Published online Dec 12, 2014. doi: 10.5528/wjtm.v3.i3.141
Revised: September 25, 2014
Accepted: October 31, 2014
Published online: December 12, 2014
Processing time: 139 Days and 12.3 Hours
The pharmacological interventions currently available to control type 2 diabetes mellitus (T2DM) show a wide interindividual variability in drug response, emphasizing the importance of a personalized, more effective medical treatment for each individual patient. In this context, a growing interest has emerged in recent years and has focused on pharmacogenetics, a discipline aimed at understanding the variability in patients’ drug response, making it possible to predict which drug is best for each patient and at what doses. Recent pharmacological and clinical evidences indicate that genetic polymorphisms (or genetic variations) of certain genes can adversely affect drug response and therapeutic efficacy of oral hypoglycemic agents in patients with T2DM, through pharmacokinetic- and/or pharmacodynamic-based mechanisms that may reduce the therapeutic effects or increase toxicity. For example, genetic variants in genes encoding enzymes of the cytochrome P-450 superfamily, or proteins of the ATP-sensitive potassium channel on the beta-cell of the pancreas, are responsible for the interindividual variability of drug response to sulfonylureas in patients with T2DM. Instead, genetic variants in the genes that encode for the organic cation transporters of metformin have been related to changes in both pharmacodynamic and pharmacokinetic responses to metformin in metformin-treated patients. Thus, based on the individual’s genotype, the possibility, in these subjects, of a personalized therapy constitutes the main goal of pharmacogenetics, directly leading to the development of the right medicine for the right patient. Undoubtedly, this represents an integral part of the translational medicine network.
Core tip: Type 2 diabetes mellitus (T2DM) is a heterogeneous complex disorder, in which predisposing genetic variants (polymorphisms) and precipitating environmental factors interact synergistically in the development of the disease. Besides being useful in identifying individuals at risk for T2DM, knowledge of the polymorphisms associated with T2DM is also useful in pharmacogenetics for correlating individual variants with individual responses to anti-diabetic drugs. To date, a wide variety of genes that influence pharmacogenetics of anti-diabetic drugs have been identified. However, with few exceptions, drug therapy has not taken into account the individual genetic diversity of treated patients, representing, this, a substantial limitation of pharmacogenetics. This review focuses on clinically important polymorphisms affecting a patient’s response to diabetic medications.
- Citation: Brunetti A, Brunetti FS, Chiefari E. Pharmacogenetics of type 2 diabetes mellitus: An example of success in clinical and translational medicine. World J Transl Med 2014; 3(3): 141-149
- URL: https://www.wjgnet.com/2220-6132/full/v3/i3/141.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v3.i3.141
The common observation that patients with type 2 diabetes mellitus (T2DM) show a great variability in the individual response to the same drug treatment suggests the importance of a personalized care approach, in which the most appropriate treatment is indicated by the genetic peculiarities of each individual[1]. The introduction, in 2007, of genome-wide association study (GWAS) has greatly enhanced the number of genes that are known to be associated with common diseases. Applied to millions of people, this method has allowed the identification of several genetic variants which are associated with T2DM[2]. However, similarly to other complex diseases, none of the individual variants identified so far is in itself sufficient to cause the disease, but most of the genetic risk for T2DM is mediated by the combined influence of more genetic variants that individually have only a small degree of risk[3,4]. This combination (haplotype) defines the genetic profile of the individual. The fact that the pathogenesis of T2DM requires the involvement of multiple genes in different combination is in line with the assumption that T2DM, far from being a disease genetically identifiable in a few specific forms, actually consists of a large number of rather different disorders[3,4], each of which is associated with a specific disease phenotype only apparently identical to one another, and in which inter-individual variability in drug response can be identified both in terms of drug efficacy and undesired drug reactions.
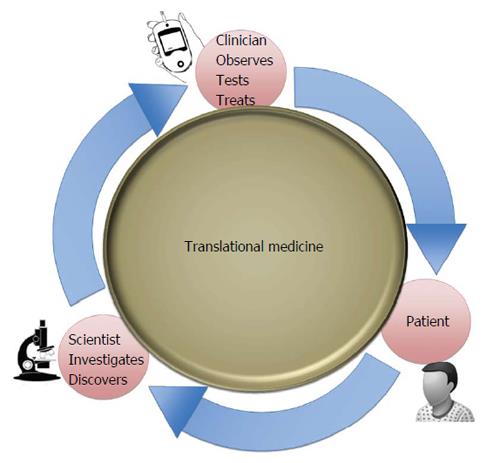
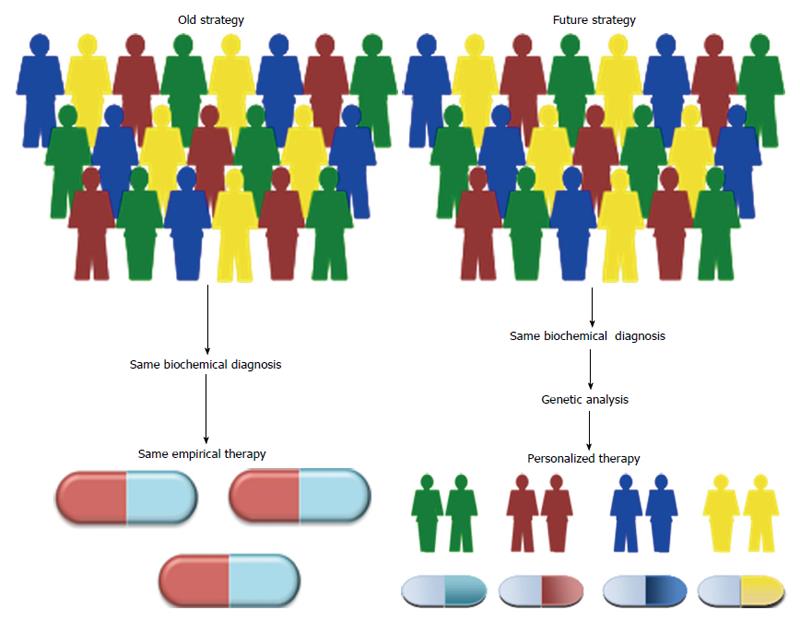
Therefore, clarifying the molecular mechanisms by which genetic variations may cause differences in phenotypic traits and in individual drug response is essential not only to determine the etiological role of gene variants, but also to identify new personalized medical solutions. Personalized therapy, based on the genetic diversity of each individual, is one of the most fascinating challenges of modern medicine, representing an integral part of the translational medicine effort, whose ultimate goal is to translate advances in biomedical research into new medical treatments and improvements in patient care (Figure 1). Herein, we provide an overview of this area and its relevance to clinical practice in T2DM.
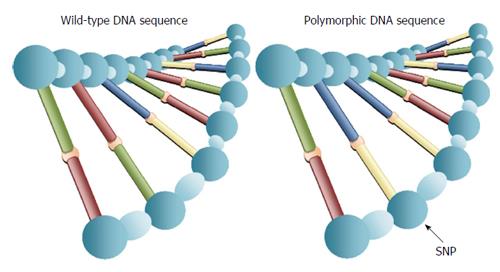
Pharmacogenetics is defined as the influence of variations in DNA sequence on drug response (http://www.ema.europa.eu). Its relevance arises from the clinical observation that patients suffering from the same disease do not necessarily respond to the same drug treatment in terms of therapeutic efficacy as well as adverse effects. The principal aim of pharmacogenetics is to provide personalized medicine, tailored to an individual’s genetic makeup, in order to optimize the effectiveness and safety of drug treatment. Although elements of pharmacogenetics can be traced back to ancient Greece (510 years BC), when it was already known the risk of hemolytic anemia in certain individuals in response to the ingestion of uncooked fava beans[5], the term “pharmacogenetics” was first coined by Vogel[6] in 1959 to indicate the importance of genetic polymorphisms on the disposition and action of drugs. The first evidence on the role of genetic variants in drug response back to the ‘70s and refers to cytochrome P-450 2D6 (CYP2D6), an enzyme of the hepatic P-450 microsomal enzyme system, which is involved in the metabolism of numerous drugs. Studies of the genetic variations within the P-450 family of enzymes provided the first direct evidence for the genetic contributions to drug therapy and efficacy, and these studies continue to be an active part of the basic and clinical research performed today. In fact, numerous other genetic variations have been identified in subsequent years, within the P-450 family of enzymes, including the biotransformation enzymes CYP3A4/5 and the CYP2C9 enzyme. It has been shown that individuals carrying genetic variants of CYP2D6 (and other P-450 isoforms resulting in poor enzymatic activity), who are concomitantly taking medications that are influenced by these enzymes, are at risk for increased or prolonged drug effect, influencing the speed and effectiveness of drug metabolism[7]. However, there is no doubt that the greatest contribution to pharmacogenetics has come from the sequencing of the entire human genome in 2003, showing that over 99% of DNA is identical in all humans and that, therefore, phenotypic differences among individuals, as well as differences in disease susceptibility and the inter-individual variability in drug response, are the result of sequence polymorphisms that affect less than 1% of 3 billion bases of human DNA. In most cases, these variants consist of the exchange of single nucleotides in both coding and noncoding DNA regions and are defined as single nucleotide polymorphisms (SNPs) (Figure 2). The ability of the SNP to influence drug response and therapeutic efficacy may rely on the capacity of the variant to induce changes in the expression of proteins that may influence either the pharmacokinetic and/or pharmacodynamic profile and hence the clinical efficacy of the drug. On the basis of these acquisitions, recent GWAS have identified several SNPs that can affect both the therapeutic efficacy and the occurrence of adverse reactions after drug intake[8-10].
In Caucasians, sulfonylureas are metabolized primarily in the liver by CYP2C9 to active metabolites, which are ultimately excreted by the kidney[11]. In previous work, it was demonstrated that polymorphisms of the CYP2C9 gene significantly affect the pharmacological response of diabetic patients to sulfonylureas[12], due to the reduction of the catalytic activity in the metabolism of these drugs[13-16], with a consequent increase in drug bioavailability. In particular, in certain diabetic patients with the variants Ile359Leu (isoleucine changes to leucine in exon 7 position 359) and Arg144Cys (arginine changes to cysteine in exon 3 position 144) in the CYP2C9 gene, the clearance of glibenclamide was reduced by 30%-80%, allowing the use of lower doses of this drug to limit the risk of hypoglycemia[12,17-20]. The risk of hypoglycemia in sulphonylurea treated patients was confirmed in a study with a larger population, in which the simultaneous presence (or the presence in homozygosity) of the variants Ile359Leu and Arg144Cys in the CYP2C9 gene was associated with the improvement in markers of glycemic control, including glycated hemoglobin A1c (HbA1c)[21]. Therefore, genotyping of the CYP2C9 gene may provide important additional information in predicting the adverse effects of these drugs and to assist physicians in prescribing oral hypoglycemic agents.
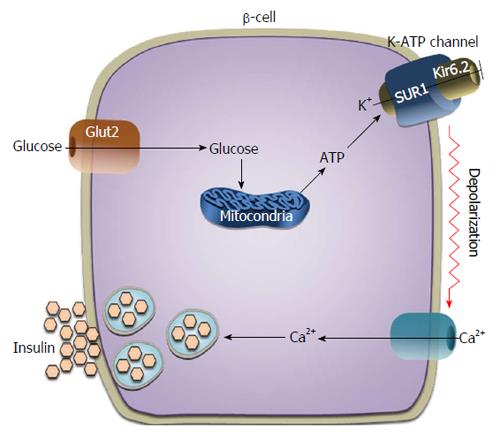
The ATP-sensitive potassium [ATP-sensitive K+ (K-ATP)] channel plays a central role in mediating glucose-stimulated insulin release from pancreatic beta-cells (Figure 3). In physiological conditions, the rapid entry of glucose into the beta-cell results in an increase in the intracellular concentration of ATP, which promotes the closure of the K-ATP channel with consequent opening of the voltage-dependent calcium channel, elevation of intracellular calcium ion concentration and insulin secretion. The K-ATP channel is composed of two subunits: the sulphonylurea receptor (SUR1) and the pore-forming inward rectifier K+ channel Kir6.2[22,23]. Genetic variants inactivating the KCNJ11 (potassium inwardly-rectifying channel, subfamily J, member 11) gene, which encodes for the protein Kir6.2, and the ATP-binding cassette, sub-family C (CFTR/MRP), member 8 (ABCC8) gene, which encodes the SUR1 protein, are responsible for neonatal diabetes mellitus; conversely, activating mutations of these two genes lead to hyperinsulinism and neonatal hypoglycemia[24]. As an example of pharmacogenetics with important clinical implications, recent studies have found that diabetic patients carrying mutations in the KCNJ11 gene respond better to treatment with sulfonylureas than to treatment with insulin[25-27].
Association of the polymorphism Ser1369Ala (serine 1369 to alanine substitution) in ABCC8 with the antidiabetic efficacy of gliclazide was found in patients with T2DM, after two months of treatment[28]. In particular, patients with the genotype alanine/alanine had a greater reduction in either fasting plasma glucose or 2 h postload plasma glucose during oral glucose tolerance test, and a greater decrease in HbA1c levels compared to patients with the Serine/Serine genotype[28]. The variant Ser1369Ala in ABCC8 is often associated in linkage disequilibrium with a variant, Glu23Lys (glutamine to lysine variant at position 23), in the KCNJ11 gene, forming a haplotype that increases the risk of developing T2DM[29]. It has been observed that this haplotype displays large differences to the therapeutic effects of various sulfonylureas: greater to gliclazide, less apparent to tolbutamide, chlorpropamide and glimepiride, invariable in the glipizide and glibenclamide treatment group[30].
Interesting results, in this context, have been obtained from the study of the transcription factor 7-like 2 (TCF7L2) gene, which encodes a nuclear transcription factor that appears to play a role in beta-cell function. Genetic variants of TCF7L2 are associated with increased risk of T2DM[3]. Recently, two variants of the TCF7L2 gene, rs7903146 (G > T), and rs7903146 (C > T), have been shown to influence the therapeutic efficacy of sulfonylureas[31-33]. In particular, the reduction in both HbA1c and fasting plasma glucose was higher in diabetic patients carrying either GG or CC genotypes[31-33]. In contrast, diabetic patients with the TT genotype in both the rs7903146 (G > T) and the rs7903146 (C > T) variants showed a lower response to sulfonylureas and appeared to be more prone to therapeutic failure[31-33].
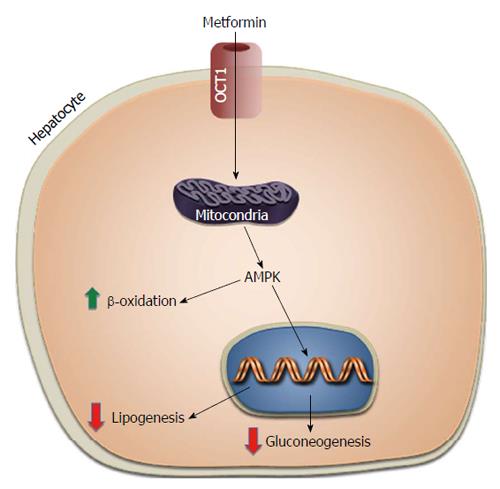
Metformin, in use for control of diabetes since 1950s, is the first-line pharmacological therapy for T2DM. After oral administration, the drug is absorbed into the blood via the gastrointestinal tract, rapidly distributed in body tissues by travelling through specific transport proteins [including the organic cation transporters 1 (OCT1) and OCT2, the multidrug and toxin extrusion 1 (MATE1) transporters and MATE2-K, and the plasma membrane monoamine transporter (PMAT)] located on the cytoplasmic membrane of many cells, especially intestinal cells, liver cells and kidney cells[34], and excreted in the urine almost unchanged from the original drug. The individual’s response to metformin is highly variable with less than 2/3 of treated patients achieving glycemic control[35]. Thus, identification of genetic variants that may influence the interindividual variability to metformin would be of major importance for the effective treatment of these patients. However, studies on the pharmacogenetics of metformin are relatively limited, mainly because its mechanism of action is still poorly defined. So far, most of the studies on this topic have involved the solute carrier family 22A1 (SLC22A1) gene, which by coding for the OCT1 transport protein, plays a key role in the cell absorption of the drug[36], and is essential for the anti-gluconeogenic effect of metformin into the liver[37] (Figure 4). It has been shown that polymorphisms of this gene (rs12208357; rs34130495; rs72552763; rs34059508), by reducing the functional capacity of OCT1, can alter the bioavailability of metformin and mitigate its hypoglycemic response in healthy people carrying these gene variants[37-39]. Recently, two polymorphisms of SLC22A1 (rs628031 and rs36056065) have been associated with gastrointestinal side effects in diabetic patients treated with metformin[40]. At the same time, other authors[41,42] have also reported that the bioavailability of metformin was increased in healthy individuals carrying mutations of the SLC22A2 gene, which encodes for the OCT2 transport protein. Variants of this gene, by adversely affecting OCT2 function, may decrease the renal clearance of metformin, and may contribute to increased plasma metformin levels with increased risk of hypoglycemic events.
Interindividual variation in metformin response has been recently reported in subjects with genetic variations in SLC47A1 and SLC47A2 genes coding for MATE1 and MATE2-K, respectively, which play important roles in the urine excretion of metformin. A better glycemic response to metformin, with lower HbA1c levels, has been reported in association with the SLC47A1 gene variant rs2252281[43-46]. In contrast, the therapeutic response to metformin was reduced in diabetic patients carriers of the variant rs12943590 in the SLC47A2 gene[45,46]. Therefore, these observations imply that genetic variants of MATE1 and MATE2-K are important determinants of the therapeutic efficacy of metformin in patients treated with this drug. The first GWAS on the efficacy of metformin on glycemic control in diabetic patients resulted in the demonstration that a gene variant near ataxia telangiectasia mutated (ATM), rs11212617, is significantly associated with metformin treatment response in T2DM, more frequently with HbA1c levels < 7%[47]. The explanation of this phenomenon lies in the role ATM, the protein product of the ATM gene, plays in the context of insulin signaling and insulin action[48].
Thus, genetic variants of SLC22A1 and SLC22A2 may be determinant in the therapeutic efficacy of metformin. Furthermore, genotyping of SLC22A1 and SLC22A2 is useful in the management of diabetic patients under metformin theraphy.
Genetic variants that can influence the pharmacogenetics of oral antidiabetic medications were also assessed in diabetic patients treated with pharmacogenetics of thiazolidinediones (TZDs) (pioglitazone and rosiglitazone). As agonists of peroxisome proliferator-activated receptor gamma (PPAR-γ), TZDs act as insulin-sensitizing, thus reducing the release of glucose from the liver and increasing glucose uptake in muscle[49]. The PPAR-γ gene has been extensively investigated in pharmacogenetic studies of TZDs, especially because genetic variants of this gene have been associated with an increased risk of T2DM[3]. However, pharmacogenetic studies with TZDs have shown conflicting results, probably due to insufficient sample size and low levels of statistical power[50]. Furthermore, it is worthy noting that the retrospective study design used in the majority of studies on pharmacogenetics has its own drawbacks, being able to expose to a variety of confounding and bias, including age, gender, ethnicity, lifestyle, concomitant use of other medications, etc. A similar discrepancy has emerged from studies on the genetic variants of the CYP2C8 gene, which is responsible for metabolizing pioglitazone[50]. A reduction in the blood glucose-lowering effect of pioglitazone was recently observed in diabetic patients carriers of the truncation variant, Ser447X, of the lipoprotein lipase gene[51]. Another study has reported that the -420 C/G variant of the resistin gene promoter can also be used as an independent predictor of the reduction of fasting plasma glucose and insulin resistance by pioglitazone in T2DM[52]. As it is known, side effects of TZDs therapy include fluid retention and peripheral edema, worsening heart failure[53]. In this context, various genetic variations have been discovered in genes known to be involved in sodium and water reabsorption. Among these, the aquaporin 2 (AQP2) rs296766 variant and the SLC12A1 rs12904216 variant, both of which have been associated with edema in T2DM patients treated with a TZD[54]. AQP2 gene codes aquaporin-2, which function as a water channel in the collecting duct of the kidney[55]. SLC12A1 encodes the kidney-specific sodium-potassium-chloride cotransporter (NKCC2), which plays an important role in both urine concentration and NaCl reabsorption[54,56]. Therefore, it is quite evident that these variants may represent both a risk factor for the development of edema in diabetic patients during treatment with TZDs.
Metiglinides (repaglinide and nateglinide) are a class of rapid-acting, short duration insulin secretagogues that act in a manner similar to that of the sulfonylureas[57]. Nateglinide is also metabolized by the CYP2C9 enzyme of the cytochrome P-450 system, and gene variants of CYP2C9 are associated with variability in glucose-lowering effect of nateglinide[58]. Repaglinide is metabolized by CYP2C8 and to a lesser degree by CYP3A4[59]. Also in this case, gene variants of CYP2C8 have been associated with increased clearance of repaglinide, although with contradictory results[60]. The solute carrier organic anion transporter family member 1B1 (SLCO1B1) gene encodes for the organic anion transporting polypeptide, OATP1B1, which regulates cellular uptake of various drugs, including statins by the liver. Recent studies have reported the role of some variants of SLCO1B1 in the pharmacokinetics of metiglinides[61-64]. For example, a more effective hypoglycemic effect of repaglinide was observed in diabetic patients carrying the Glu23Lys (E23K) polymorphism in the KCNJ11 gene[65], and the rs13266634 variant in the SLC30A8 gene[66]. Similarly, polymorphisms of neurogenic differentiation 1 (NEUROD1), also called beta2 (NEUROD1/BETA2), paired box gene 4[67] and uptake control 2[68] genes were also found to be associated with the hypoglycemic efficacy of repaglinide. An association of the variant G2677 T/A in the multidrug resistance gene, which encodes a multidrug efflux pump, with the variability in the pharmacokinetics of repaglinide was found recently in a Chinese study in healthy volunteers[69].
Glucagon-like peptide-1 (GLP-1) is part of the group of incretin hormones that are secreted from endocrine cells in the intestinal mucosa in response to meals. It mediates insulin secretion in a glucose-dependent manner and is easily inactivated after being secreted by the enzyme dipeptidyl peptidase-IV (DPP-IV). Recent pharmacological research has led to the development and synthesis of medications that are capable of acting at this level as both GLP-1 agonists (exenatide and liraglutide) and DPP-IV inhibitors (gliptins)[70]. Variants of the GLP-1 receptor gene have been shown to be associated with altered sensitivity to GLP-1[71]. Furthermore, whereas variants in the TCF7L2 (rs7903146) and wolfram syndrome 1 (rs10010131) genes have been associated with a reduced response to exogenous GLP-1, variations in the KCNQ1 (rs151290, rs2237892, and rs2237895) gene appear to alter the secretion of endogenous GLP-1[72]. The only significant study on the pharmacogenetics of gliptins showed that three novel genetic loci (transmembrane protein 114, carbohydrate sulfotransferase 3 and Chymotrypsinogen B1/2) were identified, which affect GLP-1-induced insulin release during hyperglycemic clamp in nondiabetic Caucasian subjects[73].
Pharmacogenetics is an expanding area of research which seeks to understand how variations in the genome influence medication response. Pharmacogenetics has gained increasing attention in the context of translational medicine, providing an opportunity for personalized treatment strategies based on an individual’s genetic makeup. The results obtained so far with the study of genetic variants in patients with T2DM (and other common diseases) may be used for the realization of a pharmacogenetic test, which can assist in making treatment decisions on the basis of each patient’s genetic profile, thus improving the overall management of the disease and ensuring better results in terms of safety and therapeutic efficacy. The clinical use of pharmacogenetics, through the identification of individual genetic variants (genetic polymorphisms), can contribute to move to a more evidence-based and less empiric clinical management of patients, thereby avoiding treatment failures, while reducing the incidence of adverse drug reactions (Figure 5).
| 1. | Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1175] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 2. | McCarthy MI, Zeggini E. Genome-wide association studies in type 2 diabetes. Curr Diab Rep. 2009;9:164-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Brunetti A, Chiefari E, Foti D. Recent advances in the molecular genetics of type 2 diabetes mellitus. World J Diabetes. 2014;5:128-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 4. | Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8:186-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 2004;25:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 424] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Vogel F. Moderne Probleme der Humangenetik. Ergeb Inn Med Kinderheilkd. 1959;12:52-125. [DOI] [Full Text] |
| 7. | Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977;2:584-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 764] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Crowley JJ, Sullivan PF, McLeod HL. Pharmacogenomic genome-wide association studies: lessons learned thus far. Pharmacogenomics. 2009;10:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Daly AK. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Motsinger-Reif AA, Jorgenson E, Relling MV, Kroetz DL, Weinshilboum R, Cox NJ, Roden DM. Genome-wide association studies in pharmacogenomics: successes and lessons. Pharmacogenet Genomics. 2013;23:383-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Kirchheiner J, Bauer S, Meineke I, Rohde W, Prang V, Meisel C, Roots I, Brockmöller J. Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and the insulin and glucose response in healthy volunteers. Pharmacogenetics. 2002;12:101-109. [PubMed] |
| 12. | Becker ML, Visser LE, Trienekens PH, Hofman A, van Schaik RH, Stricker BH. Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther. 2008;83:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Kirchheiner J, Brockmöller J, Meineke I, Bauer S, Rohde W, Meisel C, Roots I. Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther. 2002;71:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Elliot DJ, Suharjono BC, Gillam EM, Birkett DJ, Gross AS, Miners JO. Identification of the human cytochromes P450 catalysing the rate-limiting pathways of gliclazide elimination. Br J Clin Pharmacol. 2007;64:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Kidd RS, Curry TB, Gallagher S, Edeki T, Blaisdell J, Goldstein JA. Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics. 2001;11:803-808. [PubMed] |
| 16. | Wang R, Chen K, Wen SY, Li J, Wang SQ. Pharmacokinetics of glimepiride and cytochrome P450 2C9 genetic polymorphisms. Clin Pharmacol Ther. 2005;78:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Ragia G, Petridis I, Tavridou A, Christakidis D, Manolopoulos VG. Presence of CYP2C9*3 allele increases risk for hypoglycemia in Type 2 diabetic patients treated with sulfonylureas. Pharmacogenomics. 2009;10:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Holstein A, Plaschke A, Ptak M, Egberts EH, El-Din J, Brockmöller J, Kirchheiner J. Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol. 2005;60:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Bozkurt O, de Boer A, Grobbee DE, Heerdink ER, Burger H, Klungel OH. Pharmacogenetics of glucose-lowering drug treatment: a systematic review. Mol Diagn Ther. 2007;11:291-302. [PubMed] |
| 20. | Distefano JK, Watanabe RM. Pharmacogenetics of Anti-Diabetes Drugs. Pharmaceuticals (Basel). 2010;3:2610-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Zhou K, Donnelly L, Burch L, Tavendale R, Doney AS, Leese G, Hattersley AT, McCarthy MI, Morris AD, Lang CC. Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther. 2010;87:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 378] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Winkler M, Stephan D, Bieger S, Kühner P, Wolff F, Quast U. Testing the bipartite model of the sulfonylurea receptor binding site: binding of A-, B-, and A + B-site ligands. J Pharmacol Exp Ther. 2007;322:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Flanagan SE, Clauin S, Bellanné-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 685] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 26. | Siklar Z, Ellard S, Okulu E, Berberoğlu M, Young E, Savaş Erdeve S, Mungan IA, Hacihamdioğlu B, Erdeve O, Arsan S. Transient neonatal diabetes with two novel mutations in the KCNJ11 gene and response to sulfonylurea treatment in a preterm infant. J Pediatr Endocrinol Metab. 2011;24:1077-1080. [PubMed] |
| 27. | Dupont J, Pereira C, Medeira A, Duarte R, Ellard S, Sampaio L. Permanent neonatal diabetes mellitus due to KCNJ11 mutation in a Portuguese family: transition from insulin to oral sulfonylureas. J Pediatr Endocrinol Metab. 2012;25:367-370. [PubMed] |
| 28. | Feng Y, Mao G, Ren X, Xing H, Tang G, Li Q, Li X, Sun L, Yang J, Ma W. Ser1369Ala variant in sulfonylurea receptor gene ABCC8 is associated with antidiabetic efficacy of gliclazide in Chinese type 2 diabetic patients. Diabetes Care. 2008;31:1939-1944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Fatehi M, Raja M, Carter C, Soliman D, Holt A, Light PE. The ATP-sensitive K(+) channel ABCC8 S1369A type 2 diabetes risk variant increases MgATPase activity. Diabetes. 2012;61:241-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Lang VY, Fatehi M, Light PE. Pharmacogenomic analysis of ATP-sensitive potassium channels coexpressing the common type 2 diabetes risk variants E23K and S1369A. Pharmacogenet Genomics. 2012;22:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Schroner Z, Javorsky M, Tkacova R, Klimcakova L, Dobrikova M, Habalova V, Kozarova M, Zidzik J, Rudikova M, Tkac I. Effect of sulphonylurea treatment on glycaemic control is related to TCF7L2 genotype in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Pearson ER, Donnelly LA, Kimber C, Whitley A, Doney AS, McCarthy MI, Hattersley AT, Morris AD, Palmer CN. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes. 2007;56:2178-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Holstein A, Hahn M, Körner A, Stumvoll M, Kovacs P. TCF7L2 and therapeutic response to sulfonylureas in patients with type 2 diabetes. BMC Med Genet. 2011;12:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122:253-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1252] [Article Influence: 89.4] [Reference Citation Analysis (1)] |
| 35. | Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2133] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 36. | Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 346] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 710] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 38. | Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 39. | Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, Brosen K. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 40. | Tarasova L, Kalnina I, Geldnere K, Bumbure A, Ritenberga R, Nikitina-Zake L, Fridmanis D, Vaivade I, Pirags V, Klovins J. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients. Pharmacogenet Genomics. 2012;22:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, Shin JG. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008;84:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 43. | Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 44. | Jablonski KA, McAteer JB, de Bakker PI, Franks PW, Pollin TI, Hanson RL, Saxena R, Fowler S, Shuldiner AR, Knowler WC, Altshuler D, Florez JC, Diabetes Prevention Program Research Group. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes. 2010;59:2672-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 45. | Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, Mefford JA, Hesselson SE, Schlessinger A, Jenkins G. A common 5’-UTR variant in MATE2-K is associated with poor response to metformin. Clin Pharmacol Ther. 2011;90:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, Ramirez AH, Roden DM, Wilke RA, McCarty CA. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2013;93:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Zhou K, Bellenguez C, Spencer CC, Bennett AJ, Coleman RL, Tavendale R, Hawley SA, Donnelly LA, Schofield C, Groves CJ, Burch L, Carr F, Strange A, Freeman C, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Dronov S, Duncanson A, Edkins S, Gray E, Hunt S, Jankowski J, Langford C, Markus HS, Mathew CG, Plomin R, Rautanen A, Sawcer SJ, Samani NJ, Trembath R, Viswanathan AC, Wood NW, Harries LW, Hattersley AT, Doney AS, Colhoun H, Morris AD, Sutherland C, Hardie DG, Peltonen L, McCarthy MI, Holman RR, Palmer CN, Donnelly P, Pearson ER; The GoDARTS and UKPDS Diabetes Pharmacogenetics Study Group, The Wellcome Trust Case Control Consortium. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43:117-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 48. | Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 211] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Chen L, Yang G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 2014;2014:653017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Becker ML, Pearson ER, Tkáč I. Pharmacogenetics of oral antidiabetic drugs. Int J Endocrinol. 2013;2013:686315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Wang G, Wang X, Zhang Q, Ma Z. Response to pioglitazone treatment is associated with the lipoprotein lipase S447X variant in subjects with type 2 diabetes mellitus. Int J Clin Pract. 2007;61:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Makino H, Shimizu I, Murao S, Kondo S, Tabara Y, Fujiyama M, Fujii Y, Takada Y, Nakai K, Izumi K. A pilot study suggests that the G/G genotype of resistin single nucleotide polymorphism at -420 may be an independent predictor of a reduction in fasting plasma glucose and insulin resistance by pioglitazone in type 2 diabetes. Endocr J. 2009;56:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Karalliedde J, Buckingham RE. Thiazolidinediones and their fluid-related adverse effects: facts, fiction and putative management strategies. Drug Saf. 2007;30:741-753. [PubMed] |
| 54. | Chang TJ, Liu PH, Liang YC, Chang YC, Jiang YD, Li HY, Lo MT, Chen HS, Chuang LM. Genetic predisposition and nongenetic risk factors of thiazolidinedione-related edema in patients with type 2 diabetes. Pharmacogenet Genomics. 2011;21:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Knepper MA, Wade JB, Terris J, Ecelbarger CA, Marples D, Mandon B, Chou CL, Kishore BK, Nielsen S. Renal aquaporins. Kidney Int. 1996;49:1712-1717. [PubMed] |
| 56. | Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 670] [Cited by in RCA: 607] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 57. | Yan FF, Casey J, Shyng SL. Sulfonylureas correct trafficking defects of disease-causing ATP-sensitive potassium channels by binding to the channel complex. J Biol Chem. 2006;281:33403-33413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Kirchheiner J, Roots I, Goldammer M, Rosenkranz B, Brockmöller J. Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet. 2005;44:1209-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003;56:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Tomalik-Scharte D, Fuhr U, Hellmich M, Frank D, Doroshyenko O, Jetter A, Stingl JC. Effect of the CYP2C8 genotype on the pharmacokinetics and pharmacodynamics of repaglinide. Drug Metab Dispos. 2011;39:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. The effect of SLCO1B1 polymorphism on repaglinide pharmacokinetics persists over a wide dose range. Br J Clin Pharmacol. 2008;66:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Zhang W, He YJ, Han CT, Liu ZQ, Li Q, Fan L, Tan ZR, Zhang WX, Yu BN, Wang D. Effect of SLCO1B1 genetic polymorphism on the pharmacokinetics of nateglinide. Br J Clin Pharmacol. 2006;62:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. J Clin Pharmacol. 2008;48:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Kalliokoski A, Backman JT, Neuvonen PJ, Niemi M. Effects of the SLCO1B1*1B haplotype on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. Pharmacogenet Genomics. 2008;18:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | He YY, Zhang R, Shao XY, Hu C, Wang CR, Lu JX, Bao YQ, Jia WP, Xiang KS. Association of KCNJ11 and ABCC8 genetic polymorphisms with response to repaglinide in Chinese diabetic patients. Acta Pharmacol Sin. 2008;29:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Huang Q, Yin JY, Dai XP, Wu J, Chen X, Deng CS, Yu M, Gong ZC, Zhou HH, Liu ZQ. Association analysis of SLC30A8 rs13266634 and rs16889462 polymorphisms with type 2 diabetes mellitus and repaglinide response in Chinese patients. Eur J Clin Pharmacol. 2010;66:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Gong ZC, Huang Q, Dai XP, Lei GH, Lu HB, Yin JY, Xu XJ, Qu J, Pei Q, Dong M. NeuroD1 A45T and PAX4 R121W polymorphisms are associated with plasma glucose level of repaglinide monotherapy in Chinese patients with type 2 diabetes. Br J Clin Pharmacol. 2012;74:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Wang S, Se YM, Liu ZQ, Lei MX, Hao-BoYang ZX, Nie SD, Zeng XM, Wu J. Effect of genetic polymorphism of UCP2-866 G/A on repaglinide response in Chinese patients with type 2 diabetes. Pharmazie. 2012;67:74-79. [PubMed] |
| 69. | Xiang Q, Cui YM, Zhao X, Yan L, Zhou Y. The Influence of MDR1 G2677T/a genetic polymorphisms on the pharmacokinetics of repaglinide in healthy Chinese volunteers. Pharmacology. 2012;89:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Umpierrez GE, Meneghini L. Reshaping diabetes care: the fundamental role of dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists in clinical practice. Endocr Pract. 2013;19:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Sathananthan A, Man CD, Micheletto F, Zinsmeister AR, Camilleri M, Giesler PD, Laugen JM, Toffolo G, Rizza RA, Cobelli C. Common genetic variation in GLP1R and insulin secretion in response to exogenous GLP-1 in nondiabetic subjects: a pilot study. Diabetes Care. 2010;33:2074-2076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Smushkin G, Sathananthan M, Sathananthan A, Dalla Man C, Micheletto F, Zinsmeister AR, Cobelli C, Vella A. Diabetes-associated common genetic variation and its association with GLP-1 concentrations and response to exogenous GLP-1. Diabetes. 2012;61:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | ‘t Hart LM, Fritsche A, Nijpels G, van Leeuwen N, Donnelly LA, Dekker JM, Alssema M, Fadista J, Carlotti F, Gjesing AP. The CTRB1/2 locus affects diabetes susceptibility and treatment via the incretin pathway. Diabetes. 2013;62:3275-3281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
P- Reviewer: Fang Y, Guarneri F, Jia JH S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ