Published online Apr 12, 2016. doi: 10.5528/wjtm.v5.i1.37
Peer-review started: August 30, 2015
First decision: November 30, 2015
Revised: December 18, 2015
Accepted: January 8, 2016
Article in press: January 11, 2016
Published online: April 12, 2016
Processing time: 228 Days and 20.3 Hours
Cisplatin, a DNA crosslinking agent, is widely used for the treatment of a variety of solid tumors. Numerous studies have demonstrated that sphingolipid metabolism, which acts as a target for cisplatin treatment, is a highly complex network that consists of sphingolipid signaling molecules and related catalytic enzymes. Ceramide (Cer), which is the central molecule of this network, has been established to induce apoptosis. However, another molecule, sphingosine-1-phosphate (S1P), exerts the opposite function, i.e., serves as a regulator of pro-survival. Other sphingolipid molecules, including dihydroceramide, ceramide-1-phosphate, glucosylceramide (GluCer), and sphingosine (Sph), or sphingolipid catalytic enzymes such as Sph kinase (SphK), Cer synthase (CerS), and S1P lyase, have also attracted considerable attention, particularly Cer, GluCer, SphK, CerS, and S1P lyase, which have been implicated in cisplatin resistance. This review summarizes specific molecules involved in sphingolipid metabolism and related catalytic enzymes affecting the anticancer effect of cisplatin, particularly in relation to induction of apoptosis and drug resistance.
Core tip: Cisplatin classifies as a classical anticancer drug and DNA is identified as the most important target of cisplatin. However, increasing evidences have testified that sphingolipid metabolism is associated with the anticancer effect of cisplatin. In this mini-review, we discussed sphingolipid signaling molecules and/or related enzymes affected the anticancer effect of cisplatin, particularly in cisplatin-induced cancer cell apoptosis and drug resistance. Targeting these sphingolipid molecules and enzymes might contribute to the development of novel anticancer strategies or to increase the sensitivity of currently used drugs.
- Citation: Li YL, Lin ML, He SQ, Jin JF. Sphingolipid metabolism affects the anticancer effect of cisplatin. World J Transl Med 2016; 5(1): 37-45
- URL: https://www.wjgnet.com/2220-6132/full/v5/i1/37.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v5.i1.37
The mechanisms underlying the anticancer effect of cisplatin (cis-diamminedichloroplatinum) have been extensively investigated by researchers since the discovery of its activity in 1969[1]. It is well known that DNA is the most important target of cisplatin in a variety of cancers, especially ovarian cancer, colorectal cancer, bladder cancer, testicular cancer, head and neck cancer, and lung cancer. DNA adducts of cisplatin with covalent coordinate bonds results in DNA damage and subsequent failure to maintain normal replication and ultimately induced apoptosis[2-5]. However, increasing evidences have testified that sphingolipid metabolism is associated with cancer therapies of cisplatin[6-8]. Treatment with cisplatin in several cancer cells often results in the generation of ceramide (Cer), which has been involved in regulating the cell death response. For example, cisplatin activates acid sphingomyelinase (aSMase) and induces the production of Cer in cancer cells, which triggers a series cellular response, including redistribution of CD95 and cell apoptosis[6]. In addition, sphingolipid molecules and relative enzymes have been implicated in regulating cisplatin sensitivity[7,8]. In this review, we mainly discuss the molecules of sphingolipid metabolism and relative enzymes affecting the anticancer effect of cisplatin, particularly in the induction of apoptosis and drug resistance.
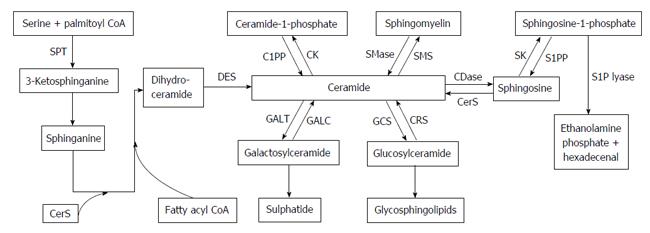
Sphingolipids are membrane lipids that are important constituents of eukaryotic cells. Sphingolipid metabolism is a highly complex network that is composed of various sphingolipid molecules and enzymes that have been identified as pivotal regulators of various cellular processes, including cell growth, migration, adhesion, apoptosis, cell arrest, senescence, autophagy, and drug resistance[8-12]. Cer and sphingosine-1-phosphate (S1P) are the most essential sphingolipid molecules, followed by sphingosine (Sph), ceramide-1-phosphate (C1P), dihydroceramide, sphingomyelin (SM), and glycosphingolipids, of which glucosylceramide (GluCer), lactosylceramide, and galactosylceramide (GalCer) have been extensively studied in various sphingolipid metabolism pathways. Cer and S1P play opposite functions in the regulation of cell fate; the former is implicated in apoptosis[10,13-16], senescence[17,18], differentiation[19,20], and autophagy[11,21,22], whereas the latter promotes cell survival and proliferation, vasculogenesis, inflammation, and resistance to widely used drugs[23-25]. In addition, Sph inhibits cell cycle progression and induces apoptosis[26], whereas C1P and GluCer induce proliferation of cells and are associated with the development of resistance to cisplatin. As we all know, Cer is thought as the central in sphingolipid pathways (Figure 1), and is the precursor of several kinds of sphingolipid molecules, including SM, C1P, GluCer and GalCer. Cer and each of these sphingolipid molecules can be reversibly converted by the action of related enzymes. Cer is generated by multiple pathways, including the synthesis by de novo, the SM hydrolysis, or the Sph recycling[11,27,28]. The main generated route is the de novo synthesis pathway, which takes place in the endoplasmic reticulum (ER). Cer is synthesized from palmitoyl-CoA and serine to form 3-ketodihydrosphingosine through the catalyst, serine palmitoyltransferase[29,30]. Subsequently, the conversion of 3-ketodihydrosphingosine to sphinganine, which is condensed with fatty acyl-CoA by six specific dihydroceramide synthase (CerS 1-6) to form dihydroceramides of different lengths[31], which is then catalyzed by dihydroceramide desaturase to generate Cer. In the hydrolysis of SM pathway, SM is catabolized to Cer through the action of the neutral or SMase, but not alkaline SMase, which is mainly due to the specific forms of phospholipase C[32-35]. The catalysis of acidic and neutral SMases plays a pivotal role in apoptotic process and cell cycle arrest[32,36]. Cer is generated via dephosphorylation of C1P by Cer-1-phosphate phosphatase. In addition, C1P can be recovered from the phosphorylation of Cer by ceramide kinase. Cer can also be formed via the degradation of the glycosphingolipids, GluCer and GalCer, which each contains a single sugar molecule linked to Cer[37], and hydrolyzed by specific β-glucosidases and galactosidases, to yield Cer[38]. Inversely, GluCer is generated by GluCer synthase (GCS) in the Golgi apparatus[39].
Cer is degraded by ceramidases (CDase) to produce Sph, Sph is subsequently phosphorylated by two sphingosine kinase isoenzymes (SK-1 and SK-2) to produce S1P, which then is decomposed under the action of S1P lyase to produce ethanolamine phosphate and hexadecenal. This is the only exit pathway of this complex network. In addition, Sph and S1P can be recycled back to Cer by CerS or dephosphorylated back to Sph, respectively[40].
Recent studies have identified the sphingolipid molecule Cer and enzymes CerS, SK, and S1P lyases as important targets for developing anticaner drugs and drug resistance. Glycosphingolipids also play important role in multidrug resistance[15,41,42].
Although DNA is regarded as the main therapeutic target of cisplatin in various tumor cells, cisplatin induces apoptosis via signaling through plasma membrane lipid rafts that contain abundant sphingolipids, and these membrane lipid rafts are perhaps the targets of cisplatin-induced apoptosis[43-45]. It has been reported that sphingolipids are the major components of lipid rafts, and sphingolipids act as pivotal roles in maintaining the structural integrity of cell membranes and in modulating apoptosis via gene regulation and signal transduction[46]. In addition, an imbalance in sphingolipid levels results in apoptosis, which may be triggered by deviant intracellular apoptotic signaling[47]. Thus, cisplatin-induced apoptosis is closely associated with sphingolipid metabolism. However, Cer, the central molecule of sphingolipids metabolism, is involved in cisplatin-induced apoptosis. The two main apoptotic pathways include the receptor-involved extrinsic pathway and the mitochondria-associated intrinsic pathway[48]. The mechanism involved the Fas death receptor-mediated pathway that contributes to cisplatin-induced apoptosis will be discussed later. In the present section, we will talk about the Cer-played role in cisplatin-induced apoptosis in the mitochondria.
A central role in the intrinsic pathway of apoptosis is played by mitochondria. Stressors such as cisplatin, a chemotherapeutic agent, targets the mitochondria, resulting in the alteration of mitochondrial outer membrane permeabilization (MOMP) that promotes some proteins of mitochondria releasing from the intermembrane space into the cytosol. Then the caspase cascade pathway is activated and cells die within minutes. Thus, MOMP is strictly regulated and is identified as an irreversible event[49-51]. Early in 1993, Obeid et al[52] firstly illustrated that Cer is a potent apoptotic inducer. Subsequently, several studies have indicated that the increase in cellular Cer early in apoptosis is a common cellular response to cisplatin[8,53,54]. Research has shown that Cer, coupled with downstream Cer metabolites that participate in apoptosis, can change the function of mitochondria and give rise to increase of MOMP[49,50,55]. Accompanying the increase in cellular Cer levels, some proteins release from the mitochondrial intermembrane space to the cytoplasm, reactive oxygen species produce more in mitochondria, and the inner membrane potential of mitochondria is decreased[56-58]. Suppressing mitochondrial function can inhibit apoptosis induced by Cer[59]. In addition, the channels formation by Cer itself facilitates apoptosis in the mitochondrial membrane with elevated Cer levels[60]. Therefore, Cer is regarded as a pro-apoptotic molecule. A study in C6 rat glioma cells revealed that cisplatin-induced apoptosis links to Cer production resulting from cisplatin-mediated neutral sphingomyelinase activation. After that, cytochrome C releases from mitochondrion to the cytosol, which is dependent upon the BCL-2 family and activation of caspases-9 and caspases-3[55,61]. These post-mitochondrial events also intrinsically trigger apoptosis. Furthermore, Cers are generally synthesized from sphingoid bases, and very long (C24) or long (C16) fatty acid chains are added by specific Cer synthases. Cers containing different acyl chain lengths may affect susceptibility to cisplatin-induced apoptosis. During cisplatin-induced apoptosis, although intracellular Cer levels are not changed, C16 Cers are specifically elevated[54,62]. In addition, the function of certain proteins involved in apoptosis, including cathepsin D, PKC-ζ, PP1, PP2A, and ceramide-activated protein kinase, were modulated by Cer. These indirect mechanisms may possibly contribute to the mechanism underlying Cer-mediated apoptosis that is involved in the mitochondria pathway[11].
Fas (also known as CD95) use the death domain that is important for protein-protein interaction inside the cell to recruit Fas-associated death domain (FADD), subsequently to recruit the proenzyme of caspase-8[63]. It is necessary to recruit FADD (the adaptor protein) and procaspase-8 to the rafts of Fas ligation in order to initiate the signaling of Fas-mediated apoptosis, disrupting the integrity of rafts fails to initiate the Fas apoptotic signaling[64,65]. Previous reports have manifested that the death receptor Fas is localized in lipid rafts constitutively or under stimulation state, the receptor clustering in lipid rafts is necessary to the cell death mediated by Fas[66,67]. It has been reported that cisplatin causes the Fas clustering at the membrane of HT29 cancer cells derived from human colon, which in turn is inhibited by an inhibitor of aSMase, imipramine[44]. Additionally, CD95 could contribute to cisplatin-induced HT29 cell apoptosis in which redistribution of CD95 played a key role; however, a cholesterol sequestering agent, nystatin through preventing aSMase translocation and Cer production, inhibits cisplatin-induced CD95 clustering and decreases cisplatin-induced HT29 apoptosis[6]. Taking together, these results show that cisplatin triggers Fas redistribution into the plasma membrane rafts by activation of aSMase and induction of Cer production. Therefore, the contribution of Fas redistribution to cell apoptosis and cell death is clearly confirmed[6,44]. Furthermore, it has been reported that apoptosis is easy to be induced by many kinds of factors, for example cisplatin, Fas, tumor necrosis factor-1, growth factor withdrawal, or hypoxia. Several of above apoptotic stimuli can regulate Cer production, that hints us Cer plays an important role in apoptotic process[14]. In addition, the levels of Cer elevate in response to cisplatin, and the Cer increase by using inhibitors of enzymes that is responsible for metabolizing Cer or by overexpressing enzymes that account for Cer production leads to apoptosis[68]. The formation of Fas capping that involves decoupling of Fas ligand and Fas receptor at the plasma membrane enriched sphingolipids especially sphingomyelin is one mechanism in Cer-mediated apoptosis[69]. In other words, cells are resistant to mitochondria-involved apoptosis if they are not sensitive to Fas-mediated apoptotic signaling[70], suggesting that cells will lose sensitivity to death signaling if their Cer-Fas pathway is disturbed. Therefore, Cer has a tight connection with apoptosis, the Fas death receptor pathway is one of the mechanisms in which cisplatin induces apoptosis.
Several other mechanisms are responsible for the induction of cisplatin-induced apoptosis. Perrotta et al[71] reported that cisplatin triggers the apoptosis of dendritic cells (DCs) through increased expression and activation of aSMase, which could be inhibited by preconditioning DCs with nitric oxide donors. Further studies involving human colon cancer cells have shown that cells’ acidification, which is depended on NHE1, appears early in the process of cisplatin-mediated apoptosis, subsequently leading to aSMase activation and fluidity elevation in cell membrane, which differs from cisplatin-induced DNA adduct formation[72]. Furthermore, de-N-acetyl-lysoglycosphingolipid, a hydrolyzed product of ganglioside GM1, inhibits the growth of various tumor cell lines, which occurs in synergy with cisplatin[73].
Although cisplatin is an extremely effective drug that induces apoptosis in cancer cells, the efficacy of cisplatin treatment in some types of cancer is often impeded by drug resistance[74]. Therefore, intrinsic and acquired resistance to cisplatin is a vital problem when using this drug clinically. The mechanisms underlying cisplatin resistance[74-77] include some classical drug resistance mechanisms such as the decrease in the concentration of intracellular cisplatin, inactivation of the drug, increase in DNA repair, and reduction in apoptotic response. However, some resistance related genes including cyclooxygenase-2, heat shock proteins, or other cell signaling pathways and molecules also play some roles in the resistance to cisplatin. Additionally, cell membrane fluidity and lipids are also associated with cisplatin resistance[78]. To investigate the underlying molecular basis of resistance to cisplatin, Alexander et al[79] used Dictyostelium discoideum (D. discoideum) as an excellent eukaryotic model for studying the mechanisms underlying cisplatin drug sensitivity[79-82]. Genome sequencing of D. discoideum has shown that various genes and pathways are highly homologous to those in human cells[79,83]. Because the pathway of sphingolipid metabolism is highly conserved between humans and D. discoideum[79], mutations in sphingolipid metabolism-related genes confer cisplatin resistance in both species[78]. The role of some of the enzymes in sphingolipid metabolism (S1P lyase and SK) in the regulation of cisplatin resistance has been investigated by establishing a D. discoideum model[78,79,84]. S1P lyase (sglA) is highly conserved in humans and this enzyme accounts for the final metabolism in the sphingolipid pathway[85]. Although the sphingolipid metabolism pathway has been extensively investigated in mammalian cells, no previous studies have indicated the relationship between this pathway and cisplatin resistance prior to 2000. SglA was found for the first time to modulate sensitivity to anticancer drug cisplatin in D. discoideum[84]. Sphingolipids are involved in regulating cell fate, and the ratio of Cer and S1P levels could be used to determine whether cells enter the pathway of cell death or survival[86-89]. Various stimuli, including γ-irradiation and anticancer drugs, have also been reported to lead Cer increase and/or to decrease S1P, which is a bioactive sphingolipid that plays a central role in apoptosis inhibition, pro-survival, or cell movement[23] in cancer cells. These effects are reversed with decreased Cer or increased S1P, which results in cell survival and proliferation. Therefore, we hypothesized that deletion of the S1P lyase increases resistance to cisplatin, whereas overexpression of this enzyme yields the opposite effect. The reports that the S1P lyase null (sglAΔ) and overexpressing cells (sglAOE) displayed decreased or increased sensitivity to cisplatin, respectively, have thoroughly proven the above hypotheses in D. discoideum[7,90,91].
Two other enzymes associated with the direct regulation of the production of S1P in D. discoideum include the sgkA and sgkB sphingosine kinases that produce S1P from sphingosine and ATP. We thought that reducing sphingosine kinase expression leads cells are more sensitive to cisplatin, whereas over-expressing this enzyme results in resistance to this drug. D. discoideum sgkA and B genes mutants were generated, which harbored disrupted single or double sphingosine kinases or overexpressed the sgkA gene. Single or double disruption of the sphingosine kinases resulted in a reduction of growth rates, whereas overexpressing mutants presented elevated growth rates. Furthermore, these two enzymes showed a capacity to modulate sensitivity to cisplatin. The null mutants of sphingosine kinase appeared elevated sensitivity to cisplatin, whereas overexpression of SgkA in these mutants would rescue this effect. The addition of S1P or using N, N-dimethylsphingosine, a sphingosine kinase inhibitor[92], counteracts these effects[90]. The effects of sensitivity of the null or sgkA-overexpressing mutants were similar to those of another platinum-based drug, carboplatin. Taken together, these findings in D. discoideum allowed us to conclude that modulation of cisplatin sensitivity can be achieved through the regulation of related enzymes of sphingolipid metabolism.
Based on the above results, considerable attention has been paid to study cisplatin resistance and related mechanisms in mammalian cells. The results of studies on the mechanism underlying the resistance to cisplatin on D. discoideum should be confirmed in mammalian cells. Researchers have investigated the effect of overexpressing or deleting S1P lyase on cisplatin sensitivity in mammalian cells. The overexpression of S1P lyase in both human lung cancer (A549) and human embryonic kidney 293 cells resulted in an increase in cisplatin sensitivity, whereas the opposite effects were obtained with the disruption of S1P lyase[93]. The role of sphingosine kinases (SphK1 and SphK2, which are the equivalent of the SgkA and SgkB on D. discoideum, respectively) affecting cisplatin resistance was also examined in mammalian cells. Although these human isoenzymes generate the same product, S1P possesses different functions in cells[94-96]. Thus, SphK1 and SphK2 also had different effects on cisplatin sensitivity. Increasing the expression of SphK1 reduced cisplatin sensitivity, whereas SphK2 generated cells that with higher cisplatin sensitivity[8]. The deletion or overexpression of S1P lyase or SphKs affects the generation of S1P, indicating that the regulation of S1P is one of the mechanisms underlying cisplatin resistance.
Cer is regarded as another sphingolipid metabolism-related molecule that influences cisplatin sensitivity. Based on the bioactivity of Cer, the alteration of Cer accumulation alters a cell’s sensitivity to cisplatin. Although Cer can be produced from various sphingolipids, de novo synthesis has proven to be the ultimate source of Cer. Each of the six key dihydroceramide synthase (CerS1 through CerS6) enzymes prefers a fatty acyl CoA with different chain length as a substrate to produce specific Cer molecules[31,97]. Three of these enzymes have yet to be tested in terms of its capacity to regulate cisplatin sensitivity. Only expression of CerS1 leads cell is more sensitive to the all tested drugs such as cisplatin, vincristine, doxorubicin, and carboplatin, accompanied by more p38 MAPK activation. Nevertheless, CerS5 expression resulted in an increased sensitivity to vincristine and doxorubicin, whereas the ovexpression of CerS4 had not similar effect on all the above mentioned reagents. The effects of CerS1 expression are implicated in its specific translocation from the ER to the Golgi apparatus, but not CerS4 or CerS5, and are reversed by the expression of SphK1, but not SphK2.
It has been previously reported that overexpression of GCS efficiently leads GluCer formation from Cer in some cancer cells, including breast cancer cells and human ovarian carcinoma cells[98-100]. Compared to sensitive cells, GluCer production is markedly higher in resistant cells[99,101,102], which is accompanied by an increase in the expression of P-glycoprotein, a membrane efflux transporter and one of the most common alterations in resistant cells[99,103,104], indicating that glucosylation of Cer is associated with drug resistance[105]. GCS is associated with multidrug resistant cancers and elevates the expression of multidrug resistance protein 1 (MDR1). Previous studies have revealed that MDR1 expression is markedly inhibited by siRNA-mediated GCS deletion, which functions as a membrane translocase and reverses drug resistance[98,99]. This finding indicates that the downregulation of GCS prevents the accumulation of glucosylceramide, which in turn increases sensitivity to anticancer drugs[15,106]. However, this phenomenon has not been observed despite the downregulation of GCS expression using specific inhibitors[107]. In addition, MDR1, as the major GluCer translocase, is required for the synthesis of neutral glycosphingolipids, but not for acid glycosphingolipids[108]. The production of glycosphingolipids with α-hydroxy fatty acids and longer carbohydrate chains is markedly higher in the human ovarian carcinoma cisplatin-resistant KF28 cells (KFr13) and taxol-resistant KF28 cells (KF28TX) compared to that of sensitive KF28 cells, suggesting that changes in the glycosphingolipid composition of cancer cells are associated with cisplatin resistance[100]. Taken together, these results suggest that the molecules related to the sphingolipid metabolic pathway can be manipulated to a certain extent by regulating the expression of related enzymes to improve cisplatin sensitivity.
In conclusion, sphingolipid metabolism may play crucial roles in the induction of apoptosis and resistance of cisplatin. In particular, Cer is closely related to cisplatin-induced apoptosis and is considered a potential target for cancer therapeutics. To study the mechanisms underlying cisplatin resistance in sphingolipid metabolism pathways, D. discoideum was established as an excellent eukaryotic model. The results obtained from this model have been extensively translated to and validated in human cells. Thus far, sphingolipid molecules particularly S1P, GluCer, and related enzymes, particularly SphK, CerS, and S1P lyase have been implicated in cisplatin sensitivity. Tumor pathogenesis is considered as an intricate process; therefore, to fully understand the mechanisms underlying the use of cisplatin as an anticancer drug targeting the sphingolipid metabolism pathway, a variety of strategies should be utilized. Targeting these essential molecules of sphingolipid metabolism may contribute to the development of novel anticancer strategies or to increase the sensitivity of currently used drugs.
| 1. | Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3056] [Cited by in RCA: 2990] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 2. | Cohen SM, Lippard SJ. Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol. 2001;67:93-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 447] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem Rev. 1999;99:2467-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2390] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 4. | Tan CP, Lu YY, Ji LN, Mao ZW. Metallomics insights into the programmed cell death induced by metal-based anticancer compounds. Metallomics. 2014;6:978-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Basu A, Krishnamurthy S. Cellular responses to Cisplatin-induced DNA damage. J Nucleic Acids. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593-3598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Min J, Stegner AL, Alexander H, Alexander S. Overexpression of sphingosine-1-phosphate lyase or inhibition of sphingosine kinase in Dictyostelium discoideum results in a selective increase in sensitivity to platinum-based chemotherapy drugs. Eukaryot Cell. 2004;3:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 2011;11:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Tirodkar TS, Voelkel-Johnson C. Sphingolipids in apoptosis. Exp Oncol. 2012;34:231-242. [PubMed] |
| 11. | Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 3016] [Article Influence: 167.6] [Reference Citation Analysis (0)] |
| 12. | Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1389] [Cited by in RCA: 1494] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 13. | Cheng L, Chen YZ, Peng Y, Yi N, Gu XS, Jin Y, Bai XM. Ceramide production mediates cinobufotalin-induced growth inhibition and apoptosis in cultured hepatocellular carcinoma cells. Tumour Biol. 2015;36:5763-5771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 601] [Article Influence: 25.0] [Reference Citation Analysis (3)] |
| 15. | Hajj C, Becker-Flegler KA, Haimovitz-Friedman A. Novel mechanisms of action of classical chemotherapeutic agents on sphingolipid pathways. Biol Chem. 2015;396:669-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Modrak DE, Leon E, Goldenberg DM, Gold DV. Ceramide regulates gemcitabine-induced senescence and apoptosis in human pancreatic cancer cell lines. Mol Cancer Res. 2009;7:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 17. | Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, Birbes H, Hannun YA, Obeid LM. Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J Biol Chem. 2001;276:24901-24910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1303] [Article Influence: 43.4] [Reference Citation Analysis (10)] |
| 20. | Jung EM, Griner RD, Mann-Blakeney R, Bollag WB. A potential role for ceramide in the regulation of mouse epidermal keratinocyte proliferation and differentiation. J Invest Dermatol. 1998;110:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA. 2008;105:17402-17407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851-25854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 435] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 24. | Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Becker KA, Riethmüller J, Zhang Y, Gulbins E. The role of sphingolipids and ceramide in pulmonary inflammation in cystic fibrosis. Open Respir Med J. 2010;4:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 26. | Nikolova-Karakashian M, Merrill AH. Ceramidases. Methods Enzymol. 2000;311:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Merrill AHJ. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chemical reviews. 2011;111:6387-6422. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 567] [Cited by in RCA: 615] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 28. | Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J lipid res. 2009;50 Suppl:S91-S96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 551] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 29. | Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847-25850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 690] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Linn SC, Kim HS, Keane EM, Andras LM, Wang E, Merrill AH. Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption. Biochem Soc Trans. 2001;29:831-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001-25005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 371] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 32. | Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Fuks Z, Haimovitz-Friedman A, Kolesnick RN. The role of the sphingomyelin pathway and protein kinase C in radiation-induced cell kill. Important Adv Oncol. 1995;19-31. [PubMed] |
| 34. | Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21:836-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Xu R, Sun W, Jin J, Obeid LM, Mao C. Role of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes. FASEB J. 2010;24:2507-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Gómez-Muñoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res. 2004;45:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Airola MV, Hannun YA. Sphingolipid metabolism and neutral sphingomyelinases. Handb Exp Pharmacol. 2013;57-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004;20:301-317. [PubMed] |
| 39. | Stefanić S, Spycher C, Morf L, Fabriàs G, Casas J, Schraner E, Wild P, Hehl AB, Sonda S. Glucosylceramide synthesis inhibition affects cell cycle progression, membrane trafficking, and stage differentiation in Giardia lamblia. J Lipid Res. 2010;51:2527-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem. 2004;92:900-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Yang L, Zheng LY, Tian Y, Zhang ZQ, Dong WL, Wang XF, Zhang XY, Cao C. C6 ceramide dramatically enhances docetaxel-induced growth inhibition and apoptosis in cultured breast cancer cells: a mechanism study. Exp Cell Res. 2015;332:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Truman JP, García-Barros M, Obeid LM, Hannun YA. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochim Biophys Acta. 2014;1841:1174-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7533] [Cited by in RCA: 7441] [Article Influence: 256.6] [Reference Citation Analysis (0)] |
| 44. | Dimanche-Boitrel MT, Meurette O, Rebillard A, Lacour S. Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat. 2005;8:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Verkleij AJ, Post JA. Membrane phospholipid asymmetry and signal transduction. J Membr Biol. 2000;178:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50:104-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Kolter T. A view on sphingolipids and disease. Chem Phys Lipids. 2011;164:590-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Patwardhan GA, Beverly LJ, Siskind LJ. Sphingolipids and mitochondrial apoptosis. J Bioenerg Biomembr. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Birbes H, El Bawab S, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002;42:113-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 52. | Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1336] [Cited by in RCA: 1412] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 53. | Noda S, Yoshimura S, Sawada M, Naganawa T, Iwama T, Nakashima S, Sakai N. Role of ceramide during cisplatin-induced apoptosis in C6 glioma cells. J Neurooncol. 2001;52:11-21. [PubMed] |
| 54. | Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hernandez-Corbacho MJ, Edinger AL, Obeid LM. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J Biol Chem. 2010;285:11818-11826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 56. | Andrieu-Abadie N, Gouazé V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 218] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 57. | Gentil B, Grimot F, Riva C. Commitment to apoptosis by ceramides depends on mitochondrial respiratory function, cytochrome c release and caspase-3 activation in Hep-G2 cells. Mol Cell Biochem. 2003;254:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Tang MJ, Chang WC, Lin YS. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. J Biol Chem. 2004;279:40755-40761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Stoica BA, Movsesyan VA, Lea PM, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640-38644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 245] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Nozawa Y. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 2000;19:3508-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Sassa T, Suto S, Okayasu Y, Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim Biophys Acta. 2012;1821:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2314] [Cited by in RCA: 2237] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 64. | Gajate C, Mollinedo F. The antitumor ether lipid ET-18-OCH(3) induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860-3863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 65. | Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002;3:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 66. | Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589-20596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 483] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 67. | Garofalo T, Misasi R, Mattei V, Giammarioli AM, Malorni W, Pontieri GM, Pavan A, Sorice M. Association of the death-inducing signaling complex with microdomains after triggering through CD95/Fas. Evidence for caspase-8-ganglioside interaction in T cells. J Biol Chem. 2003;278:8309-8315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 69. | Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954-23961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 293] [Article Influence: 11.7] [Reference Citation Analysis (4)] |
| 70. | Raisova M, Bektas M, Wieder T, Daniel P, Eberle J, Orfanos CE, Geilen CC. Resistance to CD95/Fas-induced and ceramide-mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. FEBS Lett. 2000;473:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Perrotta C, Bizzozero L, Falcone S, Rovere-Querini P, Prinetti A, Schuchman EH, Sonnino S, Manfredi AA, Clementi E. Nitric oxide boosts chemoimmunotherapy via inhibition of acid sphingomyelinase in a mouse model of melanoma. Cancer Res. 2007;67:7559-7564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann M, Kaina B. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865-7874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Tubaro E, Borelli GP, Belogi L, Cavallo G, Santoni A, Mainiero F. Effect of a new de-N-acetyl-lysoglycosphingolipid on some tumour models. Eur J Pharmacol. 1995;294:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265-7279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2560] [Article Influence: 111.3] [Reference Citation Analysis (1)] |
| 75. | Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Critical reviews in oncology/hematology. 2007;63:12-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 487] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 76. | Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer treatment reviews. 2007;33:9-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1230] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 77. | Wernyj RP, Morin PJ. Molecular mechanisms of platinum resistance: still searching for the Achilles’ heel. Drug Resist Updat. 2004;7:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Van Driessche N, Alexander H, Min J, Kuspa A, Alexander S, Shaulsky G. Global transcriptional responses to cisplatin in Dictyostelium discoideum identify potential drug targets. Proc Natl Acad Sci USA. 2007;104:15406-15411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Alexander S, Swatson WS, Alexander H. Pharmacogenetics of resistance to Cisplatin and other anticancer drugs and the role of sphingolipid metabolism. Methods Mol Biol. 2013;983:185-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Williams RS, Boeckeler K, Gräf R, Müller-Taubenberger A, Li Z, Isberg RR, Wessels D, Soll DR, Alexander H, Alexander S. Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol Med. 2006;12:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Williams JG. Dictyostelium finds new roles to model. Genetics. 2010;185:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Alexander S, Min J, Alexander H. Dictyostelium discoideum to human cells: pharmacogenetic studies demonstrate a role for sphingolipids in chemoresistance. Biochim Biophys Acta. 2006;1760:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Eichinger L, Pachebat JA, Glöckner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1000] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 84. | Li G, Alexander H, Schneider N, Alexander S. Molecular basis for resistance to the anticancer drug cisplatin in Dictyostelium. Microbiology. 2000;146:2219-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Bourquin F, Riezman H, Capitani G, Grütter MG. Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism. Structure. 2010;18:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1234] [Article Influence: 41.1] [Reference Citation Analysis (3)] |
| 87. | Van Brocklyn JR. Sphingolipid signaling pathways as potential therapeutic targets in gliomas. Mini Rev Med Chem. 2007;7:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Merrill AH. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843-25846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 478] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 89. | Pyne S. Cellular signaling by sphingosine and sphingosine 1-phosphate. Their opposing roles in apoptosis. Subcell Biochem. 2002;36:245-268. [PubMed] [DOI] [Full Text] |
| 90. | Min J, Traynor D, Stegner AL, Zhang L, Hanigan MH, Alexander H, Alexander S. Sphingosine kinase regulates the sensitivity of Dictyostelium discoideum cells to the anticancer drug cisplatin. Eukaryot Cell. 2005;4:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Alexander S, Alexander H. Lead genetic studies in Dictyostelium discoideum and translational studies in human cells demonstrate that sphingolipids are key regulators of sensitivity to cisplatin and other anticancer drugs. Semin Cell Dev Biol. 2011;22:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892-12898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 93. | Min J, Van Veldhoven PP, Zhang L, Hanigan MH, Alexander H, Alexander S. Sphingosine-1-phosphate lyase regulates sensitivity of human cells to select chemotherapy drugs in a p38-dependent manner. Mol Cancer Res. 2005;3:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 95. | Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330-40336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 297] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 96. | Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118-37129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 512] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 97. | Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62:347-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 98. | Gouazé V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861-3867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Gouazé V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633-639. [PubMed] |
| 100. | Kiguchi K, Iwamori Y, Suzuki N, Kobayashi Y, Ishizuka B, Ishiwata I, Kita T, Kikuchi Y, Iwamori M. Characteristic expression of globotriaosyl ceramide in human ovarian carcinoma-derived cells with anticancer drug resistance. Cancer Sci. 2006;97:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem. 1996;271:19530-19536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 261] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, Cabot MC. Glucosylceramide: a marker for multiple-drug resistant cancers. Anticancer Res. 1998;18:475-480. [PubMed] |
| 103. | Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2612] [Cited by in RCA: 2598] [Article Influence: 78.7] [Reference Citation Analysis (3)] |
| 104. | Chin KV, Ueda K, Pastan I, Gottesman MM. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992;255:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 569] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 105. | Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 106. | Skinner DB. The columnar-lined esophagus and adenocarcinoma. Ann Thorac Surg. 1985;40:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 107. | Norris-Cervetto E, Callaghan R, Platt FM, Dwek RA, Butters TD. Inhibition of glucosylceramide synthase does not reverse drug resistance in cancer cells. J Biol Chem. 2004;279:40412-40418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | De Rosa MF, Sillence D, Ackerley C, Lingwood C. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J Biol Chem. 2004;279:7867-7876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fritzsching B, Okada M S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ