Published online May 20, 2022. doi: 10.5662/wjm.v12.i3.132
Peer-review started: December 3, 2021
First decision: February 8, 2022
Revised: February 16, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 20, 2022
Processing time: 166 Days and 19.5 Hours
Many Ayurvedic preparations are claimed to have immune-boosting properties, as suggested in various published randomized clinical trials (RCTs)
To compile evidence on the nature and mechanism of immune system enhancement by Ayurvedic preparations in healthy and sick individuals.
After prospectively registering study protocol with PROSPERO, we searched PubMed, DOAJ, Google Scholar, three dedicated Ayurveda research portals, two specialty Ayurveda journals, and reference lists for relevant records published until February 6, 2021 using appropriate search strategies. Baseline features and data pertaining to the nature and mechanism of immune system function were extracted from all eligible records. Methodological quality was assessed using the Cochrane RoB-2 tool.
Of 12554 articles screened, 19 studies reporting 20 RCTs (17 parallel group design, three crossover design) with 1661 unique patients were included; 11/19 studies had Indian first authors. Healthy population was included in nine studies, of which one study included pregnant women and two included pediatric population; remaining studies included patients with different health conditions, including one study with coronavirus disease 2019 patients. A total of 21 Ayurvedic interventions were studied, out of which five were composite mixtures. The predominant route of administration was oral; dose and frequency of administration of the intervention varied across the studies. The results reported with five RCTs exploring five Ayurvedic interventions were incomplete, ambiguous, or confusing. Of the remaining 16 interventions, indirect evidence of immune enhancement was reported with four interventions, while lack of the same was reported with two interventions. Enhancement of T helper cells and natural killer cells was reported with three and four interventions, respectively, while the pooled results did not clearly point toward enhancement of other components of the immune system, including cytotoxic T cells, B lymphocytes, immunoglobulins, cytokines, complement components, leucocyte counts, and other components. Nine of the 20 RCTs had a high risk of bias, and the remaining 11 RCTs had some concerns according to RoB-2.
Various Ayurvedic preparations appear to enhance the immune system, particularly via enhancements in natural killer cells and T helper cells.
Core Tip: Ayurvedic preparations have been anecdotally associated with immune boosting effect in both healthy and sick individuals. Through this systematic review, we explored the nature and mechanism behind this effect by scrutinizing 20 randomized controlled trials reported in 19 articles. While we could find indirect evidence for immune enhancement (by means of reduced illness duration and severity) with some Ayurvedic preparations, the evidence was insufficient to conclude about the exact mechanisms contributing to this phenomenon, although available evidence suggests that enhancements in natural killer cells and T helper cell number and function might contribute.
- Citation: Vallish BN, Dang D, Dang A. Nature and mechanism of immune boosting by Ayurvedic medicine: A systematic review of randomized controlled trials. World J Methodol 2022; 12(3): 132-147
- URL: https://www.wjgnet.com/2222-0682/full/v12/i3/132.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i3.132
As of September 2021, the ongoing coronavirus disease 2019 (COVID-19) pandemic has seen at least two waves in almost all regions of the world, including India, with many predictions hinting at a global third wave due to new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants[1]. The remarkable trail of destruction left by the pandemic, coupled with the non-availability of a drug-of-choice to treat the condition[2], has resulted in an almost complete dependence on the development of safe and efficacious vaccines that can protect individuals against existing and potential virus variants. Despite developments in vaccinology, it is common knowledge that a complete protection against all future variants of the virus cannot be absolutely guaranteed by even the most advanced vaccines. At the same time, non-neutralizing antibodies that cause antibody-dependent enhancement of the immune system are reported to exacerbate paradoxically SARS-CoV-2 infection, leading to worsened organ damage[3]. This has led to the realization that therapies that produce a sufficiently strong, but not hyperactivated, immune system can lead to a healthier society and can contribute significantly to the fight of mankind against not only the COVID-19 pandemic but also future healthcare challenges of infectious disease origin. Consequently, healthcare systems in many parts of the world, especially India, have turned their attention toward potential therapies that can produce a non-specific and unaided enhancement of the immune system, as a complementary step to vaccine development[4,5].
Ayurveda is the Indian traditional system of medicine and has a history since the 2nd century BC. The core pathophysiological principles of Ayurveda surround the concept of loss of balance with respect to the three humors (Tridoshas), five elements (Pancha mahabhootas), and seven tissues (Saptadhatus) of the human body. Various Ayurvedic treatments are described for diseases affecting different systems of the body, and most Ayurvedic medicinal items are sourced from natural ingredients. Classical texts of Ayurveda also have mentions about management of epidemics[6,7] and has defined ‘immunity (Bala)’ as the ‘ability of the body to prevent and arrest the progression of disease for maintaining homeostasis’[4]. Few facts, such as the classification of immune system in Ayurveda into Sahaja (innate), Kalaja (chronobiologic), and Yuktikrut (acquired) closely resembling the modern medical classification of immune system[4] and Ayurvedic immune-boosting preparations such as cyavanaprasa having stood the test of time, are testaments to the near-accurate understanding of our body’s immune system by the ancient Ayurveda pioneers. Thus, it is not surprising that the Ministry of AYUSH (Ayurveda, Yoga, Unani, Siddha, and Homoeopathy) of the Indian Government released an advisory recommending various natural therapies to develop immunity against COVID-19[8]. Many components named in the said recommendation have been reported to produce immune enhancement through modulation of multiple immune system pathways[8] and also through psychoneuroimmunological mechanisms[5]. However, since most of these observations are through pre-clinical and non-human studies, we were interested to know if these pre-clinical observations hold good when the Ayurvedic preparations are studied after human administration.
With this background, we performed the present systematic literature review (SLR) with an objective for gathering evidence towards the nature and mechanism of enhancement of human immune system by the administration of Ayurvedic preparations, from published randomized clinical trials (RCTs). The research question that we were looking forward to find an answer through this SLR was: ‘what is known from published RCTs about the nature and mechanism of the impact of consuming ayurvedic medicine on enhancing the immune system activity of healthy or sick humans?’
The SLR protocol was drafted following the guidelines given in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and was subsequently refined through internal discussion. The final version of the protocol was prospectively registered with PROSPERO on July 10, 2020, with ID CRD42020191289.
To identify potentially relevant articles that answered our research question, the following eligibility criteria were drafted: ‘Population’ was all studies involving healthy or sick adults with any form of illness (acute or chronic). The protocol was subsequently amended to include humans of all age groups, including pediatric and geriatric individuals. We did not restrict the population with respect to the age or gender of the individuals, presence of any form of disease, presence of comorbidities, or presence of special situations such as pregnancy. ‘Intervention’ included any type of Ayurvedic medicine, given as a combination or single drug. Studies that did not explicitly mention whether the intervention was an ‘Ayurvedic medicine’ or not were included in the review if the intervention was found in the work ‘Indian Medicinal Plants: an Illustrated Dictionary’ by Khare[9]. We also used the same resource to compile the Ayurvedic name of various interventions. Papers that included interventions only belonging to other systems of medicine (including modern/Western medicine, traditional medicines of other regions/countries, and other systems of medicine including Unani, Siddha, Homoeopathy, Yoga, Naturopathy, Osteopathy, and Chiropractic) were excluded. We did not restrict studies based on the ‘Comparator’ and included all studies regardless of whether or not there was a comparator. ‘Outcomes’ of interest included any description of enhancement, augmentation, stimulation, increasing, or strengthening of the immune system, either directly (through observation on the effect of component/s of the immune system) or indirectly (through observation of effect of said enhancement of immune system, by means of surrogate markers such as relief from illness, quickness of recovery, etc.). Studies that described immunomodulation instead of immune enhancement and studies that did not describe any immune system effect were excluded. ‘Study design’ included only RCTs. Papers not describing primary data (such as narrative or systematic reviews, letters to editors, opinion pieces, commentaries, editorials, brief communications, news items, etc.), case studies, case reports, and studies describing non-human experiments (including in vitro studies, in vivo studies, in silico experimentation, etc), and studies not in English language were excluded from our review.
Following the eligibility criteria described above, a PubMed literature search strategy was drafted and modified through internal discussion. Using the refined search strategy, a systematic literature search was performed in PubMed/MEDLINE, from their inception till June 2, 2021. Recognizing that many research articles on Ayurveda are published in journals not indexed in PubMed, and following the recommendations by Aggithaya et al[10] (2015), we performed an additional literature search in Directory of Open Access Journals[11], AYUSH research portal[12], Digital Helpline for Ayurveda Research Articles (DHARA)[13], Annotated Bibliography of Indian Medicine (ABIM)[14], and Google Scholar. Furthermore, we also performed targeted journal search in two Ayurveda specialty journals (Ayu and Journal of Ayurveda and Integrative Medicine) using a combination of search terms and Boolean operators such as “Ayurveda”, “Ayurvedic medicine”, “Immune”, “Immune stimulating”, “Immune enhancing”, “Immunomodulatory”, and “Randomized clinical trials”. The detailed PubMed search strategy is presented as Supplementary Table 1, and a brief account of searches performed in each of the portals mentioned above is provided in Supplementary Table 2. Finally, we also scanned reference lists of relevant studies to identify potentially eligible records that were missed through database search.
After pooling all the eligible records, we identified the most eligible articles for our review and extracted relevant data from these papers after thoroughly studying the full texts of each article. Extracted data, such as data related to the study details (year of publication, country of the first author, study design, and details of the intervention and comparator), participant details (participant profile, number, age, and sex), details of the intervention and comparator (Ayurvedic, commercial, and scientific names, composition, dose, frequency, duration, and route of administration), and outcome details in terms of improvement from baseline, were entered into a predefined data entry grid. All outcomes related to the immune system (or Ojas) were considered for this study and included direct variables [such as leukocyte subtypes including T lymphocytes, B lymphocytes, natural killer (NK) cells, and various types of myelocytes, immunoglobulins, complement components, and cytokines] and indirect variables (such as absenteeism, number of healthy days and sick days, number of doctor visits, number of symptoms, Ojas score, etc.). The methodological quality of the included studies was assessed using the Cochrane RoB-2 tool[15]. We used the official Microsoft Excel tool provided by the Cochrane Foundation for implementing RoB-2, and we used separate tools for parallel group RCTs and crossover RCTs[16].
Subsequent to executing the literature search, two authors (VBN and AD) independently screened the pooled articles for their inclusion in the study, extracted data, and assessed the articles for risk of bias; any disagreements were resolved through discussion and reconciliation that was moderated by another author (DD).
Study selection and data extraction were done electronically in Microsoft Excel. To assess the inter-rater reliability (IRR) of the study inclusion and the methodological quality assessment of the included articles, Cohen’s kappa value was calculated using SPSS version 20 (Armonk, NY, United States). The cut-off points for the kappa statistic were interpreted as ≤ 0.20 = slight agreement; 0.21-0.40 = fair agreement; 0.41-0.60 = moderate agreement; 0.61-0.80 = substantial agreement; 0.81-0.99 = near-perfect agreement; and 1.00 = perfect agreement[17]. Statistical review of the study was performed by an author who is a biomedical statistician (VBN). All datasets used to derive conclusions in this study are available with the corresponding author on reasonable request.
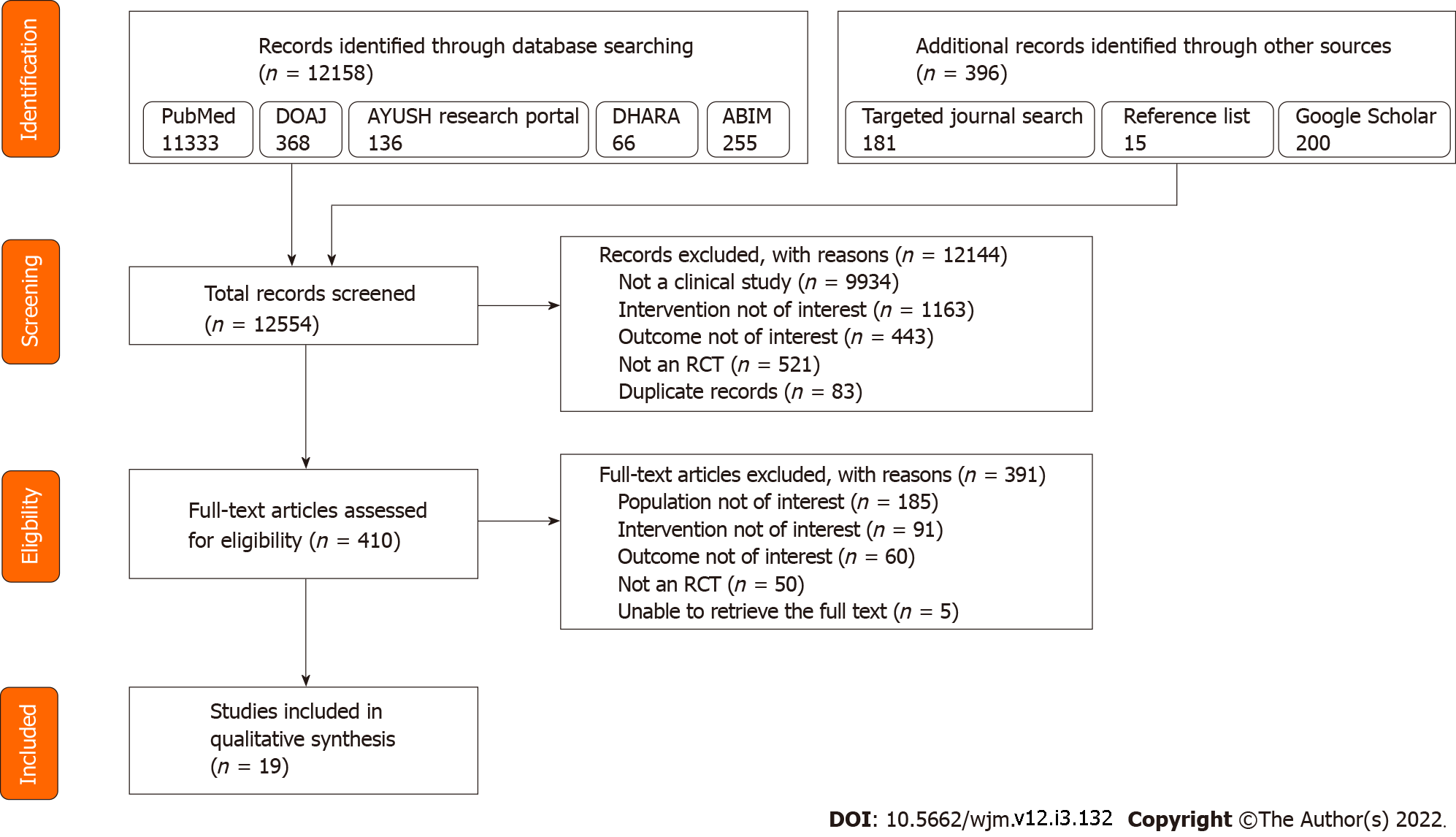
From an initial pool of 12554 potentially eligible records, 19 studies were included for data extraction and review. Figure 1 depicts the study selection process.
The 19 included studies were published from 2005 to 2021. A total of 20 RCTs were reported in the 19 included papers: 16 articles described parallel group RCTs, two described crossover trials, and one article reported two RCTs: One parallel group RCT, and one crossover RCT. The first authors of the 19 papers were from six different countries, with India being the most frequent country of affiliation (11 studies), followed by Japan and United States (two studies each); there was one study each with first authors from Germany, Iran, Romania, and Turkey. The participant profile ranged from healthy participants (six studies, healthy adults; two studies, healthy pediatric population; one study, healthy pregnant women), patients with cancer (three studies, gastrointestinal cancer; one study, head and neck cancer), patients with diabetes and human immunodeficiency virus (HIV) (two studies each), and patients with COVID-19 and allergic rhinitis (one study each). Among the 17 studies that included adult participants, two included only females, one study included only males, and the remaining 14 studies included participants of either gender. The sample size of the studies ranged from 5 to 627, with a total of 1661 unique participants across the 19 articles. The complete baseline characteristics of the included studies are summarized in Table 1.
| No | Ref. | Country of 1st author | Type of randomized controlled trial | Patient profile | Intervention1 | Comparator1 | Overall risk of bias as per RoB-2 tool2 | ||||
| Sample size | Mean age | Male (N, %) | Sample size | Mean age | Male (N, %) | ||||||
| 1 | Enesel et al[18], 2005 | Romania | Parallel group | Patients undergoing surgery for GIT cancer | 40 | 62 (range 37-87) | 23 (57.5) | 30 | NA | NA | - |
| 2 | Ishikawa et al[21], 2006 | Japan | Parallel group | Patients with inoperable colon/liver/pancreatic cancer | 25 | 63.6 ± 8.3 | 21 (84) | 25 | 65.8 ± 6.3 | 18 (72%) | ? |
| 3 | Brush et al[22], 2006 | United States | Parallel group | Healthy adult volunteers | 3 | NA | NA | 2 | NA | NA | - |
| 4 | Schink et al[27], 2007 | Germany | Parallel group | Primary/locally relapsed colorectal carcinoma patients undergoing open (complete/partial) tumour resection | 11 | 72 ± 8.2 | 7 (63.6) | 11 | 69 ± 10.4 | 5 (45.5) | ? |
| 5 | Purandare et al[33], 2007 | India | Parallel group | Patients with diabetic foot ulcer | 23 | 56.26 | 17 (73.9) | 22 | 56.32 | 19 (86.4) | ? |
| 6 | Uebaba et al[29], 2008 | Japan | Crossover | Healthy adult female volunteers | 16 | 39 ± 9 | 0 (0) | 16 | 39 ± 9 | 0 (0) | ? |
| 7 | Bhat et al[28], 2010 | India | Parallel group (I) | Healthy adult volunteers | 13 | NA | NA | 13 | NA | NA | ? |
| Crossover (II) | Healthy adult volunteers | 110 | NA | NA | 110 | NA | NA | - | |||
| 8 | Işik et al[23], 2010 | Turkey | Parallel group | Allergic rhinitis patients with house dust mite sensitivity | 12 | NA | NA | 12 | NA | NA | - |
| 9 | Kianbakht et al[30], 2011 | Iran | Parallel group | Healthy adult male volunteers | 45 | 22.5 ± 0.6 | 45 (100) | 44 | 21.1 ± 0.5 | 44 (100) | ? |
| 10 | Mondal et al[24], 2011 | India | Crossover | Healthy adult volunteers | 22 | NA | NA | 22 | NA | NA | ? |
| 11 | Nantz et al[26], 2012 | United States | Parallel group | Healthy adult volunteers | 56 | 25.4 ± 5.7 | 23 (41.1) | 56 | 26.9 ± 7.1 | 26 (46.4) | ? |
| 12 | Suprabha et al[32], 2017 | India | Parallel group | Uncomplicated pregnant women in 20-24 wk of pregnancy | 15 | NA | NA | 15 | NA | NA | ? |
| 13 | Gupta et al[34], 2017 | India | Parallel group | Healthy children aged 5-12 yr | 313 | 7.3 ± 1.8 | 161 (51.4) | 314 | 7.4 ± 1.8 | 164 (52.2) | ? |
| 14 | Rais et al[36], 2021 | India | Parallel group | 25-60 yr, asymptomatic/ uncomplicated COVID-19 RTPCR +ve, mild symptoms | 80 | NA | 57 (71.3) | 40 | NA | 30 (75) | - |
| 15 | Bhaskaran et al[31], 2019 | India | Parallel group | Healthy full-term infants (< 12 mo age), > 2.5 kg birth weight, with normal growth and development | 47 | NA | NA | 34 | NA | NA | - |
| 16 | Kumar et al[35], 2014 | India | Parallel group | Adult patients with T2DM of any stage | 56 | NA | NA | 28 | NA | NA | - |
| 17 | Ravindran et al[25], 2014 | India | Parallel group | Patients with head & neck cancer in complete remission following primary treatment | 37 | NA | NA | 38 | NA | NA | ? |
| 18 | Somarathna et al[20], 2010 | India | Parallel group | HIV +ve patients without AIDS surveillance signs as per WHO, and no concurrent illness | 21 | NA | NA | 6 | NA | NA | - |
| 19 | Gupta et al[19], 2010 | India | Parallel group | New HIV +ve patients with CD4 count not < 150/microliter and no complications or comorbidities | 12 | NA | NA | 8 | NA | NA | - |
Two of the included studies explored two interventions, and the remaining 17 studies explored one intervention each, leading to a total of 21 interventions. The predominant route of administration of the intervention was oral (n = 17), with one study each delivering the intervention through subcutaneous injection, intravenous infusion, and oil dripping on the forehead; details of route of administration of the intervention were missing in one study. Among the 17 orally administered interventions, the formulations were capsules in five studies, and Rasayana in three studies; one study each used other formulations including tablet, tea, Cyavanaprasa, Kasaya, Bhasma, Ghrita, Churna/Kalka, and tincture, and one study did not provide information about formulation. Details of dosing and duration of administration of the intervention varied across the studies. Placebo was used as the comparator in six studies. The complete details of the interventions and comparators among the included studies are summarized in Table 2. The detailed composition of the different composite preparations used as interventions in five of the studies is provided in Supplementary Table 3.
| No | Ref. | Description | Intervention: Ayurvedic name1 | Intervention: Scientific name | Intervention: Route; dose, frequency, duration2 | Comparator: Description; route, dose, frequency, duration2 |
| 1 | Enesel et al[18], 2005 | Isorel® (Mistletoe, firtree) | Bandaaka, Suvarna-bandaaka etc. | Viscum album | Subcutaneous; 2 wk pre-operatively, 2 wk post-operatively; dose details not clear | Standard care |
| 2 | Ishikawa et al[21], 2006 | Aged garlic extract | Lashuna, Rasona etc. | Allium sativum | Oral; 125 mg, 4 capsules daily, 12 wk | Matching placebo |
| 3 | Brush et al[22], 2006 | Glycyrrhizia glabra tincture | Yashtimadhu, Madhuyashtyaahvaa etc. | Glycyrrhiza glabra | Oral; 0.44 g/7.5 mL, twice daily, 7 d | Matching placebo tincture |
| 4 | Schink et al[27], 2007 | Standard care + Iscador® (Standardized mistletoe extract) | Bandaaka, Suvarna-bandaaka etc. | Viscum album | IV infusion; 5 mg, single dose | Standard care |
| 5 | Purandare et al[33], 2007 | Standard care + Tinospora cordifolia | Guduuchi, Guduuchikaa etc. | Tinospora cordifolia | Details not available | Standard care + matching Placebo |
| 6 | Uebaba et al[29], 2008 | Shirodhara oil-dripping treatment using sesame oil | Shirodhara; Tila, Snehphala | Sesamum indicum | Oil dripping on forehead; single sitting | Control supine position; single sitting |
| 7 | Bhat et al[28], 2010 | Fortified tea with Withania somnifera, Glycyrrhzia glabra, Zingiber officinale, Ocimum sanctum & Elettaria cardamomum | Ashwagandha; Yashtimadhu; Aardraka, Shunthi; Tulasi; Elaa | Withania somnifera, Glycyrrhzia glabra, Zingiber officinale, Ocimum sanctum, Elettaria cardamomum | Oral; 2.06 g, thrice daily, 2 mo | Regular tea; Oral; 2 g, thrice daily, 2 mo |
| 8 | Işik et al[23], 2010 | Specific immunotherapy + Nigella sativa | Kaalaajaaji, Kalikaa etc. | Nigella sativa | Oral; 2 g daily, 1 mo | Specific immunotherapy alone |
| 9 | Kianbakht et al[30], 2011 | Saffron tablet | Kumkuma, Rudhira, Kaashmiraka etc. | Crocus sativus | Oral; 100 mg daily, 6 wk | Matching placebo |
| 10 | Mondal et al[24], 2011 | Tulsi capsules | Tulasi, Surasa, Suravalli etc. | Ocimum sanctum | Oral; 300 mg daily, 4 wk | Matching placebo |
| 11 | Nantz et al[26], 2012 | Aged garlic extract | Lashuna, Rasona etc. | Allium sativum | Oral; 640 mg, 4 capsules daily, 90 d | Matching placebo |
| 12 | Suprabha et al[32], 2017 | Rasayana Avaleha with milk | Composite4 | Composite4 | Oral; 12 g, twice daily, 2 mo | Calcium carbonate (500 mg) with ferrous sulfate (200 mg); Oral; daily, 2 mo |
| 13 | Gupta et al[34], 2017 | Cyavanaprasa (Dabur) with milk | Composite4 | Composite4 | Oral; 6 g, twice daily, 6 mo | Milk; Oral, 100-200 mL, twice daily, 6 mo |
| 14 | Rais et al[36], 2021 | Intervention 1: Vyaghryadi Kashaya (50 mL) + Pippali (250 mg) + Samshamani vati (500 mg) | Vyaghryadi Kashaya: Kantakari, Shunthi, Guduchi; Pippali; Samshamani vati: Guduchi | Solanum xanthocarpum, Zingiber officinale, Tinospora cordifolia, Piper longum | Oral; twice daily, 10 d | Vitamin C (500 mg) twice daily, Paracetamol (500 mg) as needed |
| Intervention 2: Shunthi churna (2 g) + Rasona kalka (1 g) | Shunthi; Rasona, Lasuna | Zingiber officinale, Allium sativum | Oral; Shunti churna: Twice daily, Rasona: once daily; 10 d | |||
| 15 | Bhaskaran et al[31], 2019 | Swarna Bhasma (Calcined powder of gold), honey, ghrita | Swarna prashana, madhu, ghrita | NA | Oral; Swarna bhasma: 0.2-2.4 mg3; once daily, 4 wk | Oral Honey + ghrita; dose details not available |
| 16 | Kumar et al[35], 2014 | Intervention 1: Mamajjaka capsules | Maamajjaka, Naagjhvaa etc. | Enicostemma littorale | Oral; 500 mg, twice daily, 3 mo | Control; no details available |
| Intervention 2: Shilajatu capsules | Shilajatu | Asphaltum punjabinum | Oral; 500 mg, twice daily, 3 mo | |||
| 17 | Ravindran et al[25], 2014 | Varunadi Ghrita + Standard care | Composite4 | Composite4 | Oral; 5 g, twice daily, 1 y | Standard care |
| 18 | Somarathna | Ranahamsa Rasayana | Composite4 | Composite4 | Oral; 5 g, twice daily, 90 d | Standard care |
| 19 | Gupta et al[19], 2010 | Shilajatu Rasayana | Composite4 | Composite4 | Oral; 95 g over first 15 d, later 6 g per day for 75 d | Standard care |
Of the 19 included studies, 15 studies provided information about the impact of the intervention on at least one component of the immune system (direct evidence), three studies provided information about the impact of the intervention on the overall health status of the participant (indirect evidence), and one study provided both direct and indirect evidence. The description of the results was not uniform across various studies, with different units of measurement used to express similar outcomes; this prevented us from performing a meta-analysis of the results.
Impact on T lymphocyte subsets (excluding NK cells): CD4 lymphocyte counts were found to be significantly enhanced after perioperative mistletoe administration for 14 d among patients undergoing surgery for gastrointestinal (GI) cancer[18]. Among patients with HIV, CD4 lymphocyte counts were found to be enhanced after a 90-d consumption of Shilajatu Rasayana[19] and Ranahamsa Rasayana[20], as compared to standard care. However, the increase in CD4 lymphocyte count caused by the consumption of aged garlic extract for 12 wk was not statistically significant among patients with GI cancer and was less than that observed with matching placebo[21]. The results reported in three papers were ambiguous: Although Glycyrrhizia glabra tincture increased CD4 lymphocytes by 8.26% in 24 h in healthy individuals, the statistical significance of this finding was not reported, and the sample size was only 5 patients[22]. Significant enhancement of CD4 lymphocyte count was also not observed with Nigella sativa seed supplementation, and the data were not presented with clarity[23]. Tulsi was reported to significantly increase CD4 lymphocytes in 4 wk compared to placebo among healthy individuals, but this article did not provide any numbers to support this claim[24].
CD8 lymphocyte counts were significantly decreased with perioperative mistletoe administration for 14 d[18]. Aged garlic extract brought about a non-significant increase in CD8 lymphocyte count over 12 wk, which was also seen with matching placebo[21]. The remaining three papers presented results with less clarity: although Glycyrrhizia glabra tincture increased CD8 lymphocyte by 2.89% in 24 h in healthy individuals, the statistical significance of this finding was not reported, and the sample size was only 5 patients[22]. Significant CD8 lymphocyte count enhancement was observed with Nigella sativa seed supplementation among allergic rhinitis patients, but the presented results lacked clarity[23]. Tulsi did not have significant effect on CD8 lymphocyte counts among healthy individuals, but this paper did not provide any numbers to back this claim[24].
The impact of Ayurvedic preparations on other types/subtypes of T cells was presented in four papers. Perioperative mistletoe administration was associated with a significant increase in T lymphocyte count, CD3 lymphocyte count, and CD4/CD8 ratio among patients with GI cancer[18], and Varunadi Ghrita consumption for 1 year was found to significantly increase CD3 lymphocyte count among patients with head and neck cancer after complete remission[25]. Although aged garlic extract consumption for 45 d was reported to have a significantly higher γδ-T cell proliferation index among healthy adults compared to placebo, numbers to support this claim were not reported in this paper[26]. Finally, Nigella sativa combined with specific immunotherapy was not found to significantly alter CD3 lymphocytes among patients with allergic rhinitis, but this article did not present the results with clarity[23].
Impact on NK cell count and NK cell activity: NK cell counts were found to be significantly enhanced by perioperative administration of mistletoe for 14 d among patients undergoing surgery for GI cancer[18] and also by consumption of aged garlic extract capsules for 12 wk among patients with inoperable GI cancer[21]. Administration of Varunadi Ghrita for 1 year was associated with a marginal but significant increase in NK cell counts among patients with head and neck cancer[25]. Tulsi produced a significant increase in NK cell activity over 4 wk among healthy volunteers, but this article did not present the complete results to strengthen its claims[24]. No significant changes in NK cell counts were observed with either Glycyrrhizia glabra consumption[22] or Nigella sativa seed supplementation[23], but results presented in both these articles were incomplete.
NK cell activity was found to be significantly enhanced after 7 d of surgery with a single-dose mistletoe extract intravenous infusion perioperatively among patients undergoing colorectal carcinoma resection, compared to standard care[27]. Among patients with inoperable GI cancer, aged garlic extract administered for 12 wk was associated with a significant increase in mean NK cell activity percentage, but a non-significant reduction in mean NK cell activity per 100 cells[21]. Regular consumption of tea fortified with several Ayurvedic herbs over 2 mo by healthy individuals was associated with a significant enhancement in NK cell activity compared to regular tea; however, the crossover design of the trial did not have a sufficient wash-out period, and there were inconsistencies with the numbers in the results[28]. Next, among healthy females, a 30-min Shirodhara treatment with sesame oil was associated with a significant enhancement in NK cell activity compared to the control supine position for 30 min[29], and among healthy volunteers, the consumption of aged garlic extract for 45 d was associated with a significant enhancement of activated state of NK cells as well as NK cell proliferation index compared to placebo[26]; however, both these papers did not provide numbers to substantiate fully these claims.
Impact on B lymphocytes and immunoglobulins: While B lymphocyte counts were significantly enhanced by the consumption of Varunadi Ghrita for 1 year among patients with head and neck cancer remission[25], a significant change was not reported with consumption of mistletoe extract[18], Glycyrrhizia[22], Nigella[23], and Tulsi[24]. It is however worthwhile to note that the last three studies had methodological or reporting issues. Next, perioperative consumption of mistletoe extract was associated with a significant increase in serum levels of immunoglobulin (Ig)A and IgM, and also of IgG (significance not mentioned)[18]; however, a significant impact was not found on serum levels of IgG, IgM, and IgA by saffron consumption[30], on serum IgG levels of infants by calcined gold powder consumption[31], or on cord blood IgG levels by consumption of Rasayana Avaleha by the pregnant mother[32]. Interestingly, the last study concluded that Rasayana Avaleha enhanced fetal immunity level, despite absence of strong evidence pointing towards the same.
Impact on complement components and cytokines: While serum levels of complement C3 and C4 proteins were found to increase significantly after 14 d of perioperative administration of mistletoe extract among patients undergoing surgery for GI cancer[18], no significant changes in the serum levels of these proteins were observed after daily consumption of saffron tablets for 6 wk among healthy individuals[30]. Among healthy adult volunteers, the consumption of aged garlic extract for 45 d was associated with non-significantly reduced serum levels of tumor necrosis factor α and interferon γ levels compared to placebo; this ‘decrease in cytokine levels’ was interpreted by the authors to suggest that consumption of aged garlic extract resulted in ‘enhancement of the immune system’ in the sense that, after the consumption of aged garlic extract, ‘eradication of pathogens causing flu-like illnesses’ could be achieved by lower levels of these cytokines[26]. By contrast, consumption of Tulsi capsules for 4 wk was found to increase significantly the levels of interferon gamma and interleukin-4 in healthy adult volunteers, and this ‘increase in cytokine levels’ was also interpreted as supporting the observation that Tulsi resulted in mounting ‘an effective immune response’[24]. The latter paper also did not provide complete numbers to substantiate the claims.
Impact on white blood cell counts and granulocytes: Even though total white blood cell (WBC) counts were enhanced by perioperative mistletoe administration after 14 d, the enhancement seen with standard care was found to be numerically higher[18]. Total WBC counts were not found to be significantly enhanced by administration of aged garlic extract[21], saffron[30], or calcined gold powder[31].
Total lymphocyte counts were significantly enhanced by perioperative mistletoe administration among patients with GI cancer[18]. While calcined gold powder was found to enhance significantly lymphocyte counts among infants < 1 mo but not in older infants, this paper did not provide numbers to back their claims[31]. Saffron was not associated with a significant change in total lymphocyte counts as well[30].
While significant changes in absolute neutrophil counts were not reported with the use of saffron[30] and calcined gold powder[31], absolute neutrophil counts were significantly reduced by administration of Ranahamsa Rasayana for 90 d among patients with HIV[20]. Phagocytic function by neutrophils was found to be significantly enhanced after a 1-mo treatment with Tinospora cordifolia (among patients with diabetic foot ulcer)[33] and Nigella sativa (among patients with allergic rhinitis)[23].
No significant alterations in eosinophil or basophil levels were reported with saffron administration[30]. Absolute eosinophil count was found to decrease significantly after a 4-wk administration of calcined gold powder among infants aged < 1 mo but not among older infants; there were no significant changes in monocyte counts in either age group. However, this paper did not provide complete results to strengthen these claims[31].
Impact on other immune system variables: Perioperative subcutaneous mistletoe administration over 14 d was found to increase significantly the counts of CD2 lymphocytes (comprising of T lymphocytes and NK cells) after 14 d among GI cancer patients undergoing surgery[18]. Perioperative intravenous single-dose mistletoe infusion among GI cancer patients undergoing surgery was associated with a significant lowering of human leukocyte antigen - DR isotype expression, which was also seen among patients who received standard care[27].
Four studies reported indirect evidence of immune system enhancement following various Ayurvedic treatments.
Healthy adults who consumed capsules containing aged garlic extract for 90 d were found to have a reduced severity of cold and flu in terms of a significantly lower number of symptoms, significantly lower decrease in activity and days of reduced activity due to illness, and significantly lower number of work days missed due to illness compared to volunteers taking matched placebo capsules. However, a significant difference between these two groups was not found with respect to illness incidence, number of days with symptoms, number of symptoms per illness, and number of doctor visits. These observations were attributed to the enhancement of activities of NK cells and γδ-T cells by aged garlic extract[26].
Consumption of Cyavanaprasa with milk by healthy children aged 5-12 years for 6 mo was found to be associated with improved health compared to control in terms of significantly lower number of episodes of infection/allergy (overall number of episodes, and episodes of mild and moderate severity), significantly shorter duration of illness (overall duration of illness and illnesses of mild and moderate severity), significantly more children without infection/allergy, and significantly lower absenteeism due to illness, both in terms of children reporting absenteeism as well as the number of days of absenteeism due to illness. Other findings not reaching statistical significance included a lower number and shorter duration of severe episodes of infection/allergy and a lower number of children with infection/allergy[34].
The ‘Ojas score’, which signifies immune function, was found to be significantly improved after 3 mo of administration of both the Ayurvedic interventions studied (Mamajjaka and Shilajatu) as well as the control treatment, and the improvement caused by both the Ayurvedic interventions was found to be significantly higher than that seen with the control treatment[35]. Finally, the number of COVID-19 patients with positive reverse transcription polymerase chain reaction results was zero, after 10 d of treatment with either Vyagradhi Kashaya, ginger-garlic, or Vitamin C[36].
The complete details of the main findings of all the included studies are summarized in Table 3.
| No | Ref. | Intervention | T Helper cells | T cytotoxic cells | T cells: Other results | NK cell count/ activity | B lympho | Immuno | Comple | Cyto | WBC count/ activity | Other variables | Indirect evidence |
| 1 | Enesel et al, 2005 | Isorel® (Mistletoe, firtree) | + | - | + | + | - | + | + | NA | + | + | NA |
| 2 | Ishikawa et al, 2006 | Aged garlic extract | - | - | NA | + | NA | NA | NA | NA | - | NA | NA |
| 3 | Brush et al, 20061 | Glycyrrhizia glabra tincture | +/- | +/- | NA | +/- | +/- | NA | NA | NA | NA | NA | NA |
| 4 | Schink et al, 2007 | Iscador® (Standardized mistletoe extract) | NA | NA | NA | + | NA | NA | NA | NA | NA | - | NA |
| 5 | Purandare et al, 2007 | Tinospora cordifolia | NA | NA | NA | NA | NA | NA | NA | NA | + | NA | NA |
| 6 | Uebaba et al, 20082 | Shirodhara oil-dripping (sesame oil) | NA | NA | NA | +/- | NA | NA | NA | NA | NA | NA | NA |
| 7 | Bhat et al, 20103 | Fortified tea with multiple Ayurvedic ingredients | NA | NA | NA | +/- | NA | NA | NA | NA | NA | NA | NA |
| 8 | Işik et al, 2010 | Specific immunotherapy + Nigella sativa | +/- | +/- | +/- | +/- | +/- | NA | NA | NA | + | NA | NA |
| 9 | Kianbakht et al, 2011 | Saffron tablet | NA | NA | NA | NA | NA | - | - | NA | - | NA | NA |
| 10 | Mondal et al, 20114 | Tulsi capsules | +/- | +/- | NA | +/- | +/- | NA | NA | +/- | NA | NA | NA |
| 11 | Nantz et al, 20125 | Aged garlic extract | NA | NA | +/- | +/- | NA | NA | NA | +/- | NA | NA | + |
| 12 | Suprabha et al, 2017 | Rasayana Avaleha with milk | NA | NA | NA | NA | NA | - | NA | NA | NA | NA | NA |
| 13 | Gupta et al, 2017 | Cyavanaprasa (Dabur) with milk | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + |
| 14 | Rais et al, 2021 | Intervention 1: Vyaghryadi Kashaya + Pippali + Samshamani vati | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | - |
| Intervention 2: Shunthi churna + Rasona kalka | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | - | ||
| 15 | Bhaskaran et al, 20196 | Swarna Bhasma, honey, ghrita | NA | NA | NA | NA | NA | +/- | NA | NA | +/- | NA | NA |
| 16 | Kumar et al, 2014 | Intervention 1: Mamajjaka capsules | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + |
| Intervention 2: Shilajatu capsules | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | ||
| 17 | Ravindran et al, 2014 | Varunadi Ghrita + Standard care | NA | NA | + | + | + | NA | NA | NA | NA | NA | NA |
| 18 | Somarathna et al, 2010 | Ranahamsa Rasayana | + | NA | NA | NA | NA | NA | NA | NA | - | NA | NA |
| 19 | Gupta et al, 2010 | Shilajatu Rasayana | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| SUMMARY | + | 3 | 0 | 2 | 4 | 1 | 1 | 1 | 0 | 3 | 1 | 4 | |
| +/- | 3 | 3 | 2 | 6 | 3 | 1 | 0 | 2 | 1 | 0 | 0 | ||
| - | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 0 | 3 | 1 | 2 |
Nine of the 19 included studies did not report the safety profile of the Ayurvedic intervention, and eight studies reported not finding any new safety signals of concern. Rais et al[36] reported that 2/40 patients receiving Vyagradhi Kashaya developed loose stools and subsequently discontinued the treatment, and 3/40 patients receiving ginger-garlic developed burning sensation in the abdomen and were conservatively managed. After receiving Ranahamsa Rasayana, 2 patients were reported to have a mild burning sensation over the body, transient mouth ulceration, and mild drowsiness, which led to discontinuation of treatment[20].
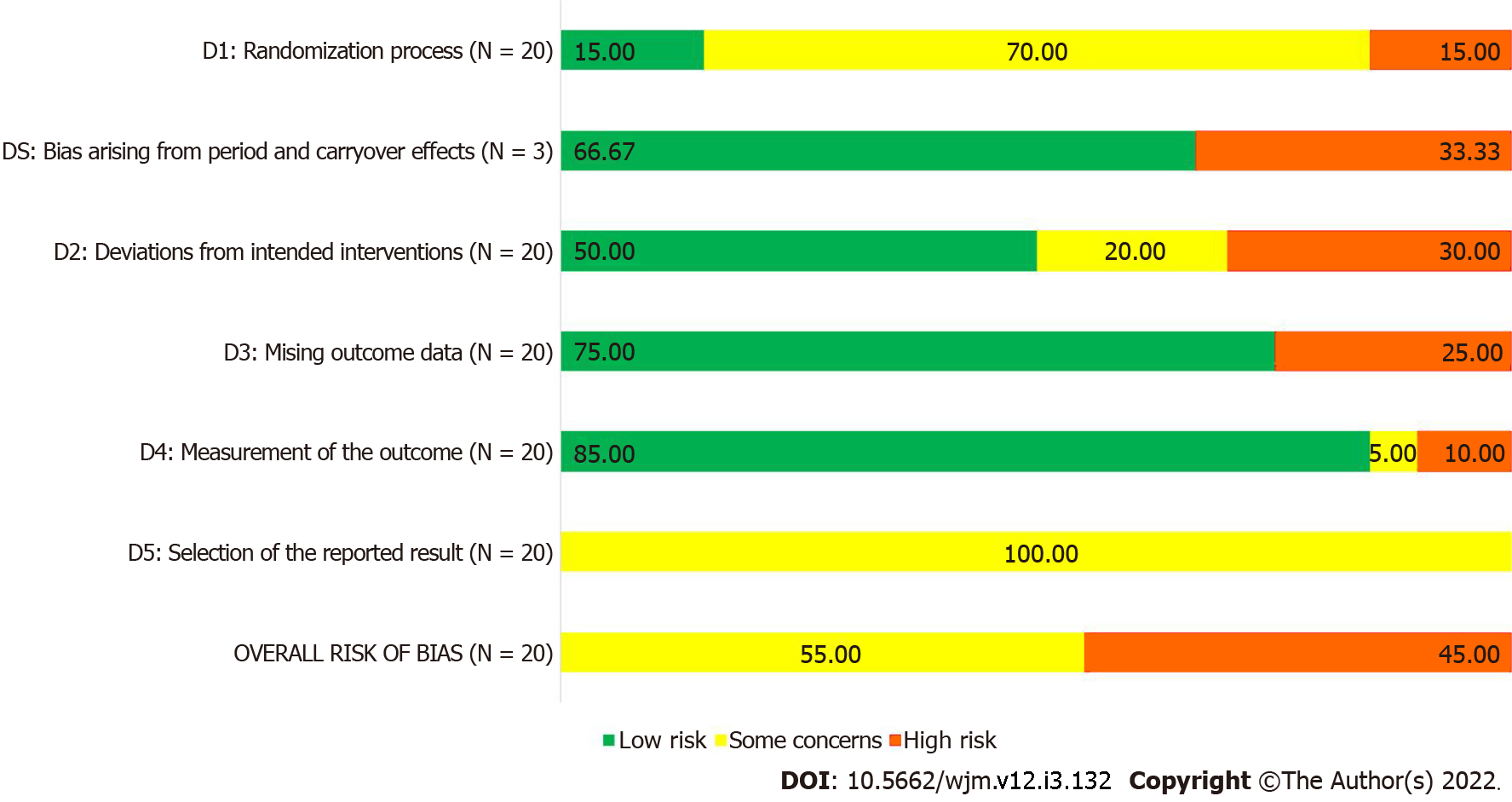
As per RoB-2 tool, nine of the 20 RCTs described in the 19 included studies had high risk of bias, and the remaining 11 RCTs had some concerns of bias. Only three RCTs gave adequate information about randomization process; most of the RCTs used standard methods to measure the outcomes, leading to domain 4 having the highest number of studies with low risk of bias. Since none of the studies provided information about pre-specified statistical analysis plan, all 20 RCTs received a ‘some concerns’ rating for domain 5, leading to a similar rating for overall risk of bias. The risk of bias summary is presented in Figure 2 and Table 1, and the complete analysis of risk of bias assessment is available in Supplementary Table 4.
The IRR for study selection between the two reviewers was substantial, with Cohen’s kappa value being 0.743 [95% confidence interval (CI): 0.714-0.772] and 0.681 (95%CI: 0.652-0.710) for title-abstract screening and full-text screening, respectively. For the methodological quality assessment, an agreement between the two reviewers with respect to the methodological quality was achieved with 16/20 RCTs, with four papers needing mediation, leading to an inter-rater agreement of 84.21%. The Cohen’s kappa value for risk of bias assessment was 0.636 (95%CI: 0.607-0.665), indicating substantial IRR.
The most prominent finding of our study is that while there is reasonably significant indirect evidence of immune system enhancement by different Ayurvedic interventions, especially Cyavanaprasa, the same cannot be said about direct evidence. In other words, we can say with some confidence that some Ayurvedic medicines have been shown to reduce the duration of illness and improve overall good health after long-term consumption, indirectly indicating that the immune system might have been boosted; however, the exact mechanism by which these effects occur is not yet clear. By looking at the summary of evidence in our review, it appears that enhancement of number and activity of NK cells and T helper cells might be responsible for such an enhancement, while the role of other components of immune system, including cytotoxic T cells, B lymphocytes, immunoglobulins, complement components, cytokines, and WBC counts is not clear. The high degree of inter-rater agreeability further points towards the accuracy of our observations.
We also observed some discomforting points about the included studies. Seven of the 20 interventions studied[19,20,25,28,32,34,36] were composite mixtures of a variety of Ayurvedic ingredients. By the nature of these individual studies, it is not possible to determine which component/s of these composite ingredients have prominent and contributory effect towards immune enhancement and which components have secondary roles such as excipients, flavoring and coloring agents or other non-essential functions. On the other hand, the exact mechanisms by which the remaining 13 individual Ayurvedic interventions impacted the immune system have been not adequately explored in the individual studies. Further contributing to the lack of clarity are the observations that there are some contradictory views wherein an apparent lack of effect in the results is claimed to have an effect (as in the case of cord blood IgG)[32] or contradictory correlations given for opposite directions of alterations in blood cytokine levels[24,26]. Next, as seen in Table 3, as many as six studies have confusing, ambiguous, or incomplete results for various reasons. The sample size of the included studies is not sufficiently large enough. None of the 20 RCTs described in the 19 studies included in the present review was able to score a ‘low risk of bias’ rating in the RoB-2 tool, had a mention of points indicative of well-conducted RCTs, such as protocol registration in a clinical trial registry, or had followed any reporting guidelines. All these factors generally tend to reduce confidence in the results presented in the studies, regardless of magnitude or statistical significance.
In the backdrop of these discouraging observations, it is apparent that, despite Ayurveda being an ancient science with an acclaimed and proven effect on immune system enhancement and despite the availability of modern medical research methodologies and technological advancements, we have not been able to know exactly how such an enhancement in immune function is brought about through the consumption of a variety of Ayurvedic medicines. The proponents of Ayurveda mostly seem to rely on the historical anecdotes, rather than using the benefits of scientific advancements to improve the general confidence of the worldwide audience in this ancient science that has stood the test of time. While this lacuna on the part of Ayurvedic researchers has been observed and commented upon for quite some time now[37], solid steps to enhance systematic evidence generation in Ayurveda seem to be lacking. In fact, ancient Ayurvedic texts have already incorporated many of the basic tenets of modern scientific discovery, such as Anumana (logical questioning), Yukti (knowledge), Tarka (argument), and Vyapti (cause and effect relationship)[38]. It should be realized that before the advent of modern medicine, Ayurveda was the ‘modern medicine’ of those times. Perhaps it is time that proponents of Ayurveda and modern medicine come together with open minds to apply modern scientific methods to generate credible and reproducible evidence, so that there is rational and evidence-based integration of Ayurveda into mainstream medicine. Also, it is apt that this is done by Indian scientists, rather than waiting for the Western scientists to do it on our behalf, as in the case of turmeric, neem, Basmati rice[39,40], Pranayama-meditation[41], and many others.
While our initial search resulted in a pool of over 12000 potentially relevant articles, we were able to identify only 19 RCTs that matched our eligibility criteria. During the process of literature screening, we observed that a large majority of research in this field is non-clinical and largely done in vitro or on animal models. It appears that a meaningful translation to clinical research is lacking for many Ayurvedic preparations, whose efficacy and safety were established in non-clinical models, for reasons unknown. It appears that Ayurvedic researchers are restricting themselves from entering clinical research, and the reasons for this hinderance should be sought out and resolved in order to boost clinical research in this field.
Our review should be interpreted in the backdrop of some limitations. We restricted to only papers published in English language, because of our familiarity with the language; we might have missed valid papers published in regional languages. We did not search other databases such as Embase or Scopus, since both these resources are paywalled, and our research was self-funded.
To conclude, various Ayurvedic preparations, both standalone and composite, appear to have an enhancing effect on the immune system, as evidenced indirectly through reduced illness variables, but the exact mechanism behind this enhancement is not fully established. There may be contributions from enhancement of NK cells and T helper cells, although the role of other immune system components is not clear. There were many inconsistencies and ambiguities with respect to the included studies. The numerous benefits of Ayurveda are being masked by a lack of proper research. The general need to improve the quality of research in Ayurveda is clearly visible, and the strong evidence thus generated, preferably by Indian researchers, will go a long way in fostering widespread acceptance of the immense knowledge of this ancient science by all stakeholders.
Ayurveda is the Indian traditional system of medicine, and has a history since the 2nd century BC. Many Ayurvedic preparations have been anecdotally claimed to have immune-boosting properties and have been used for immune enhancement and general well-being. Pre-clinical research suggests that the immune enhancement is mediated through multiple immune system modulation and psychoneuroimmunological mechanisms.
We were interested to know the exact mechanisms of immune enhancement by Ayurvedic preparations when they are administered to healthy or sick humans.
The objectives of the present systematic literature review were to gather evidence towards the nature and mechanism of enhancement of human immune system by the administration of Ayurvedic preparations from published randomized clinical trials (RCTs).
We prospectively registered the study protocol with PROSPERO. Based on predetermined eligibility criteria, search strategy was formulated and refined, and the same was used to search PubMed, DOAJ, Google Scholar, three dedicated Ayurveda research portals, two specialty Ayurveda journals, and reference lists for relevant records published until February 6, 2021. Baseline features and data pertaining to the nature and mechanism of immune system function were extracted from all eligible records. Methodological quality was assessed using the Cochrane RoB-2 tool.
Our search strategy yielded a total of 12554 articles, and we found 19 studies reporting 20 RCTs (17 parallel group design, three crossover design) with 1661 unique patients to be eligible for inclusion. Healthy population was included in nine studies, of which one study included pregnant women and two included pediatric population; remaining studies included patients with different health conditions. A total of 21 Ayurvedic interventions were studied, out of which five were composite mixtures. Through indirect evidence, four interventions were seen to be associated with immune enhancement, and two interventions were associated with a lack of such an enhancement. The role of T helper cell and natural killer cell enhancement was reported to contribute to the enhancement of immune systems by three and four interventions, respectively. Evidence pointing to enhancement of other immune system components, including cytotoxic T cells, B lymphocytes, immunoglobulins, cytokines, complement components, leucocyte counts, and other components, was not found. Risk of bias was 'high' in 9/20 RCTs, and 'some concerns' of bias were found in the remaining 11/20 RCTs, according to RoB-2.
Various Ayurvedic preparations, both standalone and composite, appear to have an enhancing effect on the immune system, as evidenced indirectly through reduced illness variables, but the exact mechanism behind this enhancement is not fully established. There may be contributions from enhancement of natural killer cells and T helper cells, although the role of other immune system components is not clear.
There is a need to improve the quality of research in Ayurveda. Ayurvedic scholars should team up with experts of modern clinical research and generate credible and reproducible evidence towards immune system enhancement. This will enable widespread acceptance of the immense knowledge of Ayurveda, leading to its increased usage, and ultimately, a healthier society.
| 1. | Mandal S, Arinaminpathy N, Bhargava B, Panda S. Plausibility of a third wave of COVID-19 in India: A mathematical modelling based analysis. Indian J Med Res. 2021;153:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Bhowmick S, Dang A, Vallish BN, Dang S. Safety and Efficacy of Ivermectin and Doxycycline Monotherapy and in Combination in the Treatment of COVID-19: A Scoping Review. Drug Saf. 2021;44:635-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2972] [Article Influence: 495.3] [Reference Citation Analysis (0)] |
| 4. | Golechha M. Time to realise the true potential of Ayurveda against COVID-19. Brain Behav Immun. 2020;87:130-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Rajkumar RP. Ayurveda and COVID-19: Where psychoneuroimmunology and the meaning response meet. Brain Behav Immun. 2020;87:8-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Samal J. Fundamental tenets of epidemiology in Ayurveda and their contemporary relevance. Ind J Health Sci Biomed Res 2016;9: 20-26 . [DOI] [Full Text] |
| 7. | Jaiswal YS, Williams LL. A glimpse of Ayurveda - The forgotten history and principles of Indian traditional medicine. J Tradit Complement Med. 2017;7:50-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Khanal P, Duyu T, Patil BM, Dey YN, Pasha I, Wanjari M, Gurav SS, Maity A. Network pharmacology of AYUSH recommended immune-boosting medicinal plants against COVID-19. J Ayurveda Integr Med. 2022;13:100374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Khare CP. Indian medicinal plants : an illustrated dictionary. Berlin: Springer; 2007. |
| 10. | Aggithaya MG, Narahari SR. Literature searches on Ayurveda: An update. Ayu. 2015;36:238-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Directory of Open Access Journals. Available from: https://doaj.org/. |
| 12. | AYUSH Research Portal: Ministry of AYUSH, Govt of India. Available from: https://ayushportal.nic.in/. |
| 13. | DHARA: Digital Helpline for Ayurveda Research Articles: AVP Research Foundation. Available from: http://www.dharaonline.org/Forms/Home.aspx. |
| 14. | ABIM: An Annoted Bibliography of Indian Medicine. Available from: https://indianmedicine.eldoc.ub.rug.nl/. |
| 15. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18895] [Article Influence: 2699.3] [Reference Citation Analysis (0)] |
| 16. | Current version of RoB-2 [updated 22 Aug 2019]. Available from: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2. |
| 17. | Dang A, Chidirala S, Veeranki P, Vallish BN. A Critical Overview of Systematic Reviews of Chemotherapy for Advanced and Locally Advanced Pancreatic Cancer using both AMSTAR2 and ROBIS as Quality Assessment Tools. Rev Recent Clin Trials. 2021;16:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Enesel MB, Acalovschi I, Grosu V, Sbarcea A, Rusu C, Dobre A, Weiss T, Zarkovic N. Perioperative application of the Viscum album extract Isorel in digestive tract cancer patients. Anticancer Res. 2005;25:4583-4590. [PubMed] |
| 19. | Gupta GD, Sujatha N, Dhanik A, Rai NP. Clinical Evaluation of Shilajatu Rasayana in patients with HIV Infection. Ayu. 2010;31:28-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Somarathna KI, Chandola HM, Ravishankar B, Pandya KN, Attanayake AM. A short-term intervention trial on HIV positive patients using a Sri Lankan classical rasayana drug - Ranahamsa Rasayanaya. Ayu. 2010;31:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr. 2006;136:816S-820S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Brush J, Mendenhall E, Guggenheim A, Chan T, Connelly E, Soumyanath A, Buresh R, Barrett R, Zwickey H. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD69 expression and immune cell activation in humans. Phytother Res. 2006;20:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Işik H, Cevikbaş A, Gürer US, Kiran B, Uresin Y, Rayaman P, Rayaman E, Gürbüz B, Büyüköztürk S. Potential adjuvant effects of Nigella sativa seeds to improve specific immunotherapy in allergic rhinitis patients. Med Princ Pract. 2010;19:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Mondal S, Varma S, Bamola VD, Naik SN, Mirdha BR, Padhi MM, Mehta N, Mahapatra SC. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J Ethnopharmacol. 2011;136:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Ravindran D, Hariharan I, Muwonge R, Kumar RR, Pillai MR, Ramadas K. Efficacy of Varunadi Ghritha (polyherbal compound) in treated head and neck cancer cases as a biological response modifier. Ayu. 2014;35:168-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Nantz MP, Rowe CA, Muller CE, Creasy RA, Stanilka JM, Percival SS. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: a randomized, double-blind, placebo-controlled nutrition intervention. Clin Nutr. 2012;31:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Schink M, Tröger W, Dabidian A, Goyert A, Scheuerecker H, Meyer J, Fischer IU, Glaser F. Mistletoe extract reduces the surgical suppression of natural killer cell activity in cancer patients. a randomized phase III trial. Forsch Komplementmed. 2007;14:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Bhat J, Damle A, Vaishnav PP, Albers R, Joshi M, Banerjee G. In vivo enhancement of natural killer cell activity through tea fortified with Ayurvedic herbs. Phytother Res. 2010;24:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Uebaba K, Xu FH, Ogawa H, Tatsuse T, Wang BH, Hisajima T, Venkatraman S. Psychoneuroimmunologic effects of Ayurvedic oil-dripping treatment. J Altern Complement Med. 2008;14:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Kianbakht S, Ghazavi A. Immunomodulatory effects of saffron: a randomized double-blind placebo-controlled clinical trial. Phytother Res. 2011;25:1801-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Bhaskaran JK, Patel KS, Srikrishna R. Immunomodulatory activity of Swarna Prashana (oral administration of gold as electuary) in infants - A randomized controlled clinical trial. Ayu. 2019;40:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Suprabha K, Mamatha KV. A clinical trial to evaluate the effect of Rasayana Avaleha during pregnancy W.S.R. to the fetal outcome. Ind J Health Sci Biomed Res. 2017;10:74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Purandare H, Supe A. Immunomodulatory role of Tinospora cordifolia as an adjuvant in surgical treatment of diabetic foot ulcers: a prospective randomized controlled study. Indian J Med Sci. 2007;61:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Gupta A, Kumar S, Dole S, Deshpande S, Deshpande V, Singh S, Sasibhushan V. Evaluation of Cyavanaprāśa on Health and Immunity related Parameters in Healthy Children: A Two Arm, Randomized, Open Labeled, Prospective, Multicenter, Clinical Study. Anc Sci Life. 2017;36:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Kumar S, Singh G, Pandey AK, Singh RH. A clinical study on the Naimittika Rasayana effect of Silajatu and Mamajjaka in type-2 Diabetes Mellitus. Ayu. 2014;35:404-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Rais A, Negi DS, Yadav A, Arya H, Verma R, Galib R, Ahmad A, Kumar Yadav M, Ahirwar PN. A Randomized open label parallel group pilot study to evaluate efficacy of Ayurveda interventions in the management of Asymptomatic and Mild COVID-19 patients-Experiences of a Lucknow based Level 2 hospital of Uttar Pradesh, India. J Ayurveda Integr Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 38. | Panja AK, Upadhyaya OP, Chattopadhyaya A. A critical review of the philosophical concepts of Carakopaskara commentary. Ayu. 2011;32:422-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Balasubramanian S. India: Traditional Knowledge And Patent Issues: An Overview Of Turmeric, Basmati, Neem Cases. 2017. Available from: https://www.mondaq.com/india/patent/586384/traditional-knowledge-and-patent-issues-an-overview-of-turmeric-basmati-neem-cases. |
| 40. | Kumar S. India wins battle with USA over turmeric patent. Lancet. 1997;350:724. [DOI] [Full Text] |
| 41. | McCraty R, Zayas MA. Cardiac coherence, self-regulation, autonomic stability, and psychosocial well-being. Front Psychol. 2014;5:1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Maghazachi AA, United Arab Emirates; Peterfalvi A, Hungary S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH