Published online Jun 26, 2022. doi: 10.5528/wjtm.v10.i1.1
Peer-review started: January 12, 2022
First decision: February 8, 2022
Revised: March 5, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: June 26, 2022
Processing time: 163 Days and 0.9 Hours
Diabetes has been one of the major concerns in recent years, due to the increasing rate of morbidity and mortality worldwide. The available treatment strategies for uncontrolled diabetes mellitus (DM) are pancreas or islet transplantation. However, these strategies are limited due to unavailability of quality pancreas/ islet donors, life-long need of immunosuppression, and associated complications. Cell therapy has emerged as a promising alternative options to achieve the clinical benefits in the management of uncontrolled DM. Since the last few years, various sources of cells have been used to convert into insulin-producing β-like cells. These extrapancreatic sources of cells may play a significant role in β-cell turnover and insulin secretion in response to environmental stimuli. Stem/progenitor cells from liver have been proposed as an alternative choice that respond well to glucose stimuli under strong transcriptional control. The liver is one of the largest organs in the human body and has a common endodermal origin with pancreatic lineages. Hence, liver has been proposed as a source of a large number of insulin-producing cells. The merging of nanotechnology and 3D tissue bioengineering has opened a new direction for producing islet-like cells suitable for in vivo transplantation in a cordial microenvironment. This review summarizes extrapancreatic sources for insulin-secreting cells with reference to emerging technologies to fulfill the future clinical need.
Core tip: The currently accepted available treatment strategies for uncontrolled diabetes mellitus (DM) are pancreas and islet transplantation. However, these strategies are limited due to unavailability of quality pancreas/islet donors, life-long need of immunosuppression, and associated complications. Exogenous insulin administration is one of the clinically established options for patients with type 1 DM. Liver could be an appropriate extrapancreatic source to isolate insulin-producing cells due to their similar embryonic developmental origin. The need to develop more effective diabetes cell-based therapies lies in the advancements of robust sensitive micro- and nanodevices for exogenous insulin delivery.
- Citation: Habeeb MA, Vishwakarma SK, Habeeb S, Khan AA. Current progress and emerging technologies for generating extrapancreatic functional insulin-producing cells. World J Transl Med 2022; 10(1): 1-13
- URL: https://www.wjgnet.com/2220-6132/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v10.i1.1
Currently diabetes mellitus (DM) and associated complications have been a major concern due to the continuous increasing rate of morbidity and mortality worldwide. DM is caused by insufficient insulin production due to autoimmune destruction of functional β-cells [insulin-dependent type 1 DM (T1DM)] and insulin resistance [non-insulin-dependent type 2 DM (T2DM)] and subsequent β-cell apoptosis within the pancreas[1]. The pancreas is an acinar gland and a critical controller of blood glucose levels. It has both exocrine and endocrine functions. The endocrine part of the pancreas consists of small islands of cells, called the islets of Langerhans, that encompass α-cells (involved in secretion of glucagon), β-cells (involved in insulin secretion), δ-cells (involved in somatostatin secretion), ε-cells (involved in release of ghrelin), and PP cells (release of pancreatic polypeptide). These hormones upregulate or downregulate depending upon the blood glucose levels and inadequate secretion of these hormones may result in uncontrolled glycemic regulation in our circulation causing T1DM or T2DM[2].
The currently available treatment approaches for uncontrolled DM are pancreas and islet transplantation[3,4]. However, these strategies are limited due to unavailability of quality pancreas or islet donors, life-long need of immunosuppression, and associated complications. Exogenous insulin administration is one of the clinically established approaches for patients with T1DM[5]; however, this strategy does not completely control blood glucose level and impaired insulin release, resulting in continuous abnormal functioning of β-cells. Hence, there is an urgent need to develop clinically relevant and scalable strategies to replenish the loss of β-cell function to provide long-term recovery. Cell therapy has emerged as one of the promising alternatives to achieve the required clinical benefits in both T1DM and T2DM.
Since the last decade, various sources other than pancreas-derived cells have been used to generate insulin-producing β-like cells[6-8]. These extrapancreatic sources of cells may play a significant role in β-cell turnover and insulin secretion in response to environmental stimuli. However, the most critical limitation with such extrapancreatic source of cells is that they do not respond to glucose stimuli, and do not mimic clinical conditions. In addition, the production of insulin-producing cells (InPCs) in vitro as well as in vivo requires complicated protocols which should be glucose responsive. Among various extrapancreatic sources, the liver has been considered as one of the most appropriate options for isolating large numbers of cells with the potential to dedifferentiate into InPCs due to their similar embryonic developmental origin from the endoderm. The stem/progenitor cells from the liver respond well to glucose stimuli in vitro and in vivo. Hence, the liver could be one of the most suitable sources of large numbers of functional InPCs. This review summarizes the few crucial extrapancreatic sources that are currently proposed to generate InPCs, along with other advancements in this field that may fulfill the current clinical needs for the welfare of DM patients.
Studies have demonstrated that liver cells can be converted into InPCs under the influence of several growth factors and a high-glucose environment[9-11]. However, several questions remain to be addressed regarding which cell type undergoes differentiation and the minimum cascade of genes essential to activate or inactivate to generate fully functional β-cells. An initial study by Zalzman et al[12] in 2003 reported successful reversal of hyperglycemia in mice using fetal liver progenitor cells that were converted into InPCs. This study gave a boost for utilization of human fetal liver progenitors to obtain significant numbers of InPCs and develop relevant protocols to be applied in clinical settings. In support of the above study, Cao et al[13] in 2004 reported the importance of liver cells in reversal of hyperglycemia in diabetic mice, using a rat liver cell line cultured in high-glucose medium. However, cells failed to produce enough insulin when injected in vivo, which is comparatively very less to desired insulin content (< 1%) obtained from the native β–cells. Hence, it was assumed that primary cells are of utmost important for further in vivo studies.
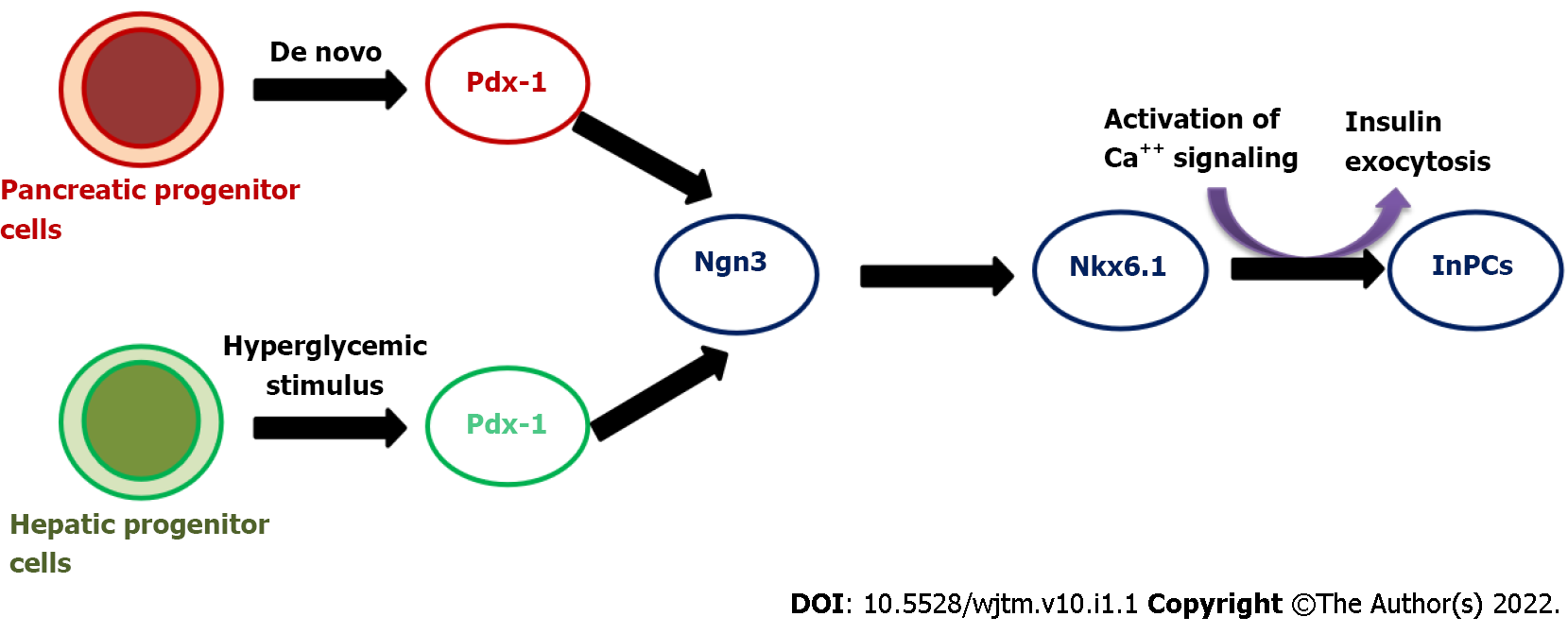
In our preliminary study, we have demonstrated the successful production of InPCs from human fetal liver-derived EpCAM+ stem/progenitor cells in response to glucose stimuli in vitro[14]. Following to this study, we also reported a cascade of transcription factors that are required to convert EpCAM+ human fetal liver-derived stem/progenitor cells into InPCs in vitro under a high-glucose microenvironment (Figure 1). This study revealed that activation of master regulator Pdx-1 with β-cell-specific transcription factor Nkx-6.1 in combination with Ngn-3, Pax-4, Pax-6 and Isl-1 is required to transdifferentiate hepatic stem/progenitor cells into InPCs under hyperglycemic challenge without the need of any genetic manipulation, which is crucial for its potential clinical relevance. The study also showed that the amount of insulin production was higher compared to that with other developed protocols. This particular strategy has the additional advantage for its clinical potential of scalability and lack of genetic manipulation.
Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocysts. ESCs are pluripotent and can produce cells present in all three germ layers, including InPCs, when placed in appropriate in vitro or in vivo conditions. Due to self-renewal and differentiation potential, ESCs can be used to produce large numbers of desired cell types for downstream applications. Different derivatives of ESCs have shown restoration of functional and structural benefits in injured organs/tissues[15]. Hence ESCs are considered a good source for the generation of large numbers of InPCs. D’Amour et al[16] in 2006 demonstrated a stepwise protocol involving five stages for the conversion of ESCs into InPCs in response to a number of secretagogues in vitro. Recently, Vegas et al[17] in 2016 also developed a multistep protocol for converting ESCs into β-cells and achieved long-term glycemic control in immunocompetent mice using an encapsulation approach. However, these strategies are not clinically relevant due to their non-responsive nature to glucose stimuli, and ethical issues. Zhang et al[18] in 2009 developed a four-step protocol for converting ESCs into insulin/C-peptide-producing cells in response to glucose stimuli. Based on the clinical applicability of this protocol, Hua et al[19] in 2014 developed a four-step protocol to convert human ESCs into pancreatic InPCs and successfully corrected the hyperglycemia in immunodeficient mice. These studies have boosted our knowledge for the experimental conversion of ESCs into functional β-cells; however, the clinical applicability of these approaches is still questionable due to ethical concerns and complicated protocols, and importantly, these experiments were conducted in immunocompetent mice that do not mimic clinical conditions. Hence, there is still a need to discover alternative sources of human β-cells that can mimic clinical conditions and can be applied in clinical settings without the need for immunosuppression. More studies are desired to obtain homogeneous populations of functional human InPCs from ESCs and further to answer whether an inductive or selective mechanism of differentiation can be clinically relevant or not.
Bone-marrow-derived stem cells (BMSCs) are considered one of the potential sources for generating functional pancreatic β-cells[20,21]. Several studies have demonstrated experimental conversion of BMSCs into InPCs[22-25]. However, the amount of insulin/C-peptide secreted by these cells was less compared to that from isolated pancreatic islets and was not enough to reverse hyperglycemia in diabetic rats. Several other studies have reported similar problems with BMSCs[26,27]. Some of the results of these studies were nonreproducible, which warrants their further investigation for clinical applicability.
Human umbilical cord blood (UCB) is known to contain stem/progenitor cell populations that can be converted into different types of organ-specific cells including InPCs. UCB is considered one of the most appropriate sources of stem/progenitor cells because of fewer ethical concerns and biological waste materials. Human UCB can also be readily available in sufficient amounts with low risk of graft rejection[28,29]. A few studies have successfully reported the conversion of human UCB-derived stem cells into functional InPCs by activating several crucial pancreatic transcription factors (Pdx-1, Isl-1, Pax-4 and Ngn-3), which is capable of correcting hyperglycemia in diabetic mice[30-33]. However, for their clinical applicability, more appropriate protocols need to be developed with suitable transplantation strategies to support long-term cell survival and function post-transplantation in vivo.
A recent study by Zhu et al[34] in 2016 demonstrated that fibroblasts of adults or neonates can generate precursors of endodermal lineages following the cell activation and signaling directed transdifferentiation paradigm. They developed conditions for expansion of glucose responsive β-like transdifferentiated pancreatic endodermal cells into the progenitor stage. These transdifferentiated cells can control hyperglycemia in mice and provide a new approach for the production of patient-specific InPCs for studying unresolved questions in pancreatic biology, disease modeling and drug testing strategies.
Patient-specific cell lines can be generated using induced pluripotent stem cells (iPSCs), which can then be converted into cells of interest for disease models or cell replacement therapy. Current advances in iPSCs research have evolved several robust protocols for the production of patient specific functional β-cells. These differentiation protocols resemble with the same developmental stages ultimately targeting the final differentiated β-cells[35]. To trigger the pathways required for differentiation phases, a mixture of signaling molecules and growth factors are often utilized, with the aim of mimicking embryonic stages of developmental. For converting somatic cells towards iPSCs, the Yamanaka factors (OCT4, KLF4, SOX2 and c-MYC) are commonly used[36].
Several studies have examined the role of iPSCs-derived β-cells in the context of T1DM[37,38]. Maehr et al[39] in 2009, for the first time demonstrated conversion of iPSCs derived from T1DM patients into glucose-responsive functional InPCs[39]. Millman et al[40] in 2016 compared differentiation potential of iPSCs derived from diabetic and nondiabetic individuals and concluded that both the sources of iPSCs encompass similar expression patterns of the specific surface markers, and capacity of insulin secretion both in vitro and in vivo[40]. Despite progress towards potential applicability of iPSC-derived β-cells, there are technical difficulties and financial concerns related to iPSC therapy in clinical settings[41]. To alleviate the financial concern, iPSC biobanks with ability to match the majority of HLA types and occasional blood types are continuously being explored. However, the genomic instability involving chromosomal aberrations or mutations, as well as tumor formation remain obstacles for bench to bedside clinical translation of iPSCs.
In addition to the above extrapancreatic sources, several other source of tissues have also been investigated that contain progenitor cell populations which can be converted into InPCs. Among these sources, spleen[42], adipose tissues[43], blood[44], amniotic membrane, and central nervous system[45] have showed β-cell-specific markers during in vitro or in vivo transdifferentiation (Table 1). In particular, Kodama et al[42] in 2003 demonstrated that injected splenocytes can be converted into InPCs and minimize the onset of autoimmunity. Transplantation of these cells combined with Freund’s adjuvant improves diabetes in nonobese diabetic (NOD) mice. However, subsequent reports[46,47] found no such evidence which questions their clinical applicability.
| Characteristics | Target cell | ||||
| Liver | Pituitary | Muscle | Kidney | Bone marrow | |
| Endodermal origin | + | - | - | - | + |
| Possession of β-cell transcription factors | - | - | - | - | + |
| Glucose sensing system | + | - | - | + | - |
| Processing enzymes | - | + | - | + | - |
| Glucose-regulatable promoter | + | - | - | + | - |
| Exocytosis system | - | + | - | + | - |
| Autologous use | + | + | + | + | + |
| Allogenic use | - | - | - | - | + |
Cell encapsulation technology is based on the concept of immunoisolation. Because islet cells can be effectively harvested and transplanted, encapsulation technology is promising for future clinical transplantation. Therefore, during the last three decades, various encapsulation strategies have been demonstrated in different animals such as mice[48], rats[49], dogs[50] and monkeys[51]. Encapsulation technology also offers enhanced cell survival post-transplantation without the use of immunosuppressive drugs. This technology involves an artificial compartment of semipermeable membrane as a capsule that contains cells and allows oxygen and nutrient supply. The capsule protects cells from injuries against antibodies, proteins, and potent immune cells. Hence, these capsules are also referred to as an immunoisolation device. Diffusion of insulin, glucose, nutrients and oxygen across the capsule has additional advantages that allows efficient glucose homeostasis. Moreover, additional intravascular devices have been designed that contain a small planar or tubular diffusion chamber which is directly connected with the host vascular system and also referred to as an encapsulation device[52]. This device does not require anastomosis after implantation, and encompasses better clinical application compared to the intravascular device.
In a recent study of Vegas et al[17] in 2016, long-term glycemic control was achieved in immunocompetent hyperglycemic mice using polymer-encapsulated human stem-cell–derived β-cells. This study highlighted the decreased obscurity in successful immunoprotection against xenogenic human cell implants in diabetic mice. This report provided groundwork for future studies in autoimmune animal models using xenogenic cells transplantation with the goal of achieving long-term glycemic control and cell survival to offer insulin independence in patients with DM. However, this study had the limitation of generating β-cells using complex long duration protocols and was conducted in immunodeficient mice. Hence, further investigations are required to prove that this technology is clinically acceptable using a wild-type diabetic model without the need of immunosuppression.
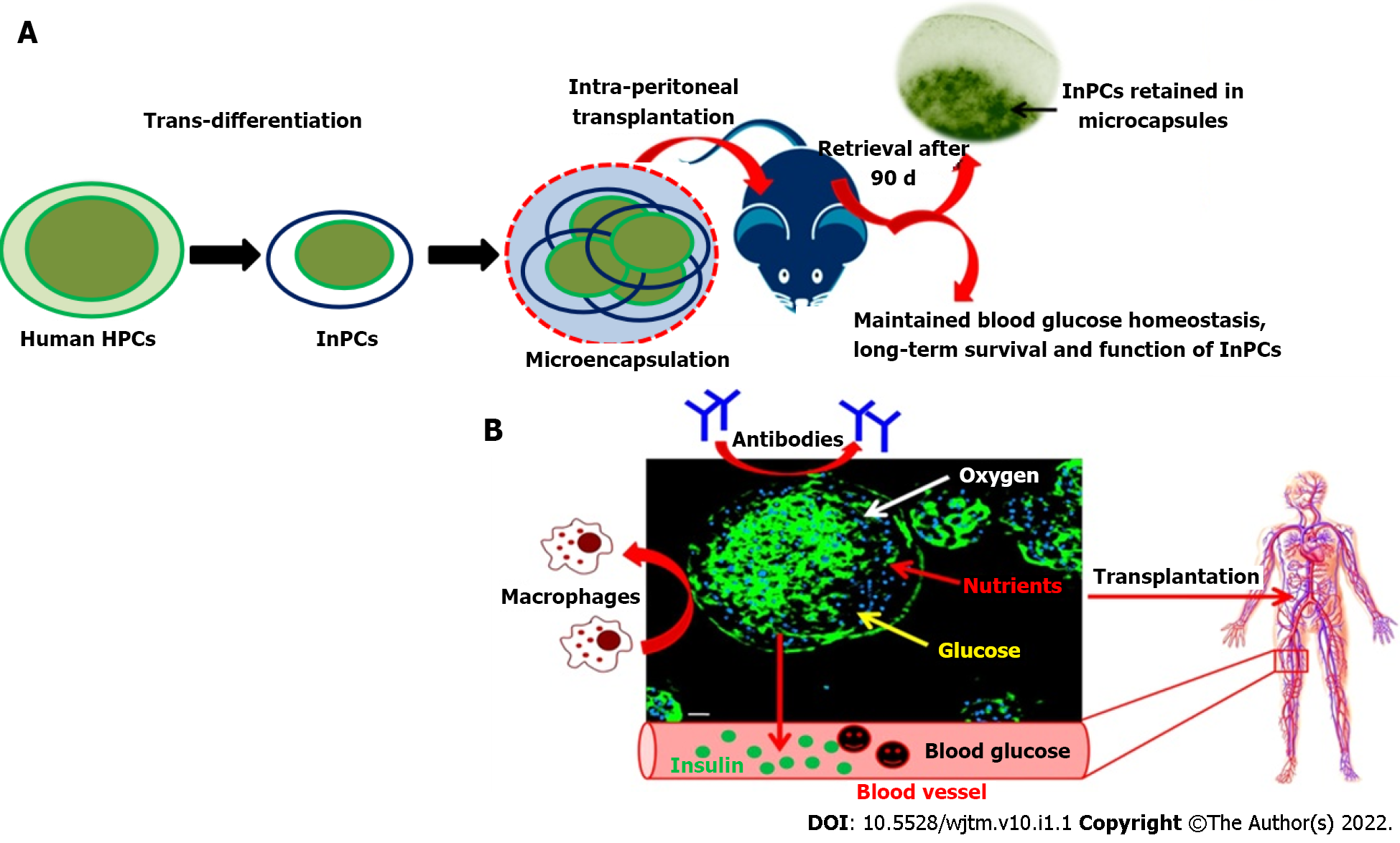
In a similar direction, our group has also been working to generate functional insulin-producing β-like cells in vitro through transdifferentiation of human fetal liver-derived EpCAM+ enriched progenitor cells in response to high glucose concentration (Figure 2). This protocol has been simplified and encapsulated functional β-like cells in alginate beads (with modified protocol) have been transplanted into C57BL6 hyperglycemic wild-type mice without the need of immunosuppression. The mice were able to restore the blood glucose level within 30 d after transplantation of encapsulated cells and maintained for up to 90 d (unpublished data). In our view, this is the only study where xenogenic human functional β-like cells have been transplanted into wild-type mice without immunosuppression. The encouraging results of this study have shown a path to further investigate their potential in clinical applications.
Suspending viable cells in an aqueous medium of hydrogel precursors, infusing the cocktail to target areas, and stimulating crosslinking (gelation) to generate 3D gel matrices in situ are the three primary phases in cell transplantation utilizing hydrogels. Cell transplantation using a hydrogel-based strategy has several crucial advantages. Hydrogels are injectable, and can be used to construct cell-encapsulated gels that have a uniform distribution of cells in the transplanted space. They have water content that can be used to construct different shapes and sizes. Hydrogels can be used for cell therapy when they are constructed with limited pore size that can facilitate diffusion and metabolic wastes but prevent leakage in the cells[53]. Hydrogel scaffolds can be coated with encapsulated InPCs and placed at the site of transplantation[54].
Despite these advances, using hydrogels to control the fate of transplanted therapeutic cells still poses significant obstacles including unsatisfactory cell survival rate due to their death in the new microenvironment post-transplantation. Cells that survive in the hydrogels have uncontrolled interactions, proliferation and differentiation. The overall therapeutic efficacy was unsatisfactory using this method. The nonautologous cells were rejected by the host immune system and the cells were strictly confined. The encapsulated cells were not able to proliferate freely, the cell viability varied for different cell types and in many cases it decreased to nearly 50% when assessed after 10 wk. Hence, the methodologies were modified to improve the drug delivery and integration with the target tissue for successful delivery of the cells.
To improve the functional response and stability of the implanted cells, dual-layer hydrogels as well as silk hydrogels are being tested for efficient transfer of islet cells to the required site. Silk hydrogels are formed using extracellular matrix (ECM) proteins such as collagen IV and laminin. These hydrogels have been tested for cell signaling and survival by immunostaining and oscillatory theometry and found to be stable. The silk hydrogels encapsulated with islet cells in laminin had high insulin response compared with nonencapsulated cells when stimulated over a 7-d period. Silk hydrogels allow coencapsulation of other ECM proteins and cells that can ultimately be used as a transplant device for the treatment of T1DM[55].
The generation of functional β-cells by genetic engineering necessitates a thorough knowledge of pancreas development and a good understanding of the organ. The production of a cascade of transcriptional regulators, as well as their involvement in regulating endocrine cell fate and maturation, are important considerations for creating artificial β-cells. Furthermore, the type of vector used for gene delivery and the selection of suitable cell type candidates for differentiation into functional β-cells are key challenges.
Selecting an ideal cell type: The ultimate aim of gene therapy is to produce cells with the ability to produce insulin and maintain blood glucose level in vivo. Different types of cells have been investigated over the last few years, in particular, pituitary cells that contain both the proinsulin processing enzymes and the secretory granules. However, these cells were non-glucose responsive and later became glucose responsive after transfection with glucose transporter 2 and glucokinase genes. However, in vivo production of adenocorticotropic hormone inhibits insulin function, which limits its clinical efficacy[56]. Muscle cells, liver cells, mesenchymal stem cells from UCB and bone marrow cells have also been investigated by gene transfer technology and demonstrated the reversal of hyperglycemia in immunodeficient diabetic mice. More recently, a combination of gene and cell therapy was used to produce glucose-responsive InPCs after retroviral transfection with Pdx-1. Transplantation of this combination reversed hyperglycemia in immunodeficient diabetic mice[57].
Choice of vector for gene delivery: The ideal method of gene delivery in cells relies on integrating viral vectors for sustained gene transfer into the daughter cells to provide sustained therapeutic benefits throughout the life of the patient. Four main different kinds of viral vectors have been used in in vitro and in vivo models for gene delivery purposes: (1) Retroviral vectors[58]; (2) Adenoviral vectors[59]; (3) Adeno-associated vectors[60]; and (4) Lentiviral vectors[61]. Among these, lentiviral vectors have become a popular choice for gene delivery in animal models of diabetes. However, the clinical applicability of these strategies is limited due to virus-mediated complications and resultant pancreatic transdifferentiation.
β-cell transcription factors: Transcription factors play a significant role in determining the phenotype of β-cells. Homeobox factor Pdx-1 is considered to be the master regulator and has a crucial role in early development of the pancreas[62]. Hes-1 and Neurogen-3 are present in pancreatic progenitor cells and direct the respective compartmental fates through Notch signaling[63]. However, for subsequent differentiation into respective pancreatic cell lineages, a cascade of transcription factors are required. All endocrine cells express Neurogen-3, which activates NeuroD1, and maintains the endocrine cell differentiation program[64]. As soon as the endocrine cell differentiation program is activated, Pax-4 and Pax-6 direct the differentiation into different kinds of endocrine cells[62]. In these two transcription factors, Pax-4 is responsible for the fate of β- and γ-cells, and Pax-6 is essential for α-cell fate. More importantly, NK homeobox factors Nkx2.2 and Nkx6.1 are responsible for driving the β-cell fate. Both these transcription factors are imperative in β-cell differentiation. Nkx6.1 is also responsible for preservation of β-cell function during its interaction with Pdx-1, the master regulator, and the other transcription factor NeuroD1 that modulates insulin transcription. Alteration in the expression of these transcription factors and their subcellular localization alters the cellular processes related to β-cell differentiation, and cell cycle modulation and function.
Direct gene transfer: In relation to β-cell replacement therapy, direct gene delivery represents one of the potential techniques to obtain β-like cell phenotype in autologous tissues[65]. Although in past decades a lot of work was focused on direct transcription factor/gene transfer in hepatocytes, the emergence of stem cells has changed the way to look into the cells and needs further investigation due to their clinical suitability. However, before developing a suitable strategy, one needs to consider the appropriate type of transcription factor for direct delivery and true conversion into functional β-cells without causing future complications.
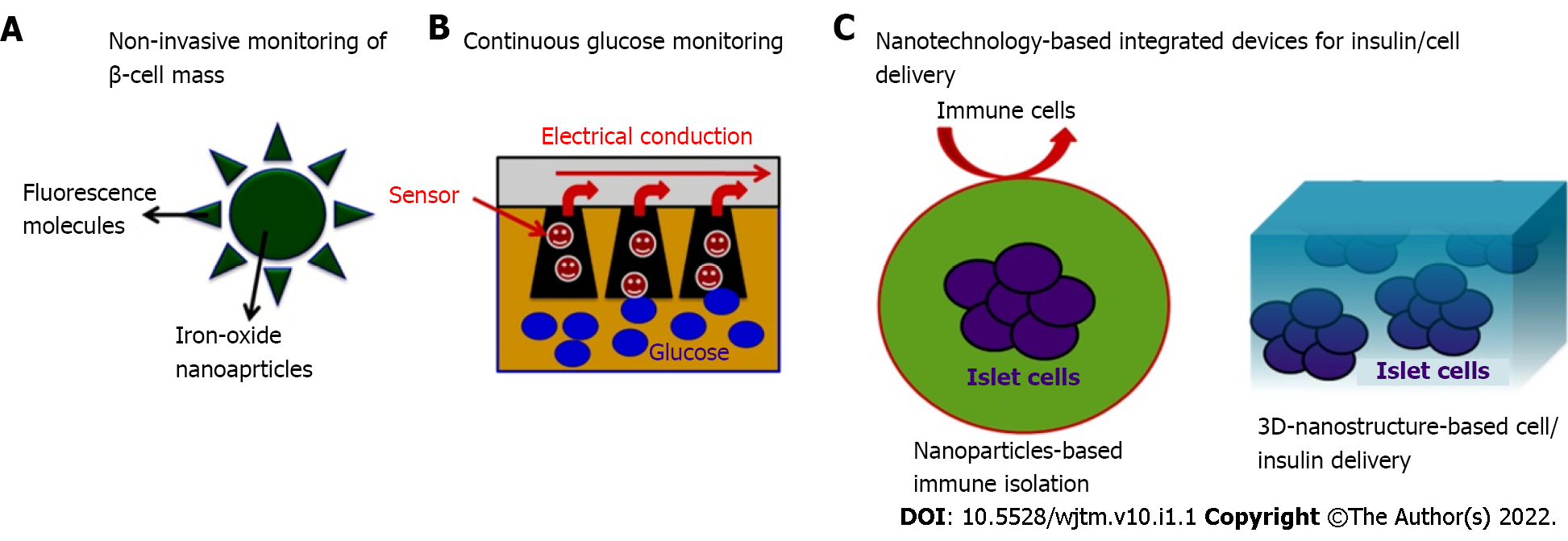
Nano-bioengineering is an emerging field that has the potential to revolutionize DM treatment. Merging of nanotechnology with medical biology has given a new direction to create smart delivery systems that can regulate blood glucose levels within the body and could produce the desired amount of insulin. Recent advances have been made in this field and novel blood glucose measurement and insulin delivery methods are being developed to improve the quality of life of DM patients (Figure 3).
Nano-bioengineering has been used to develop a novel smart insulin patch that can deliver glucose-responsive insulin with the help of a painless microneedle-array patch. This device is based on the glucose-responsive enzymatic mechanism that can regulate the blood glucose level in T1DM faster than the commonly used pH-sensitive formulations. In addition, it can also avoid the risk of developing hypoglycemia. The study by Lee et al[66] in 2016 showed the diversified application of a graphene-based electrochemical device to monitor DM and efficient transcutaneous delivery of drugs to reduce blood glucose levels in hyperglycemic mice.
In our recent study, we have demonstrated the applicability of an unique strategy that enables effective transdifferentiation of human hepatic progenitor cells (hHPCs) into InPCs on 3D-nanostructured TiO2 substrate developed on conducting surfaces[67]. This 3D-TiO2 cellularized chip was able to reverse hyperglycemia in wild-type mice (C57BL6) when transplanted into the peritoneal cavity. We also observed enhanced cell survival and insulin production, and long-term glycemic control in hyperglycemic animals where it does not elicit significant immunological response after ectopic trans
Neo-organ bioengineering is one of the most promising approaches, which includes decellularization and repopulation of whole organs harvested from human or xenogenic sources. This approach generates a whole functional neo-organ construct for future clinical applications as a bridge therapy for organ transplantation. It offers a unique strategy of using natural organ platforms to produce natural organ systems using different sources of InPCs. Therefore, this particular technology can overcome the above-mentioned critical concerns and has attracted a lot of attention to generate different organs, including the pancreas[68].
A few recent studies have reported the use of decellularized pancreas to produce functional neo-organs for ex vivo insulin secretion[69-71]. However, a critical stumbling block is the lack of sufficient pancreata for decellularization and repopulation. Furthermore, the mechanical integrity of the neopancreas has not been reported. The developing concept of using heterografts to create viable humanized neo-organ systems has opened a new avenue for meeting transplant demand[72,73]. Nevertheless, due to the small amount of research conducted, some important questions remained unanswered. To date, no effective decellularization and repopulation of xenogeneic spleen sources with endocrine cells has been documented. As a result, it is still unknown if decellularized spleen can be repopulated with human InPCs and perform similarly to islets or the entire pancreas.
Our recent study has demonstrated a heterograft approach of using whole decellularized xenogenic scaffold of spleen to generate functional constructs that are capable of producing the desired amount of insulin in response to hyperglycemic stimulus[74,75]. In our preliminary study, we standardized a unique strategy for activating pancreatic transcription factors of EpCAM+-enriched human hepatic progenitor cells repopulated within acellular spleen harvested from rats[74]. This indicates that 3D, intact acellular splenic scaffolds can provide a superior microenvironment for long-term survival of cells, activation of crucial transcription factors, and transdifferentiation of hHPCs into functional InPCs. Our subsequent study demonstrated that the heterograft approach developed in our previous study generates secondary neo-organoids during ectopic transplantation in diabetic rats and is capable of transporting insulin into the bloodstream, which is essential to manage uncontrolled blood glucose levels[75]. Moreover, this study provides the first proof-of-concept for creating bio/immunocompatible, humanized insulin-producing neo-organoids, which could evolve into more acceptable functional biological implantable devices for long-term diabetes management.
The ideal choice for cell transplantation offers optimum engraftment and long-term cell function. The appropriate site for cell transplantation should include: (1) Membrane drainage for the permeabilization of blood glucose and to avoid systemic hyperinsulinemia; (2) Rich arterial supply; (3) Minimal invasive infusion; (4) Access for morphological and functional follow-up of the transplant; (5) Microenvironment with maximum cell survival; and (6) Immunological tolerance. Such types of transplantation sites need to be defined. Over the last few years, various sites have been attempted for islet cell transplantation[76] to manage DM in different animal models. Among different proposed sites/routes, the portal vein has been the site of choice for clinical transplantation (Table 2). However, inflammatory reactions and low oxygen tension lead to cell loss and varied responses among patients. Peritoneal transplantation has the advantage of being an immunologically privileged site, and offers enough space for housing the cells. Hence, the peritoneum overcomes the limitations of other identified transplantation sites and could be an ideal choice for ectopic transplantation. In our recent study, we have demonstrated the usefulness of the peritoneal site in diabetic animal models and have reported that this site increases animal survival and faster recovery of normoglycemia within 30 d post-transplantation without the need for immunosuppression (unpublished data).
| Transplantation site | Pros | Cons | Ref. |
| Liver | Ease of transplant, Portal insulin delivery | Portal hypertension, bleeding, portal vein thrombosis, inflammation, portal circulation | Kim et al[77], 2010 |
| Kidney subcapsule | Graft retrieval, immunological tolerance | Not ideal site, invasive procedure, graft tissue oxygen tension | Reece-Smith et al[78], 1981 |
| Spleen | Good vascular supply, portal vein insulin delivery | Rich in lymphocytes, risk of bleeding | Gray[79], 1990; Alderson et al[80], 1984 |
| Pancreas | Home of islets, ideal oxygen tension | Autoimmune destruction, invasive procedure | Stagner et al[81], 2008 |
| Omental pouch | High vascular density, neoangiogenesis, good blood supply, impure islets can be implanted | Large graft unstable, islet cells estimation is empirical | Gustavson et al[82], 2005 |
| Gastro intestinal wall | Ease of accessibility, portal vein delivery of insulin | Thickening of gastric walls after islet cells transplantation | Wszola et al[83], 2009 |
| Bone marrow | Ease of accessibility, no graft rejection | Conserved differentiation into α and β cells | Salazar-Bañuelos et al[84], 2008; Cantarelli et al[85], 2009 |
The major challenges in DM cell therapy are: (1) Identifying clinically acceptable sources of cells that can be used to produce homogeneous populations and therapeutic doses of insulin-secreting cells; (2) Prevention of immunological rejection post-transplantation; (3) in vivo glucose responsiveness of transplanted cells; (4) Long-term cell survival and function post-transplantation; and (5) No need for immunosuppression. Other clinical challenges include: Safety of the transplantation procedure; determining the cell delivery and engraftment efficiency using live clinical imaging systems; cell delivery at the targeted site within a clinically relevant time; identification of ways to promote regeneration of resident β-cells; ease of source tissue collection and clinical grade cell isolation; and cost-effectiveness of procedures. These key considerations and challenges need to be resolved to successfully translate the stem-cell-based therapeutic possibilities for timely management of T1DM into clinical practice. Given the current debate on such issues, clinical applicability of stem-cell-based therapies for the treatment of DM is still a future goal.
In summary, in the next decade, we expect stem-cell-based therapeutic strategies in combination with nanotechnology and other potential areas of science for improved management of DM. Recent developments in neo-organ bioengineering and United States Food and Drug Administration-approved nanotechnology-based formulations with the success of insulin-delivery are encouraging and provide newer opportunities for DM treatment. In our view, the need to develop more effective microencapsulation, neo-organ bioengineering, and nanotechnology-based diabetes therapies lies in the development of robust sensitive micro- and nanodevices for insulin delivery, using clinically acceptable platforms.
| 1. | Atkinson MA, Eisenbarth GS. Type 1 diabetes: new prespective on disease pathogenesis and treatment. Lancet. 2001;358:221-229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 937] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Kaur H, Bhaskar N, Ishaq S, Najeeb Q. Stem Cells: Source for diabetes cell therapy. J Diabet. 2012;3:3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Gunnarsson R, Klintmalm G, Lundgren G, Wilczek H, Ostman J, Groth CG. Deterioration in glucose metabolism in pancreatic transplant recipients given cyclosporin. Lancet. 1983;2:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3879] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 5. | Oriando G, Stratta RJ, Light J. Pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant. 2011;16:110-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Pan G, Mu Y, Hou L, Liu J. Examining the therapeutic potential of various stem cell sources for differentiation into insulin-producing cells to treat diabetes. Ann Endocrinol (Paris). 2019;80:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, Loyal J, Kim PTW, Warnock GL, Levings MK, Kieffer TJ. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021;28:2047-2061.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 8. | Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, Wilensky J, Daniels M, Wang RM, Brandon EP, Jaiman MS, Kroon EJ, D’Amour KA, Foyt HL. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2:100466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 9. | Yang LJ. Liver stem cell-derived beta-cell surrogates for treatment of type 1 diabetes. Autoimmun Rev. 2006;5:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Meivar-Levy I, Zoabi F, Nardini G, Manevitz-Mendelson E, Leichner GS, Zadok O, Gurevich M, Mor E, Dima S, Popescu I, Barzilai A, Ferber S, Greenberger S. The role of the vasculature niche on insulin-producing cells generated by transdifferentiation of adult human liver cells. Stem Cell Res Ther. 2019;10:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Lee YN, Yi HJ, Seo EH, Oh J, Lee S, Ferber S, Okano T, Shim IK, Kim SC. Improvement of the therapeutic capacity of insulin-producing cells trans-differentiated from human liver cells using engineered cell sheet. Stem Cell Res Ther. 2021;12:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253-7258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ. High glucose is necessary for complete maturation of pdx 1-VP16-expressing hepatic cells into functional insulin producing cells. Diabetes. 2004;53:3168-3178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Khan AA, Rajendraprasad A, Parveen N, Shaik MV, Tiwari SK, Srinivas G, Raj TA, Habeeb MA, Pande G, Habibullah CM. In vitro insulin production and analysis of pancreatic transcription factors in induced human hepatic progenitor cells. Diabetes Technol Ther. 2010;12:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Solis MA, Moreno Velásquez I, Correa R, Huang LLH. Stem cells as a potential therapy for diabetes mellitus: a call-to-action in Latin America. Diabetol Metab Syndr. 2019;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1429] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 17. | Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 537] [Cited by in RCA: 510] [Article Influence: 51.0] [Reference Citation Analysis (1)] |
| 18. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 19. | Hua XF, Wang YW, Tang YX, Yu SQ, Jin SH, Meng XM, Li HF, Liu FJ, Sun Q, Wang HY, Li JY. Pancreatic insulin-producing cells differentiated from human embryonic stem cells correct hyperglycemia in SCID/NOD mice, an animal model of diabetes. PLoS One. 2014;9:e102198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 421] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Mathews V, Hanson PT, Ford E, Fujita J, Polonsky KS, Graubert TA. Recruitment of bone marrow-derived endothelial cells to sites of pancreatic regeneration. Diabetes. 2004;53:91. [RCA] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant Proc. 2003;35:2140-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 207] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Li G, Peng H, Qian S, Zou X, Du Y, Wang Z, Zou L, Feng Z, Zhang J, Zhu Y, Liang H, Li B. Bone Marrow-Derived Mesenchymal Stem Cells Restored High-Fat-Fed Induced Hyperinsulinemia in Rats at Early Stage of Type 2 Diabetes Mellitus. Cell Transplant. 2020;29:963689720904628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | He J, Kong D, Yang Z, Guo R, Amponsah AE, Feng B, Zhang X, Zhang W, Liu A, Ma J, O’Brien T, Cui H. Clinical efficacy on glycemic control and safety of mesenchymal stem cells in patients with diabetes mellitus: Systematic review and meta-analysis of RCT data. PLoS One. 2021;16:e0247662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 26. | Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Lechner A, Habener JF. Stem/progenitor cells derived from adult tissues: potential for the treatment of diabetes mellitus. Am J Physiol Endocrinol Metab. 2003;284:E259-E266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1730] [Cited by in RCA: 1648] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 30. | Pessina A, Eletti B, Croera C, Savalli N, Diodovich C, Gribaldo L. Pancreas developing markers expressed on human mononucleated umbilical cord blood cells. Biochem Biophys Res Commun. 2004;323:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Zhao Y, Wang H, Mazzone T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp Cell Res. 2006;312:2454-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Päth G, Perakakis N, Mantzoros CS, Seufert J. Stem cells in the treatment of diabetes mellitus – Focus on mesenchymal stem cells. Metabolism. 2019;90:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Chen S, Du K, Zou C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther. 2020;11:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Zhu S, Russ HA, Wang X, Zhang M, Ma T, Xu T, Tang S, Hebrok M, Ding S. Human pancreatic beta-like cells converted from fibroblasts. Nat Commun. 2016;7:10080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Maxwell KG, Millman JR. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep Med. 2021;2:100238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, Goland RS, Leibel RL, Solomon SL, Benvenisty N, Sauer MV, Egli D. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Kalra K, Chandrabose ST, Ramasamy TS, Kasim NHBA. Advances in the Generation of Functional β-cells from Induced Pluripotent Stem Cells As a Cure for Diabetes Mellitus. Curr Drug Targets. 2018;19:1463-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Kim MJ, Lee EY, You YH, Yang HK, Yoon KH, Kim JW. Generation of iPSC-derived insulin-producing cells from patients with type 1 and type 2 diabetes compared with healthy control. Stem Cell Res. 2020;48:101958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106:15768-15773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 408] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 40. | Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 41. | Leite NC, Sintov E, Meissner TB, Brehm MA, Greiner DL, Harlan DM, Melton DA. Modeling Type 1 Diabetes In Vitro Using Human Pluripotent Stem Cells. Cell Rep. 2020;32:107894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 42. | Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Müller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 44. | Ruhnke M, Ungefroren H, Nussler A, Martin F, Brulport M, Schormann W, Hengstler JG, Klapper W, Ulrichs K, Hutchinson JA, Soria B, Parwaresch RM, Heeckt P, Kremer B, Fändrich F. Differentiation of in vitro-modified human peripheral blood monocytes into hepatocyte-like and pancreatic islet-like cells. Gastroenterology. 2005;128:1774-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Hori Y, Gu X, Xie X, Kim SK. Differentiation of insulin-producing cells from human neural progenitor cells. PLoS Med. 2005;2:e103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Suri A, Calderon B, Esparza TJ, Frederick K, Bittner P, Unanue ER. Immunological reversal of autoimmune diabetes without hematopoietic replacement of beta cells. Science. 2006;311:1778-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Nishio J, Gaglia JL, Turvey SE, Campbell C, Benoist C, Mathis D. Islet recovery and reversal of murine type 1 diabetes in the absence of any infused spleen cell contribution. Science. 2006;311:1775-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Foster JL, Williams G, Williams LJ, Tuch BE. Differentiation of transplanted microencapsulated fetal pancreatic cells. Transplantation. 2007;83:1440-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Meyer T, Höcht B, Ulrichs K. Xenogeneic islet transplantation of microencapsulated porcine islets for therapy of type I diabetes: long-term normoglycemia in STZ-diabetic rats without immunosuppression. Pediatr Surg Int. 2008;24:1375-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Wang T, Adcock J, Kühtreiber W, Qiang D, Salleng KJ, Trenary I, Williams P. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation. 2008;85:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005;37:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 54. | Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33:6691-6697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 56. | Hughes SD, Johnson JH, Quaade C, Newgard CB. Engineering of glucose-stimulated insulin secretion and biosynthesis in non-islet cells. Proc Natl Acad Sci U S A. 1992;89:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 58. | Xu J, Lu Y, Ding F, Zhan X, Zhu M, Wang Z. Reversal of diabetes in mice by intrahepatic injection of bone-derived GFP-murine mesenchymal stem cells infected with the recombinant retrovirus-carrying human insulin gene. World J Surg. 2007;31:1872-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Zhou HS, Liu DP, Liang CC. Challenges and strategies: the immune responses in gene therapy. Med Res Rev. 2004;24:748-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Sugiyama A, Hattori S, Tanaka S, Isoda F, Kleopoulos S, Rosenfeld M, Kaplitt M, Sekihara H, Mobbs C. Defective adenoassociated viral-mediated transfection of insulin gene by direct injection into liver parenchyma decreases blood glucose of diabetic mice. Horm Metab Res. 1997;29:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Ren B, O’Brien BA, Swan MA, Koina ME, Nassif N, Wei MQ, Simpson AM. Long-term correction of diabetes in rats after lentiviral hepatic insulin gene therapy. Diabetologia. 2007;50:1910-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Chakrabarti SK, Mirmira RG. Transcription factors direct the development and function of pancreatic beta cells. Trends Endocrinol Metab. 2003;14:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1106] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 64. | Gasa R, Mrejen C, Lynn FC, Skewes-Cox P, Sanchez L, Yang KY, Lin CH, Gomis R, German MS. Induction of pancreatic islet cell differentiation by the neurogenin-neuroD cascade. Differentiation. 2008;76:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 550] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 66. | Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T, Choi SH, Kim DH. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol. 2016;11:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 918] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 67. | Vishwakarma SK, Jaiswal J, Park KH, Lakkireddy C, Raju N, Bardia A, Habeeb MA, Paspala SAB, Khan AA, Dhayal M. Glycemic Stimulation with Conducting Substrate Enhances Insulin Secretion of Transdifferentiated Human Hepatic Progenitor Cells Adherent on Nanostructured TiO2 Chips for Hyperglycemia Reversal in Diabetic Mice without Immunosuppression. Adv Therapeutics. 2020;900205:1-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Khan AA, Vishwakarma SK, Bardia A, Venkateshwarulu J. Repopulation of decellularized whole organ scaffold using stem cells: an emerging technology for the development of neo-organ. J Artif Organs. 2014;17:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C, Pileggi A. Bioengineering the Endocrine Pancreas: Intraomental Islet Transplantation Within a Biologic Resorbable Scaffold. Diabetes. 2016;65:1350-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 70. | Katsuki Y, Yagi H, Okitsu T, Kitago M, Tajima K, Kadota Y, Hibi T, Abe Y, Shinoda M, Itano O, Takeuchi S, Kitagawa Y. Endocrine pancreas engineered using porcine islets and partial pancreatic scaffolds. Pancreatology. 2016;16:922-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Napierala H, Hillebrandt KH, Haep N, Tang P, Tintemann M, Gassner J, Noesser M, Everwien H, Seiffert N, Kluge M, Teegen E, Polenz D, Lippert S, Geisel D, Reutzel Selke A, Raschzok N, Andreou A, Pratschke J, Sauer IM, Struecker B. Engineering an endocrine Neo-Pancreas by repopulation of a decellularized rat pancreas with islets of Langerhans. Sci Rep. 2017;7:41777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 72. | Gao R, Wu W, Xiang J, Lv Y, Zheng X, Chen Q, Wang H, Wang B, Liu Z, Ma F. Hepatocyte culture in autologous decellularized spleen matrix. Organogenesis. 2015;11:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Zheng XL, Xiang JX, Wu WQ, Wang B, Liu WY, Gao R, Dong DH, Lv Y. Using a decellularized splenic matrix as a 3D scaffold for hepatocyte cultivation in vitro: a preliminary trial. Biomed Mater. 2015;10:045023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Vishwakarma SK, Lakkireddy C, Bardia A, Raju N, Paspala SAB, Habeeb MA, Khan AA. Molecular dynamics of pancreatic transcription factors in bioengineered humanized insulin producing neoorgan. Gene. 2018;675:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Vishwakarma SK, Lakkireddy C, Bardia A, Nagarapu R, Paspala SAB, Habeeb MA, Khan AA. Biofabricated Humanized Insulin Producing Neo-Organs Generates Secondary Neo-Organoids Through Ectopic Transplantation. Cellul Mol Bioengin. 2019;12:569-582. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Rajab A. Islet transplantation: alternative sites. Curr Diab Rep. 2010;10:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Kim HI, Yu JE, Park CG, Kim SJ. Comparison of four pancreatic islet implantation sites. J Korean Med Sci. 2010;25:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Reece-Smith H, Du Toit DF, McShane P, Morris PJ. Prolonged survival of pancreatic islet allografts transplanted beneath the renal capsule. Transplantation. 1981;31:305-306. [PubMed] |
| 79. | Gray DW. Islet isolation and transplantation techniques in the primate. Surg Gynecol Obstet. 1990;170:225-232. [PubMed] |
| 80. | Alderson D, Walsh TN, Farndon JR. Islet cell transplantation in diabetic dogs: studies of graft function and storage. Br J Surg. 1984;71:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Stagner J, Ahren B, Sundler F, White K. Reconstructing the pancreas: restoration of normoglycemia, exocrine function, and islet innervation by islet transplantation to the pancreas. Transplant Proc. 2008;40:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Gustavson SM, Rajotte RV, Hunkeler D, Lakey JR, Edgerton DS, Neal DW, Snead WL, Penaloza AR, Cherrington AD. Islet auto-transplantation into an omental or splenic site results in a normal beta cell but abnormal alpha cell response to mild non-insulin-induced hypoglycemia. Am J Transplant. 2005;5:2368-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Wszola M, Berman A, Fabisiak M, Domagala P, Zmudzka M, Kieszek R, Ptasinska AP, Sabat M, Pawelec K, Kownacki L, Kownacka DP, Ostrowski K, Januchta M, Klucinski W, Rowinski O, Kwiatkowski A, Chmura A. Trans Endoscopic Gastric SubMucosa Islet Transplantation (eGSM-Itx) in pigs with streptozotocine induced diabetes – technical aspects of the procedure – preliminary report. Ann Transplant. 2009;14:45-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Salazar-Bañuelos A, Wright J, Sigalet D, Benítez-Bribiesca L. The bone marrow as a potential receptor site for pancreatic islet grafts. Arch Med Res. 2008;39:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Cantarelli E, Melzi R, Mercalli A, Sordi V, Ferrari G, Lederer CW, Mrak E, Rubinacci A, Ponzoni M, Sitia G, Guidotti LG, Bonifacio E, Piemonti L. Bone marrow as an alternative site for islet transplantation. Blood. 2009;114:4566-4574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Grawish ME, Egypt; Liu L, China; Liu D, China; Mrozikiewicz-Rakowska B, Poland; Rasoulinejad A, Iran A-Editor: Soriano-Ursúa MA, Mexico S-Editor: Fan JR L-Editor: Kerr C P-Editor: Fan JR