©The Author(s) 2024.
World J Methodol. Jun 20, 2024; 14(2): 91319
Published online Jun 20, 2024. doi: 10.5662/wjm.v14.i2.91319
Published online Jun 20, 2024. doi: 10.5662/wjm.v14.i2.91319
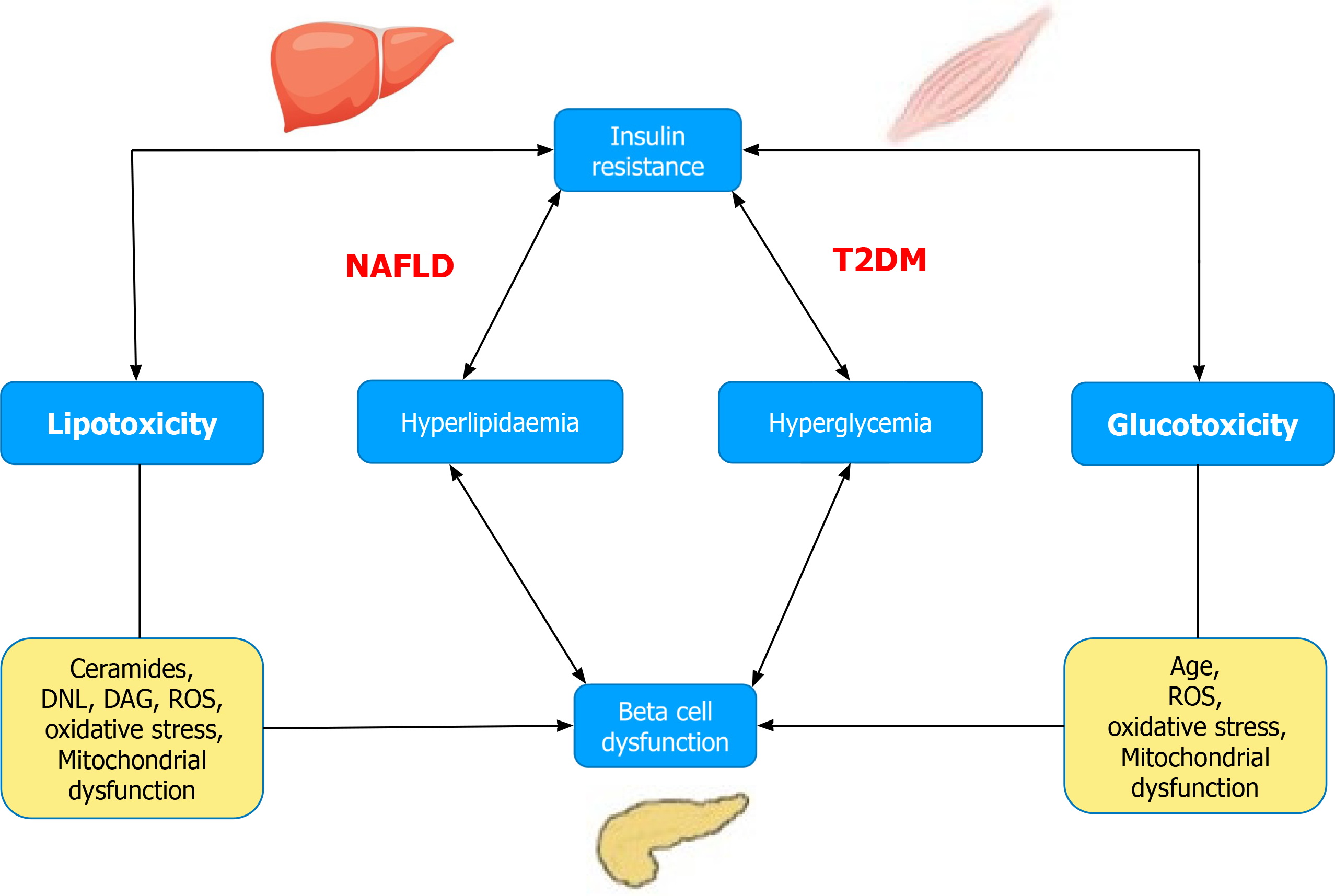
Figure 1 Relationship between lipo- and glucotoxicity, insulin resistance, and beta-cell function.
DAG: Diacylglycerol; DNL: De novo lipogenesis; NAFLD: Non-alcoholic fatty liver disease; ROS: Reactive oxygen species; T2D: Type 2 diabetes.
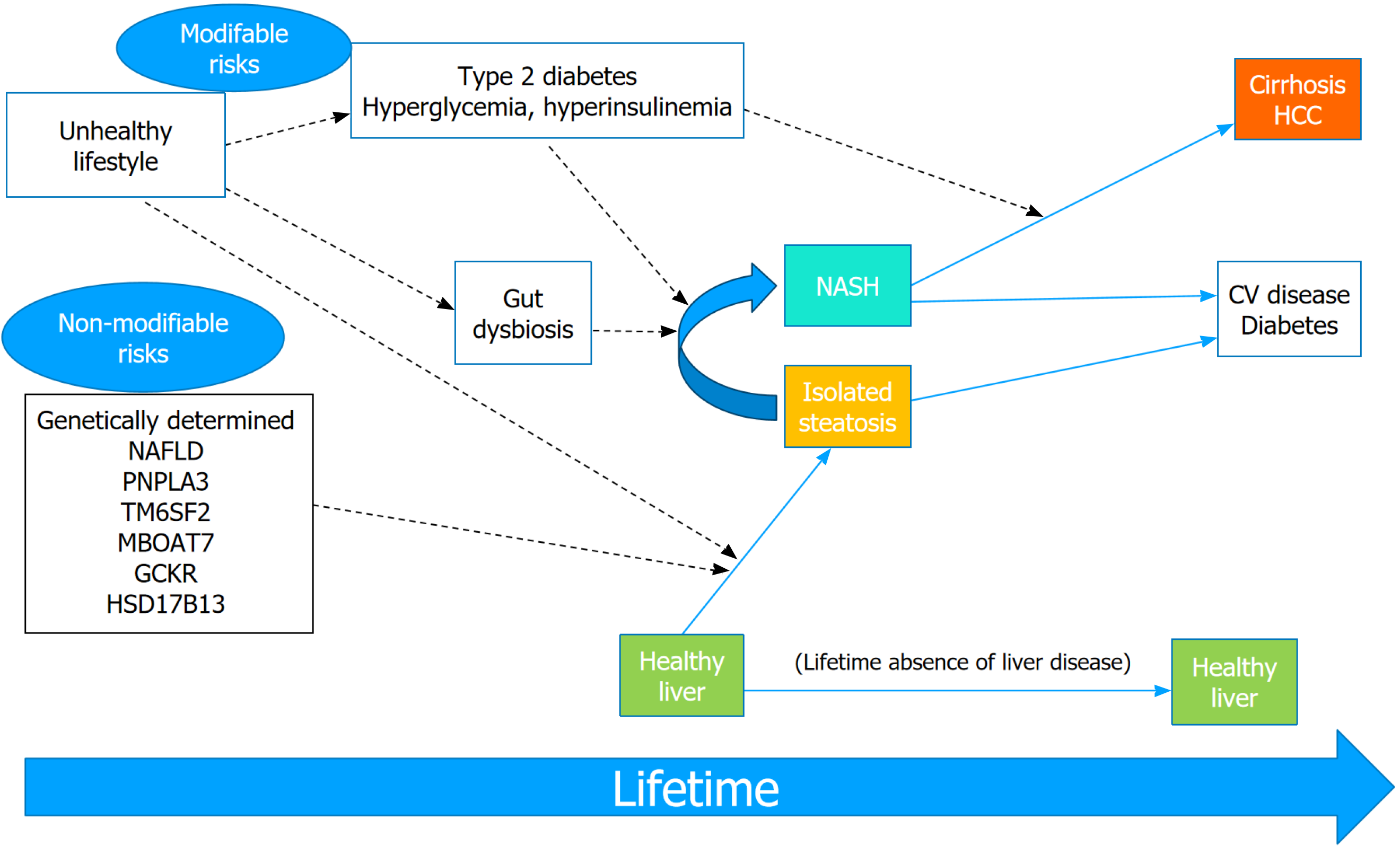
Figure 2 Modifiable and non-modifiable risk factors of non-alcoholic fatty liver disease and its progression to severe liver disease.
Dashed arrows indicate factors that promote or predispose to disease. Among non-modifiable risk factors, most important genetic variants of nonalcoholic fatty liver disease are being extensively studied. Type 2 diabetes-associated hyperglycemia induces progression from nonalcoholic steatohepatitis to hepatic fibrosis and HCC. CV: Cardiovascular; HCC: Hepatocellular carcinoma; NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis.
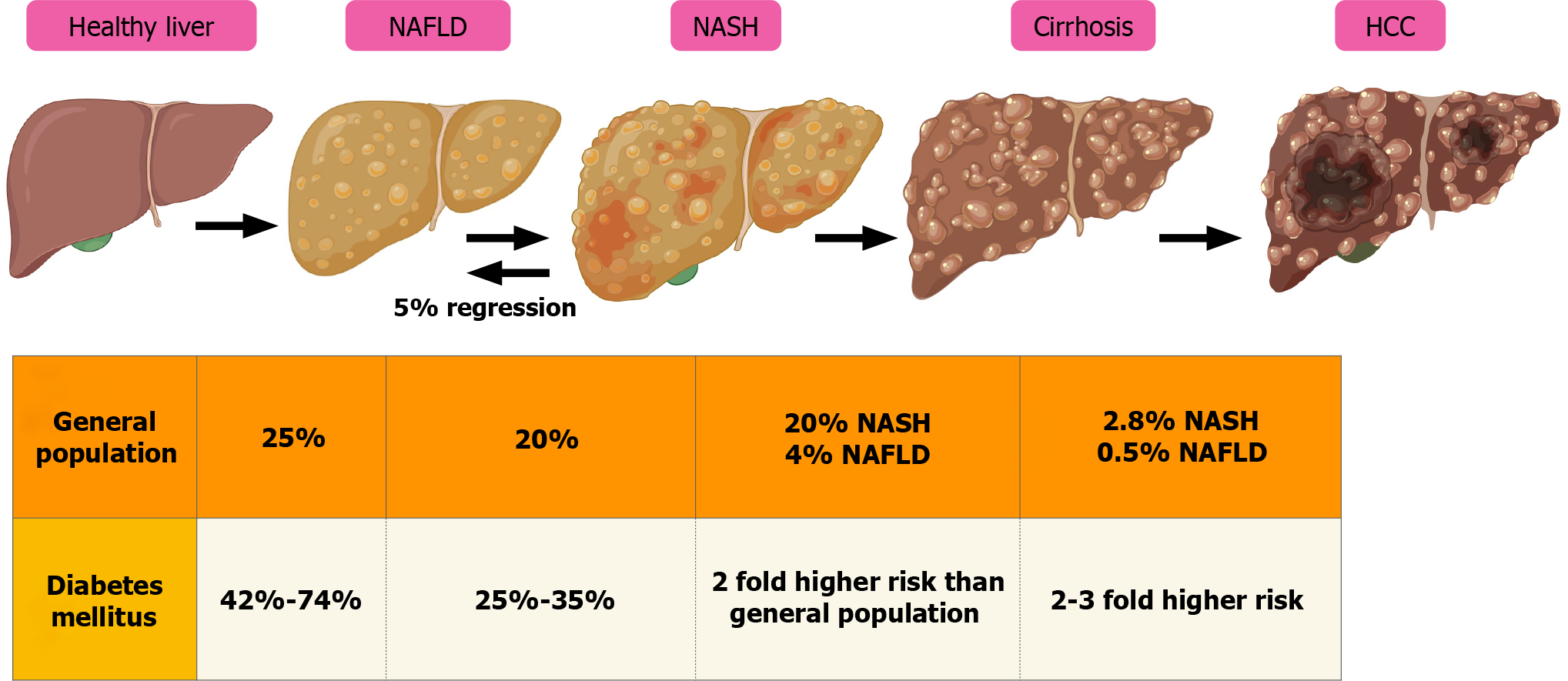
Figure 3 Natural history of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
HCC: Hepatocellular carcinoma; NASH: Nonalcoholic steatohepatitis; NAFLD: Nonalcoholic fatty liver disease.
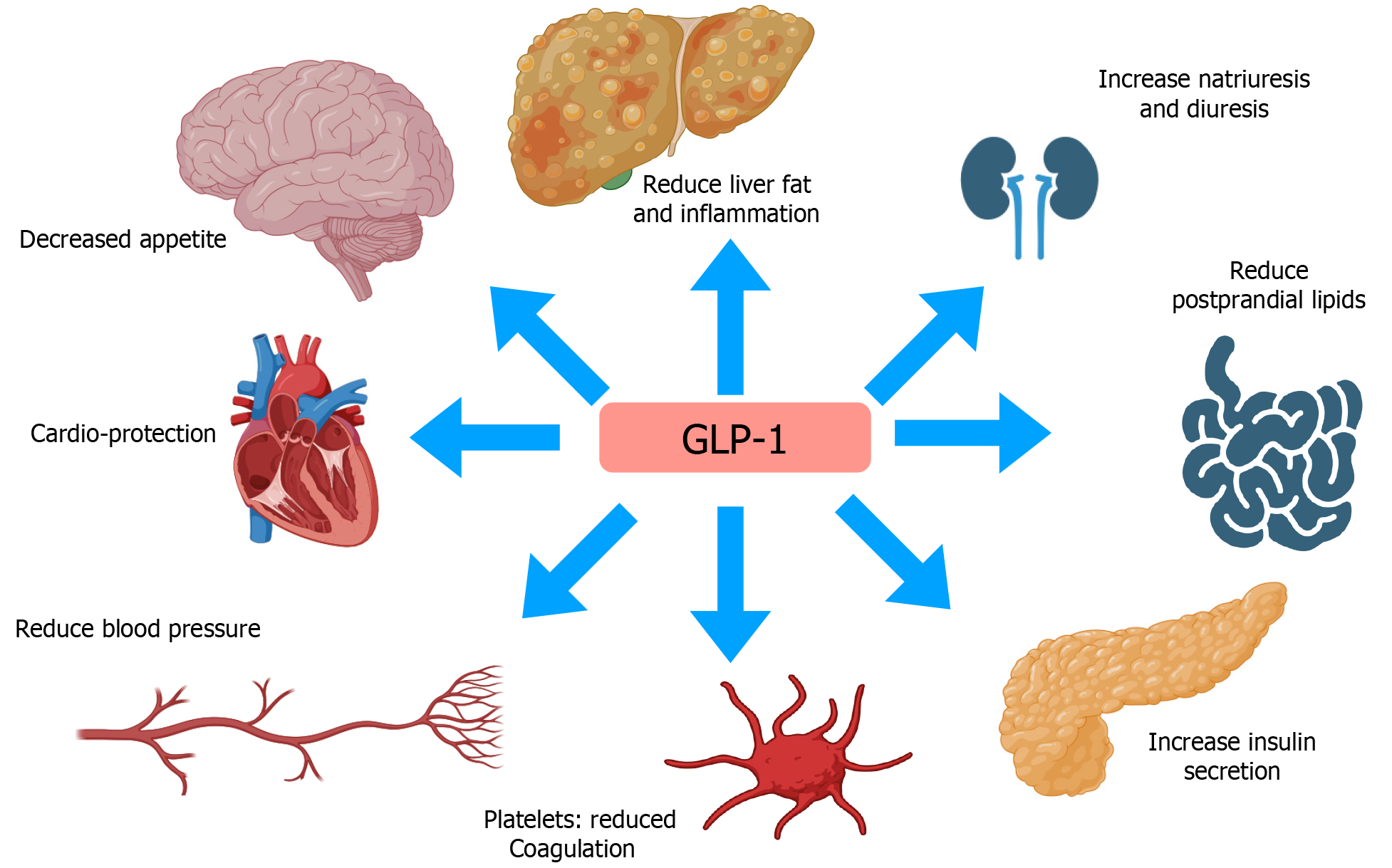
Figure 4 Pleotropic effects of glucagon-like peptide 1 and its effect of several organ systems in systematic inflammation characteristic of nonalcoholic steatohepatitis.
GLP-1: Glucagon-like peptide 1.
- Citation: Pramanik S, Pal P, Ray S. Non-alcoholic fatty liver disease in type 2 diabetes: Emerging evidence of benefit of peroxisome proliferator-activated receptors agonists and incretin-based therapies. World J Methodol 2024; 14(2): 91319
- URL: https://www.wjgnet.com/2222-0682/full/v14/i2/91319.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i2.91319