Published online Apr 12, 2016. doi: 10.5528/wjtm.v5.i1.53
Peer-review started: October 5, 2015
First decision: November 24, 2015
Revised: December 14, 2015
Accepted: January 29, 2016
Article in press: January 31, 2016
Published online: April 12, 2016
Processing time: 188 Days and 20.2 Hours
AIM: To investigate the effect of prednisolone, a synthetic glucocorticoid used in inflammatory diseases, on the growth of cultured osteosarcoma cells.
METHODS: Two osteosarcoma cell lines with different degree of differentiation were used. SaOS2 show a rather mature phenotype, while U2OS are negative for almost all osteoblastic markers. The cells were exposed to different concentrations of prednisolone (1-9 μmol/L) with or without antioxidants or the inhibitor of inducible nitric oxide synthase (iNOS) l-N6-(iminoethyl)-lysine-HCl (L-NIL). Cell growth was assessed by counting viable cells. The production of nitric oxide (NO) was measured in the conditioned media by the Griess method. The production of reactive oxygen species was quantified using 2’-7’-dichlorofluorescein diacetate. Western blot with specific antibodies against NOSs was performed on cell extracts.
RESULTS: Prednisolone inhibited SaOS2 cell growth in a dose dependent manner. No significant effects were observed in U2OS. The inhibition of SaOS2 growth is not due to oxidative stress, because antioxidants do not rescue cell proliferation. Since high concentrations of NO inhibit bone formation, we also measured NO and found it induced in SaOS2, but not in U2OS, exposed to prednisolone, because of the upregulation of iNOS as detected by western blot. Therefore, we treated SaOS2 with prednisolone in the presence or in the absence of L-NIL. L-NIL prevented NO release induced by prednisolone at all the concentrations apart from 9 μmol/L. At the same concentrations, we found that L-NIL rescued SaOS2 growth after exposure to prednisolone. In U2OS cells, prednisolone did not induce NO production nor affected cell growth. All together, these data indicate that a link exists between increased amounts of NO and growth inhibition in response to prednisolone in SaOS2.
CONCLUSION: Prednisolone inhibited SaOS2 proliferation by increasing the release of NO through the upregulation of iNOS, while no effect was exerted on U2OS.
Core tip: Since prednisolone, a widely used synthetic glucocorticoid, inhibits osteoblast proliferation, we evaluated its effects on osteosarcoma cells. In particular, we used two osteoblastic osteosarcoma cell lines with different degree of differentiation, i.e., SaOS2, which have a rather mature phenotype, and U2OS, which are less differentiated. We found that prednisolone inhibited SaOS2 proliferation by increasing the release of nitric oxide (NO) through the upregulation of inducible NO synthase (iNOS). Indeed, pharmacological inhibition with the iNOS inhibitor l-N6-(iminoethyl)-lysine-HCl restored the normal proliferation rate of the SaOS2. On the contrary, prednisolone did not modulate NO production nor cell growth in U2OS.
- Citation: Cazzaniga A, Maier JAM, Castiglioni S. Prednisolone inhibits SaOS2 osteosarcoma cell proliferation by activating inducible nitric oxide synthase. World J Transl Med 2016; 5(1): 53-58
- URL: https://www.wjgnet.com/2220-6132/full/v5/i1/53.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v5.i1.53
Osteosarcomas are aggressive primary malignant tumors of the bone characterized by the deposition of immature bone by the neoplastic cells which most likely arise from mesenchymal stem cells. Osteosarcomas mostly affect teenagers and frequently metastasize. Nowadays, systemic multidrug chemotherapy and surgery are successful in 60%-70% of patients. Therefore, novel approaches are foreseen.
Since glucocorticoids participate to the regulation of survival, differentiation, and proliferation of many cell types, including osteoblasts and bone mesenchymal stem cells[1-3], we asked whether glucocorticoids might control the growth of osteosarcoma cells. Glucocorticoids act by binding their cognate receptor which functions as a hormone-regulated transcription factor. In addition, glucocorticoids interact with transcription factors such as AP1 and nuclear factor kappa B (NF-κB) and inhibit their activity. They can also modulate some intracellular signalling pathways, one of which is the MAP kinase cascade. Because of their effects on cell cycle progression and apoptosis[4], they are also used in the treatment of lymphoid malignancy and of some solid cancers[5,6].
In this study, we evaluate the effect of prednisolone, a synthetic glucocorticoid widely used to treat inflammatory diseases, on cultured osteosarcoma cells. It is well known that cultured neoplastic cells have been the basis of cancer biology and the chase to identify drug treatments[7]. Two human osteosarcoma cell lines are particularly intriguing, i.e., SaOS2 and U2OS, which are among the first generated cell lines used for anticancer research[8]. U2OS were derived from a moderately differentiated sarcoma of a 15-year-old girl, and SaOS2 from an osteogenic sarcoma of an 11-year-old girl. SaOS2 are relatively resistant to drugs because of the mutation of major oncosuppressors, i.e., p53 and Rb[9], which are functional in U2OS. While SaOS2 show a mature phenotype, U2OS are negative for almost all osteoblastic markers but positive for cartilage markers like collagen II, IX and X and for type IV collagen, which is only expressed in very early differentiation stages but not by mature osteoblasts. These two cell lines were selected for this study because of their different degree of differentiation and gene expression.
SaOS2 and U2OS (American Type Culture Collection) were cultured in DMEM containing 10% fetal bovine serum. Proliferation assays were performed on cells at low density (7000/cm2) with different concentrations of prednisolone. After trypsinization and staining with trypan blue solution (0.4%), the viable cells were counted. In some experiments cells were exposed to apocynin (10 μg/mL), trolox (40 μmol/L), or l-N6-(iminoethyl)-lysine-HCl (L-NIL) (100 μmol/L), a selective inhibitor of inducible nitric oxide synthase (iNOS).
Intracellular oxidative stress was quantified using 2’-7’-dichlorofluorescein diacetate (DCFH). Cells were seeded into black bottomed 96 plates (Greiner Bio-One) and 24 h later exposed for 30 min to different concentrations of prednisolone dissolved in a 20 μmol/L DCFH solution. The rate of intracellular oxidative stress was evaluated by monitoring the emission at 529 nm of the DCFH dye using Promega Glomax Multi Detection System[10]. Data are shown as the mean of three independent experiments in triplicate ± SD. H2O2 (50 μmol/L) was used as a positive control.
NOS activity was measured in the conditioned media by the Griess method as described[11]. Data are shown as the mean of four independent experiments in triplicate ± SD.
Western blot was performed using anti-iNOS, total endothelial nitric oxide synthase (eNOS) and p-eNOSSer1177 antibodies (Cell Signalling Technology) followed by incubation with secondary antibodies labelled with horseradish peroxidase (GE Healthcare). Anti-actin antibodies (Sigma-Aldrich) were used to show that equal amounts of proteins were loaded per lane. The SuperSignal chemiluminescence kit (Thermo Fisher Scientific) was utilized to detect immunoreactive proteins. Densitometry was performed using ImageJ software and results are shown as the mean ± SD of three separate experiments. A representative blot is shown.
Statistical significance was determined using the student’s t test and set at P values less than 0.05. In the figures aP < 0.05; bP < 0.01; dP < 0.001.
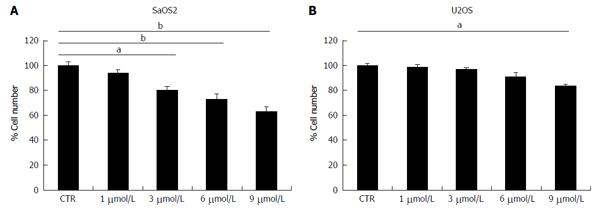
SaOS2 were cultured in media containing different concentrations of prednisolone and counted after 4 d. Figure 1A shows that prednisolone inhibits SaOS2 cell growth in a dose dependent manner. No effect is observed in cells treated with 1 μmol/L prednisolone, while growth inhibition is significant with 3, 6, and 9 μmol/L. Similar results were obtained when the MTT assay was used (data not shown). Under the same experimental conditions U2OS were less sensitive to prednisolone than SaOS2 since a modest growth inhibition was observed only with 9 μmol/L of prednisolone (Figure 1B).
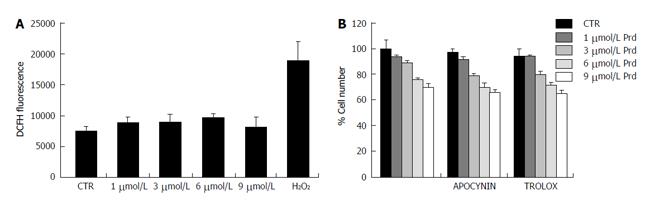
We focused on SaOS2 to understand the mechanisms underlying the inhibitory effect of prednisolone. Since the detrimental effects of glucocorticoids in osteoblasts are mediated by the induction of oxidative stress[12], we measured intracellular reactive oxygen species (ROS) by DCFH fluorescence in SaOS2. Prednisolone did not significantly affect the basal levels of DCFH-detectable ROS (Figure 2A). Accordingly, antioxidants, i.e., apocynin, a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor, and trolox, a water soluble analog of α-tocopherol, did not prevent growth inhibition by prednisolone (Figure 2B).
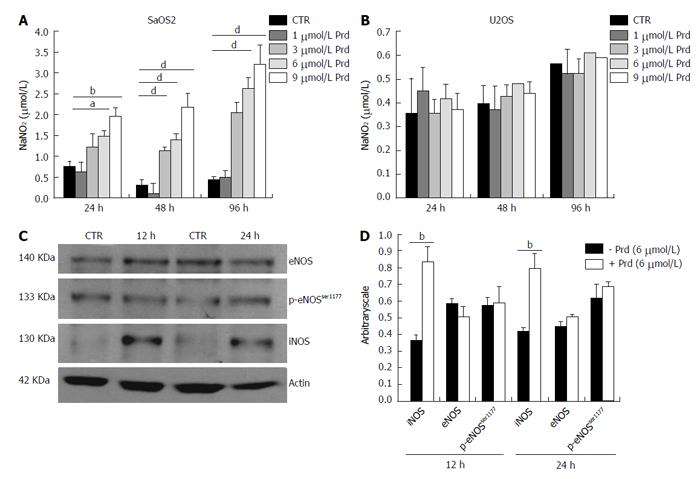
Because of the role of nitric oxide (NO) in bone homeostasis[13], we evaluated whether prednisolone affectedNOS activity. After 24, 48 and 96 h of culture in various concentrations of prednisolone, we found that NOS activity was higher in SaOS2 treated with the glucocorticoid as detected by Griess assay (Figure 3A), while no increase of NO was detected in U2OS (Figure 3B). Since iNOS and eNOS were described in cultured osteoblast-like cells from various species[14], we evaluated the amounts of these enzymes by western blot in SaOS2. The phosphorylation of p-eNOSSer1177 was also investigated because it enhances enzyme activity[11]. After 12 and 24 h exposure to prednisolone (6 μmol/L), iNOS was up-regulated (Figure 3C), while the amounts of total eNOS and p-eNOSSer1177 remained almost unvaried.
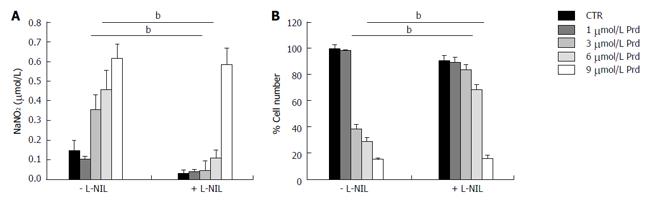
To study whether an increased activity of NOS was responsible for SaOS2 growth retardation by prednisolone, the cells were cultured in medium containing various concentrations of prednisolone in the presence or in the absence of the iNOS inhibitor L-NIL (100 μmol/L) for 96 h. Figure 4A shows that L-NIL (100 μmol/L) prevented NO release induced by prednisolone up to 6 μmol/L, but not at 9 μmol/L. We then counted the cells and found that L-NIL prevents prednisolone-dependent growth inhibition up to 6 μmol/L (Figure 4B).
High levels of glucocorticoids impact on the generation and lifespan of osteoblasts[15]. In humans, prednisolone, even at low doses[16], causes significant bone loss and increases the risk of fractures through a direct action mainly on osteoblasts and osteocytes[17]. Because of the inhibitory effect of prednisolone on osteoblast proliferation and viability, we asked whether prednisolone might inhibit also osteosarcoma cell proliferation. Indeed, the outcome of antineoplastic therapies in osteosarcoma is not satisfactory and the quest for novel treatments continues. Here, we investigated the effects of prednisolone on two human osteoblastic osteosarcoma cell lines that reveal a different degree of differentiation, i.e., SaOS2 and U2OS[8]. We found that SaOS2 are growth inhibited by prednisolone while U2OS are not. We therefore investigated the mechanisms underlying prednisolone inhibition of SaOS2 cell growth, which also means to understand why U2OS are far less sensitive to the drug. Glucocorticoids are known to alter redox balance. Indeed, the administration of prednisolone to mice increased ROS production in the bone and dexamethasone had similar effects on osteoblastic cells in vitro[12]. Moreover, prednisolone enhanced the formation of superoxide by augmenting NADPH oxidase activity in pulmonary endothelial cells[18]. We found no significant induction of ROS production in prednisolone-treated SaOS2. In agreement with this result, two anti-oxidants with different mechanisms of action have no effect in preventing SaOS2 cell growth inhibition by prednisolone.
Also NO has a role in bone homeostasis. Low NO levels stimulate, while high concentrations inhibit bone formation. It is eNOS, constitutively expressed in the bone, that is implicated in maintaining the basal levels of NO[19]. Accordingly, eNOS-/- mice show defective bone formation and are osteopenic[11]. Also iNOS null mice show imbalances in bone osteogenesis and abnormalities in bone healing[11]. It is interesting to note that iNOS pathway is crucial in bone resorption upon inflammatory stimuli and also mediates the negative effects of estrogen depletion on bones[20]. Indeed, once activated, iNOS is capable of generating high levels of NO locally for many hours. It should be recalled that NO is also an inducer of stress signaling, owing to its ability to damage proteins and DNA. We here show that SaOS2 exposed to prednisolone upregulate iNOS and, because of this, produce higher amounts of NO than untreated cells. Indeed, pharmacological inhibition of iNOS reduced NO release to basal levels and restored the normal proliferation rate. The mechanisms implicated in iNOS induction are still a matter of investigation. It is known that iNOS is regulated through the activation of several signaling pathways among which NF-κB and MAPK. We can rule out a role of NF-κB, since glucocorticoids suppress NF-κB activity. More studies are necessary to reveal the pathways responsible for the increase of iNOS activity.
It is noteworthy that prednisolone does not induce NO in U2OS and this might account for the different behavior of the two cell lines. It is noteworthy that NO impairs also U2OS proliferation as shown in a study that links the increased activity of iNOS and the detrimental effects of benzyl isothiocyanate and phenethyl isothiocyanate on these cells[21]. It is also possible that the different response of SaOS2 and U2OS to prednisolone is due to the many differences of their proteomic profile[9,22]. Alternatively, since the glucocorticoid receptor gene generates several splice and translation protein variants that lead to different genomic and non genomic effects, the different response of U2OS and SaOS2 might result from the expression of various isoforms of glucocorticoid receptors.
We have previously shown that increased iNOS activity mediates SaOS2 growth inhibition by low magnesium[11]. Therefore NO is emerging as a relevant signaling molecule to control SaOS2 cell proliferation.
Our results indicate that prednisolone impairs SaOS2 cell proliferation through the upregulation of iNOS and consequent induction of NO release.
Glucocorticoids control the growth and differentiation of osteoblasts and bone mesenchymal stem cells. Little is known about the effects of glucocorticoids on osteosarcoma cells. The authors therefore evaluated the response to prednisolone of two human osteosarcoma cell lines, i.e., SaOS2, which show a mature phenotype, and U2OS, which are rather undifferentiated.
Prednisolone inhibited SaOS2 cell growth through the induction of inducible nitric oxide (NO) synthase with consequent increase of NO production. No effects were observed in U2OS.
NO is emerging as a relevant signaling molecule to control SaOS2 cell proliferation under different experimental conditions. This result also highlights the different sensitivity to prednisolone of osteosarcoma cells with different degree of differentiation.
More than one cell line should be used when in vitro experiments are performed to test the response to various compounds. The possibility of using glucocorticoids in animal models of osteosarcoma should be fostered.
The manuscript by Cazzaniga et al analyses the effects of prednisolone on two different osteosarcoma cell lines. The data are novel and the experiments have been competently performed.
| 1. | Li H, Li T, Fan J, Li T, Fan L, Wang S, Weng X, Han Q, Zhao RC. miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ. 2015;22:1935-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Li T, Li H, Li T, Fan J, Zhao RC, Weng X. MicroRNA expression profile of dexamethasone-induced human bone marrow-derived mesenchymal stem cells during osteogenic differentiation. J Cell Biochem. 2014;115:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 755] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 4. | Schlossmacher G, Stevens A, White A. Glucocorticoid receptor-mediated apoptosis: mechanisms of resistance in cancer cells. J Endocrinol. 2011;211:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Pirotte B, Levivier M, Goldman S, Brucher JM, Brotchi J, Hildebrand J. Glucocorticoid-induced long-term remission in primary cerebral lymphoma: case report and review of the literature. J Neurooncol. 1997;32:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E. Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv Cancer Res. 2008;101:127-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Borrell B. How accurate are cancer cell lines? Nature. 2010;463:858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004;24:3743-3748. [PubMed] |
| 9. | Niforou KM, Anagnostopoulos AK, Vougas K, Kittas C, Gorgoulis VG, Tsangaris GT. The proteome profile of the human osteosarcoma SaOS2 cell line. Cancer Genom Proteom. 2006;3:325-346. |
| 10. | Castiglioni S, Caspani C, Cazzaniga A, Maier JA. Short- and long-term effects of silver nanoparticles on human microvascular endothelial cells. World J Biol Chem. 2014;5:457-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Leidi M, Dellera F, Mariotti M, Banfi G, Crapanzano C, Albisetti W, Maier JA. Nitric oxide mediates low magnesium inhibition of osteoblast-like cell proliferation. J Nutr Biochem. 2012;23:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem. 2011;286:44326-44335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Wimalawansa SJ. Nitric oxide and bone. Ann N Y Acad Sci. 2010;1192:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | MacPherson H, Noble BS, Ralston SH. Expression and functional role of nitric oxide synthase isoforms in human osteoblast-like cells. Bone. 1999;24:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1184] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 16. | Ton FN, Gunawardene SC, Lee H, Neer RM. Effects of low-dose prednisone on bone metabolism. J Bone Miner Res. 2005;20:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Henneicke H, Gasparini SJ, Brennan-Speranza TC, Zhou H, Seibel MJ. Glucocorticoids and bone: local effects and systemic implications. Trends Endocrinol Metab. 2014;25:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Prednisolone augments superoxide formation in porcine pulmonary artery endothelial cells through differential effects on the expression of nitric oxide synthase and NADPH oxidase. Br J Pharmacol. 2005;145:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Klein-Nulend J, van Oers RF, Bakker AD, Bacabac RG. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int. 2014;25:1427-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | van Bezooijen RL, Van der Bent C, Papapoulos SE, Löwik CW. Oestrogenic compounds modulate cytokine-induced nitric oxide production in mouse osteoblast-like cells. J Pharm Pharmacol. 1999;51:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Wu CL, Huang AC, Yang JS, Liao CL, Lu HF, Chou ST, Ma CY, Hsia TC, Ko YC, Chung JG. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29:1199-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Niforou KM, Anagnostopoulos AK, Vougas K, Kittas C, Gorgoulis VG, Tsangaris GT. The proteome profile of the human osteosarcoma U2OS cell line. Cancer Genomics Proteomics. 2008;5:63-78. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Angoules A, Guerado E, Lawen A S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ