©The Author(s) 2023.
World J Methodol. Dec 20, 2023; 13(5): 466-474
Published online Dec 20, 2023. doi: 10.5662/wjm.v13.i5.466
Published online Dec 20, 2023. doi: 10.5662/wjm.v13.i5.466
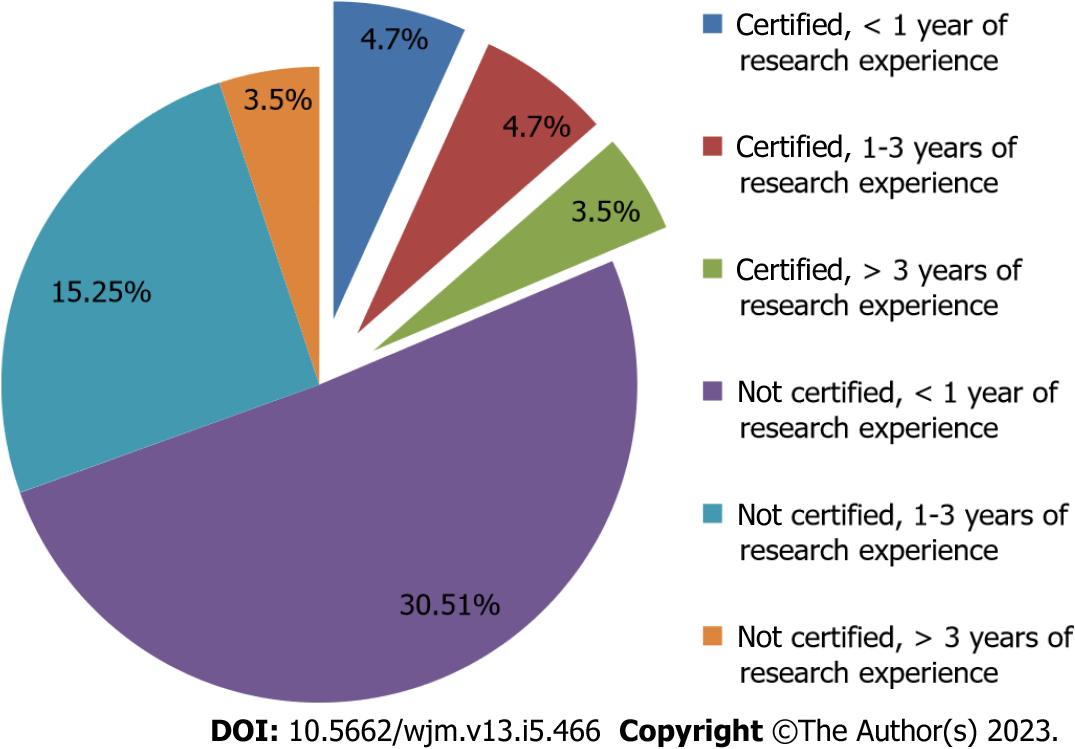
Figure 1 Research experience with respect to certification of good clinical practice.
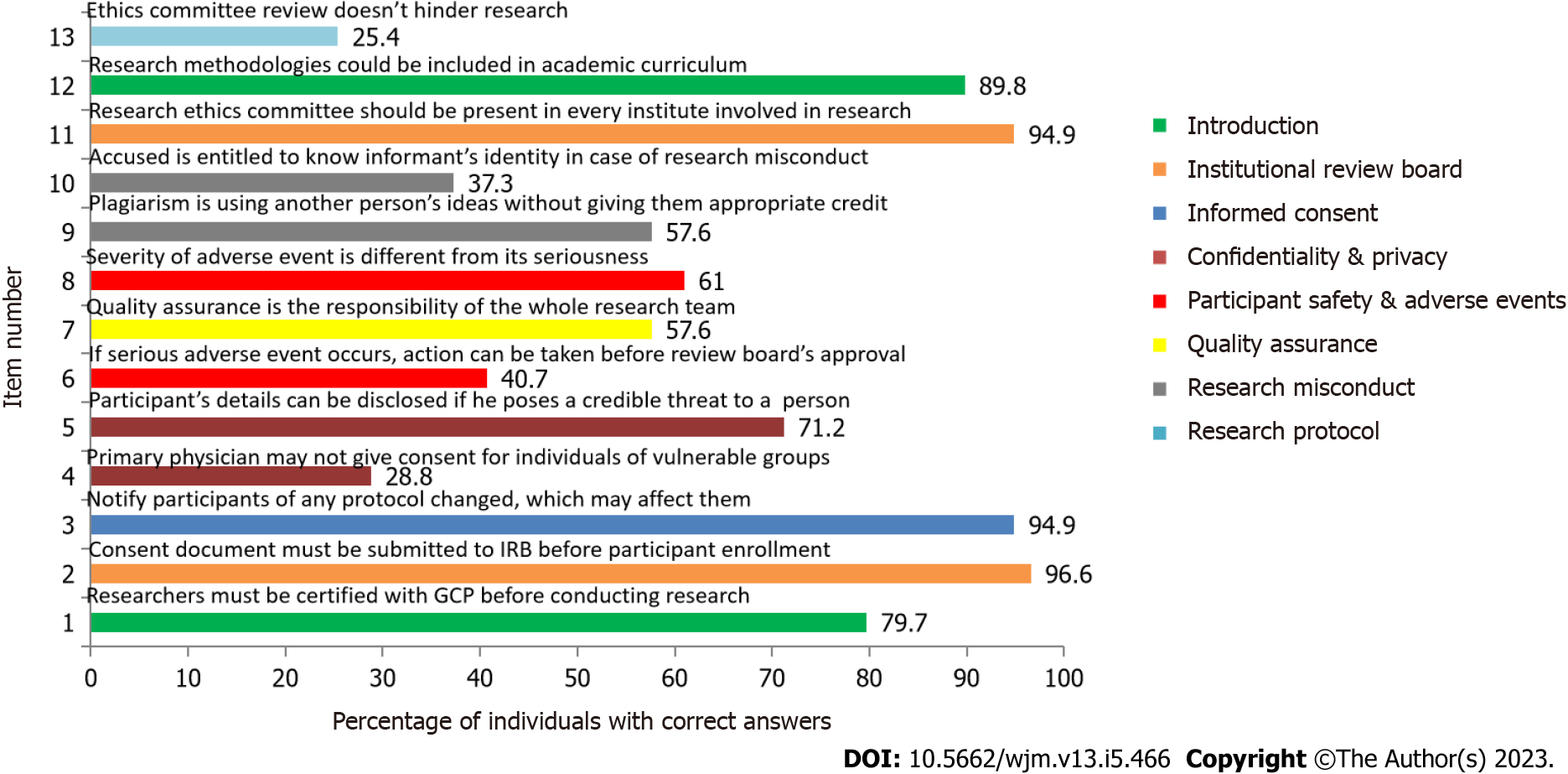
Figure 2 Module-wise arrangement of questions assessing knowledge.
GCP: Good clinical practice.
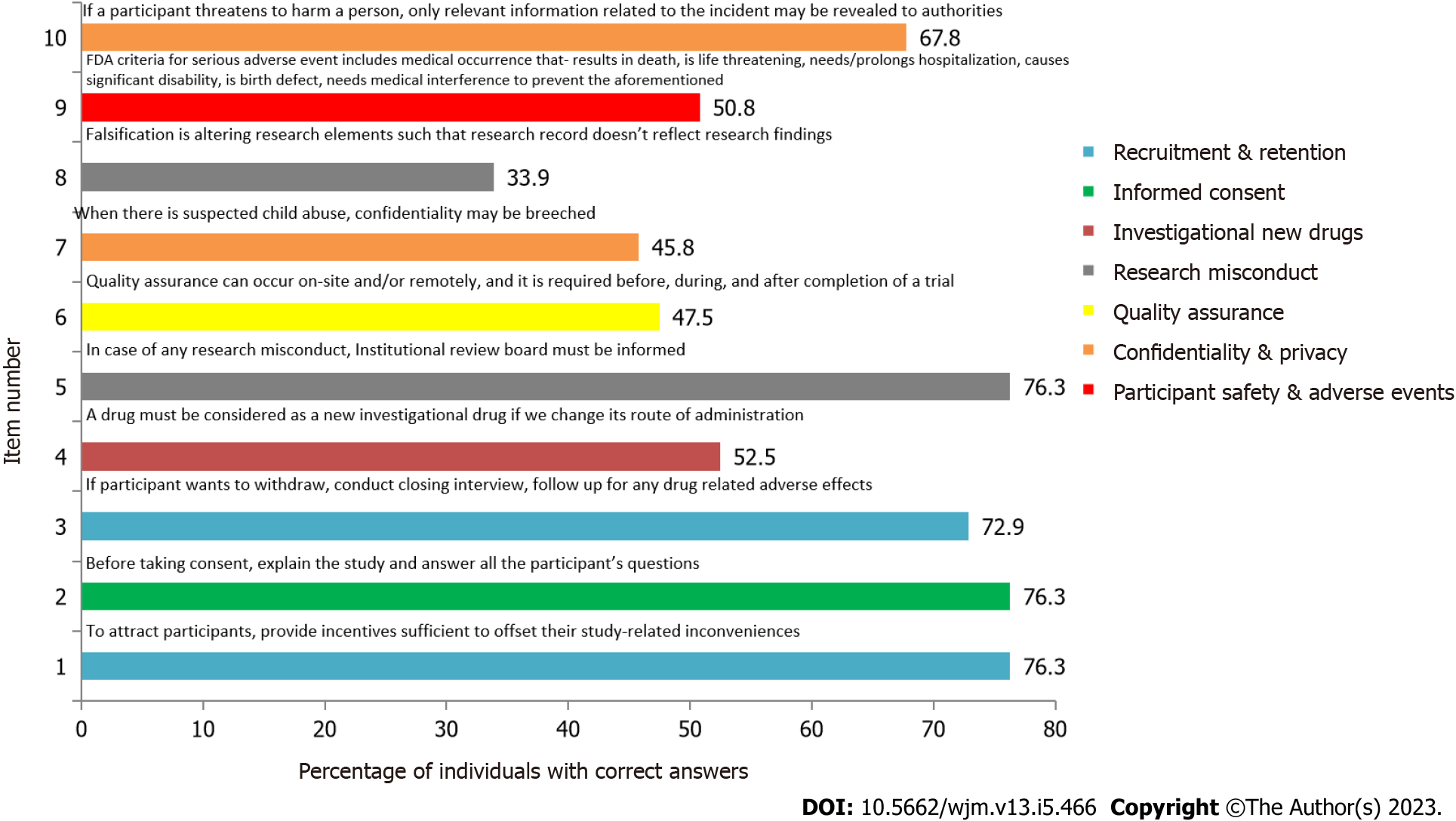
Figure 3 Module-wise arrangement of questions assessing practices.
FDA: Food and drug administration.
- Citation: Harshita H, Panda PK. Study on good clinical practices among researchers in a tertiary healthcare institute in India. World J Methodol 2023; 13(5): 466-474
- URL: https://www.wjgnet.com/2222-0682/full/v13/i5/466.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i5.466