Published online Nov 12, 2019. doi: 10.5527/wjn.v8.i7.109
Peer-review started: June 10, 2019
First decision: August 2, 2019
Revised: September 4, 2019
Accepted: September 22, 2019
Article in press: September 22, 2019
Published online: November 12, 2019
Processing time: 158 Days and 17.1 Hours
Interferons (IFNs) are characterized by a wide range of biological effects, which justifies their potential therapeutic use in several pathologies, but also elicit a wide array of adverse effects in almost every organ system. Among them, renal involvement is probably one of the most complex to identify.
We describe four cases of kidney damage caused by different IFN formulations: IFN-β-related thrombotic microangiopathy, IFN-β-induced systemic lupus erythematosus, and two cases of membranous nephropathy secondary to pegylated-IFN-α 2B. In each case, we carefully excluded any other possible cause of renal involvement. Once suspected as the casual relationship between drug and kidney damage, IFN treatment was immediately discontinued. In three cases, we observed a complete and persistent remission of clinical and laboratory abnormalities after IFN withdrawal, while the patient who developed thrombotic microangiopathy, despite IFN withdrawal and complement-inhibitor therapy with eculizumab, showed persistent severe renal failure requiring dialysis.
This case series highlights the causal relationship between IFN treatment and different types of renal involvement and enables us to delineate several peculiarities of this association.
Core tip: Different patterns of kidney damage can occur in patients treated with different interferon types, even after years of well-tolerated therapy. Interferon can cause renal dysfunction which ranges from subclinical to severe dysfunction and, regardless the mechanism of renal failure, the usual pathological finding includes either podocytopathies, interstitial nephritis, systemic lupus erythematosus-like disease or thrombotic microangiopathy. We describe four cases of interferon-related nephropathies and for each case we highlight clinical features, laboratory work-up, histological findings, treatment and follow-up. Moreover, we report for the first time a case of drug-induced systemic lupus erythematosus with renal involvement after the use of interferon-β.
- Citation: Gianassi I, Allinovi M, Caroti L, Cirami LC. Broad spectrum of interferon-related nephropathies-glomerulonephritis, systemic lupus erythematosus-like syndrome and thrombotic microangiopathy: A case report and review of literature. World J Nephrol 2019; 8(7): 109-117
- URL: https://www.wjgnet.com/2220-6124/full/v8/i7/109.htm
- DOI: https://dx.doi.org/10.5527/wjn.v8.i7.109
Interferons (IFNs) are a family of pleiotropic mediators of the immune system which collaborate in the genesis of numerous physiological processes, such as anti-viral, immunomodulatory and anti-proliferative activities[1]. This wide range of biological effects justifies their therapeutic use in several pathologies (Table 1). Class I IFNs (IFN-α and -β), in accordance to their huge biological effects, are the most widely used, while class II IFNs (IFN-γ) is mainly oriented to the macrophage response but have been less used. A wide array of adverse effects of IFNs have been described in almost every organ system[2]. Among them, renal involvement is probably one of the most complex to identify. Two potential mechanisms can be involved in IFN-related kidney injury: (1) Dose-dependent, caused by increasing cumulative drug doses and likely reversible at dose reduction; or (2) Idiosyncratic, which in general is not directly correlated with the drug dose and not necessarily reversible upon drug discontinuation[3]. Therefore, kidney damage can occur shortly after IFN-treatment beginning or after a long period of apparently well-tolerated therapy.
| Type of IFN | Hepatological indications | Immunological indications | Neurological indications | Haematological indications | Neoplastic indications |
| IFN-α 2A and IFN-α 2B | HBV-CH, HCV-CH, HDV-CH | BS, ECD | No current indications | ET, PV, CML, NHL, HCL, CTCL, WM | aRCC, MM stage II, AIDS-associated Kaposi sarcoma |
| IFN-β 1A and IFN-β 1B | No current indications | No current indications | RRSM | No current indications | No current indications |
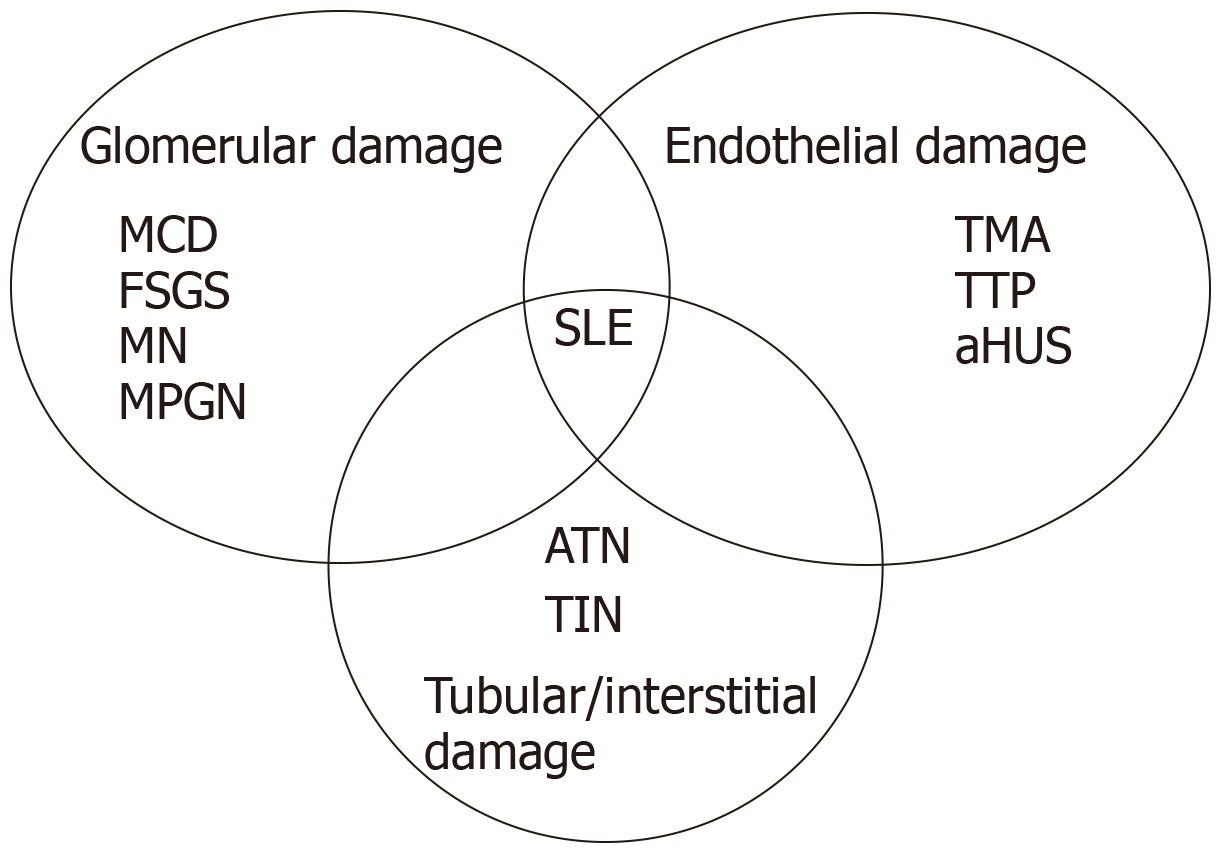
In preclinical surveys and in clinical trials, IFN-induced renal side effects were not infrequently reported but usually limited to mild proteinuria in about 20% of patients, mild abnormalities in urinalysis, and transient increase in serum creatinine[4,5]. Different types of renal toxicity have been associated with IFN therapy, mostly in case reports and including glomerulonephritis, tubulo-interstitial nephritis, systemic lupus erythematosus (SLE)-like syndrome or thrombotic microangiopathy (TMA)[6,7] (Figure 1). The causal relationship between IFN and several nephropathies is still underestimated and the incidence of renal involvement during IFN therapy remains undetermined. We herein report our case series of four patients affected by a broad spectrum of IFN-related nephropathies, among whom we report the first case with IFN-β-related SLE-like syndrome to be described in the literature.
We describe four cases of renal involvement during IFN therapy, diagnosed at our Nephrology Department during the last 10 years. Two patients were affected by relapsing-remitting multiple sclerosis (RRMS) and two patients by hepatitis C virus (HCV)-related chronic hepatitis, treated with different IFN formulation: IFN-β and pegylated (Peg)-IFN-α 2B, respectively. All patients were regularly followed up by the Multiple Sclerosis Centre of the Department of Neurosciences and Hepatitis Centre of the Department of Gastroenterology, at the Careggi University Hospital, Florence. Information on our patients, including age at onset, duration of the disease, type of IFN treatment, physical examination at the time of diagnosis, laboratory and clinical data were retrieved from medical records. We also reviewed the cases characterized by IFN-related kidney damage reported in the literature. A PubMed literature search was conducted to identify the publications discussing renal involvement in patients on IFN therapy from 2010 to 2013, using the medical subject heading terms: “interferon”, “beta-interferon”, "alpha-interferon", “nephritis”, “glomerulonephritis”, “nephropathy”, “proteinuria”, “acute renal failure”, "thrombotic microangiopathy", "haemolytic uremic syndrome", "thrombotic thrombocytopenic purpura," and "malignant hypertension." We excluded cases in whom other possible etiological factors or triggers of kidney damage were present. In cases of diabetes mellitus, monoclonal gammopathy or uncertain significance and HCV infection, which are potential causes of nephropathies but very prevalent in the elderly population, we have carefully investigated their causal role or simply their random coexistence.
Chief complaint and history of present illness: A 56-year-old woman was referred to the emergency department of our hospital due to hypertensive crisis (blood pressure 210/120 mmHg) and headache.
History of past illness: Past medical history revealed RRMS treated with IFN-β for 10 years with good response and no complications.
Physical examination: The patient presented with dyspnoea, oedema of lower limbs and purpura.
Laboratory examinations: On admission, her laboratory workup showed normocytic anaemia, thrombocytopenia, haemolysis signs and severe acute kidney injury (serum creatinine 4.5 mg/dL). Furthermore, we observed an activation of the alternative complement pathway, with low C3 (79 mg/dL; n.v. 90-180 mg/dL) and normal C4 levels. Immunological tests [cryoglobulins, anti-nuclear antibody (ANA), anti-dsDNA, antineutrophil cytoplasmic antibody (ANCA), anti-phospholipid antibodies, complement levels] as well as infectious disease tests (hepatitis B virus, HCV, human immunodeficiency virus, cytomegalovirus, Epstein–Barr virus, parvovirus B19, Escherichia coli serotype O157:H) and cancer markers were normal.
Imaging examinations: Chest x-ray showed mild pulmonary congestion, while ultrasonography of the kidney revealed a normal kidney size but increased echogenicity in the renal cortex.
Final diagnosis: The clinical and laboratory findings were compatible with the diagnosis of TMA (Table 2). Subsequent tests, carried out for suspected TMA, showed no mutations in genes of regulatory complement factors (CFH, CFI, CFB, MCP, C3, THBD) and normal ADAMTS-13 activity. The patient underwent a kidney biopsy that confirmed our first hypothesis of TMA [IF: Minimal alone nonspecific entrapment of C3 (+)].
| Case 1 | Case 2 | Case 3 | Case 4 | |
| Age in yr | 56 | 56 | 65 | 66 |
| Sex | F | F | F | M |
| Type of IFN and time of exposure | IFN-β, 10 yr | IFN-β, 2 mo | IFN-α 2B, 4 yr; Peg-IFN-α 2B, 1 yr | Peg-IFN-α 2B, 2 yr |
| Indication for IFN treatment | RRMS | RRMS | HCV-related chronic hepatitis | HCV-related chronic hepatitis |
| Clinical presentation | Dyspnoea, severe hypertension, oedema of lower limbs, purpura | Chest pain and dyspnoea | Oedema of lower limbs and oliguria | Dyspnoea, lower limbs oedema, peripheral purpura, and itch |
| Serum creatinine at onset in mg/dL | 4.5 | 2.5 | 3 | 0.83 |
| Need for dialysis | Yes | No | No | No |
| Proteinuria in g/24 h | 4 | 1.5 | 24 | 3.5 |
| Renal biopsy | Thrombotic microangiopathy | Class IV-S (A/C) Lupus nephritis | Membranous nephropathy | Membranous nephropathy |
| Treatment, in addition to IFN discontinuation | Eculizumab | IV steroids, azathioprine | ACTH, RASS-B | No |
| Recurrence after IFN withdrawal | No | No | No | No |
| Outcome | ESRD | Remission | Remission | Remission |
| Length of follow-up in yr | 3 | 4.5 | 12.5 | 5.5 |
Treatment: IFN-β was discontinued and eculizumab therapy was then started. The patient was given intravenous eculizumab dose of 900 mg weekly for 4 wk, followed by 1200 mg 1 wk later as the 5th.
Outcome and follow-up: Despite IFN withdrawal and complement-inhibitor therapy, the severe renal failure persisted. Despite persistent normal haematological values, (3 years later) our patient is still undergoing a thrice-weekly intermittent dialysis.
Chief complaint and history of present illness: A 56-year-old woman was referred to our department due to dyspnoea that had started few hours before.
History of past illness: The patient was treated with IFN-β for 2 mo because of RRMS, which was recently diagnosed.
Physical examination: The patient reported moderate dyspnoea and positional chest pain. Her physical examination was otherwise unremarkable.
Laboratory examinations: Laboratory tests showed mild thrombocytopenia, inflammation (C-reactive protein of 31 mg/dL), acute kidney injury (serum creatinine of 2.5 mg/dL) and red cell casts on urine examination. Immunological tests showed ANA (1:640), anti-dsDNA (1:320) and anti-ENA (anti-RNP/sm, anti-nucleosome, anti-SM, anti-histone) positivity, low C3 and C4 levels (Table 2).
Imaging examinations: Echocardiography and chest-x ray showed pleural and pericardial effusions.
Final diagnosis: We performed kidney biopsy, that demonstrated a pattern of diffuse proliferative nephritis, involving more than 50% of the total number of glomerula with active and chronic lesions [class IV–S(A/C) lupus nephritis (LN)]. The electron microscopy showed tubuloreticular inclusions widespread, typically identified in LN as expression of endogenous IFNs but also suspected for being linked to the exposure of excess exogenous IFNs.
Treatment: We started treatment with corticosteroids (methylprednisolone 3 intravenous pulses) and oral corticosteroids, followed by maintenance therapy (azathioprine at 2 mg/kg per os) and concomitantly; IFN-β therapy was discontinued.
Outcome and follow-up: The patient showed a rapid clinical recovery, with progressive improvement of her renal function. Immunological tests exhibited a total remission. After 6 mo, we progressively suspended immunosuppressive treatment, without any clinical and laboratory relapse. Actually, 4 and ½ years later, she is regularly followed up at our nephrology centre, exhibiting a normal kidney function and a total immunological remission without steroids or immunosuppressive drugs.
Chief complaint and history of present illness: A 65-year-old man was admitted to the emergency department due to progressive symptomatic fatigue.
History of past illness: The patient had an HCV-related chronic hepatitis, previously treated with IFN-α 2B and ribavirin for 4 years, shifted to Peg-IFN-α 2B 1 year before.
Physical examination: At presentation, the patient had fatigue, oedema of lower limbs and oliguria.
Laboratory examinations: Laboratory examinations showed acute kidney injury (serum creatinine of 3 mg/dL). Urinary tests detected nephrotic proteinuria (24 g/die) and microhaematuria. Immunological tests did not show any pathological findings, in particular cryoglobulins and anti-PLA2R antibodies were negative. Furthermore blood exams revealed a stable hepatic status, with no abnormalities suspected for HCV-related disease, and virology tests excluded any sign of HCV viremia (HCV PCR RNA-negative) (Table 2).
Imaging examinations: Chest x-ray showed minimal basal bilateral opacities, while abdominal echography was unremarkable.
Final diagnosis: We performed a kidney biopsy, which was compatible with membranous nephropathy (MN) stage I/II.
Treatment: After IFN withdrawal, the patient started double renin-angiotensin-aldosterone system (commonly known as RAAS)-blockade and immunosuppressive therapy with long-acting adrenocorticotropic hormone, which was interrupted early due to appearance of complications (hyperaldosteronism and meta-steroid diabetes) after about 1 mo.
Outcome and follow up: After 12 and ½ years, our patient is still under conservative treatment with double RAAS-block and has had no recurrence of MN.
Chief complaint, history of present illness, and relapse of clinical manifestation: A 66-year-old man presented to the emergency department with purpura and itch, which progressively appeared 1 wk before. Despite the initial clinical and laboratory recovery, 2 mo later, after IFN dose increase, our patient experienced fatigue, dyspnoea and lower limbs’ oedema.
History of past illness: The patient had HCV-related chronic hepatitis and had been treated with Peg-IFN-α 2B and ribavirin for the past 2 years with good response and no complications.
Physical examination: His physical examination revealed lower limbs’ oedema, in addition to peripheral purpura.
Laboratory examinations: Laboratory workup showed normal liver function with no signs of HCV viremia (HCV PCR RNA-negative). Nephrotic-range proteinuria (3.5 g/die) was detected at urinalysis. The patient was transferred to our nephrology ward, where we performed secondary tests that showed nephrotic syndrome (nephrotic-range proteinuria, hypoalbuminemia, lipid abnormalities, thyroid dysfunction) with normal serum creatinine (0.83 mg/dL) (Table 2). Immunological tests were all normal and, in particular, cryoglobulins and anti-PLA2R antibodies were negative.
Imaging examinations: Chest x-ray, echocardiography, and abdominal echography were unremarkable.
Diagnosis and treatment: At the beginning, the IFN daily dose was reduced because an IFN overdose had been hypothesized. Therefore, we performed kidney biopsy, which demonstrated a typical pattern of MN stage I. After IFN withdrawal, our patient showed a rapid kidney recovery with a complete remission, without immunosuppressive treatment. The patient definitively stopped IFN treatment, according with our gastroenterological centre.
Outcome and follow up: Currently, 5 and ½ years later, our patient is regularly followed up at the hepatitis centre of the department of gastroenterology, showing normal kidney function.
The case series we have presented here, together with the previously published cases on IFN-related nephropathies we reported, highlight the causal relationship between IFN treatment and different types of renal involvement, ruling out the hypothesis of a fortuitous association. Two patients with HCV-related chronic hepatitis were treated with Peg-IFN-α 2B and two patients with RRMS were treated with IFN-β, showing potentially early or late onset renal toxicity after IFN initiation. Evidence now supports the concept that IFNs contribute to several types of kidney diseases[8]. A causal association between IFN and renal toxicity was often recognized late or not diagnosed in the past. In many of these cases, the cause of this missed diagnosis was the presence of other possible etiological factors or triggers of kidney damage. This study enables us to delineate several peculiarities of this association.
IFN has been widely described to be associated with development of TMA in the scientific literature[9]. It is well known that direct toxic reactions in the course of type I IFN treatment are typically dose-dependent[10]. In fact, quantitative histopathological analyses identified a dose-dependent spectrum of microvascular disease, including endothelial hypertrophy, intimal thickening, microaneuryms, perivascular inflammatory infiltrates and reduced intracellular nitric oxide generation[10,11]. Our patient developed TMA after 10 years of IFN treatment, confirming the dose-dependent toxic effect and also indicating that long-term IFN therapy can induce late-onset TMA. The development of renal lesions after several years of well-tolerated IFN therapy may be the result of a cumulative effect in renal and systemic microvessels.
A recent review analysed 24 cases of TMA occurring in multiple sclerosis patients treated with IFN-β and the authors observed TMA-onset after a mean time of 7 years[12]. We ruled out any other possible cause of TMA, such as primary forms (typical and atypical haemolytic-uremic syndrome, thrombotic thrombocytopenic purpura) and other secondary forms (malignant hypertension, cancers, autoimmune conditions, other causative drugs). In this context, an extensive list of drugs has been associated with TMA[13]. The first case of TMA associated with IFN-α was reported in 1993[14], and the first case associated with IFN-β was reported in 1998[15]. Since then, many cases of both IFN-α- and IFN-β-related TMA have been reported in the literature, mostly as individual case reports[16-20].
First line therapy in drug-related TMA is drug discontinuation and supportive treatments. Plasma exchange, immunosuppressive drugs and also eculizumab have been described as effective therapies on refractory forms of IFN-related TMA, although mainly in case reports. Nevertheless, the prognosis of drug-related TMA and particularly IFN-related TMA is particularly severe. It is generally characterized by fulminating presentation, even after years of well-tolerated IFN treatment, with severe clinical course, poor response to therapy and bad renal prognosis. In fact, despite treatment with eculizumab, our patient developed renal dysfunction that progressed to end-stage renal disease requiring dialysis.
Several drugs can induce SLE, a condition collectively referred as drug-induced lupus (DIL)[21]. It generally shows only some manifestations of full-blown SLE (e.g., sicca syndrome, Raynaud’s phenomenon, arthritis and serositis) and tends to resolve, requiring weeks to months, after drug withdrawal. Less frequently, DIL shows major organ involvement (e.g., LN, central nervous system disease, severe haematologic abnormalities), but if these are present drug cessation should be combined with systemic immunosuppressive therapies. Idiopathic SLE and DIL differ not only for the extent of organ involvement and for the frequency and type of disease manifestations but also for the serological autoimmune profile[22]. The main immunological pattern in DIL is ANA and anti-histone antibodies positivity and, only in some cases, anti-dsDNA are present[23], depending on the type of the causative drugs (e.g., TNFα inhibitors).
IFN-related DIL is generally associated with silent SLE or lupus-like syndromes, neither with systemic symptoms nor major organ involvement[24-26]. It is well known that type I IFNs, and particularly IFN-α, play a major role in SLE pathogenesis[27] and a proportion of patients displays increased serum levels of IFN-α or an upregulation of the IFN-regulated genes[28]. Type I IFNs prime an adaptive immune response and, if IFN sensitivity or its production are increased, a self-reactive immune response may be favoured. Neutrophils can respond to exposure to type I IFNs and immune complexes with extrusion of nucleic acids and their associated proteins concurring to disease flares. In most of the cases, DIL was caused by IFN-α treatment and less frequently by IFN-γ treatment[29]. Our patient was treated with IFN-β because of RRMS and, to the best of our knowledge, our case is the first report of LN-induced by IFN-β therapy. Interestingly, in our patient the immunological tests showed anti-dsDNA (1:320), ANA (1:640) and anti-histone positivity, an immunological pattern similar to idiopathic SLE and TNFα inhibitors-related SLE. Furthermore, even if not completely understood, type I IFNs and TNFα cross-regulation is well accepted in immune-mediated inflammatory diseases[30].
Our two patients developed nephrotic syndrome with a histological pattern of membranous nephropathy while on Peg-IFN-α therapy. Cases of nephritic or nephrotic syndrome have been reported during treatment with IFN associated with different underling nephropathies, such as focal segmental glomerulosclerosis[31-34], minimal change disease[35,36], IgA nephropathy[37], and membranoproliferative glomerulonephritis[38,39]. Among these, MN is an uncommon but important and previously described pattern[40,41]. Different clinical features at onset have been described, varying from a full-blown nephritic or nephrotic syndrome, to a severe oligoanuria requiring dialysis[42,43].
In our patients, Peg-IFN-α was used as HCV-related chronic hepatitis treatment. Certainly, kidney damage is one of the most important HCV-related extra-hepatic manifestations and membranoproliferative glomerulonephritis is the most considerable histopathological pattern. However, we have to consider a broad spectrum of histopathological features, with possible glomerular and/or interstitial lesions[44]. For these reasons, to diagnose a drug-induced nephritis in patients with HCV can be difficult. In addition, IFN treatment may produce an exacerbation of a previous nephropathy associated with the HCV infection[45], making the diagnosis even more difficult. We found these two possible pathogenic explanations (HCV as a direct cause or a trigger of nephropathy) very unlikely in our patients; in fact, laboratory workup did not show any sign of viremia (HCV PCR RNA-negative at different time points) and immunological tests (cryoglobulins, complement) did not exhibit any pathological finding.
As largely known, the pathogenetic mechanism of HCV-related kidney damage includes direct invasion of pathogens (justified by HCV PCR RNA positivity) or deposition of antigen-antibody complex by immunological reaction[44,46], neither detected in our cases. Moreover, after IFN withdrawal and with no immunosuppressive drugs, we observed a clinical and laboratory complete remission, persistent after a very long follow-up. For this reason, we excluded a form of HCV-related kidney injury, suggesting a causal relationship between IFN treatment and the appearance/disappearance of the kidney damage in the context of a casual HCV coexistence.
Different pattern of kidney damage can occur in patients treated with different IFN types, even after years of well-tolerated therapy. IFN can cause renal dysfunction which ranges from subclinical to severe dysfunction and, whatever the mechanism of renal failure, the usual pathological finding includes either podocytopathies, acute tubular necrosis, interstitial nephritis, SLE-like picture, TMA, or some features of focal sclerosis. This is the first report of drug-induced SLE with renal involvement after the use of IFN-β. We recommend that a new onset of severe headache, hypertension, proteinuria or renal dysfunction in patients on IFN therapy should be identified early and evaluated thoroughly, also with consideration of a renal biopsy. Once it is suspected, interruption of IFN treatment is warranted, while the role of additional immunosuppressive therapy remains undefined. Despite IFN withdrawal, renal prognosis is bad in IFN-related TMA and kidney damage can persist and also evolve to end-stage renal disease.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Haggar M, Fujigaki Y S-Editor: Yan JP L-Editor: Filipodia E-Editor: Qi LL
| 1. | Gresser I. Biologic effects of interferons. J Invest Dermatol. 1990;95:66S-71S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Raanani P, Ben-Bassat I. Immune-mediated complications during interferon therapy in hematological patients. Acta Haematol. 2002;107:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Nerrant E, Charif M, Ramay AS, Perrochia H, Patrier L, de Champfleur NM, Renard D, Labauge P. Hemolytic uremic syndrome: an unusual complication of interferon-β treatment in a MS patient. J Neurol. 2013;260:1915-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Kurschel E, Metz-Kurschel U, Niederle N, Aulbert E. Investigations on the subclinical and clinical nephrotoxicity of interferon alpha-2B in patients with myeloproliferative syndromes. Ren Fail. 1991;13:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Quesada JR, Talpaz M, Rios A, Kurzrock R, Gutterman JU. Clinical toxicity of interferons in cancer patients: a review. J Clin Oncol. 1986;4:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 413] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Markowitz GS, Bomback AS, Perazella MA. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol. 2015;10:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Kundra A, Wang JC. Interferon induced thrombotic microangiopathy (TMA): Analysis and concise review. Crit Rev Oncol Hematol. 2017;112:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Ben-Amor AF, Trochanov A, Fischer TZ. Cumulative Review of Thrombotic Microangiopathy, Thrombotic Thrombocytopenic Purpura, and Hemolytic Uremic Syndrome Reports with Subcutaneous Interferon β-1a. Adv Ther. 2015;32:445-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kavanagh D, McGlasson S, Jury A, Williams J, Scolding N, Bellamy C, Gunther C, Ritchie D, Gale DP, Kanwar YS, Challis R, Buist H, Overell J, Weller B, Flossmann O, Blunden M, Meyer EP, Krucker T, Evans SJ, Campbell IL, Jackson AP, Chandran S, Hunt DP. Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood. 2016;128:2824-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Jia H, Thelwell C, Dilger P, Bird C, Daniels S, Wadhwa M. Endothelial cell functions impaired by interferon in vitro: Insights into the molecular mechanism of thrombotic microangiopathy associated with interferon therapy. Thromb Res. 2018;163:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Allinovi M, Cirami CL, Caroti L, Antognoli G, Farsetti S, Amato MP, Minetti EE. Thrombotic microangiopathy induced by interferon beta in patients with multiple sclerosis: three cases treated with eculizumab. Clin Kidney J. 2017;10:625-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Reese JA, Bougie DW, Curtis BR, Terrell DR, Vesely SK, Aster RH, George JN. Drug-induced thrombotic microangiopathy: Experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015;90:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Stratta P, Canavese C, Dogliani M, Thea A, Degani G, Mairone L, Vercellone A. Hemolytic-uremic syndrome during recombinant alpha-interferon treatment for hairy cell leukemia. Ren Fail. 1993;15:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ubara Y, Hara S, Takedatu H, Katori H, Yamada K, Yoshihara K, Matsushita Y, Yokoyama K, Takemoto F, Yamada A, Takagawa R, Endo Y, Hara M, Koida I, Kumada H. Hemolytic uremic syndrome associated with beta-interferon therapy for chronic hepatitis C. Nephron. 1998;80:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Demoulin N, Jadoul M. Interferon-α-induced thrombotic microangiopathy in patients with chronic myelogenous leukemia. Kidney Int. 2014;85:213-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Zuber J, Martinez F, Droz D, Oksenhendler E, Legendre C; Groupe D'stude Des Nephrologues D'ile-de-France (GENIF). Alpha-interferon-associated thrombotic microangiopathy: a clinicopathologic study of 8 patients and review of the literature. Medicine (Baltimore). 2002;81:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Vosoughi R, Marriott JJ. Thrombotic microangiopathy in Interferon Beta treated multiple sclerosis patients: Review of literature and report of two new cases. Mult Scler Relat Disord. 2014;3:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Hunt D, Kavanagh D, Drummond I, Weller B, Bellamy C, Overell J, Evans S, Jackson A, Chandran S. Thrombotic microangiopathy associated with interferon beta. N Engl J Med. 2014;370:1270-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Yam C, Fok A, Mclean C, Butler E, Kempster P. Interferon-beta in multiple sclerosis causing thrombotic microangiopathy. Intern Med J. 2019;49:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci. 2007;1108:166-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Vaglio A, Grayson PC, Fenaroli P, Gianfreda D, Boccaletti V, Ghiggeri GM, Moroni G. Drug-induced lupus: Traditional and new concepts. Autoimmun Rev. 2018;17:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Bonanni A, Vaglio A, Bruschi M, Sinico RA, Cavagna L, Moroni G, Franceschini F, Allegri L, Pratesi F, Migliorini P, Candiano G, Pesce G, Ravelli A, Puppo F, Martini A, Tincani A, Ghiggeri GM. Multi-antibody composition in lupus nephritis: isotype and antigen specificity make the difference. Autoimmun Rev. 2015;14:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Morris LF, Lemak NA, Arnett FC, Jordon RE, Duvic M. Systemic lupus erythematosus diagnosed during interferon alfa therapy. South Med J. 1996;89:810-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Boonen A, Stockbrügger RW, van der Linden S. Pericarditis after therapy with interferon-alpha for chronic hepatitis C. Clin Rheumatol. 1999;18:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Conlon KC, Urba WJ, Smith JW 2nd, Steis RG, Longo DL, Clark JW. Exacerbation of symptoms of autoimmune disease in patients receiving alpha-interferon therapy. Cancer. 1990;65:2237-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192:5459-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 414] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 28. | Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1745] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 29. | Graninger WB, Hassfeld W, Pesau BB, Machold KP, Zielinski CC, Smolen JS. Induction of systemic lupus erythematosus by interferon-gamma in a patient with rheumatoid arthritis. J Rheumatol. 1991;18:1621-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Cantaert T, Baeten D, Tak PP, van Baarsen LG. Type I IFN and TNFα cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res Ther. 2010;12:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Coroneos E, Petrusevska G, Varghese F, Truong LD. Focal segmental glomerulosclerosis with acute renal failure associated with alpha-interferon therapy. Am J Kidney Dis. 1996;28:888-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Bremer CT, Lastrapes A, Alper AB, Mudad R. Interferon-alpha-induced focal segmental glomerulosclerosis in chronic myelogenous leukemia: a case report and review of the literature. Am J Clin Oncol. 2003;26:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Markowitz GS, Nasr SH, Stokes MB, D'Agati VD. Treatment with IFN-{alpha}, -{beta}, or -{gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 34. | Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, Sagrinati C, Ballerini L, Peired A, Shankland SJ, Liapis H, Romagnani P, Anders HJ. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol. 2013;183:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Dizer U, Beker CM, Yavuz I, Ortatatli M, Ozguven V, Pahsa A. Minimal change disease in a patient receiving IFN-alpha therapy for chronic hepatitis C virus infection. J Interferon Cytokine Res. 2003;23:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Rettmar K, Kienast J, van de Loo J. Minimal change glomerulonephritis with reversible proteinuria during interferon alpha 2a therapy for chronic myeloid leukemia. Am J Hematol. 1995;49:355-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Gordon A, Menahem S, Mitchell J, Jenkins P, Dowling J, Roberts SK. Combination pegylated interferon and ribavirin therapy precipitating acute renal failure and exacerbating IgA nephropathy. Nephrol Dial Transplant. 2004;19:2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Kimmel PL, Abraham AA, Phillips TM. Membranoproliferative glomerulonephritis in a patient treated with interferon-alpha for human immunodeficiency virus infection. Am J Kidney Dis. 1994;24:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Cardineau E, Le Goff C, Henri P, Reman O, Lobbedez T, Hurault de Ligny B, Leporrier M, Ryckelynck JP. [Nephropathies caused by interferon alpha: apropos of 2 cases]. Rev Med Interne. 1995;16:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Tsai MS, Chen JH, Fang YW, Yang AH, Chang CH. Membranous nephropathy induced by pegylated interferon alpha-2a therapy for chronic viral hepatitis B. Clin Nephrol. 2012;77:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Auty A, Saleh A. Nephrotic syndrome in a multiple sclerosis patient treated with interferon beta 1a. Can J Neurol Sci. 2005;32:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Nishimura S, Miura H, Yamada H, Shinoda T, Kitamura S, Miura Y. Acute onset of nephrotic syndrome during interferon-alpha retreatment for chronic active hepatitis C. J Gastroenterol. 2002;37:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Miranda-Guardiola F, Fernández-Llama P, Badia JR, Botey A, Estruch R, Darnell A, Rozman C, Revert L. Acute renal failure associated with alpha-interferon therapy for chronic hepatitis B. Nephrol Dial Transplant. 1995;10:1441-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Ferri C, Giuggioli D, Colaci M. Renal Manifestations of Hepatitis C Virus. Clin Liver Dis. 2017;21:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Ohta S, Yokoyama H, Wada T, Sakai N, Shimizu M, Kato T, Furuichi K, Segawa C, Hisada Y, Kobayashi K. Exacerbation of glomerulonephritis in subjects with chronic hepatitis C virus infection after interferon therapy. Am J Kidney Dis. 1999;33:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Fabrizi F, Aghemo A, Messa P. Hepatitis C treatment in patients with kidney disease. Kidney Int. 2013;84:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |