Published online May 6, 2016. doi: 10.5527/wjn.v5.i3.274
Peer-review started: July 31, 2015
First decision: August 14, 2015
Revised: February 2, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: May 6, 2016
Processing time: 271 Days and 16.2 Hours
Chronic inflammation and nutritional imbalance are important comorbid conditions that correlate with poor clinical outcomes in children with chronic kidney disease (CKD). Nutritional disorders such as cachexia/protein energy wasting, obesity and growth retardation negatively impact the quality of life and disease progression in children with CKD. Inadequate nutrition has been associated with growth disturbances in children with CKD. On the other hand, over-nutrition and obesity are associated with poor outcomes in children with CKD. The exact mechanisms leading to these unfavorable conditions are not fully elucidated and are most likely multifactorial. In this review, we focus on the pathophysiology of nutrition disorders and inflammation and their impact on clinical outcomes in children with CKD.
Core tip: Nutritional imbalances, such as protein energy wasting, cachexia, obesity and growth retardation, have been associated with poor clinical outcomes in children with chronic kidney disease (CKD). Chronic inflammation may lead to further deterioration of nutritional imbalance in advanced CKD patients. Results of recent studies have increased awareness of the importance of chronic inflammation and nutritional imbalance in children with CKD.
- Citation: Tu J, Cheung WW, Mak RH. Inflammation and nutrition in children with chronic kidney disease. World J Nephrol 2016; 5(3): 274-282
- URL: https://www.wjgnet.com/2220-6124/full/v5/i3/274.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i3.274
Nutritional imbalance is prevalent in children with chronic kidney disease (CKD) and may influence clinical outcomes. Wasting, defined as low weight proportion to height, is the consequence of inadequate nutrition intake, and highly prevalent in children with CKD. The term cachexia or wasting syndrome has been defined as the pathological combination of a dramatic decrease in appetite and increase in the metabolism of fat and lean body mass[1]. The International Society of Renal Nutrition and Metabolism expert panel defined the term protein energy wasting (PEW) as a state of decreased body stores of protein and energy fuels (body protein and fat masses)[2]. PEW/cachexia, a complex condition of metabolic and nutritional derangement, has been associated with not only malnutrition, but also maladaptive responses, such as anorexia, increased metabolic rate, decreased protein store, reduced body weight and muscle mass. PEW/cachexia cannot be reversed nutritionally. In contrast to PEW/cachexia, malnutrition is the consequence of insufficiency of energy intake and is accompanied by adaptive responses, including hunger, a protective decrease in energy expenditure, preferential use of fat stores for energy and preservation of lean body mass. Nutrition supplementation cannot reverse nutritional deficiency in malnourished patients. Thus, PEW/cachexia and malnutrition are not identical. In addition to PEW/cachexia, two common features of nutritional imbalance in children with CKD are obesity and growth failure[3-7]. This review focuses on these nutrition disorders and the pathophysiological role of chronic inflammation in children with CKD.
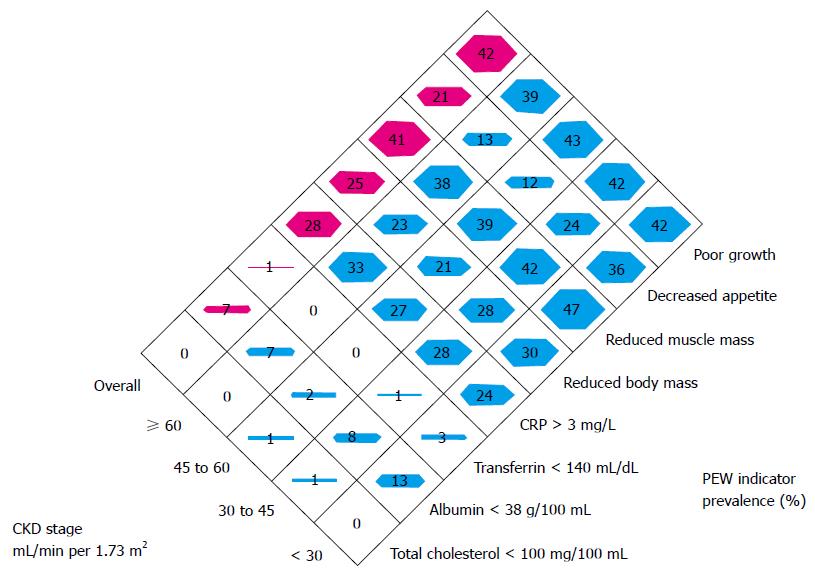
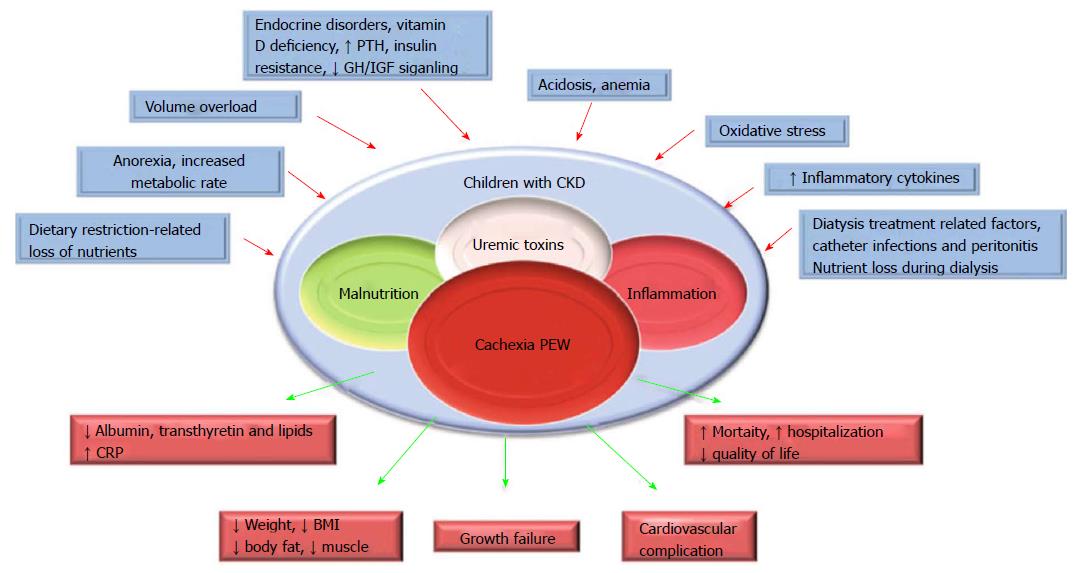
PEW and cachexia is highly prevalent in CKD patients. PEW was evident in 20% to 75% adult dialysis patients. Studies of CKD in children (CKiD), a multi-center prospective cohort study of children aged 1 to 16 in United States, revealed the prevalence of PEW estimates ranged from 6% to 65%. The wide range of prevalence of PEW in this cohort is likely due to difference in diagnostic criteria[5]. To better define PEW in children with CKD, we evaluated prevalence of PEW using different diagnostic criteria. The incidence of PEW in CKiD ranged from 7% to 20% by applying 3 different diagnostic criteria, namely, a minimal, a standard and a modified PEW definition (Figure 1). Our results suggested that only the modified PEW diagnostic criteria, which included growth retardation as a criterion, showed modest significance. Our modified PEW diagnostic criteria for children with CKD is defined as the standard ≥ 3 of the 4 criteria as described in adults PEW (biochemical parameters, body and muscle mass assessments and anorexia) with the additional incorporation of growth retardation as a diagnostic criteria. The etiology of CKD-associated PEW is complex. Common risk factors for PEW in CKD, such as poor nutrition, systemic inflammation, endocrine disorder, comorbid condition, fluid overload and metabolic acidosis have been listed (Figure 2)[1,3,5]. Of the many complications of CKD-associated PEW/cachexia, CKD patients are prone to muscle weakness and as a result, have difficulties performing their daily routine of activities. Other systemic consequences of PEW in children with CKD comprise increased risk of cardiovascular disease, infection, depression, prolonged hospitalization and mortality, and growth retardation[3,5]. The incidence rates of hospitalization were almost 2-fold higher for CKD children with PEW[3]. Mortality rate in patients with CKD is 100-200 times higher than the general population[8] and represents a major burden to health systems. Importantly, high mortality in patients with CKD has been associated with components of risk factor of PEW/cachexia as listed in Figure 2.
Anorexia is prevalent and has contributed to the nutritional imbalance and growth failure in children with CKD[5]. Ironically, another nutritional disorder - over-nutrition and obesity, is also prevalent in children with CKD[9,10]. Prevalence of overweight or obesity (34%) exceeds the prevalence of PEW in CKiD cohort. Prevalence of overweight or obesity in children with glomerular and non-glomerular CKD was 46% and 32%, respectively[11]. In a large cohort of European pediatric renal replacement therapy (RRT) population, the prevalence of overweight and obesity far exceeded the prevalence of underweight (20.8%, 12.5% vs 3.5%, respectively)[12]. There was a significant increase in body mass index (BMI) after the initiation of RRT in this study cohort. Short stature and glucocorticoid treatment were further associated with an increased risk of overweight and obesity in this transplanted population. Other risk factors strongly associated with increased BMI in patients with RRT were lower initial BMI and higher age at the initiation of RRT, longer duration of dialysis as well as a longer time with a functioning graft[12].
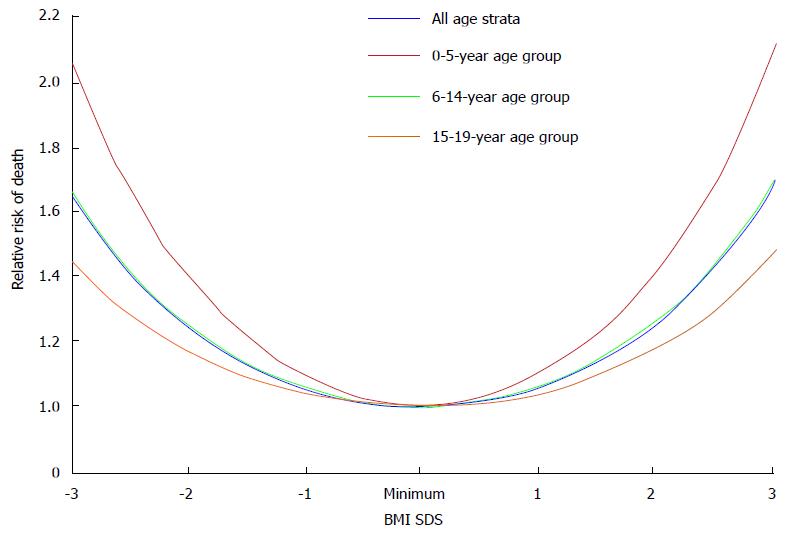
The obesity paradox or reverse epidemiology is a controversial hypothesis[13]. It proposes that obesity may, contrary to conventional wisdom, be related to decreased morbidity and mortality in some populations. This hypothesis has been reported in patients with heart failure, myocardial infarction, and acute coronary syndrome[13,14]. Nevertheless, it was not consistently supported by data in end-stage renal disease (ESRD) patients. Indeed, initial analysis of epidemiologic studies have shown a strong survival advantage of obesity in dialysis patients with the primary outcomes of all-cause and cardiovascular mortality[14]; and low BMI values are associated with increased mortality rate. However, there is a fundamental flaw in the study design as those investigators compared short-term mortality rate in dialysis patients vs long-term mortality rate in the general population. No evidence of reverse epidemiology of BMI and survival advantage was found in dialysis patients when both patients and general population were analyzed with the same time frame for outcomes, even with multivariable adjustments for age and race[15]. More recently, association of BMI values with all-cause of mortality rate and disease progression was analyzed in a large cohort of adult predialysis CKD patients. BMI showed a U-shaped relationship with clinical outcomes, with the best outcomes observed in overweight and mildly obese patients[16]. Similar findings were observed in children with ESRD, the showing of a U-shaped relationship between BMI values and the risk of all-cause mortality rate (Figure 3). Higher mortality rate was observed in obese children relative to non-obese children with CKD after renal transplantation (27% vs 17%, respectively)[17]. Furthermore, childhood and adolescent obesity have negative impacts on the cardiovascular health. Obesity in adolescence was positively associated with death rate in future decades[18]. Obesity per se is a strong and independent risk factor for the progression of CKD. Obesity hastens the deterioration of renal function among patients with IgA nephropathy and unilateral renal agenesis[19-21]. In another study, progression of CKD is increased by 1.23 fold for each standard deviation increment of BMI values[22].
Poor nutrition contributes to the high prevalence of growth retardation in children with CKD but growth retardation may still persist despite improvement of nutritional status in this population. Recent data from International Pediatric Peritoneal Dialysis Network registry suggested that enteral feeding by nasogastric or gastrostomy tube improved nutritional status, as indicated by an increment of BMI values in pediatric patients with stage 5 CKD. Nevertheless, nutritional supplementation did not attenuate growth failure in this population[23]. Growth failure has been associated with poor clinical outcomes of increased morbidity and mortality rate in children with CKD. About one third of children enrolled in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry in 2005 had severe short status[24]. Similar findings were observed in a recent report from the same registry in 2011[25]. Prevalence of growth retardation was 29.3% for children enrolled in the Serbian Pediatric Registry of CKD[26].
The etiology of poor growth in CKD is multifactorial and can be associated with poor nutritional status as well as other comorbidities such as metabolic acidosis, anemia, bone and mineral disorders, genetic factors and perturbations in growth hormone (GH) and insulin-like growth factor (IGF)-I axis signaling pathways. Data from NAPRTCS showed that the greatest height deficits were observed in youngest CKD patients prior to entering RRT programs. After renal transplantation, the greatest height improvement was observed for those youngest patients with the greatest height deficits prior to their RRT[9]. Growth retardation is associated with poor clinical outcomes in children with ESRD. Five year mortality rate for children on hemodialysis with severe growth failure, moderate growth failure and normal growth was 16.2%, 11.5% and 5.6%, respectively. Moreover, higher hospitalization rate was observed in ESRD children with severe and moderate growth failure relative to those with normal growth[27].
Levels of serum inflammatory markers such as C-reactive protein (CRP), IL-6 and TNF-α were elevated in CKD patients[28]. The etiology of CKD-associated inflammation is multifactorial. Important factors include decreased glomerular filtration rate, underlying disorders and other complications of CKD[29].
Chronic inflammation is important for the pathogenesis of PEW/cachexia in patients with CKD through various mechanisms including leptin and melanocortin signaling modulation, inflammatory cytokines and nuclear factor kappa B (NFκB) signaling.
Leptin is an anorexigenic hormone. Leptin is mainly secreted by adipose tissues and modulates energy homeostasis through melanocortin signaling. Leptin signaling in the hypothalamus nuclei is enabled by inhibiting neuropeptide Y (NPY) and agouti related peptide (AgRP) neurons and by stimulating pro-opiomelanocortin neurons, which in turn activates the release of α-melanocyte-stimulating hormone and stimulates the type 4 melanocortin receptor signaling (MC4R)[30,31]. Transgenic mice over-expressing leptin had reduced energy consumption relative to controls[32]. Leptin is degraded from the circulation in the renal tubules. Serum levels of leptin were elevated in CKD patients with the decline in renal glomerular filtration function[33,34]. We have shown that leptin/melanocortin signaling is an important mechanism underlying CKD-associated cachexia. Transgenic mice with deletion of leptin receptor (db/db) and MC4-R knockout attenuated aberrant metabolic effects of CKD-associated cachexia[35]. Administration of AgRP, a natural MC4R antagonist, normalized food intake, total weight gain, improved lean mass content as well as basal metabolic rate in CKD mice relative to control mice[36]. We also evaluated the effects of leptin receptor antagonism in CKD mice. Administration of pegylated leptin receptor antagonist (PLA) attenuated food intake, weight gain, improved lean mass and in vivo muscle function as well as normalized basal metabolic rate in mice with CKD. In addition, the administration of PLA significantly decreased expression of uncoupling proteins and corrected aberrant muscle mass signaling pathway as well as normalized muscle protein levels of IL-1α, IL-1β, IL-6, and TNF-α in CKD mice[37]. Thus, inhibition of the leptin/melanocortin signal pathway may represent a novel therapeutic approach for CKD-associated cachexia. Elevated serum levels of leptin were associated with higher prevalence of PEW/cachexia in patients. Malnourished patients had higher serum levels of leptin than those without malnutrition[38]. Increases in serum leptin levels have been associated with inflammation and a decrease in lean mass content in dialysis patients[39].
Increased levels of serum inflammatory cytokines were associated with poor clinical outcomes in patients with CKD[40]. Loss of kidney function, uremia and dialysis treatment per se are important causes of inflammation in this population. In addition, gene polymorphisms of inflammatory cytokines have been implicated in CKD patients[41]. Polymorphisms of TNF-α gene predisposed malnutrition and inflammation in patients with ESRD[42]. Robust evidence supports a direct pathologic role of IL-1α, IL-6, and TNF-α in the development of PEW. Muscle wasting is a cardinal feature of CKD. Elevation of pro-inflammatory cytokines stimulates muscle catabolism. In animal models of CKD, IL-1, IL-6 and TNF-α stimulate inflammation in animal models of CKD. Increased serum levels of IL-6 correlated with increased muscle catabolism while the antagonist of IL-6 receptor attenuated CKD-associated muscle wasting[43].
PI3K-Akt signal transduction pathway mediates muscle metabolism in response to various extracellular signals. Aberrant PI3K/Akt pathway has been implicated in the etiology of muscle wasting. In skeletal muscle, Akt signaling mediates muscle fast/glycolytic fiber metabolism and muscle atrophy in CKD is associated with reduced Akt signaling in skeletal muscle tissue. In a mouse model of CKD, reduced Akt signaling was associated with skeletal muscle wasting. In contrast, skeletal muscle-specific Akt1 transgenic mice promoted skeletal muscle growth[44]. Akt1 transgenic mice attenuated renal fibrosis, apoptosis, and inflammation in unilateral ureteral obstruction-induced CKD mice. Importantly, maintenance of muscle mass is associated with favorable clinical outcomes while muscle wasting is related to deterioration of renal function in patients with CKD[45].
Pro-inflammatory cytokines signal through the central nervous system and induce anorexia[46]. A meta-analysis of 22 studies with 924 participants (anorexia nervosa = 512, health controls = 412) has shown that compared to controls, the serum level of TNF-α, IL-1β, IL-6 and TNF-receptor-II were elevated in anorexia nervosa[47]. An animal study demonstrated that anorectic effects were observed following acute administration of exogenous TNF-α and IL-1β to mice[48]. Cytokines regulates energy expenditure. Infusion of IL-1 increased resting energy expenditure in rats and administration of recombinant TNF-α increased energy expenditures in patients with disseminated cancer[49,50].
Activation of intracellular NFκB system has been correlated with PEW/cachexia in CKD[51]. Several recent articles provide comprehensive reviews for the NFκB family of transcription factors and its regulation[52]. Cytokines induce muscle wasting via activation of NFκB while blockade of NFκB signaling attenuates muscle atrophy. Denervation-induced muscle atrophy was significantly improved in muscle specific IKK knockout mice[53]. What are the underlying mechanisms by which activation of NFκB induce significant muscle atrophy? First, ubiquitin-proteasome system (UPS) promoted muscle protein degradation and activation of NFκB stimulated expression of protein levels of several components of UPS. Second, NFκB increased the expression of several NFκB-regulated molecules, especially pro-inflammatory cytokines. This positive feedback loop resulted in the over-stimulation of NFκB and the subsequent muscle atrophy. Third, NFκB suppressed myogenic differentiation likely through the activation of transcription factor YY1[54]. And fourth, NFκB may suppress energy intake likely through the suppression of NPY. Phenylpropanolamin (PPA), a synthetic sympathomimetic amine, suppressed food intake likely via the signaling of hypothalamic NPY. Cerebral NFκB knockdown attenuated the anorexic effects in PPA-treated rats by decreasing the expression of NPY and antioxidants[55].
Adipose tissue is an important energy reservoir and an active metabolic organ secreting numerous hormones. The adipokines are cell signaling proteins secreted by adipose tissue, including leptin, adiponectin, IL-6, TNF-α, and monocyte chemotactic protein-1[56]. Adipose tissue is an important source of inflammation in CKD patients. Adipokines mediate inflammation and accelerate the progression of vascular disease in patients with CKD[57]. Chronic inflammation may accelerate the progression of renal dysfunction in CKD patients. Elevated expression of adipokines was associated with increased numbers of infiltrated immunocompetent cells in adipose tissue in obese CKD patients[58]. Elevated serum inflammatory markers such as IL-6, TNF-α and CRP are correlated with thickness of carotid intima media and associated with high mortality rate in CKD patients. Increased expression of inflammatory cytokines in adipose tissue may accelerate atherosclerosis and induce deterioration of renal function in obese CKD patients[9,59].
Perturbation in the GH/IGF-I axis is an important cause of growth failure in CKD children. GH/IGF-I mediated postnatal growth, body composition and renal function. GH binds to its receptor (GHR) and subsequently regulates the expression of GH-regulated genes, including the IGF-I gene. GH insensitivity is commonly observed in growth retarded CKD children, as serum levels of GH were normal or even elevated in this population. Pharmacological or endogenous GH treatments have diminished growth-promoting effects in children with CKD. CKD caused a post-receptor defect in GH pathway via the JAK/STAT signaling which in turn, resulted in reduced expression of IGF-I[60]. GH induces the expression of suppressors of cytokine signaling (SOCS) via the JAK-STAT signaling pathway. SOCS proteins, in turn, inactivates GHR/JAK2 complex, thus establishing a feedback loop for GH activity. CKD-induced GH insensitivity was mediated by activation of GH-JAK2 via STAT transduction and the overexpression of SOCS proteins[61].
IGF-I stimulates longitudinal growth at the growth plate. Circulating IGF-I complex constitutes of IGF-I, IGF binding protein (IGFBP) and acid labile subunit. Decline in renal function in CKD patients is associated with elevated serum IGFBP1 levels and the concomitant diminished IGF-I bioactivity. Increased IGFBPs levels have been associated with decreased longitudinal growth in CKD children. A recent study further exploited the underlying mechanism of CKD-induced GH insensitivity. In CKD rats with acute inflammation, endotoxin aggregates GH resistance and reduced IGF-I gene expression, and this effect is related to the increased production of pro-inflammatory cytokines[62,63].
Maternal malnutrition negatively influences the fetal and early life development. This critical period of pre- and early postnatal development exerts long-term effects on body weight and growth. An inadequate or excess maternal nutritional environment may activate multiple fetal responses which persist postnatally and have been correlated with the development of chronic diseases, including CKD and nutritional disorders[64]. Low birth weight (LBW) was associated with impaired renal reserve (a reduction in the number of nephrons) and structure per se (smaller renal size)[65-67]. Results from animal studies strongly support the notion that maternal malnutrition caused intrauterine growth retardation and a nephron deficit[66]. LBW was correlated with increased prevalence of early-onset CKD. The odds ratio for ESRD was 1.4 in adults who were born underweight[68]. LBW was correlated with deterioration of renal function in CKD patients[69]. Low nephron numbers was a risk factor for hypertension, likely due to the effect of compensatory hypertrophy in the setting of a low nephron number.
Intrauterine and early-life environment substantially impact the development of obesity in childhood and in adulthood. Animal and human studies suggested that an adverse in utero environment such as intrauterine growth restriction (IUGR) was closely associated with postnatal development of obesity. Studies also showed that IUGR fetuses exhibited increased body fat accumulation, reduced serum levels of leptin and aberrant epigenomic properties, which subsequently promoted obesity in adult life. Food restriction during rat pregnancy produced hypoglycemic IUGR pups. Subsequently, for those IUGR pups permitted rapid catch-up growth, they exhibited aberrant metabolic responses including hypertriglyceridemia and adult obesity with insulin-resistance. The concept of developmental origins of health and disease has been generally recognized. Infants born to obese, overweight, and diabetic mothers as well as infants born to malnourished mothers are associated with a higher risk of chronic illnesses in adult life. High birth weight enhances the risk of developing obesity and CKD in adult life[64]. On the other hand, LBW accompanied by an accelerated catch-up growth has also correlated with an increased risk of obesity and CKD in adulthood. In an observational study, LBW and small gestational age in infants were associated with poor growth outcomes in children with mild to moderate CKD[70].
Nutritional disorders, including PEW, cachexia, obesity and growth failure, have major impacts on clinical outcomes in children with CKD. Chronic inflammation is important for the pathogenesis of nutritional disorders in CKD. Increased awareness of nutritional status is needed for CKD children. Further research into the pathophysiology may yield novel therapies for CKD-associated nutritional disorders.
| 1. | Mak RH, Ikizler TA, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Erratum to: Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1382] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 3. | Abraham AG, Mak RH, Mitsnefes M, White C, Moxey-Mims M, Warady B, Furth SL. Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2014;29:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Nourbakhsh N, Rhee CM, Kalantar-Zadeh K. Protein-energy wasting and uremic failure to thrive in children with chronic kidney disease: they are not small adults. Pediatr Nephrol. 2014;29:2249-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Rees L, Mak RH. Nutrition and growth in children with chronic kidney disease. Nat Rev Nephrol. 2011;7:615-623. [PubMed] |
| 6. | Mehls O, Lindberg A, Nissel R, Haffner D, Hokken-Koelega A, Ranke MB. Predicting the response to growth hormone treatment in short children with chronic kidney disease. J Clin Endocrinol Metab. 2010;95:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Mastrangelo A, Paglialonga F, Edefonti A. Assessment of nutritional status in children with chronic kidney disease and on dialysis. Pediatr Nephrol. 2014;29:1349-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Peev V, Nayer A, Contreras G. Dyslipidemia, malnutrition, inflammation, cardiovascular disease and mortality in chronic kidney disease. Curr Opin Lipidol. 2014;25:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Stolic R. Obesity in renal failure--health or disease? Med Hypotheses. 2010;75:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Tullus K. Is there an obesity-related epidemic of CKD starting already in childhood? Nephrol Dial Transplant. 2013;28 Suppl 4:iv114-iv116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Rodig NM, McDermott KC, Schneider MF, Hotchkiss HM, Yadin O, Seikaly MG, Furth SL, Warady BA. Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol. 2014;29:1987-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Bonthuis M, van Stralen KJ, Verrina E, Groothoff JW, Alonso Melgar Á, Edefonti A, Fischbach M, Mendes P, Molchanova EA, Paripović D, Peco-Antic A, Printza N, Rees L, Rubik J, Stefanidis CJ, Sinha MD, Zagożdżon I, Jager KJ, Schaefer F. Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol Dial Transplant. 2013;28 Suppl 4:iv195-iv204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 876] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433-442. [PubMed] |
| 15. | de Mutsert R, Snijder MB, van der Sman-de Beer F, Seidell JC, Boeschoten EW, Krediet RT, Dekker JM, Vandenbroucke JP, Dekker FW. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol. 2007;18:967-974. [PubMed] |
| 16. | Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25:2088-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Hanevold CD, Ho PL, Talley L, Mitsnefes MM. Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2005;115:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 19. | Bonnet F, Deprele C, Sassolas A, Moulin P, Alamartine E, Berthezène F, Berthoux F. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2001;37:720-727. [PubMed] |
| 20. | González E, Gutiérrez E, Morales E, Hernández E, Andres A, Bello I, Díaz-González R, Leiva O, Praga M. Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 2005;68:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Praga M, Hernández E, Herrero JC, Morales E, Revilla Y, Díaz-González R, Rodicio JL. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Kalantar-Zadeh K, Kopple JD. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:701; author reply 701-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Rees L, Azocar M, Borzych D, Watson AR, Büscher A, Edefonti A, Bilge I, Askenazi D, Leozappa G, Gonzales C. Growth in very young children undergoing chronic peritoneal dialysis. J Am Soc Nephrol. 2011;22:2303-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | North American Pediatric Renal Trials and Collaborative Studies Online. NAPRTCS 2005 Annual Report: Renal Transplantation, Dialysis, Chronic Renal Insufficiency [online]. Available from: https: //web.emmes.com/study/ped/annlrept/annlrept2005.pdf. |
| 25. | North American Pediatric Renal Trials and Collaborative Studies Online. NAPRTCS 2011 Annual Report: Renal Transplantation, Dialysis, Chronic Renal Insufficiency [online]. Available from: https: //web.emmes.com/study/ped/annlrept/annlrept2011.pdf. |
| 26. | Salević P, Radović P, Milić N, Bogdanović R, Paripović D, Paripović A, Golubović E, Milosević B, Mulić B, Peco-Antić A. Growth in children with chronic kidney disease: 13 years follow up study. J Nephrol. 2014;27:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR. Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol. 2002;17:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Impellizzeri D, Esposito E, Attley J, Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD). Pharmacol Res. 2014;81:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Kalantar-Zadeh K, Stenvinkel P, Pillon L, Kopple JD. Inflammation and nutrition in renal insufficiency. Adv Ren Replace Ther. 2003;10:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Alix PM, Guebre-Egziabher F, Soulage CO. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie. 2014;105:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Engineer DR, Garcia JM. Leptin in anorexia and cachexia syndrome. Int J Pept. 2012;2012:287457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Cumin F, Baum HP, Levens N. Mechanism of leptin removal from the circulation by the kidney. J Endocrinol. 1997;155:577-585. [PubMed] |
| 34. | Daschner M, Tönshoff B, Blum WF, Englaro P, Wingen AM, Schaefer F, Wühl E, Rascher W, Mehls O. Inappropriate elevation of serum leptin levels in children with chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. J Am Soc Nephrol. 1998;9:1074-1079. [PubMed] |
| 35. | Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest. 2005;115:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Cheung WW, Mak RH. Melanocortin antagonism ameliorates muscle wasting and inflammation in chronic kidney disease. Am J Physiol Renal Physiol. 2012;303:F1315-F1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Cheung WW, Ding W, Gunta SS, Gu Y, Tabakman R, Klapper LN, Gertler A, Mak RH. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. J Am Soc Nephrol. 2014;25:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Besbas N, Ozaltin F, Coşkun T, Ozalp S, Saatçi U, Bakkaloğlu A, El Nahas AM. Relationship of leptin and insulin-like growth factor I to nutritional status in hemodialyzed children. Pediatr Nephrol. 2003;18:1255-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Stenvinkel P, Lindholm B, Lönnqvist F, Katzarski K, Heimbürger O. Increases in serum leptin levels during peritoneal dialysis are associated with inflammation and a decrease in lean body mass. J Am Soc Nephrol. 2000;11:1303-1309. [PubMed] |
| 40. | Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 351] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | Braosi AP, de Souza CM, Luczyszyn SM, Dirschnabel AJ, Claudino M, Olandoski M, Probst CM, Garlet GP, Pecoits-Filho R, Trevilatto PC. Analysis of IL1 gene polymorphisms and transcript levels in periodontal and chronic kidney disease. Cytokine. 2012;60:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Sharma R, Agrawal S, Saxena A, Sharma RK. Association of IL-6, IL-10, and TNF-α gene polymorphism with malnutrition inflammation syndrome and survival among end stage renal disease patients. J Interferon Cytokine Res. 2013;33:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Ikizler TA. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 45. | Hanatani S, Izumiya Y, Araki S, Rokutanda T, Kimura Y, Walsh K, Ogawa H. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol. 2014;25:2800-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Meuwese CL, Carrero JJ, Stenvinkel P. Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contrib Nephrol. 2011;171:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Solmi M, Veronese N, Favaro A, Santonastaso P, Manzato E, Sergi G, Correll CU. Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (2)] |
| 48. | Wu W, Zhang H. Role of tumor necrosis factor-α and interleukin-1β in anorexia induction following oral exposure to the trichothecene deoxynivalenol (vomitoxin) in the mouse. J Toxicol Sci. 2014;39:875-886. [PubMed] |
| 49. | Tocco-Bradley R, Georgieff M, Jones CT, Moldawer LL, Dinarello CA, Blackburn GL, Bistrian BR. Changes in energy expenditure and fat metabolism in rats infused with interleukin-1. Eur J Clin Invest. 1987;17:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Starnes HF, Warren RS, Jeevanandam M, Gabrilove JL, Larchian W, Oettgen HF, Brennan MF. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988;82:1321-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 201] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Rangan G, Wang Y, Harris D. NF-kappaB signalling in chronic kidney disease. Front Biosci (Landmark Ed). 2009;14:3496-3522. [PubMed] |
| 52. | Jackman RW, Cornwell EW, Wu CL, Kandarian SC. Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol. 2013;98:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest. 2006;116:2945-2954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 54. | Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl). 2008;86:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 55. | Kuo DY, Chen PN, Chu SC, Hsieh YS. Knocking down the transcript of NF-kappaB modulates the reciprocal regulation of endogenous antioxidants and feeding behavior in phenylpropanolamine-treated rats. Arch Toxicol. 2012;86:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Kwan BC, Beddhu S. A story half untold: adiposity, adipokines and outcomes in dialysis population. Semin Dial. 2007;20:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Kahraman S, Yilmaz R, Akinci D, Arici M, Altun B, Erdem Y, Yasavul U, Turgan C. U-shaped association of body mass index with inflammation and atherosclerosis in hemodialysis patients. J Ren Nutr. 2005;15:377-386. [PubMed] |
| 58. | Teplan V, Vyhnánek F, Gürlich R, Haluzík M, Racek J, Vyhnankova I, Stollová M, Teplan V. Increased proinflammatory cytokine production in adipose tissue of obese patients with chronic kidney disease. Wien Klin Wochenschr. 2010;122:466-473. [PubMed] |
| 59. | Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 60. | Mahesh S, Kaskel F. Growth hormone axis in chronic kidney disease. Pediatr Nephrol. 2008;23:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Schaefer F, Chen Y, Tsao T, Nouri P, Rabkin R. Impaired JAK-STAT signal transduction contributes to growth hormone resistance in chronic uremia. J Clin Invest. 2001;108:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Chen Y, Biada J, Sood S, Rabkin R. Uremia attenuates growth hormone-stimulated insulin-like growth factor-1 expression, a process worsened by inflammation. Kidney Int. 2010;78:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Ingulli EG, Mak RH. Growth in children with chronic kidney disease: role of nutrition, growth hormone, dialysis, and steroids. Curr Opin Pediatr. 2014;26:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Yim HE, Yoo KH. Early life obesity and chronic kidney disease in later life. Pediatr Nephrol. 2015;30:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr. 2007;137:1066-1072. [PubMed] |
| 66. | Lelièvre-Pégorier M, Merlet-Bénichou C. The number of nephrons in the mammalian kidney: environmental influences play a determining role. Exp Nephrol. 2000;8:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Merlet-Bénichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelièvre-Pégorier M. Nephron number: variability is the rule. Causes and consequences. Lab Invest. 1999;79:515-527. [PubMed] |
| 68. | Simeoni U, Ligi I, Buffat C, Boubred F. Adverse consequences of accelerated neonatal growth: cardiovascular and renal issues. Pediatr Nephrol. 2011;26:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med. 2000;160:1472-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 70. | Greenbaum LA, Muñoz A, Schneider MF, Kaskel FJ, Askenazi DJ, Jenkins R, Hotchkiss H, Moxey-Mims M, Furth SL, Warady BA. The association between abnormal birth history and growth in children with CKD. Clin J Am Soc Nephrol. 2011;6:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Friedman EA, Trkulja V S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK