 2021
First decision: January 25, 2022
Revised: January 31, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: July 20, 2022
Processing time: 225 Days and 19.2 Hours
There are three main forms of leishmaniasis in humans: cutaneous leishmaniasis (CL), visceral leishmaniasis (VL), and mucocutaneous leishmaniasis. The prevalence of human leishmaniasis varies widely in different countries and different regions of the same country. To date, there is no overall estimation of the prevalence of human leishmaniasis in Sudan.
To determine the pooled prevalence of human leishmaniasis and the disease risk factors among Sudanese citizens.
From all articles written in English or Arabic languages conducted before the 4th of August 2021 from [Scopus, Web of Science, PubMed, and MEDLINE, African Journals Online (AJOL), ResearchGate, direct Google search, Google Scholar, and universities websites], just 20 articles with a total of 230960 participants were eligible for this study. Data synthesis and analysis were done using STATA software, version 16. EndNote citation manager version X9.3.3 and Reference Citation Analysis (RCA) were used to remove the duplicated studies and manage the citation respectively.
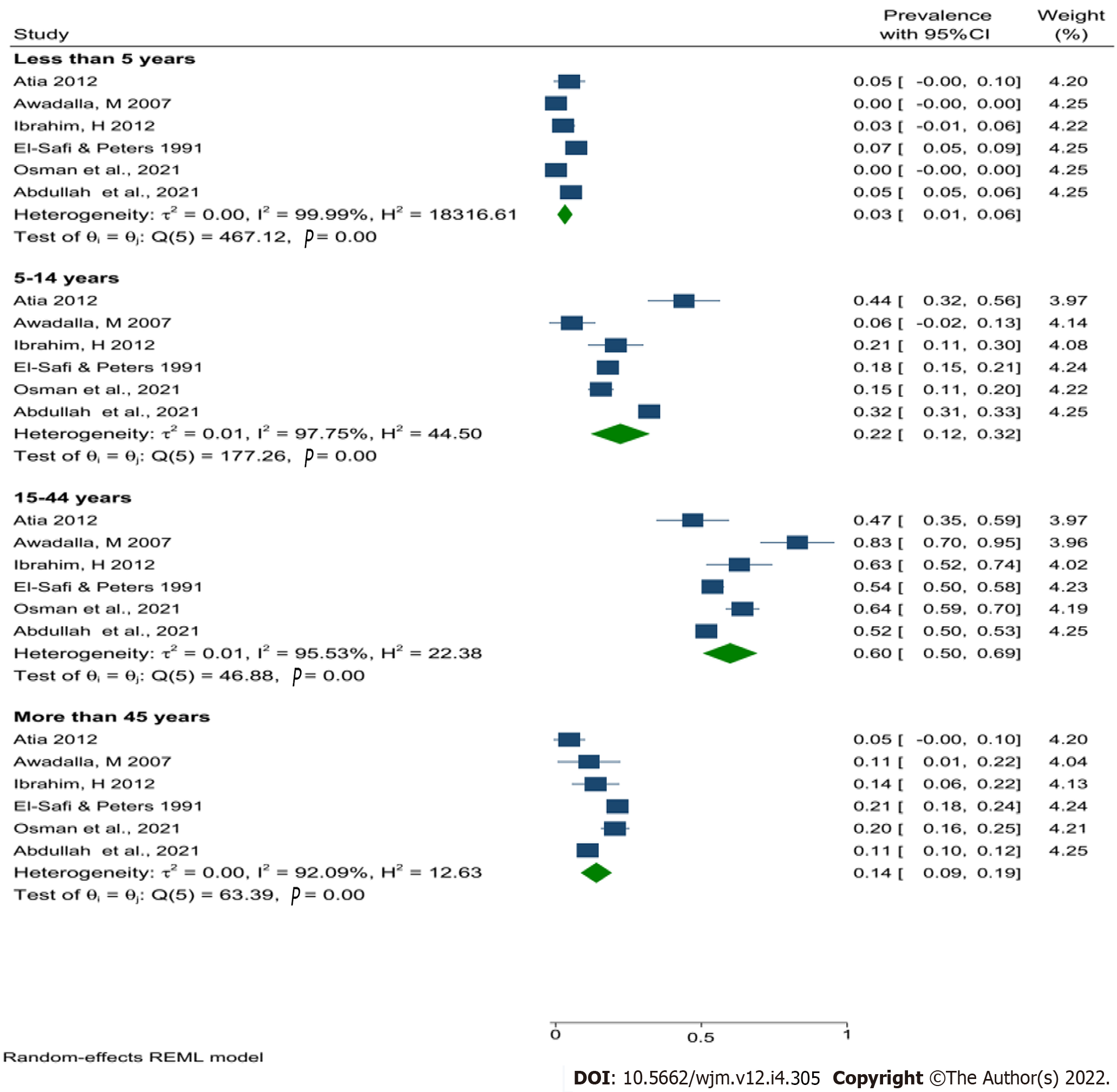
The overall pooled prevalence of human leishmaniasis in Sudan was 21% (with confidence interval 12%-30%). CL was the most common type of leishmaniasis in Sudan, with a pooled prevalence of 26% followed by VL (18%). Nevertheless, the pooled prevalence of human leishmaniasis in Sudan was higher in males compared with females (60% vs 40%). The current results revealed that the people in the age group between 15 and 44 were the most affected group (60%), and central Sudan has the highest pooled prevalence of human leishmaniasis (27%) compared with other regions of Sudan. Finally, the prevalence of human leishmaniasis seems to decrease with time.
This study showed that human leishmaniasis infection is still endemic in many regions in Sudan and highly prevalent in central and eastern Sudan, and CL is the most prevalent in the country. Males and adults were more susceptible to infection compared with females and children. However, the human leishmaniasis prevalence decreased relatively over time.
Core Tip: A comprehensive systematic review and meta-analysis study was conducted to find the pooled prevalence of leishmaniasis and its associated factors among Sudanese citizens. After applying all required quality check-ups for the individual studies, 20 studies were included in this study. The pooled prevalence of human leishmaniasis in Sudan was 21%, and cutaneous leishmaniasis was the commonest form of leishmaniasis in Sudan. Finally, the results of this study showed that human leishmaniasis infection is still endemic in many regions in Sudan.
- Citation: Ahmed M, Abdulslam Abdullah A, Bello I, Hamad S, Bashir A. Prevalence of human leishmaniasis in Sudan: A systematic review and meta-analysis. World J Methodol 2022; 12(4): 305-318
- URL: https://www.wjgnet.com/2222-0682/full/v12/i4/305.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i4.305
Neglected tropical zoonotic diseases (NTZDs) are endemic diseases in many developing countries of Africa, Asia, and Latin America[1]. The WHO's annual report for 2021, revealed that leishmaniasis is set among the top ten NTZDs worldwide[2].
In addition to the zoonotic nature of the disease, leishmaniasis is transmitted to humans by the infected female sandflies with Leishmania parasite, when it feeds on the human's blood[3]. There are three main forms of the disease in humans: cutaneous leishmaniasis (CL), which mainly features skin lesions, visceral leishmaniasis (VL), or Kala-azar, which can affect the spleen, liver, and bone marrow leading to some serious symptoms, and mucocutaneous leishmaniasis (ML)[3]. Of the three leishmaniasis forms, VL is the most lethal with a fatality rate of 95% if it is left untreated, while CL is the most common form[2]. In general, the high incidence and prevalence of human leishmaniasis have been highly associated with the prevalence of conditions that leads to a weak immune response, such as AIDS or tuberculosis. Studies also found a strong association between leishmaniasis prevalence and poor household status, poverty, population displacement, and recent climate change[4-7].
Evidence showed that the annual incidence of human leishmaniasis was 700000 to 1 million new cases. Although the disease was reported in 89 countries all around the world, East Africa, Southeast Asia, and South America countries, have the highest incidence rates[8]. Nevertheless, almost all reported outbreaks of human leishmaniasis were from East African countries, namely Sudan, South Sudan, and Ethiopia[9-13].
Sudan has a long history of leishmaniasis which was firstly discovered by Neave in the early 1900s[14]. Moreover, in the late twentieth century, several leishmaniasis (CL & VL) outbreaks were reported in the eastern and central parts of the country[15]. The geographical distribution study of human leishmaniasis in Sudan found a high relationship between disease occurrence and vector distribution[16,17]. Reports from Sudan found that the VL is endemic in the country, especially in the savannah area in the eastern and central parts of the country, which lies between four states (White Nile State in the west, Gadarif state in the east, Blue Nile State in the south, and Kassala state in the northeast)[18]. Moreover, VL was reported outside the savannah area in some scattered foci in the western parts of the country in Darfur states and Kordofan states[19]. Furthermore, national-wide epidemiological studies, report the endemic presence of the CL, especially in the northern, central, and western parts of the country[15]. For all the above reasons, it can be said that human leishmaniasis (both CL & VL) is endemic in Sudan, and the disease represents a serious health problem that affects the whole healthcare system[20].
Despite the importance of the disease in Sudan and the many published studies across the country that described the epidemiology of human leishmaniasis, no study estimated the overall prevalence of the disease at the national level exists to date. The lack of evidence about the disease in the country may prevent the health care policymakers and stakeholders from developing and adopting a suitable prevention program. Thus, the current study aimed to investigate the pooled prevalence of human leishmaniasis (both CL and VL) in Sudan.
The following were the eligibility criteria of this study: (1) All human observational studies; (2) Done on the Sudanese population; (3) Published in Arabic or English; (4) Reported the prevalence of human leishmaniasis (CL and VL); and (5) The positive cases of leishmaniasis were detected using the standards' diagnostics methods (serological and molecular tests). Moreover, studies were not eligible for this study (1) If they were reviews, letters, editorials, animal studies; and (2) If the full text was not available and has been requested from the author(s) through email but no feedback was received after 2 wk.
This meta-analysis study was conducted according to the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[21]. The relevant information was retrieved from the electronic databases sources, namely Scopus, Web of Science, PubMed, MEDLINE, African Journals Online (AJOL), ResearchGate, direct Google search, Google Scholar, and universities websites. All indicated databases were searched from their inception to the 4th of August 2021, for human studies published in English and/or Arabic.
To achieve the current study objectives, a research strategy was developed using the Boolean search terms (AND, OR, NOT). The final search strategy included the use of Title/Abstract related to ((human leishmaniasis) AND ((prevalence) OR (epidemiology) OR (frequency)) OR (Risk factors)) AND Sudan) taken from the study questions. In addition, a manual search was done by the investigators for the grey literature and unpublished thesis/papers.
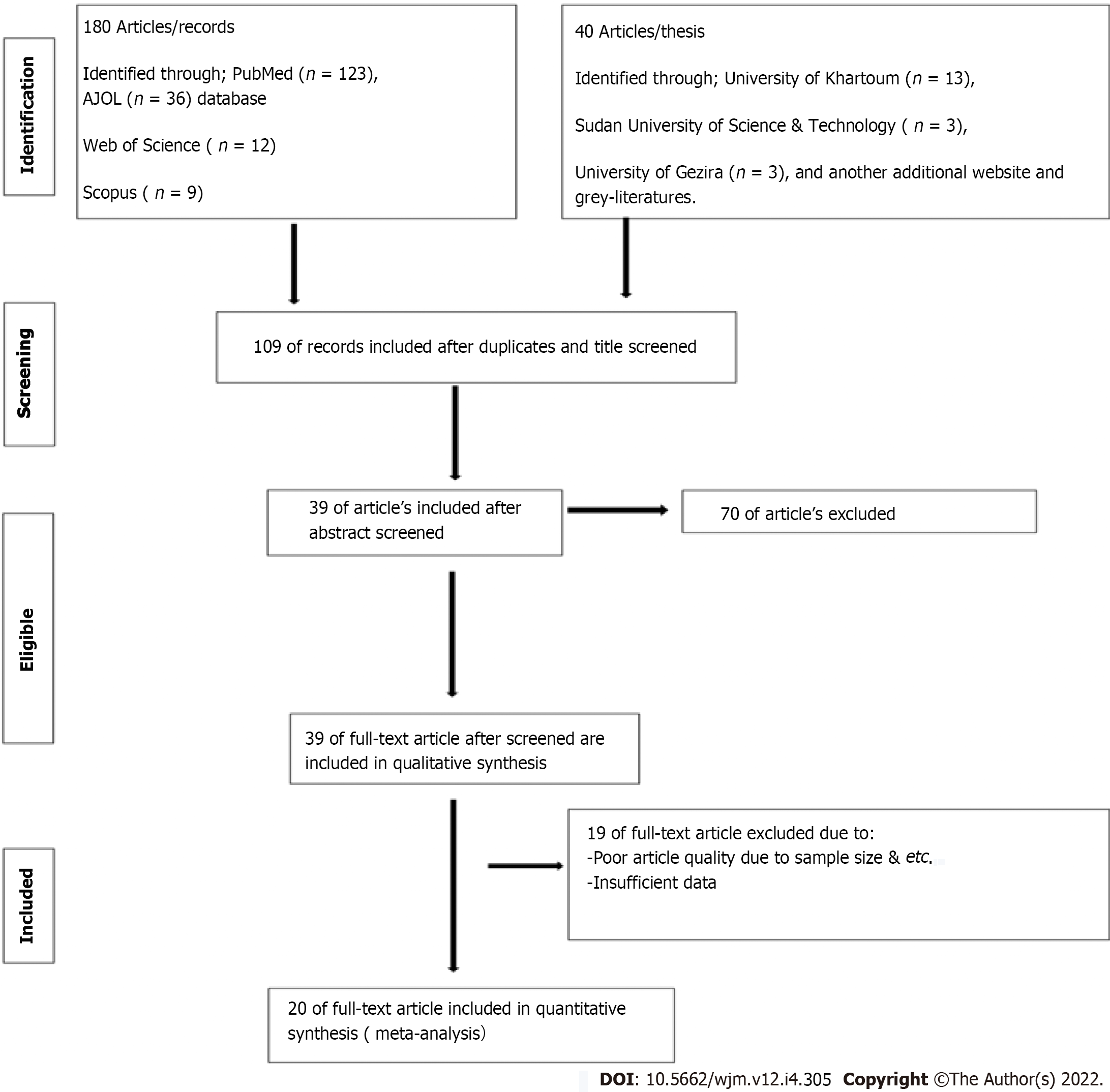
Initially, primary screening was done based on the inclusion and exclusion criteria. Thereafter, all retrieved studies were exported to the EndNote citation manager version X9.3.3, to remove the duplicated studies. After that, the remaining articles were screened and evaluated by two investigators (Ahmed M and Abdulslam Abdullah A) independently. The investigators carefully have read the title, abstract, and full text of each article to eliminate the unrelated studies to prior defined objectives. Furthermore, the remaining articles were considered for further quality checkups against the checklist of Joanna Briggs Institute quality assessment tools[22]. Any discrepancy in the study findings was resolved by discussion between the two authors (Ahmed M and Abdulslam Abdullah A) or by consulting Hamad S. Figure 1 shows the selection process using the PRISMA statement flow diagram. Finally, Reference Citation Analysis (RCA) were used to manage the citation.
Following the selection process, the relevant data were extracted using a Microsoft word 2016 data extraction template.
Two investigators (Ahmed M and Abdulslam Abdullah A) contacted the corresponding author of any study that failed to report the information required for the eligibility criteria indicated above (via email) to get the original data; however, if the missing data were not obtained after 2 wk, a sensitivity analysis was carried out to remove the studies with the missing information. The extraction template contains (author/s name and publication year, study period, study design, study setting, geographical location (based on state names), type of leishmaniasis (VL & CL), sample size, diagnostic method, and the prevalence of leishmaniasis in (overall and male and female) (Table 1). The accuracy of the data extraction process was verified by comparing the extraction results of 2 authors (Ahmed M and Abdulslam Abdullah A), who extracted the data independently, in a randomly- chosen set of papers (30% of the total).
| Ref. | Sample size | Method | Type of leishmaniasis | Geographical location | Study design | Study setting | Prevalence n (%) | ||
| Overall, n % | Male | Female | |||||||
| Hashim[24], 1997 | 126 | PCR & LST | VL/CL | Central Sudan | CS | HB | 43 (34.1) | NR | NR |
| El Dawi[25], 1994 | 44 | DAT | VL | Central Sudan | PS | HB | 19 (43.2) | NR | NR |
| Ibrahim[26], 2012 | 734 | LST | CL | Central Sudan | CS | CB | 73 (9.9) | NR | NR |
| Sharief et al[27], 2019 | 1781 | DAT & LST | VL | Western Sudan | ES | CB | 238 (13) | NR | NR |
| Osman[28], 2011 | 332 | PCR | CL | Western Sudan | CS | CB | 32 (9.6) | NR | NR |
| Noraldaim[29], 2012 | 110 | DAT & ELISA | VL | Central Sudan | PS | CB | 46 (41.8) | NR | NR |
| Mohamed et al[30], 2019 | 95 | DAT | VL | Eastern Sudan | CS | CB | 5 (5.3) | NR | NR |
| Dereure et al[31], 2003 | 79 | Culture | VL | Eastern Sudan | NR | CB | 23 (29.1) | NR | NR |
| EL-Safi et al[18], 2002 | 947 | DAT & LST | VL | Eastern Sudan | CS | CB | 132 (13.9) | NR | NR |
| El-Safi and Peters[32], 1991 | 9657 | DAT | CL | Central Sudan | RS | HB | 736 (7.6) | 449 (61) | 287 (39) |
| Atia[23], 2012 | 373 | DAT | VL | Eastern Sudan | CS | CB | 64 (17.2) | 29 (45.3) | 35 (54.7) |
| Abdallah[34], 2015 | 352 | DAT & ELISA | VL | Eastern Sudan | PS | HB | 71 (20.2) | 43 (60.6) | 28 (39.4) |
| Ebrahim[19], 2016 | 48972 | Mixed | VL | Western Sudan | RS | HB | 815 (1.7) | (62) | (38) |
| Awadalla[35], 2007 | 399 | DAT | VL | Eastern Sudan | CS | CB | 35 (8.8) | 23 (65.7) | 12 (34.3) |
| Muawyia et al[36], 2021 | 40 | DAT | CL | Central Sudan | NR | HB | 13 (32.5) | 10 (76.9) | 3 (23.1) |
| Osman et al[37], 2021 | 410 | LST | CL | Northern Sudan | CS | CB | 290 (70.7) | 91 (31.4) | 199 (68.6) |
| Abdullah et al[38], 2021 | 162443 | Mixed | VL/CL | Western Sudan | RS | HB | 7131 (4.4) | 4657 (65.3) | 2474 (34.7) |
| Ahmed[39], 2011 | 50 | Mixed | VL | Central Sudan | CS | HB | NR | 38 (76) | 12 (24) |
| Ahmed[40], 2017 | 215 | Mixed | VL | Eastern Sudan | R-CC | HB | NR | 140 (65.1) | 75 (34.9) |
| Collis et al[41], 2019 | 3801 | LST | CL | Nationwide | RS | HB | NR | 2178 (57.3) | 1599 (42.1) |
The prevalence of human leishmaniasis in Sudan was the main outcome of the current study. Moreover, the prevalence was measured from the individual studies by the direct report. To quantify the outcome, studies that reported the prevalence of VL and/or CL in their statistics were considered. Finally, the result was interpreted by the proportions of the population who tested positive for leishmaniasis compared with the total population studied.
The risk of bias for this study was checked through several steps: firstly, by appraising the eligibility criteria for all retrieved articles by checking the title and abstract for each retrieved study; secondly, the full-text for each included study from step one was screened using the quality assessment criteria to identify their quality before the final selection. The quality assessment criteria used to determine if the study could be included were: (1) The presence of Leishman parasite in the patient was identified after performing the appropriate diagnostic tests; and (2) From the statistical point of view, the study sample was representative of the study population. To minimize the risk of bias two strategies were followed: (1) A comprehensive search for all electronic and non-electronic databases; and (2) A critical appraisal tool (Joanna Briggs Institute Quality Assessment Tool)[22] was used by two investigators (Ahmed M and Abdulslam Abdullah A) independently to critically appraise the included studies. The publication bias in the current review was checked primarily by Egger’s regression test, which is a test of statistical symmetry of the funnel plot. Also, visualizing the inspection of the funnel plot was used to check the publication bias.
This review was developed based on the PRISMA guideline[21]. The review protocol has been registered by the International prospective register of systematic reviews at https://www.crd.york.ac.uk/Prospero/#recordDetails (No. CRD42021270418).
The collected study data were synthesized and analyzed using the STATA software, version 16.0 (Stata Corp LLC, 77845 Texas, United States). Statistically significance was set for P values < 0.05. The heterogeneity test was conducted using the degree of inconsistency (I2), which is a percentage, and range from (0%-100%), moreover, Higgins et al[23] described the heterogeneity to be low, medium, and high, for the (I2) values of 25%, 50%, and 75% respectively. Two statistical measurements were used to calculate the result of this study: effect size with a 95% confidence interval (CI) and standard error (SE). The prevalence of leishmaniasis (proportion) was considered as the effect size of this study, and the binomial distribution was used to calculate it.
The standard error was calculated using the following data: sample size (n) and the proportion of leishmaniasis positive case among the overall population (p) using the SE formula: SE = sqrt[p (1-p) / n).
In the final meta-analysis model, the outcome of each individual study, as well as the pooled outcome of all included studies, were presented as forest plots [reported as effect size (prevalence) with a 95%CI]. The visual symmetry of the funnel plot and the result of Egger’s Regression were used to check the potential publication bias; however, unlike other statistical tests reported here, the Egger’s test was considered significant if the P values were less than 0.10.
A meta-regression test was conducted (univariate and multivariate regression) to investigate the possible relationship between study variables (study year/s, sample size, diagnostic method, type of leishmaniasis, study region, study design, and study setting) and the prevalence of human leishmaniasis. Sensitivity analysis and subgroup analysis were performed to check the potential heterogeneity among the included studies and possible sources of bias.
Finally, the findings of this study were reported according to the PRISMA guidelines[21], and the results were presented using a narrative synthesis and followed by the full meta-analysis chart.
After applying the search strategies of the current study, a total of 220 articles were identified and retrieved from the major electronic databases sources. From the 220 retrieved articles, 111 of them were removed due to duplication. Meanwhile, the remaining 109 articles underwent further individual screening by title and abstract to appraise the eligibility criteria for each included study. Only 39 records were eligible for full-text quality assessment. Of the remaining 39 articles, 19 were excluded due to the article's poor quality and insufficient study data. Eventually, only 20 studies with good quality assessment scores that fulfilled the eligibility criteria were included in this review. Figure 1 showed the full process of study selection.
As shown in Table 1, twenty studies with a total of 230960 participants, were included in the quantitative analysis. Of these 20 studies, 10 were community-based studies, and the remaining 10 studies were hospital-based. The overall prevalence of human leishmaniasis in Sudan was reported in 17 studies, and the association between sex and leishmaniasis was reported in 11 studies. Meanwhile, two types of human leishmaniasis were reported (CL & VL). The geographical distributions of included studies revealed that the most frequent study areas in the included studies were central and eastern Sudan (7 for each), followed by western Sudan (4), with only one study from northern Sudan, and no study from southern Sudan. From all available diagnostic tests for leishmania parasite, only five were mentioned in the included studies: (1) Direct agglutination test (DAT) - 11 times; (2) Leishmania skin test (LST) - 5 times; (3) Polymerase chain reaction (PCR) - 2 times; (4) Enzyme-linked immunosorbent assay test (ELISA) - 2 times; and (5) Culture method - 1 time.
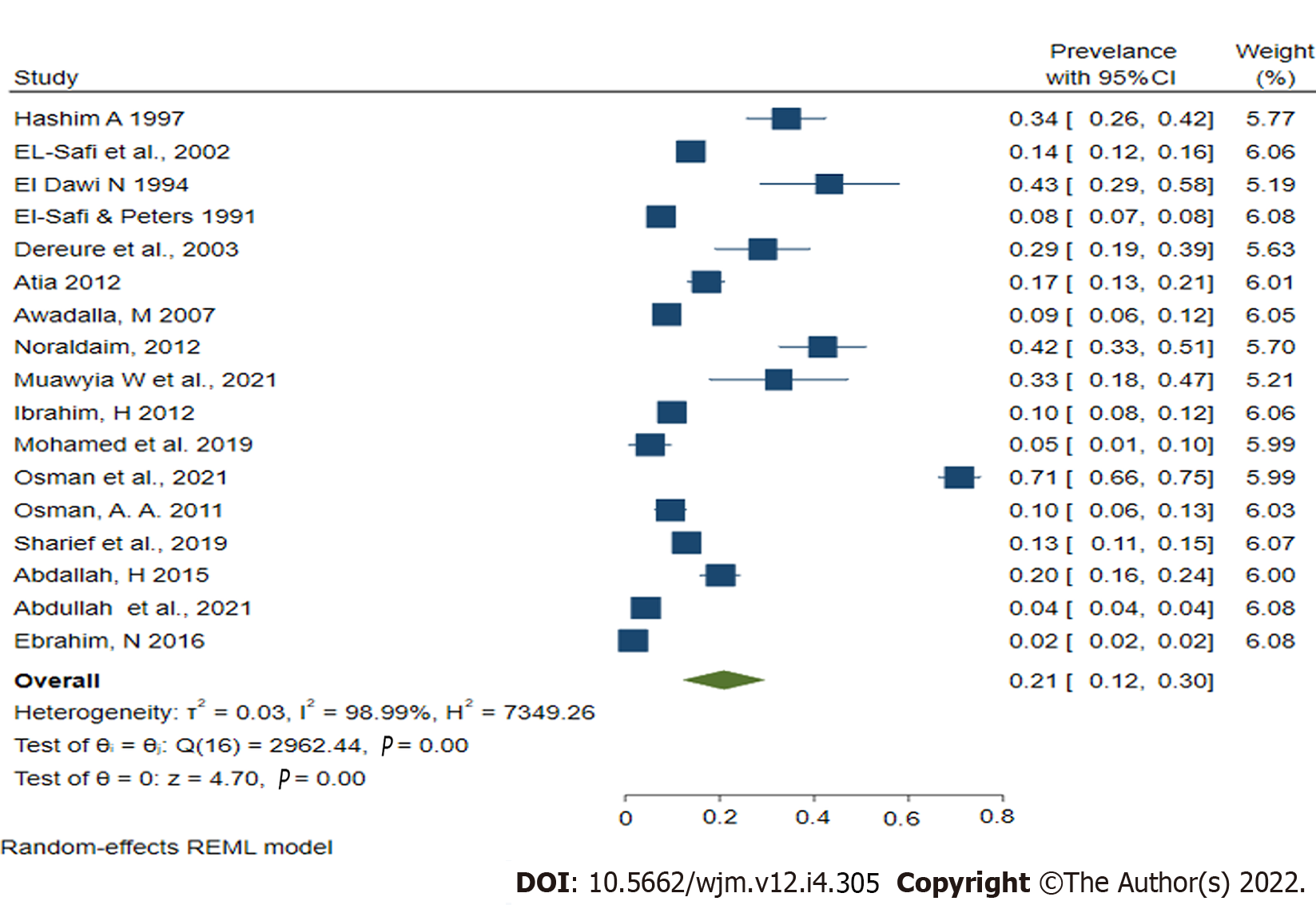
The current comprehensive study found a wide range of human leishmaniasis prevalence in Sudan in the twenty included studies. The lowest prevalence of human leishmaniasis, 1.7 (95%CI: 1, 2.8) was reported in a study in North Darfur state[19], whereas, the highest prevalence, 70.7% (95%CI: 66, 75), was reported in a study done in Al-tragma Village, River Nile state[37]. From the included studies, the pooled prevalence of human leishmaniasis in Sudan was 21% (CI: 12%-30%), and the heterogeneity across studies was substantially high (with P < 0.00001; I2 = 98.9%); therefore, the random effect model (REML) was employed for the final analysis (Figure 2).
A meta-regression test was conducted (both univariate and multivariate regression) to investigate the possible relationship between study variables (study year/s, sample size, diagnostic method, type of leishmaniasis, study region, study design, and study setting) and the prevalence of human leishmaniasis. Nevertheless, all examined variables were not found to be statistically significant (Table 2), and from that, it can concluded that these study variables did not affect the heterogeneity. Alongside, the meta-regression, a sensitivity analysis was performed to identify the possible sources of the heterogeneity among the included studies. This study was done by sequentially excluding studies from the analysis model, but again the results did not find any significant difference in the analysis model. Thus, it can be concluded that the meta-analysis result of this study was stable. Furthermore, Egger's test for publication bias was statistically insignificant P = 0.128.
| Variables | Coefficient | SE | t | P > |t| | 95%CI |
| Study yr/s | -0.0183371 | 0.0892299 | -0.21 | 0.841 | (-0.2171537, 0.1804794) |
| Sample size | -1.46e-06 | 1.10e-06 | -1.33 | 0.204 | (3.80e-06, 8.83e-07) |
| Diagnostic method | 0.0152374 | 0.0500373 | 0.30 | 0.767 | (-0.0962528, 0.1267275) |
| Type of leishmaniasis | -0.0271858 | 0.0653937 | -0.42 | 0.686 | (-0.172892, 0.1185204) |
| Study region | -0.0472426 | 0.0775729 | -0.61 | 0.556 | (-0.2200857, 0.1256005) |
| Study design | 0.0029982 | 0.0584459 | 0.05 | 0.960 | (-0.1272273, 0.1332237) |
| Study setting | -0.0381169 | 0.1762884 | -0.22 | 0.833 | (-0.4309118, 0.354678) |
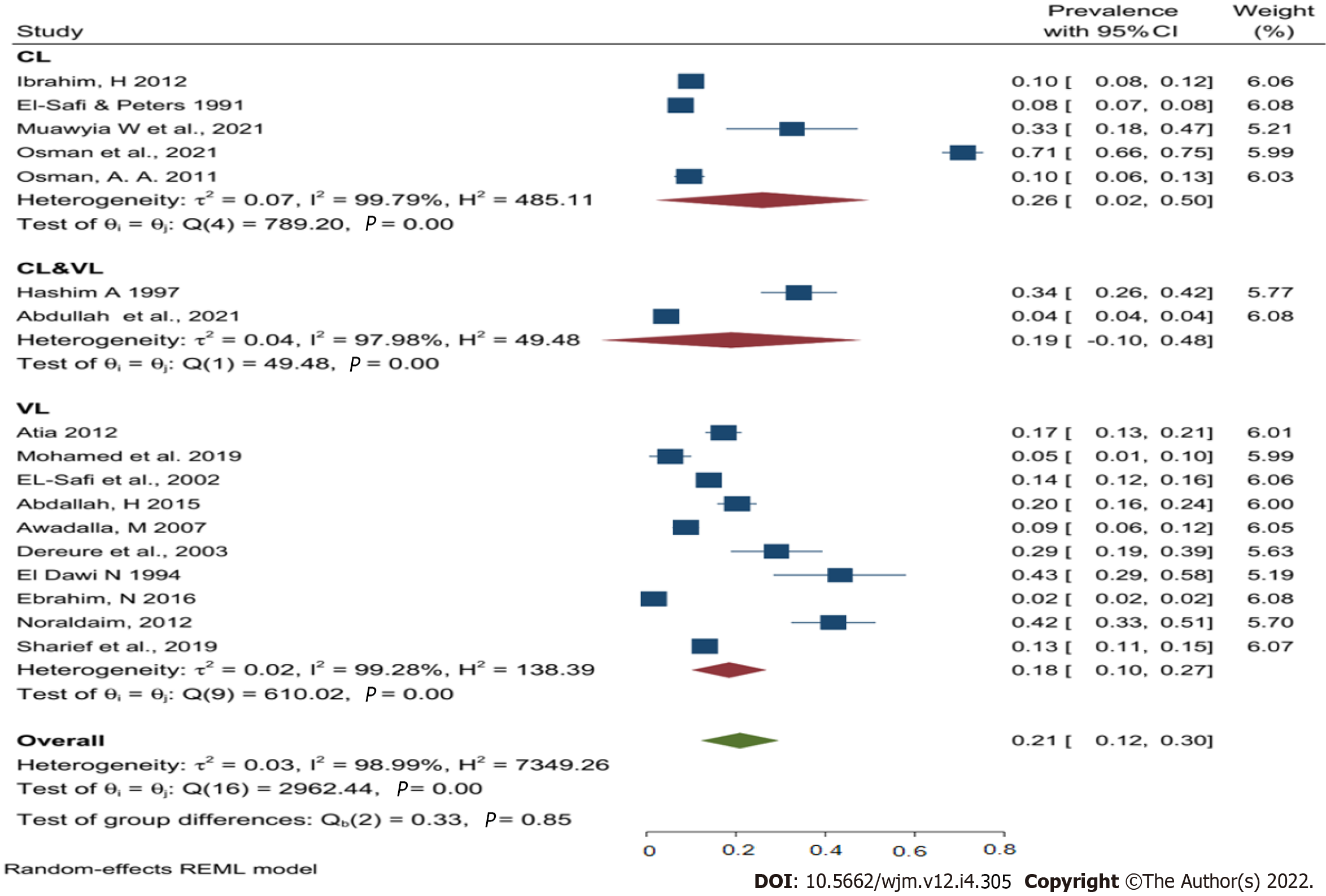
Given the very high heterogeneity level presented in the analyses of human leishmaniasis, a subgroup analysis was done to find the effect of the sex, age, study year's, type of leishmaniasis, study region, study design, and study setting on the pooled prevalence of human leishmaniasis (Table 3). Using the above-mentioned factors as risk factors, the study results found that CL was the most common type of leishmaniasis in Sudan, with a pooled prevalence of 26% followed by combined infection (VL & CL) 19%, and then VL at 18%. Despite this, no data were found about ML prevalence in Sudan (Figure 3).
| Analysis of leishmaniasis | Number of studies/pooled sample size | Pooled prevalence % (95%CI) | T2 | I2% | H2 | P value | |
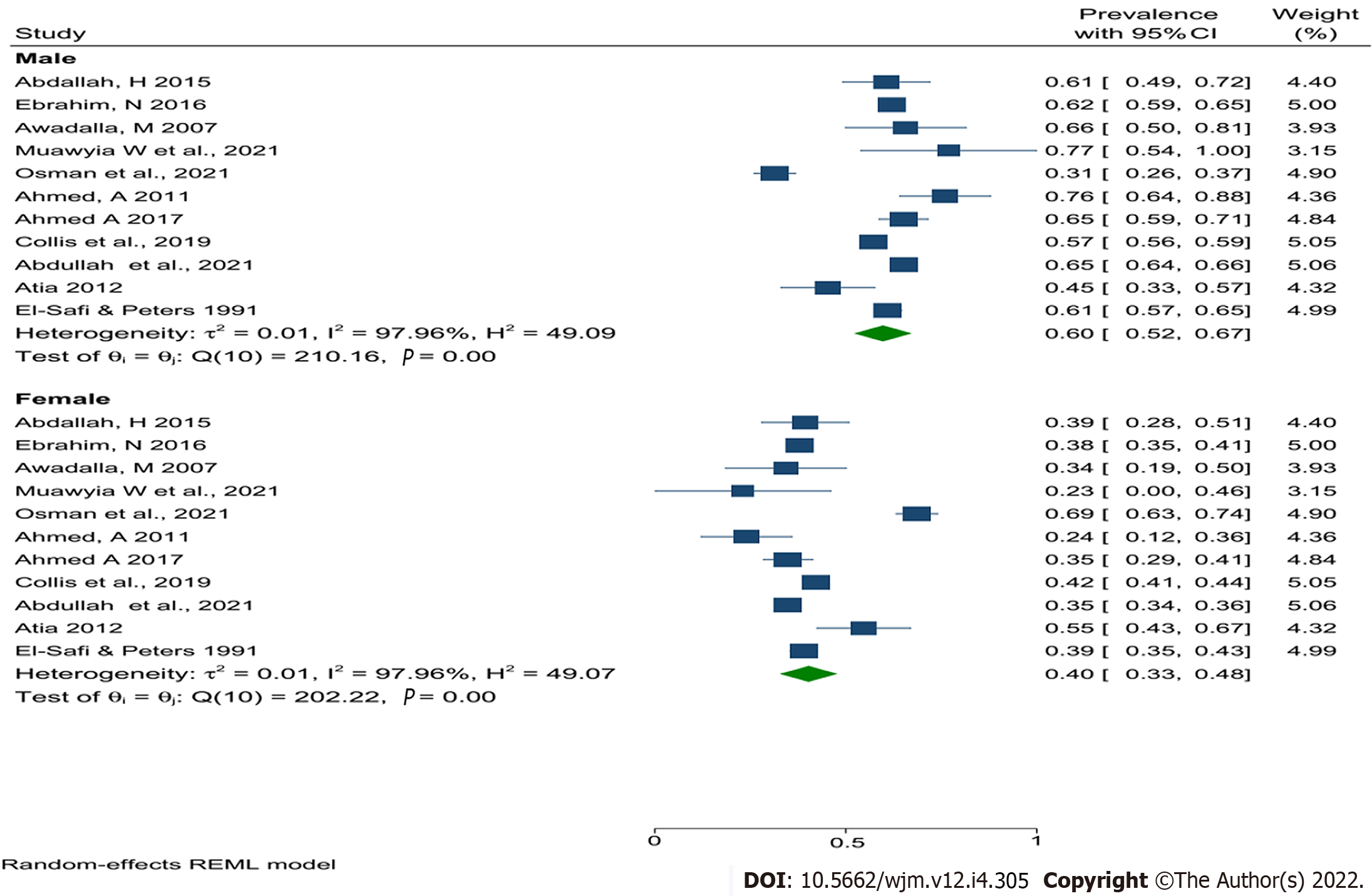
| Sex | Male | 10/13218 | 60 (52-67) | 0.01 | 97.96 | 49.09 | < 0.001 |
| Female | 10/13218 | 40 (33-48) | 0.01 | 97.96 | 49.07 | < 0.001 | |
| Age group | < 5 | 5/8326 | 3 (1-6) | 0.001 | 99.99 | 18316.61 | < 0.001 |
| 5-14 | 5/8326 | 22 (12-32) | 0.01 | 97.76 | 44.50 | < 0.001 | |
| 15-44 | 5/8326 | 60 (50-69) | 0.01 | 95.53 | 22.38 | < 0.001 | |
| ≥ 45 | 5/8326 | 14 (9-19) | 0.001 | 92.09 | 12.63 | < 0.001 | |
| Types of human leishmaniasis | VL | 10/53152 | 18 (10-27) | 0.02 | 99.28 | 138.39 | < 0.001 |
| CL | 5/11173 | 26 (2-50) | 0.07 | 99.79 | 485.11 | < 0.001 | |
| VL/CL | 2/162569 | 19 (10-48) | 0.04 | 97.98 | 49.48 | < 0.001 | |
| Study region | Central Sudan | 6/10711 | 27 (14-40) | 0.02 | 98.86 | 87.63 | < 0.001 |
| Eastern Sudan | 6/2245 | 15(9-21) | 0.01 | 93.84 | 16.23 | < 0.001 | |
| Northern Sudan | 1/410 | 71(66-75) | - | - | - | - | |
| Western Sudan | 4/213528 | 7 (2-12) | 0.00 | 99.97 | 2882.28 | < 0.001 | |
| Study yr/s | Before 2000 | 5/10853 | 24 (12-37) | 0.02 | 98.8 | 83.02 | < 0.001 |
| Between 2001 to 2010 | 4/922 | 24 (9-39) | 0.02 | 96.83 | 31.54 | < 0.001 | |
| After 2011 | 8/215119 | 17 (1-32) | 0.05 | 100 | 24190.74 | < 0.001 | |
| Study setting | Hospital-based study | 8/221713 | 20 (10-31) | 0.02 | 99.99 | 11092.03 | < 0.001 |
| Community-based study | 9/5181 | 21 (7-35) | 0.05 | 99.54 | 218.75 | < 0.001 | |
Nevertheless, the pooled prevalence of human leishmaniasis in Sudan was higher in males (60%) compared with females (40%) (Figure 4). In addition, the current results revealed that the people in the age group between 15 and 44 were the most affected group (60%) (Figure 5), central Sudan has the highest pooled prevalence of human leishmaniasis (27%) compared with other regions of Sudan, and the prevalence of human leishmaniasis seem to decrease over time (Table 3).
The United Nations Environment Programme 2020 annual report revealed that the majority of the Sudanese population live in the river Nile bank, forest zones, and savannah[42,43]. These areas are the natural areas for the presence of the carrier host (Sandfly)[17]. Also, the unique geographical location of Sudan, which is characterized by long staggered borders with some of leishmaniasis endemic areas on the southern and eastern sides of the country, together with the fact that the majority of the population are either nomad or farmers, make it very hard to control the disease in the country. Thus, human leishmaniasis poses an important challenge for the health and economic sectors in Sudan.
Based on a REML, the overall pooled prevalence of human leishmaniasis in Sudan was 21% (95%CI: 12%-30%). Assefa (2018), in Ethiopia, found almost the same result 21% (95%CI: 15%-27%)[44]. However, another Ethiopian study in 2021 found a lower result 9.13% (95%CI: 5-13.27)[45]. This difference between the two Ethiopian studies may be large because of the difference in the number of included studies between them, which was 27 and 11, for Assefa[44], 2018, and Haftom et al[45], 2021, respectively. Although both Ethiopia and Sudan are endemic countries, the overall prevalence showed a clear discrepancy. The current findings showed variations in the pooled prevalence of human leishmaniasis between different geographical regions, age groups, sex, study settings, and years of publication, as well as between the different forms of human leishmaniasis. However, these findings showed no statistical difference in all subgroup analyses.
Two forms of human leishmaniasis were reported in Sudan, CL & VL, and between them, CL had the highest pooled prevalence of 26%, followed by mixed infection (CL & VL) (19%), and VL (18%). These results are in agreement with WHO findings[2] and Assefa's (2018) findings[44]. In contrast, Haftom and his colleagues (2021)[45] found a higher pooled prevalence of VL compared with CL in Ethiopia. Furthermore, the pooled prevalence of VL in Sudan was significantly higher than in Iran (2%)[46,47] and lower than it is in Latin America at 38.8%[48]. However, the current results seem to have one the highest reported pooled prevalence of CL worldwide, with only Sabzevari and his colleagues (2021)[49] in Iran reporting a higher pooled prevalence (45%); all other studies reported a lower pooled prevalence of CL compared with the current findings, including 22.1% in Mali[50], and 6.03%[45], and 19%[44] in Ethiopia.
The reported difference in the results between the other studies and this study may be due to differences in the climate of the study area, the study population, the absence of routine treatment or vaccinations for the definitive host, sample size, sampling procedure, and/or diagnostics method[51,52].
In Sudan usually, men work in agriculture and/or livestock sectors more than women and during the hot evenings and nights, men wear fewer clothes than women. These two main reasons may explain increased prevalence of leishmaniasis in Sudanese males compared with females (60% vs 40%), as these likely an increased risk of sand flies biting. These findings are in agreement with Haftom et al[45] (2021) in Ethiopia, Belo et al[53] (2013) in the Americas, and Kone et al[50] (2016) in Mali. However, two Iranian studies[47,49] disagreed with the current findings, with both studies reporting that the pooled prevalence of human leishmaniasis (CL & VL) was higher in females than in males. The sex-related difference in the pooled prevalence of human leishmaniasis between the current study and the Iranian studies may be due to differences in the cultural and work patterns between Sudan and Iran, Whereby, Iranian women were more involved in agricultural and livestock activities than men which would increase their risk of being bitten by sand flies[49,54].
The association between human leishmaniasis and age was reported in very few studies[26,32,35,37,38]; however, the pooled result reveals that people of workforce age had the highest pooled prevalence, followed by school-aged children and the infants. This makes sense because people who work in the agriculture and/or livestock sectors are at a higher risk of being bitten by sand flies. Similar results were found in Iran[47,49], Mali[50], and the Americas[53].
This meta-analysis study found that central Sudan has the highest reported pooled prevalence of human leishmaniasis compared with other parts of the country, and, generally, the pooled prevalence of human leishmaniasis in Sudan was decreasing over time. This result is corresponding with Al-Salem et al[6] (2016), who stated that “between 1985 and 2005, many epidemics of VL and CL were reported in Sudan, especially in central Sudan”, and resulting from that, a high overall prevalence of human leishmaniasis in the same period of time in central Sudan. The relatively high prevalence of human leishmaniasis in Sudan may be due to the negative effects of the Sudanese civil war. Consequently, the overall prevalence of human leishmaniasis and VL were significantly decreased after the leaders of the two war parties [The federal government of Sudan and the Sudan People's Liberation Army (SPLA)] signed the Comprehensive Peace Agreement on January 9, 2005 to stop the ongoing civil war[55].
Despite the seriousness of human leishmaniasis in Sudan, as presented in the current comprehensive study, no data is available about the economic impact of the disease on the livestock sector and public health sector in the country; thus, work needs to be done to cover the gap in this area. In addition, in our humble opinion, a collaborative effort and immediate action need to be taken from the policymakers and governments (federal and state government), to adopt a national wide epidemiological program to clarify the design of regional strategies and to guide the development of prevention and eradication programs in light of the one health concept during and beyond the COVID-19 pandemic.
The strengths of this study were the use of comprehensive search strategies to ensure that all published and unpublished studies related to the study objectives were included, and the use of standardized quality tools to evaluate the quality of the included studies. Finally, studies with abstracts were only included in this study.
The absence of data about patient places of residence, Leishman parasite species, and other potential risk factors in some included studies, are considered as limitations of the current study.
To the best of our knowledge, the current study is the first systematic review and meta-analysis study regarding the epidemiology of leishmaniasis in Sudanese citizens. Unluckily, there are very few published meta-analysis studies on the overall prevalence of human leishmaniasis, particularly in developing countries to compare with.
This systematic review and meta-analysis showed that human leishmaniasis infection is still endemic in many regions in Sudan and highly prevalent in central and eastern Sudan, and cutaneous leishmaniasis is the most prevalent in Sudan. Males and adults were more susceptible to infection compared with females and children. However, the human leishmaniasis prevalence decreased relatively over time. The presence of the high heterogeneity among the included studies should be considered when interpreting this study's findings. There is a lack of published research about human leishmaniasis in northern and southern regions Sudan. Research need to be updated and more research needs to be conducted in many regions in Sudan to provide adequate information.
The prevalence of human leishmaniasis varies widely in different countries and in different regions of the same country. To date, there is no overall estimation of the prevalence of human leishmaniasis in Sudan
The lack of evidence about human leishmaniasis in Sudan may prevent health care policymakers and stakeholders from developing and adopting a suitable prevention program.
The objective of this study was to find the pooled prevalence of leishmaniasis and its associated factors among Sudanese citizens.
A systematic literature search was conducted before the 4th of August 2021, from Scopus, Web of Science, PubMed, and MEDLINE, African Journals Online (AJOL), ResearchGate, direct Google search, Google Scholar, and universities websites.
A total of 20 articles were included in this meta-analysis after 220 articles had been subjected to full-text evaluations, and the overall pooled prevalence of human leishmaniasis in Sudan was 21% (with confidence interval 12%-30%).
Human leishmaniasis infection is still endemic in many regions in Sudan and is highly prevalent in central and eastern Sudan, and cutaneous leishmaniasis is the most prevalent in the country.
More studies need to be done in Sudan to cover all epidemiological aspects of the disease in humans and animals under the umbrella of one health approach, with special emphasis on the health and economic impacts of the disease.
| 1. | Mableson HE, Okello A, Picozzi K, Welburn SC. Neglected zoonotic diseases-the long and winding road to advocacy. PLoS Negl Trop Dis. 2014;8:e2800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Centre for Disease Control and Prevention. Parasites – Leishmaniasis 2020. [cited 2 December 2021]. Available from: https://www.cdc.gov/parasites/Leishmaniasis. |
| 4. | Shirzadi MR, Javanbakht M, Vatandoost H, Jesri N, Saghafipour A, Fouladi-Fard R, Omidi-Oskouei A. Impact of Environmental and Climate Factors on Spatial Distribution of Cutaneous Leishmaniasis in Northeastern Iran: Utilizing Remote Sensing. J Arthropod Borne Dis. 2020;14:56-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 5. | Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 6. | Al-Salem W, Herricks JR, Hotez PJ. A review of visceral leishmaniasis during the conflict in South Sudan and the consequences for East African countries. Parasit Vectors. 2016;9:460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J. Visceral Leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis. 2014;8:e2869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3243] [Cited by in RCA: 3759] [Article Influence: 268.5] [Reference Citation Analysis (1)] |
| 9. | Seaman J, Pryce D, Sondorp HE, Moody A, Bryceson AD, Davidson RN. Epidemic visceral leishmaniasis in Sudan: a randomized trial of aminosidine plus sodium stibogluconate versus sodium stibogluconate alone. J Infect Dis. 1993;168:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Seaman J, Mercer AJ, Sondorp E. The epidemic of visceral leishmaniasis in western Upper Nile, southern Sudan: course and impact from 1984 to 1994. Int J Epidemiol. 1996;25:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Ashford RW, Seaman J, Schorscher J, Pratlong F. Epidemic visceral leishmaniasis in southern Sudan: identity and systematic position of the parasites from patients and vectors. Trans R Soc Trop Med Hyg. 1992;86:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Gebre-Michael T, Balkew M, Alamirew T, Gudeta N, Reta M. Preliminary entomological observations in a highland area of Amhara region, northern Ethiopia, with epidemic visceral leishmaniasis. Ann Trop Med Parasitol. 2007;101:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Zijlstra EE. Visceral leishmaniasis: a forgotten epidemic. Arch Dis Child. 2016;101:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Abdalla RE. Serodiagnosis of visceral leishmaniasis in an endemic area of the Sudan. Ann Trop Med Parasitol. 1980;74:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Osman OF, Kager PA, Oskam L. Leishmaniasis in the Sudan: a literature review with emphasis on clinical aspects. Trop Med Int Health. 2000;5:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Elnaiem DA, Hassan HK, Ward RD. Phlebotomine sandflies in a focus of visceral leishmaniasis in a border area of eastern Sudan. Ann Trop Med Parasitol. 1997;91:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Lambert M, Dereure J, El-Safi SH, Bucheton B, Dessein A, Boni M, Feugier E, Dedet JP. The sandfly fauna in the visceral-leishmaniasis focus of Gedaref, in the Atbara-River area of eastern Sudan. Ann Trop Med Parasitol. 2002;96:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | EL-Safi SH, Bucheton B, Kheir MM, Musa HA, EL-Obaid M, Hammad A, Dessein A. Epidemiology of visceral leishmaniasis in Atbara River area, eastern Sudan: the outbreak of Barbar El Fugara village (1996-1997). Microbes Infect. 2002;4:1439-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ebrahim NAA. Occurrence of Visceral Leishmaniasis and its Determinants in North Darfur State, Sudan (2013). M.Sc. Thesis, University of Gezira. 2016. Available from: http://repo.uofg.edu.sd/handle/123456789/1427. |
| 20. | Meheus F, Abuzaid AA, Baltussen R, Younis BM, Balasegaram M, Khalil EAG, Boelaert M, Musa AM. The economic burden of visceral leishmaniasis in Sudan: an assessment of provider and household costs. Am J Trop Med Hyg. 2013;89:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 5683] [Article Influence: 1136.6] [Reference Citation Analysis (0)] |
| 22. | Jordan Z, Lockwood C, Munn Z, Aromataris E. The updated Joanna Briggs Institute Model of Evidence-Based Healthcare. Int J Evid Based Healthc. 2019;17:58-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 254] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 23. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48631] [Article Influence: 2114.4] [Reference Citation Analysis (4)] |
| 24. | Hashim AOY. The Polymerase chain reaction as a tool of molecular diagnosis of Leishmania infection in the Sudan. M.Sc. Thesis, University of Khartoum. 1997. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi9s7myfT0AhXMzaQKHRURCNYQFnoECAIQAQ&url=https%3A%2F%2Fwww.osti.gov%2Fetdeweb%2Fservlets%2Fpurl%2F320124&usg=AOvVaw1xM352FNDH7vhLAalZfF5h. |
| 25. | El Dawi NIA. Parasitological and Serological Studies on Visceral Leishmaniasis in the Sudan. M.D. Thesis, University of Khartoum. 1994. Available from: http://khartoumspace.uofk.edu/items/2210d792-b772-414c-a8b7-cc1202fc3411. |
| 26. | Ibrahim HMO. Prevalence of Leishmaniasis in Surogia village, Khartoum North. M.Sc. Thesis, University of Khartoum. 2012. Available from: http://khartoumspace.uofk.edu/items/bc33d20b-4167-465a-b736-a91237d2d2ad/full. [DOI] [Full Text] |
| 27. | Sharief A, Khalil E, Elmagzoub R, Omer S. Spectrum of Leishmania donovani infection in the Southwest of Sudan: a rapid epidemiological mapping. Ann Syst Biol. 2019;2:008-011. [DOI] [Full Text] |
| 28. | Osman A. Epidemiology of leishmaniasis in south Kordofan region, western Sudan. Res J Med Scien. 2011;5:108-111. [DOI] [Full Text] |
| 29. | Noraldaim HAM. Monitoring of Anti Leishmania Antibody Responses for Early Diagnosis and Prognosis of Visceral Leishmaniasis in Dinder National Park. M.Sc. Thesis, University of Khartoum. 2012. Available from: http://khartoumspace.uofk.edu/handle/123456789/8988. |
| 30. | Mohamed NS, Osman HA, Muneer MS, Samy AM, Ahmed A, Mohammed AO, Siddig EE, Abdel Hamid MM, Ali MS, Omer RA, Elaagip AH. Identifying asymptomatic Leishmania infections in non-endemic villages in Gedaref state, Sudan. BMC Res Notes. 2019;12:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, Pratlong F, Feugier E, Lambert M, Dessein A, Dedet JP. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | el-Safi SH, Peters W. Studies on the leishmaniases in the Sudan. 1. Epidemic of cutaneous leishmaniasis in Khartoum. Trans R Soc Trop Med Hyg. 1991;85:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Atia MAE. Visceral Leishmaniasis Situation Analysis: Seroprevalence and Associated Factors with Emphasis on Vector Control in Tabark Allah Village, Eastern Sudan 2010. PhD. Thesis, University of Khartoum. 2012. Available from: http://khartoumspace.uofk.edu/items/3c54189f-b318-4de3-81e0-517db480271e. [DOI] [Full Text] |
| 34. | Abdallah HAMA. Comparison between DAT and rK39 used in the Diagnosis of Visceral Leishmaniasis and their Potential Role in the Diagnosis of the Disease Progress and PKDL. M.Sc. Thesis, University of Gezira. 2015. Available from: http://repo.uofg.edu.sd/handle/123456789/1674. |
| 35. | Awadalla M. Epidemiology of Visceral leishmaniasis among the Population at El Howata Town. University of Khartoum; 2007. M.Sc. Thesis, University of Khartoum. 2012. Available from: http://khartoumspace.uofk.edu/items/a88a780d-fe8f-43d1-8ddb-5af8f673dc53. |
| 36. | Muawyia W, Satti AB, Allseed BAA, Al-Toom THK, Mohammed NMS. Prevalence of Cutaneous leishmaniasis in Khartoum State-Sudan. Health Sciences. 2021;2:443-445. [DOI] [Full Text] |
| 37. | Osman AM, Abakar AD, Abdalla NM, Hussain K, Hassan RSE-d, Mohamedahmed KA. Role of Leishmania Skin Test (LST) as Epidemiological Indicator for Cutaneous Leishmaniasis in Al-tragma Village, River Nile State, Sudan. Endocrinol Metab. 2021;5:175-180. [DOI] [Full Text] |
| 38. | Abdulslam Abdullah A, Ahmed M, Gadeed A, Eltayeb A, Ahmed S, Hamad S. A Five-year retrospective hospital-based study on epidemiological data regarding human Leishmaniasis in West Kordofan state - Sudan, 28 December 2021, PREPRINT (Version 1) available at Research Square. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Ahmed AMF. Ultrasound findings in Sudanese patients with Visceral Leishmaniasis in Omdurman Tropical Diseases Teaching Hospital (March–August 2011). M.Sc. Thesis, Sudan University of Science and Technology. 2011. Available from: http://repository.sustech.edu/handle/123456789/2303. |
| 40. | Ahmed AMB. Evaluation of Visceral Leishmaniasis in Gadarif State Using Ultrasonography. M.Sc. Thesis, Sudan University of Science and Technology. 2017. Available from: http://repository.sustech.edu/handle/123456789/16590. |
| 41. | Collis S, El-Safi S, Atia AA, Bhattacharyya T, Hammad A, Den Boer M, Le H, Whitworth JA, Miles MA. Epidemiological and molecular investigation of resurgent cutaneous leishmaniasis in Sudan. Int J Infect Dis. 2019;88:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 42. | Abdalla IF. Socioeconomic aspects of urban and peri-urban agriculture: A diagnostic study in Khartoum, Sudan. PhD. Thesis, University of Kassel. 2012. Available from: https://www.uni-kassel.de/ub/publizieren/kassel-university-press/verlagsprogramm?h=9783862192687. [DOI] [Full Text] |
| 43. | United Nations Environment Programme. Sudan: First State of Environment and Outlook Report 2020. [DOI] [Full Text] |
| 44. | Assefa A. Leishmaniasis in Ethiopia: A systematic review and meta-analysis of prevalence in animals and humans. Heliyon. 2018;4:e00723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Haftom M, Petrucka P, Gemechu K, Nesro J, Amare E, Hailu T, Ashebir Y, Gebreheat G, Hagos H, Gebremedhin D, Gebremariam A. Prevalence and Risk Factors of Human Leishmaniasis in Ethiopia: A Systematic Review and Meta-Analysis. Infect Dis Ther. 2021;10:47-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Rahmanian V, Rahmanian K, Sotoodeh-Jahromi A, Bokaie S. Systematic review and meta-analysis of human visceral leishmaniasis in Iran. Acta Fac Medicae Naissensis. 2019;36:279-293. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Rostamian M, Bashiri H, Yousefinejad V, Bozorgomid A, Sohrabi N, Raeghi S, Khodayari MT, Ghadiri K, Rezaeian S. Prevalence of human visceral leishmaniasis in Iran: A systematic review and meta-analysis. Comp Immunol Microbiol Infect Dis. 2021;75:101604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Gutiérrez-Ocampo E, Villamizar-Peña R, Cortes-Bonilla I, García-Zuluaga LM, Holguin-Rivera Y, Ospina-Arzuaga HD, Cardona-Trujllo MC, Trejos-Mendoza AE, Perez-Vargas S, Arteaga-Livias K, Zambrano LI, Bonilla-Aldana DK, Perez-Garcia LA, Hernandez-Pereira CE, Rodriguez-Morales AJ, Paniz-Mondolfi A, Delgado OM. Human visceral leishmaniasis prevalence by different diagnostic methods in Latin America: a systematic review and meta-analysis. Infez Med. 2021;29:199-208. [PubMed] |
| 49. | Sabzevari S, Teshnizi SH, Shokri A, Bahrami F, Kouhestani F. Cutaneous leishmaniasis in Iran: A systematic review and meta-analysis. Microb Pathog. 2021;152:104721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Kone AK, Niare DS, Thera MA, Kayentao K, Djimde A, Delaunay P, Kouriba B, Giudice PD, Izri A, Marty P, Doumbo OK. Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis. Asian Pac J Trop Med. 2016;9:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | de Ruiter CM, van der Veer C, Leeflang MM, Deborggraeve S, Lucas C, Adams ER. Molecular tools for diagnosis of visceral leishmaniasis: systematic review and meta-analysis of diagnostic test accuracy. J Clin Microbiol. 2014;52:3147-3155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 52. | Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, Modabber F, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Smith PG. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine. 2009;27:4747-4753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Belo VS, Werneck GL, Barbosa DS, Simões TC, Nascimento BW, da Silva ES, Struchiner CJ. Factors associated with visceral leishmaniasis in the americas: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Zare S, Baghestani S. Cutaneous leishmaniasis in Hormozgan, Iran. Int J Dermatol. 2001;40:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious Diseases
Country/Territory of origin: Sudan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasabo EA, Sudan; Xiong A, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Qi WW