Published online Apr 12, 2016. doi: 10.5528/wjtm.v5.i1.46
Peer-review started: August 27, 2015
First decision: October 27, 2015
Revised: November 12, 2015
Accepted: December 13, 2015
Article in press: December 14, 2015
Published online: April 12, 2016
Processing time: 229 Days and 22.6 Hours
The major histocompatibility complex (MHC) is the exclusive chaperone that presents intracellular antigens, either self or foreign to T cells. Interestingly, aberrant expression of MHC molecules has been reported in various autoimmune target tissues such as thyroid follicular cells in Grave’s disease. Herein, we review the discovery of an unexpected effect of cytosolic double-stranded DNA (dsDNA), despite its origins, to induce antigen processing and presenting genes, including MHC molecules, in non-immune cells. Moreover, we highlight several recent studies that suggest cell injury endows thyroid epithelial cells with a phenotype of mature antigen presenting cells by inducing multiple antigen processing and presenting genes via releasing genomic DNA fragments into the cytosol. We discuss the possibility that such cytosolic dsDNA, in naked form without binding to histone proteins, might be involved in the development of cell damage-triggered autoimmune responses. We also discuss the possible molecular mechanism by which cytosolic dsDNA can induce MHC molecules. It is reasonable to speculate that cytosolic dsDNA-induced MHC class I is partially due to an autocrine/paracrine effect of type I interferon (IFN). While the mechanism of cytosolic dsDNA-induced MHC class II expression appears, at least partially, distinct from that mediated by IFN-γ. Further in-depth are required to clarify this picture.
Core tip: We reviewed the discovery of an unexpected effect of cytosolic double-stranded DNA (dsDNA) to induce antigen processing and presenting genes including major histocompatibility complex (MHC) molecules in non-immune cells. We also focus on the current status quo of the overall research in the field with highlight on our recent findings that suggest cell injury-induced self-cytosolic dsDNA is a potential trigger in the development of autoimmunity. Meanwhile, we provide in-depth discussion of the molecular signals responsible for the effect of dsDNA to induce MHC molecules, based on the current opinion of dsDNA-mediated signal pathways.
- Citation: Luo Y, Yoshihara A, Oda K, Ishido Y, Hiroi N, Suzuki K. Naked DNA in cells: An inducer of major histocompatibility complex molecules to evoke autoimmune responses? World J Transl Med 2016; 5(1): 46-52
- URL: https://www.wjgnet.com/2220-6132/full/v5/i1/46.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v5.i1.46
The major histocompatibility complex (MHC) is the exclusive chaperone that presents intracellular antigens (more specifically, peptides), either synthesized by the cell itself or internalized from the extracellular environment, to T cells. Upon binding to the MHC-peptide complex via the T cell receptor and CD4/CD8 co-receptors, T cells should in principle tolerate self antigens. In contrast, activation should occur if the T cells had not been trained to recognize the antigen during thymus positive selection. If this principle, known as immune tolerance, is violated, an autoimmune response will occur. Interestingly, aberrant expression of MHC molecules has been reported in endocrine epithelial cells in autoimmune target tissues (Table 1) such as pancreatic beta cells of insulin-dependent diabetes[1] and thyroid follicular cells of Grave’s disease[2-5]. Transgenic mouse strains harboring MHC linked to insulin promoter overexpress MHC molecules in pancreatic beta cells and spontaneously develop insulin-dependent diabetes[6-8]. Many attempts have been made to artificially induce Grave’s disease in mice. Although not very successful, these efforts have demonstrated that autoantibodies developed only when the animals were immunized with cells co-expressing the self antigen, thyroid stimulating hormone receptor (TSHR), and MHC class II molecules[9]. The few successful mouse models of Grave’s disease were generated by vaccination of TSHR-expressing plasmids or by infection of TSHR-expressing adenovirus[10,11]. Significantly, both DNA vaccine and adenovirus are primarily double-stranded DNAs (dsDNAs) that should be able to induce MHC molecules in the cells at the site of inoculation. Does this “adjuvant-effect” contribute to the successful generation of the autoimmune mouse model?
| Disease | Cells with aberrant expression of MHC molecules |
| Insulin-dependent diabetes | Pancreatic beta cells[1] |
| Grave's disease | Thyroid epithelial cells[54] |
| Rheumatic carditis | Valvular fibroblasts[55] |
| Primary biliary cirrhosis | Bile duct epithelial cells[56] |
| Sjögren's syndrome | Salivary acinar and ductal epithelial cells[57] |
| Acute lymphoproliferative disorders | Bone marrow-derived mesenchymal stromal cells[58] |
| Asthma1 | Bronchial epithelial cells[59] |
| Dilated cardiomyopathy1 | Endothelial and endocardial cells[60] |
| Tubulointerstitial nephritis1 | Renal tubular epithelial cells[61] |
| Biliary atresia1 | Intrahepatic bile ducts[62] |
In 1999, Suzuki et al[12] reported that the expression of MHC molecules, including MHC class I and MHC class II, could be strongly induced by the transfection of dsDNA, regardless of their origins. Diverse dsDNAs were tested in vitro, including bacterial DNA, viral DNA, salmon sperm DNA, calf thymus DNA, host genomic DNA, plasmid DNA and artificially synthesized DNA fragments. Remarkably, all induced MHC expression, whereas single-stranded DNA (ssDNA) could not[12]. To exert this effect, dsDNA needs to be introduced into the cytosol, as free dsDNA in extracellular medium was not sufficient to induce MHC expression[13], indicating that this effect is unlikely mediated by cell surface receptors. The method of introducing dsDNA into cytosol is not critical. Different transfection procedures, including lipofection, electroporation and diethylaminoethyl-dextran similarly increased MHC levels[12]. The first study that explicitly and thoroughly described such effects specific to cytosolic dsDNA was not reported until 1999. This was surprisingly late, considering that cell culture transfection methods were developed in the 1970s and became widespread during the 1980s, although it had been previously observed that fibroblasts could somehow respond to nucleic-acids derived from pathogens or the host[14,15].
The effect of cytosolic dsDNA does not require professional antigen presenting cells (APCs). In addition to professional APCs, including primary cultures of mouse dendritic cells (DCs) and macrophages, the induction of MHC molecules by cytosolic dsDNA was reproduced in non-professional APCs such as rat thyroid follicular cells, human and mouse fibroblasts, human and mouse muscle cells and human endothelial cells[12,13,16,17]. These results imply the possibility that a potential APC pool consisting of various non-immune cells in vivo can be activated upon their exposure to cytosolic dsDNA (possibly derived from invasive pathogens or dying host cells). In particular, direct evidence has shown that MHC-expressing thyroid epithelial cells are potentially competent APCs to present antigens to activate T cells. MHC class II-positive human thyroid follicular cells were able to present a influenza-specific peptide to a human T-cell clone, a reaction which was abrogated by anti-MHC class II antibodies[3]. Lectin-induced MHC class II-positive human thyroid cells in monolayer culture were able to induce a proliferative reaction in autologous T cells, a phenomenon not found with MHC class II-negative cells[18]. Wistar rats are susceptible to the induction of experimental autoimmune thyroiditis. A cloned Wistar thyroid epithelial cell line was shown to be directly recognized by Wistar rat lymphoid T cells that were both MHC class I- and class II-restricted[19]. When CBA mouse lymphoblasts generated on co-culture with monolayer syngeneic thyroid epithelial cells were injected either intravenously or into the thyroid lobes of intact CBA recipients, thyroiditis appeared within three weeks[20]. All these evidence suggest that the potential ability of thyroid epithelial cells as APCs to directly interact with T cells in a MHC-restricted manner likely precipitates autoimmune response in the thyroid, although whether exposure to cytosolic dsDNA would substantialize such potential in non-immunes cells needs to be further tested by experiments.
Unmethylated CpG motifs, which commonly exist within bacterial DNA and viral DNA, but not in vertebrates, have been shown to activate innate immunity via CpG sensor toll-like receptor 9 (TLR9)[21]. However, CpG motif-containing ssDNA failed to induce MHC molecules whereas methylase-treated CpG dsDNA induced MHC expressions to a level comparable to untreated CpG dsDNA[12]. These results indicated that the induction of MHC molecules by cytosolic dsDNA is not mediated by CpG motifs. In contrast, DNase treatment, as predicted, completely abolished the induction of MHC molecules following dsDNA transfection[12,13]. Later, it was shown that the effect of cytosolic dsDNA to stimulate a host innate immune response was independent of TLRs, but required a classic double-stranded right-handed helix sense (B-DNA)[22] with a native sugar-phosphate backbone[16]. Although the effect of dsDNA on MHC appears sequence-independent, MHC expression was shown to be induced by dsDNA in a length-dependent manner[12,13]. Nevertheless, a DNA fragment as short as 25 bp was capable of exerting a reproducible concentration-dependent effect on the expression of MHC molecules[12]. It was shown in later studies that cytosolic dsDNA activated innate immune responses in a length-dependent manner. This result might indicate an increasing binding affinity by putative cytosolic dsDNA sensors for longer dsDNA[22-24].
Antigen processing and presenting is a multiple-step process involving numerous molecules with diverse functions, such as proteasome proteins responsible for antigen degradation to generate peptides, e.g., LMP2 and cathepsin; molecules required for transporting and loading peptides onto MHC molecules, e.g., transporter associated with antigen processing, MHC II-associated invariant chain (Ii), and HLA-DMB; cell surface co-stimulators indispensable for fully activating T cells, e.g., CD80, CD86, CD40; and cell adhesion molecules for stabilizing the binding with lymphocytes, e.g., CD54 (also known as intracellular adhesion molecule 1)[25,26]. In addition to MHC molecules, many of these essential participants in antigen processing and presenting, as well as the transcription factors for MHC expression, including signal transducers and activators, interferon regulatory factor 1, nuclear factor κB (NF-κB), and class II MHC transactivator (CIITA), have also been shown to be induced/activated by cytosolic dsDNA, but not ssDNA, in both professional APCs and non-professional APCs[12,13,16,17]. Overall, these results suggest that in the presence of cytosolic dsDNA, even non-immune cells can acquire full capability to present antigens (so called APC maturation). Theoretically, T cells should have a much greater chance to be activated by antigens under this condition. In agreement with such a prediction, production of interleukin-2 (IL-2) and interferon-γ (IFN-γ) in T cells was significantly increased (approximately 3-fold) by mixing with peptide-challenged DCs containing cytosolic dsDNA, compared to those without cytosolic dsDNA, or those containing cytosolic ssDNA[13].
DNA, normally sequestered within the nucleus or in mitochondria, can be internalized into the cytosol of phagocytes from apoptotic bodies released from dying cells in vivo. Phagocytes engulf the apoptotic bodies (also the nuclei expelled from erythroid precursor cells) in extracellular medium and digest the DNA contents using DNase in the phagolysosomes[27,28]. Indeed, a large amount of cytosolic DNA has been demonstrated to accumulate in DNase-deficient murine macrophages that were presented apoptotic cells, but only a small amount in wild-type macrophages after the same treatment[29]. Defective clearance of self DNA due to mutations in DNase genes is associated with the development of human autoimmune diseases such as systemic lupus erythematosus[30,31] and Aicardi-Goutieres syndrome[32,33], thus revealing self DNA as a potential trigger for autoimmune responses.
Despite the presence of normal DNase, tissue injury, depending on the severity, can generate more dying cells than the intrinsic digestive capacity of phagocytes can process, inevitably leading to cytosolic DNA accumulation in phagocytes. It has been shown that electric pulse-mediated cell injury under sterile conditions induced cytosolic DNA in a current intensity-dependent manner in rat thyroid epithelial cells[17], supporting the notion that tissue injury is a possible cause for the presence of cytosolic DNA in vivo. Intriguingly, electric pulse-caused cell injury also induced the expression of MHC II and its transactivator CIITA in a current intensity-dependent manner in rat thyroid epithelial cells[12]. Such cell injury endows thyroid epithelial cells with a phenotype of mature APC by inducing multiple antigen processing and presenting genes that largely overlapped with cytosolic DNA-inducible molecules such as MHC I, MHC II, CD40, CD54, CD80, CD86, RFX5 and CIITA[12,17].
APC maturation characterized by increased expression of CD40 was also observed in primary cultures of immature murine CD11c+ bone marrow dendritic cells (BMDCs) when cultured with necrotic fibroblasts derived from the same animal[13]. Proteinase-K-treated necrotic fibroblasts reproduced the same effect, inducing APC maturation in BMDCs to a similar extent[13], indicating self protein was unlikely the essential factor. However, additional DNase treatment significantly abrogated the ability of necrotic fibroblasts to induce APC maturation[13], suggesting it is likely that the self DNA derived from the dying cells had contributed to the activation of APCs. However, the profile and kinetics of cell injury-induced genes was not completely duplicated by that induced by dsDNA transfection[12,17], indicating additional factors other than cytosolic DNA may be involved in a cell injury event.
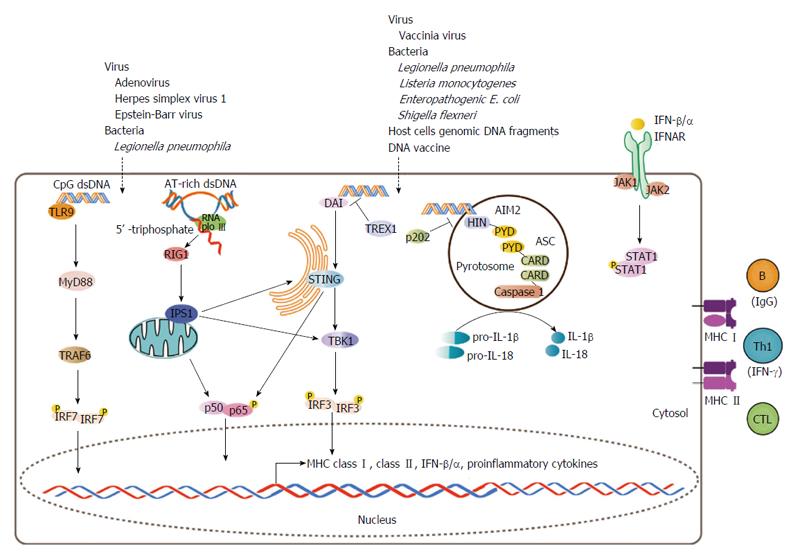
Cytosolic DNA-mediated signal pathways have been extensively investigated (well-reviewed in[34]). Two independent responses can be simultaneously induced by cytosolic dsDNA, one characterized by the production of type I IFNs (IFN-α and -β) as well as type I IFN-inducible molecules, and a second pro-inflammatory response characterized by the production of IL-1β and IL-18, both of which have been implicated in the activation of the immune response[34] (Figure 1).
It is reasonable to speculate that cytosolic dsDNA-induced APC maturation is partially due to an autocrine/paracrine effect involving secreted type I IFNs, mediated by the cell surface type I IFN receptor (IFNAR) (Figure 1). Such a process could stimulate APC maturation in DCs and precipitate T cell activation in vitro and in vivo, concomitantly with increased expression of antigen processing and presenting genes, including MHC I, CD40 and CD86[35-37] (Figure 1). Repeated low-dose chemotherapy or radiation could also trigger an autoimmune response. These cellular insults can induce MHC I expression in cancer cells via the IFN-β/IFNAR signal pathway[38] and enhance CD8+ T cell-mediated antitumor immune responses to tumor vaccine in vivo[39]. As a therapeutic strategy to restore autoimmune surveillance in cancer cells, low-dose chemotherapy is given to metastatic pancreatic cancer patients before receiving a cell-based cancer vaccine[40]. Thus, cell damage-induced autoimmunity may not be entirely harmful if wisely used.
Moreover, it is possible that the induction of type I IFNs and antigen processing and presenting genes share some upstream signals in common, such as STAT and NF-κB signal pathways that could be directly activated upon the recognition of cytosolic dsDNA as a “danger signal”[12,17,34,41]. Thus, it is likely that the signal pathways that mediate type I IFN production and the induction of antigen processing and presenting genes cross-talk with one another (Figure 1). Stimulator of IFN genes (STING)[42,43] and TANK-binding kinase 1 (TBK1)[44,45], which have been shown to mediate cytosolic dsDNA-induced type I IFN production (Figure 1), may also be required for the induction of antigen processing and presenting genes by cytosolic dsDNA, as DNA vaccine-mediated T cell activation was abolished in STING-knockout mice that were challenged with antigen peptides[43]. Moreover, TBK1-deficiency abrogated cytosolic dsDNA-induced APC maturation in primary mouse bone marrow macrophages[45]. Further studies are required to clarify this picture.
Cytosolic dsDNA-induced MHC II expression should be IFNAR-independent, as type I IFNs do not induce MHC II, but rather suppress its expression by acting as an antagonist of IFN-γ[46], especially in non-professional APCs[47]. Indeed, cytosolic dsDNA prominently induced MHC I rather than MHC II in rat thyroid epithelial cells in vitro[12]. Knockout mice have revealed something more in vivo. Both MHC I and MHC II induction occurred in areas of tissue injury in IFN-γ-deficient mice, but with 50% less induction than that in the wild-type[48], suggesting that the IFN-γ signal contributed half of the effect to induce MHC molecules triggered by tissue injury in vivo, while IFN-γ-independent signals were also at play. It is possible in vivo that the activated T cells secrete IFN-γ (Figure 1), which in turn induces more MHC molecules on APCs to facilitate antigen presentation to further accelerate T cells activation and IFN-γ secretion, thus forming a positive-feedback loop in the area of injury.
Cytosolic dsDNA-induced production of IL-1β and IL-18 is mediated by a rather independent upstream signal pathway that involves absent in melanoma 2, apoptosis-associated speck-like protein complex and caspase 1 cleavage[49-52] (Figure 1). Nevertheless, the contribution of a pro-inflammatory extracellular milieu to the development of autoimmunity should never be underestimated.
Numerous factors must be working together to trigger an autoimmune response: Environmental stimuli (e.g., those that can cause tissue injury) and genetic predisposition (e.g., having a specific human leukocyte antigen haplotype increases the risk of autoimmune diseases[53]). Studies have indicated that cytosolic naked dsDNA (either foreign or self orign) could be a universal factor that activates both innate and acquired immune responses in any tissue and cell type to trigger unfavorable immune responses in autoimmune-prone individuals.
| 1. | Foulis AK, Farquharson MA. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986;35:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;2:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 817] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Londei M, Lamb JR, Bottazzo GF, Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984;312:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 308] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Singer DS, Mozes E, Kirshner S, Kohn LD. Role of MHC class I molecules in autoimmune disease. Crit Rev Immunol. 1997;17:463-468. [PubMed] |
| 5. | Todd I, Londei M, Pujol-Borrell R, Mirakian R, Feldmann M, Bottazzo GF. HLA-D/DR expression on epithelial cells: the finger on the trigger? Ann N Y Acad Sci. 1986;475:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Allison J, Campbell IL, Morahan G, Mandel TE, Harrison LC, Miller JF. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988;333:529-533. [PubMed] |
| 7. | Sarvetnick N, Liggitt D, Pitts SL, Hansen SE, Stewart TA. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988;52:773-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 350] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Lo D, Burkly LC, Widera G, Cowing C, Flavell RA, Palmiter RD, Brinster RL. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988;53:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 246] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Shimojo N, Kohno Y, Yamaguchi K, Kikuoka S, Hoshioka A, Niimi H, Hirai A, Tamura Y, Saito Y, Kohn LD. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci USA. 1996;93:11074-11079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | McLachlan SM, Nagayama Y, Rapoport B. Insight into Graves’ hyperthyroidism from animal models. Endocr Rev. 2005;26:800-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Nagayama Y. Animal models of Graves’ hyperthyroidism. Endocr J. 2005;52:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, Krause PR, Kohn LD. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci USA. 1999;96:2285-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Ishii KJ, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn LD, Klinman DM. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Jensen KE, Neal AL, Owens RE, Warren J. Interferon responses of chick embryo fibroblasts to nucleic acids and related compounds. Nature. 1963;200:433-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Rotem Z, Cox RA, Isaacs A. Inhibition of virus multiplication by foreign nucleic acid. Nature. 1963;197:564-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Kawashima A, Tanigawa K, Akama T, Wu H, Sue M, Yoshihara A, Ishido Y, Kobiyama K, Takeshita F, Ishii KJ. Fragments of genomic DNA released by injured cells activate innate immunity and suppress endocrine function in the thyroid. Endocrinology. 2011;152:1702-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Davies TF. Cocultures of human thyroid monolayer cells and autologous T cells: impact of HLA class II antigen expression. J Clin Endocrinol Metab. 1985;61:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kimura H, Davies TF. Thyroid-specific T cells in the normal Wistar rat. II. T cell clones interact with cloned wistar rat thyroid cells and provide direct evidence for autoantigen presentation by thyroid epithelial cells. Clin Immunol Immunopathol. 1991;58:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Charreire J, Michel-Bechet M. Syngeneic sensitization of mouse lymphocytes on monolayers of thyroid epithelial cells. III. Induction of thyroiditis by thyroid-sensitized T lymphoblasts. Eur J Immunol. 1982;12:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4803] [Cited by in RCA: 4864] [Article Influence: 187.1] [Reference Citation Analysis (13)] |
| 22. | Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 629] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 741] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 24. | Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 683] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 25. | York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Pieters J. MHC class II restricted antigen presentation. Curr Opin Immunol. 1997;9:89-96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene. 2003;322:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Yoshida H, Kawane K, Koike M, Mori Y, Uchiyama Y, Nagata S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Möröy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 620] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 31. | Yasutomo K, Horiuchi T, Kagami S, Tsukamoto H, Hashimura C, Urushihara M, Kuroda Y. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet. 2001;28:313-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 468] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1051] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 33. | Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 34. | Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1227] [Cited by in RCA: 1186] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 36. | Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 240] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263-3271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 38. | Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. 2012;7:e32542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 39. | Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, Gillanders WE, Cole DJ. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30:40-53. [PubMed] |
| 40. | Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 41. | Kawashima A, Yamazaki K, Hara T, Akama T, Yoshihara A, Sue M, Tanigawa K, Wu H, Ishido Y, Takeshita F. Demonstration of innate immune responses in the thyroid gland: potential to sense danger and a possible trigger for autoimmune reactions. Thyroid. 2013;23:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2812] [Cited by in RCA: 2857] [Article Influence: 158.7] [Reference Citation Analysis (1)] |
| 43. | Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788-792. [PubMed] |
| 44. | Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725-729. [PubMed] |
| 45. | Miyahira AK, Shahangian A, Hwang S, Sun R, Cheng G. TANK-binding kinase-1 plays an important role during in vitro and in vivo type I IFN responses to DNA virus infections. J Immunol. 2009;182:2248-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Yoshida R, Murray HW, Nathan CF. Agonist and antagonist effects of interferon alpha and beta on activation of human macrophages. Two classes of interferon gamma receptors and blockade of the high-affinity sites by interferon alpha or beta. J Exp Med. 1988;167:1171-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Harris PE, Malanga D, Liu Z, Hardy MA, Souza F, Del Pozzo G, Winchester RJ, Maffei A. Effect of interferon alpha on MHC class II gene expression in ex vivo human islet tissue. Biochim Biophys Acta. 2006;1762:627-635. [PubMed] |
| 48. | Halloran PF, Goes N, Urmson J, Ramassar V, Hobart M, Sims T, Lui SL, Miller LW. MHC expression in organ transplants: lessons from the knock-out mice. Transplant Proc. 1997;29:1041-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 858] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 50. | Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1506] [Cited by in RCA: 1500] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 51. | Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2094] [Cited by in RCA: 2108] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 52. | Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1094] [Cited by in RCA: 1090] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 53. | Bowness P. HLA B27 in health and disease: a double-edged sword? Rheumatology (Oxford). 2002;41:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Hanafusa T, Pujol-Borrell R, Chiovato L, Russell RC, Doniach D, Bottazzo GF. Aberrant expression of HLA-DR antigen on thyrocytes in Graves’ disease: relevance for autoimmunity. Lancet. 1983;2:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 448] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Amoils B, Morrison RC, Wadee AA, Marcus R, Ninin D, King P, Sareli P, Levin S, Rabson AR. Aberrant expression of HLA-DR antigen on valvular fibroblasts from patients with active rheumatic carditis. Clin Exp Immunol. 1986;66:88-94. [PubMed] |
| 56. | Ballardini G, Mirakian R, Bianchi FB, Pisi E, Doniach D, Bottazzo GF. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984;2:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 220] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Nakamura S, Hiroki A, Shinohara M. [Aberrant expression of HLA-DR antigens on acinar and ductal epithelial cells of salivary glands in Sjögren’s syndrome]. Nihon Rinsho. 1995;53:2407-2411. [PubMed] |
| 58. | Lanza F, Campioni D, Moretti S, Ferrari L, Rizzo R, Baricordi R, Cuneo A. Aberrant expression of HLA-DR antigen by bone marrow-derived mesenchymal stromal cells from patients affected by acute lymphoproliferative disorders. Leukemia. 2007;21:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Vachier I, Godard P, Michel FB, Descomps B, Damon M. [Aberrant expression of HLA-DR antigens of the MHC class II in bronchial epithelial cells in asthmatic patients]. C R Acad Sci III. 1990;311:341-346. [PubMed] |
| 60. | Caforio AL, Stewart JT, Bonifacio E, Burke M, Davies MJ, McKenna WJ, Bottazzo GF. Inappropriate major histocompatibility complex expression on cardiac tissue in dilated cardiomyopathy. Relevance for autoimmunity? J Autoimmun. 1990;3:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Yokoyama H, Kida H, Ogi M, Naito T, Ikeda K, Takasawa K, Goshima S, Katagiri M, Takeda S, Yoshimura M. [Aberrant expression of major histocompatibility complex class II. (HLA-DR/DQ) antigens and proliferative nuclear antigen. (Ki-67) in renal tubular epithelial cells]. Nihon Jinzo Gakkai Shi. 1989;31:1125-1132. [PubMed] |
| 62. | Feng J, Li M, Gu W, Tang H, Yu S. The aberrant expression of HLA-DR in intrahepatic bile ducts in patients with biliary atresia: an immunohistochemistry and immune electron microscopy study. J Pediatr Surg. 2004;39:1658-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Thurmond RL S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ