Published online Sep 25, 2025. doi: 10.5527/wjn.v14.i3.107177
Revised: April 9, 2025
Accepted: May 7, 2025
Published online: September 25, 2025
Processing time: 184 Days and 4.6 Hours
End-stage kidney disease is a growing global health burden with many patients requiring urgent kidney replacement therapy. Urgent-start peritoneal dialysis (PD) has emerged as a viable alternative to hemodialysis particularly in resource-limited settings. However, concerns remain regarding catheter-related complications associated with early initiation of PD. Automated PD (APD) offers en
To evaluate the clinical outcomes and biochemical changes associated with urgent-start APD with a shortened break-in period.
This was a single center, observational study that included 62 patients with end-stage kidney disease who required urgent-start dialysis, underwent PD catheter placement, and received APD. Patients were stratified based on catheter opening time (< 12 hours vs > 12 hours). Catheter-related complications, biochemical parameters, and dialysis efficacy were analyzed.
The median catheter opening time was 11 h (interquartile range: 8-14 hours). No significant differences in catheter-related complications were observed between groups (P > 0.05). Catheter dysfunction, migration, leakage, and replacement occurred in 14.5%, 9.7%, 12.9%, and 11.3% of patients, respectively. APD led to significant reductions in serum creatinine, blood urea nitrogen, urea, phosphorus, and potassium (P < 0.05), alongside correction of metabolic acidosis. No cases of peritonitis or hemoperitoneum were observed.
Urgent-start APD with shortened break-in appears safe with low complication rates and improved biochemical outcomes.
Core Tip: Urgent-start peritoneal dialysis (PD) with a shortened break-in period is increasingly recognized as a safe and effective alternative to hemodialysis in patients with end-stage kidney disease requiring immediate kidney replacement therapy. This study evaluated the clinical outcomes and biochemical changes associated with automated PD (APD) initiated within ≤ 12 hours of catheter placement. Our findings demonstrated low complication rates, significant metabolic improvements, and potential cost benefits, supporting the expansion of urgent-start PD with APD as a feasible and resource-efficient strategy for urgent dialysis initiation.
- Citation: Bastida-Castro LA, Martínez-Cuautle J, Corredor-Nassar MJ, Reyes-Torres BE, Alonso-Lobato SI, Balderas-Juarez J, Salinas-Ramirez MA, Hernandez-Castillo JL, Martínez-Sánchez FD. Automated peritoneal dialysis with shortened break-in periods in urgent-start scenarios: A retrospective cohort study. World J Nephrol 2025; 14(3): 107177
- URL: https://www.wjgnet.com/2220-6124/full/v14/i3/107177.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i3.107177
End-stage kidney disease (ESKD) represents a significant global health burden with a rising incidence and substantial socioeconomic impact[1,2]. Patients with ESKD often require urgent initiation of kidney replacement therapy (KRT) due to late referral or unexpected deterioration of kidney function[3,4]. While hemodialysis (HD) remains the predominant modality for urgent dialysis initiation, it is frequently associated with vascular complications, catheter-related blood
Peritoneal dialysis (PD) is an alternative dialysis modality that offers several advantages, including better preservation of residual kidney function, lower infection risks, and improved quality of life[2,4-6]. Urgent-start PD (USPD) is defined as initiating PD within 2 weeks of catheter placement. It has become a viable option for unplanned dialysis starts and demonstrates comparable safety and efficacy to conventional PD initiation[5,6]. The ability to initiate PD urgently using low fill volumes and a gradual increase in exchange volumes makes it an attractive choice for patients with acute dialysis needs, even in health facilities with limited resources[2-4].
The coronavirus disease 2019 pandemic and other global health epidemics have placed an unprecedented strain on healthcare systems, limiting access to medical care for vulnerable populations[7,8]. These health crises have particularly impacted patients with ESKD who require urgent dialysis initiation[7]. The surge in patients who are critically ill led to resource shortages, including dialysis supplies and trained personnel, making conventional HD less accessible[5,7]. Furthermore, the high risk of infection transmission in HD units heightened concerns regarding in-center HD, emphasizing the need for alternative dialysis modalities[5]. In response, USPD gained traction as a viable solution, providing a home-based therapy that minimizes hospital exposure[2,5]. Nevertheless, within USPD, it became essential to optimize dialysis techniques further[2,4]. Automated PD (APD) has emerged as an effective alternative, offering improved treatment flexibility and better fluid management[3,9]. This change highlights the necessity for flexible dialysis approaches that guarantee ongoing care for patients with ESKD, especially during healthcare strain[5,7].
The break-in period in USPD traditionally refers to the time between catheter insertion and the start of full-dose dialysis, typically lasting several days to minimize mechanical complications[6,10]. However, recent studies have explored shortened break-in periods (≤ 24 hours), suggesting their feasibility without significantly increasing complications[6,11,12]. Despite this, the optimal break-in duration remains uncertain, particularly in APD[11-13]. Given the limited evidence of a shortened break-in period in APD, this study aimed to evaluate the clinical outcomes and complications associated with its use in USPD in a referral center in Mexico City.
This was a single center, observational, retrospective cohort study that included 62 patients with ESKD who required urgent-start KRT from January 2024 to February 2025 at the Department of Internal Medicine, Hospital General Dr. Manuel Gea González in Mexico City, Mexico. The inclusion criteria were individuals aged between 18 and 90 years of both sexes, diagnosed with chronic kidney disease, urgently presenting to the emergency department due to ESKD, and who required urgent-start KRT. Some patients received HD before a trained nephrologist placed the PD catheter with an ultrasound-guided percutaneous technique, as reported previously[2]. The study was approved by the HGDMGG Research Committee and Research Ethics Committee (REF 14-69-2024), and patient anonymity was guaranteed per the 1975 Declaration of Helsinki.
Furthermore, an additional group of 277 patients undergoing continuous ambulatory PD (CAPD) for USPD, whose PD catheters were also placed at our institution, was retrospectively included to compare demographic characteristics and catheter-related complication rates between APD and CAPD. For these patients, only sex, age, length of hospital stay, and the presence or absence of catheter-related complications (dysfunction, migration, leakage, hemoperitoneum, and catheter replacement) were available for analysis.
Catheter dysfunction was defined as any complication that resulted in prolonged in-and-out dialysis solution times during PD. Catheter migration was defined as the displacement of the PD catheter from its intended position in the pelvic cavity. To resolve this issue, we administered prokinetic drugs or encouraged patients to walk or move out of bed. This was identified using imaging techniques, such as X-ray, when the catheter was found outside the pelvic cavity. Pericatheter leakage was defined as the escape of dialysis solution around the PD catheter insertion site.
All patients in this study underwent shortened break-in periods with catheter opening times ranging from a minimum of 7 hours to a maximum of 72 hours. Catheter dysfunction and migration were managed with non-surgical interventions, such as ambulation and prokinetic agents, and imaging was used to confirm the position. Catheter replacement was performed only when conservative measures failed.
Statistical analysis was performed using SPSS 26 (SPSS Inc., Chicago, IL, United States). Data were screened for outliers and normality assumptions. The normality of continuous variables was assessed with the Shapiro-Wilk normality test and visually using histograms and Q-Q plots. Values were expressed as mean ± SD, median (interquartile range), or frequencies (%). Means and medians were compared using the Student’s t-test or the Mann-Whitney U test and frequencies with the χ2 test when needed. For paired comparisons before and after APD, the Wilcoxon signed-rank test or the paired Student’s t-test was used, depending on the data distribution. A P value < 0.05 was considered statistically significant.
A total of 62 patients with ESKD requiring urgent-start dialysis were included, all of whom underwent PD catheter placement and received APD. They had a mean age of 49.3 ± 13.4 years and a mean body mass index of 26.2 ± 4.7 kg/m2, and 27.4% (n = 17) were female. The median length of hospital stay was 11 (8-15) days. The median Acute Physiology and Chronic Health Evaluation score at admission was 12.5 (8.0-17.0) with a median Sequential Organ Failure Assessment score of 5 (4-7). Overall, 61.3% (n = 38) had type 2 diabetes, 74.2% (n = 46) had hypertension, and 91.9% (n = 57) had a prior diagnosis of chronic kidney disease. A history of abdominal surgery was reported in 22.6% (n= 14) of patients. Moreover, 37.7% of patients (n = 23) received HD before PD catheter placement.
The median time elapsed between admission and PD catheter placement was 72 (48-120) hours, with 79.0% (n = 49) of patients undergoing catheter placement after 48 hours of admission. All patients had a shortened break-in period, with a median catheter opening time of 11 (8-14) hours. Most patients (54.8%, n = 34) started PD < 12 h after catheter placement, 33.9% (n = 21) started PD within 12-24 hours, and 11.3% (n = 7) started PD after 24 hours. The minimum and maximum catheter opening times were 7 h and 72 h, respectively.
Regarding catheter-related complications, only 14.5% (n = 9) experienced catheter dysfunction, 9.7% (n = 6) had catheter migration, 12.9% (n = 8) developed PD fluid leakage, and 11.3% (n = 7) required PD catheter replacement. No patient experienced peritonitis related to PD or APD nor hemoperitoneum. All PD-related complications were transient and resolved at discharge.
Biochemical data obtained before APD initiation showed a mean hemoglobin level of 8.4 ± 1.5 g/dL and a mean serum albumin of 3.1 ± 0.6 g/dL. Median serum creatinine was 13.90 (9.35–18.37) mg/dL, while median blood urea nitrogen (BUN) and serum urea were 104 (80-144) mg/dL and 223 (170–308) mg/dL, respectively. Mean serum sodium, potassium, and chloride levels were 133.0 ± 17.5 mEq/L, 4.9 ± 0.9 mEq/L, and 97.8 ± 13.7 mEq/L, respectively. Mean serum calcium and phosphorus levels were 7.6 ± 0.9 mg/dL and 7.7 ± 2.4 mg/dL, respectively. Acid-base balance before APD initiation showed a mean arterial pH of 7.34 ± 0.09, serum bicarbonate of 18.2 ± 5.1 mEq/L, and pCO2 of 34.2 ± 9.5 mmHg. The median serum lactate level was 0.8 (0.7–1.2) mmol/L.
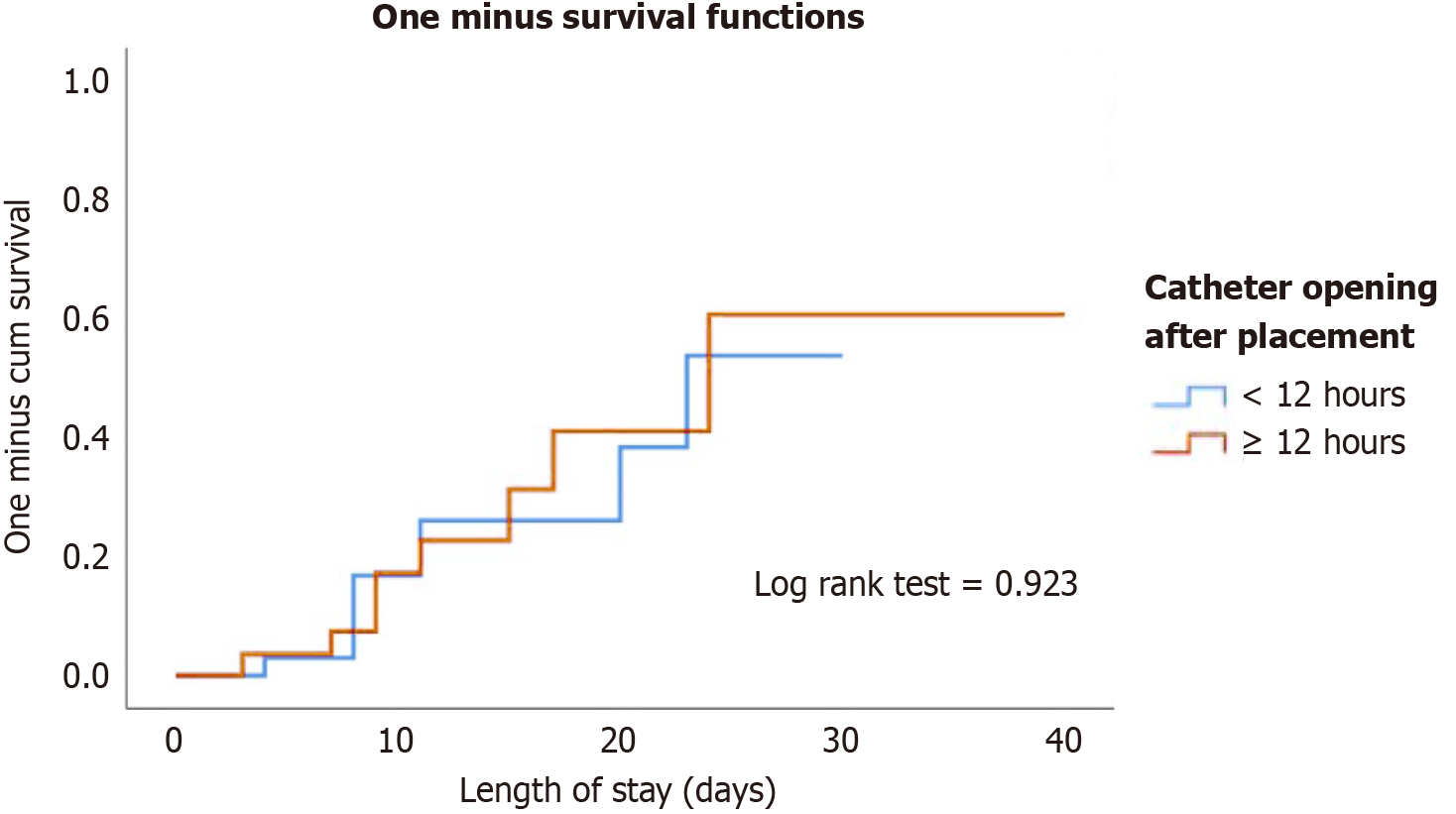
Table 1 shows patients’ baseline characteristics and clinical outcomes stratified by catheter opening time (< 12 hours vs > 12 hours). No significant differences were observed in age, sex, body mass index, Acute Physiology and Chronic Health Evaluation score, Sequential Organ Failure Assessment score, or comorbidities between both groups. However, a higher proportion of patients in the > 12-h group had received prior HD before PD (53.6% vs 24.2%, P = 0.033) and experienced a longer time from admission to catheter placement (median 96 hours vs 72 hours, P = 0.033). The incidence of catheter-related complications, including dysfunction, migration, PD fluid leakage, and the need for catheter replacement, was similar between groups. Figure 1 shows the cumulative incidence of catheter-related complications over time stratified by catheter opening time (< 12 hours vs > 12 hours). The Kaplan-Meier survival curves showed a comparable progression of complications between both groups, with no significant difference observed.
| Variables | Catheter opening after placement | ||
| < 12 hours, n = 34 | ≥ 12 hours, n = 28 | P value | |
| Female | 9 (26.5) | 8 (28.6) | 0.854 |
| Age in years | 48.6 ± 13.3 | 50.2 ± 13.6 | 0.652 |
| BMI in kg/m2 | 25.8 ± 4.3 | 26.6 ± 5.2 | 0.523 |
| Length of stay in days | 11 (8-14) | 11 (8-16) | 0.904 |
| APACHE | 11 (7-17) | 13 (10-17) | 0.574 |
| SOFA | 5 (3-7) | 5 (4-6) | 0.553 |
| Type 2 diabetes | 23 (67.6) | 15 (53.6) | 0.302 |
| Hypertension | 27 (79.4) | 19 (67.9) | 0.386 |
| CKD | 30 (88.2) | 27 (96.4) | 0.366 |
| History of abdominal surgery | 11 (32.4) | 3 (10.7) | 0.066 |
| HD prior to PD catheter placement | 8 (24.2) | 15 (53.6) | 0.033 |
| Time elapsed between admission and catheter placement in h | 72 (24-96) | 96 (48-144) | 0.033 |
| Time between admission and catheter placement | 0.327 | ||
| < 24 h | 7 (20.6) | 2 (7.1) | |
| 24-48 h | 2 (5.9) | 2 (7.1) | |
| > 48 h | 25 (73.5) | 24 (85.7) | |
| Dysfunction | 5 (14.7) | 4 (14.3) | 0.963 |
| Catheter migration | 5 (14.7) | 1 (3.6) | 0.209 |
| PD fluid leakage | 5 (14.7) | 3 (10.7) | 0.719 |
| PD catheter replacement | 4 (11.8) | 3 (10.7) | 0.897 |
Table 2 presents the changes in laboratory parameters before and after APD initiation. A significant increase in hemoglobin levels was observed following APD, with marked reductions in serum creatinine, BUN, and urea levels (P < 0.05 for all), reflecting improved clearance. Similarly, serum potassium, chloride, and phosphorus levels significantly decreased after APD, while serum calcium levels increased. Acid-base parameters showed significant improvement, with increased pH and bicarbonate levels alongside a rise in pCO2, indicating correction of metabolic acidosis. Conversely, a slight but significant decrease in serum sodium and a mild increase in lactate levels were observed. No significant difference was found in serum albumin levels after APD.
| Variables | Before APD | After APD | P value |
| Hemoglobin (g/dL) | 8.37 ± 1.49 | 9.11 ± 1.59 | 0.007 |
| Serum albumin (g/dL) | 3.09 ± 0.56 | 2.79 ± 0.68 | 0.265 |
| Serum creatinine (mg/dL) | 13.90 (9.35-18.37) | 9.23 (7.43-12.84) | < 0.001 |
| BUN (mg/dL) | 104 (80-143) | 69 (53-82) | < 0.001 |
| Urea (mg/dL) | 223 (171-307) | 147 (115-175) | < 0.001 |
| Serum sodium (mEq/L) | 135 (132-139) | 133 (131-136) | 0.033 |
| Serum potassium (mEq/L) | 4.80 (4.30-5.40) | 4.00 (3.50-4.40) | < 0.001 |
| Serum chloride (mEq/L) | 99 (96-103) | 94 (92-96) | < 0.001 |
| Serum calcium (mEq/L) | 7.59 (7.10-7.96) | 8.00 (7.40-8.40) | 0.001 |
| Serum phosphorus (mEq/L) | 7.94 (5.61-9.19) | 4.80 (4.32-5.93) | < 0.001 |
| pH | 7.35 (7.29-7.39) | 7.44 (7.40-7.46) | < 0.001 |
| Bicarbonate (mEq/L) | 18.7 (15.2-21.9) | 26.3 (24.7-28.5) | < 0.001 |
| pCO2 (mmHg) | 33 (29-38) | 40 (36-42) | < 0.001 |
| Lactate (mmol/L) | 0.80 (0.70-1.20) | 1.30 (1.00-1.60) | 0.001 |
Table 3 compares the patients who received APD and CAPD in USPD. Patients undergoing CAPD were significantly older, were more likely to be female, and had a longer in-hospital stay. While catheter-related complications, including dysfunction, migration, leakage, hemoperitoneum, and the need for catheter replacement, were numerically lower in the APD group, these differences did not reach statistical significance. The overall rate of catheter-related complications was comparable between both groups.
| Variables | APD | CAPD | P value |
| Age (years) | 49.3 ± 13.4 | 54.5 ± 14.6 | 0.008 |
| Female | 17 (27.4) | 137 (49.5) | 0.002 |
| Length of stay in days | 11 (8-15) | 15 (11-22) | < 0.001 |
| Dysfunction | 9 (14.5) | 66 (23.8) | 0.129 |
| Catheter migration | 6 (9.7) | 34 (19.1) | 0.113 |
| PD fluid leakage | 8 (12.9) | 26 (9.4) | 0.481 |
| Hemoperitoneum | 0 (0.0) | 5 (1.8) | 0.589 |
| PD catheter replacement | 7 (11.3) | 46 (16.6) | 0.340 |
| PD catheter-related complications | 17 (27.4) | 84 (30.3) | 0.759 |
The growing global burden of ESKD is a significant challenge for all healthcare systems, with an increasing number of patients requiring KRT to sustain life[1,4]. Kidney transplantation, HD, and PD remain the primary modalities for KRT, each with distinct advantages and limitations[5,14]. Among these, PD has emerged as a safe, effective, and patient-centered option, offering comparable clinical outcomes to in-center HD, particularly in the early years of treatment[15,16]. Likewise, USPD provides a safe and effective alternative to HD in patients requiring immediate KRT[2-5]. By enabling early dialysis initiation without vascular access, USPD reduces the risk of catheter-related bloodstream infections and other vascular complications associated with urgent-start HD[5,7]. It offers a patient-centered approach that facilitates a smoother transition to home-based therapy, preserves residual kidney function, and improves quality of life. In this context, APD presents an attractive option, providing greater flexibility in treatment schedules, optimizing fluid management, and improving adherence to therapy[4,16].
Additionally, USPD with APD remains a cost-effective and logistically feasible strategy in resource-limited settings, ensuring timely dialysis initiation while minimizing hospital-related complications[6,9-11]. Our study supports the feasibility and safety of urgent-start APD with shortened break-in periods, demonstrating that early catheter use does not increase the risk of major catheter-related complications.
Despite a median catheter opening time of 11 (8-14) hours, the incidence of dysfunction, migration, fluid leakage, and the need for catheter replacement was low and comparable between groups, regardless of whether PD was initiated before or after 12 hours post-catheter placement. Importantly, no APD-related or catheter-related peritonitis or hemoperitoneum cases were observed, and all complications were transient and resolved before discharge. Additionally, biochemical markers improved significantly following APD initiation, with reductions in serum creatinine, BUN, urea, potassium, chloride, and phosphorus alongside a correction of metabolic acidosis, as indicated by increased pH, bicarbonate, and pCO2 Levels. These findings reinforce that urgent-start APD with a shortened break-in period is a practical, safe, and effective strategy, enabling timely dialysis initiation without compromising patient safety. Hence, these results could partially suggest that USPD using APD modality could be a safe alternative for managing patients requiring urgent KRT and might reduce the burden of ESKD.
These findings align with a multicenter study that evaluated 871 patients undergoing USPD. The study reported no significant difference in mechanical complications, catheter migration, or infectious complications between the two groups across multiple follow-up time points (2 weeks, 1 month, 3 months, and 6 months). Logistic regression analysis further confirmed that a break-in period of ≤ 24 hours was not an independent risk factor for these complications[11].
A major concern of patients with ESKD is the long-term cost of dialysis, especially in middle-income and low-income countries[17]. The cost of dialysis imposes a significant financial burden on healthcare systems, particularly in low-income and middle-income countries where treatment expenses often surpass the average individual’s ability to pay, limiting access to life-sustaining therapy and straining public health resources[17,18].
Moreover, dialysis costs vary significantly between Latin America and the United States, primarily influenced by healthcare infrastructure, reimbursement models, and resource allocation[17-20]. In Mexico, the Instituto Mexicano del Seguro Social data estimates that the annual cost per patient for CAPD is approximately MXN 41931.20 (USD about 2100)[18]. In comparison, APD costs MXN 64123.20 (USD about 3200). In contrast, intramural HD costs MXN 33301.32 (USD about 1700), whereas extramural HD, which includes private facilities, escalates significantly to MXN 165360.00 (USD about 8300) per year. These figures highlight that APD remains a cost-effective home-based therapy, offering clinical benefits while remaining competitive[18,20,21].
Meanwhile, in the United States, dialysis expenses are substantially higher. A recent Journal of Medical Economics review reported that in 2020 Medicare spending per patient for HD reached USD 95932 annually. PD costs were approximately USD 81525 per year, representing a 15% lower cost than HD[19-22]. However, private insurance payers face considerably higher expenses, with outpatient dialysis costing up to USD 10149 per patient per month compared with USD 3364 for Medicare beneficiaries[19]. Additionally, vascular access-related costs for HD contribute to a substantial financial burden, further reinforcing the economic advantage of PD. Although the cost gap between HD and PD has narrowed over the past decade, transitioning from PD to HD has been associated with a marked increase in overall healthcare expenditures[19,21]. Given the economic constraints in Latin America and the escalating dialysis costs in the United States, APD presents itself as an efficient and cost-effective alternative, particularly in urgent-start scenarios where timely dialysis initiation is critical[4,20,21]. Expanding APD use could lead to significant long-term healthcare savings while optimizing patient-centered care by reducing hospital-based expenses, vascular access complications, and inpatient dialysis costs[19,20].
Our data showed that the overall incidence of catheter-related complications was low, with catheter dysfunction occurring at 14.5%, migration at 9.7%, leakage at 12.9%, and catheter replacement at 11.3%. These findings align with a multicenter study evaluating break-in periods of less than 24 hours in 871 patients, which reported no significant increase in catheter-related complications compared with longer break-in periods[11]. Specifically, mechanical and infectious complication rates were comparable between the break-in period ≤ 24 hours group (470 patients) and the > 24 hours group (401 patients) across multiple follow-up points, including 2 weeks, 1 month, 3 months, and 6 months.
Similarly, Hu et al[6] specifically investigated patients with diabetes undergoing USPD. They found that a break-in period of ≤ 24 hours was not associated with an increased risk of mechanical or infectious complications. Over 60% of our cohort had type 2 diabetes yet did not show increased complications compared with other reports.
Moreover, it is important to highlight a recent randomized clinical trial comparing APD vs HD in USPD[13]. This trial found that APD was associated with a significantly lower incidence of dialysis-related complications (25.9% vs 56.9%, P = 0.001) compared with HD, supporting APD as a viable first-line option in urgent-start settings[13]. Additionally, APD was more cost-effective, reducing initial hospitalization costs while maintaining similar catheter survival and patient outcomes.
The economic advantage of APD is crucial for healthcare systems, especially in resource-limited settings, and aligns with our findings where APD was safely implemented without excess hospitalization due to complications. Finally, our study demonstrated a significant improvement in metabolic parameters following APD initiation, including reduced BUN, creatinine, and phosphorus levels and correction of metabolic acidosis. These biochemical improvements mirror findings from previous studies indicating that APD facilitated better solute clearance and hemodynamic stability than HD. Although no infectious complications were observed during hospitalization, our study did not include long-term follow-up data to evaluate late-onset peritonitis, catheter failure, or peritoneal membrane dysfunction. These outcomes will be essential to assess in future analyses to better understand the long-term safety and durability of APD in urgent-start settings.
A key strength of this study was its real-world application of urgent-start APD with a shortened break-in period, demonstrating its feasibility and safety in a clinical setting with limited resources. The study provided valuable insights into catheter-related complications, metabolic improvements, and cost-effectiveness, reinforcing APD as a viable first-line option for urgent dialysis initiation. Additionally, this study contributed to the growing body of evidence supporting early PD initiation, aligning with findings from recent multicenter studies and randomized controlled trials[9-13].
However, this study had several limitations. First, the single center, observational design may limit the generalizability of the findings to other healthcare settings, particularly those with different dialysis protocols or patient populations. Second, the sample size was relatively small, limiting the ability to detect fewer common complications. Third, although this study provided short-term clinical outcomes, long-term follow-up data on catheter survival, peritonitis rates, and patient-reported quality of life are needed to further assess the benefits and risks of APD in urgent-start scenarios. Fourth, while our study included a general cost comparison based on national estimates and institutional reports, it did not reflect a detailed patient-level cost analysis. Direct and indirect costs were not individually captured, including hospitalization length, supply usage, staff training, and complication management. A comprehensive economic evaluation will be crucial in future studies to assess the true cost-effectiveness of APD in urgent-start scenarios, particularly in resource-constrained settings.
Our findings suggested that urgent-start APD with a shortened break-in period is a safe and effective alternative to HD for patients requiring immediate KRT. The low incidence of catheter-related complications combined with significant metabolic improvements and the potential cost benefits of APD supports its wider implementation in clinical practice, particularly in resource-limited settings. Given the growing burden of ESKD and the need for efficient dialysis strategies, expanding APD in urgent-start settings could improve access to care, reduce hospital-related complications, and enhance patient outcomes. Future multicenter studies and long-term follow-up are needed to refine APD protocols further, evaluate patient-reported outcomes, and optimize dialysis initiation strategies.
The authors would like to thank the head of the Department of Internal Medicine for acquiring the Siemens ACUSON Juniper Ultrasound System. Previously, the authors placed catheters using the blind Seldinger technique.
| 1. | Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 2. | Balderas-juarez J, Salinas-ramírez MA, Hernández-castillo JL, Moreno-novales R, Cortina-marquez RA, Martínez-sánchez FD. Ultrasound-Guided Percutaneous Peritoneal Dialysis Catheter Insertion for Urgent-Start Dialysis: Technique Description and Experience of a Single Center in Mexico City. IJN. 2024;0:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Hernández-Castillo JL, Balderas-Juárez J, Jiménez-Zarazúa O, Guerrero-Toriz K, Loeza-Uribe MP, Tenorio-Aguirre EK, Mendoza-García JG, Mondragón JD. Factors Associated With Urgent-Start Peritoneal Dialysis Catheter Complications in ESRD. Kidney Int Rep. 2020;5:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Yaxley J, Scott T. Urgent-start peritoneal dialysis. Nefrologia (Engl Ed). 2023;43:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Rajora N, Shastri S, Pirwani G, Saxena R. How To Build a Successful Urgent-Start Peritoneal Dialysis Program. Kidney360. 2020;1:1165-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Hu X, Yang L, Sun Z, Zhang X, Zhu X, Zhou W, Wen X, Liu S, Cui W. Break-in Period ≤24 Hours as an Option for Urgent-start Peritoneal Dialysis in Patients With Diabetes. Front Endocrinol (Lausanne). 2022;13:936573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Nassiri AA, Ronco C, Kazory A. Resurgence of Urgent-Start Peritoneal Dialysis in COVID-19 and Its Application to Advanced Heart Failure. Cardiorenal Med. 2021;11:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Martínez-Sánchez FD, Bastida-Castro LA, Torres-Cuevas JL, Vasquez-Vasquez JA, Diaz-Jarquin A, Moreno-Novales R, Balderas-Juarez J, Salinas-Ramírez MA, Hernández-Castillo JL, Tenorio-Aguirre EK. COVID-19 and Acute Kidney Injury Outcomes in Hospitalized Patients Following SARS-CoV-2 Vaccination: A Case-Control Study. Can J Kidney Health Dis. 2024;11:20543581241297369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 9. | Cnossen TT, Usvyat L, Kotanko P, van der Sande FM, Kooman JP, Carter M, Leunissen KM, Levin NW. Comparison of outcomes on continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis: results from a USA database. Perit Dial Int. 2011;31:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Scalamogna A, Nardelli L, Cicero E, Castellano G. Analysis of mechanical complications in urgent-start peritoneal dialysis. J Nephrol. 2022;35:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Wen X, Yang L, Sun Z, Zhang X, Zhu X, Zhou W, Hu X, Liu S, Luo P, Cui W. Feasibility of a break-in period of less than 24 hours for urgent start peritoneal dialysis: a multicenter study. Ren Fail. 2022;44:450-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Liu S, Zhuang X, Zhang M, Wu Y, Liu M, Guan S, Liu S, Miao L, Cui W. Application of automated peritoneal dialysis in urgent-start peritoneal dialysis patients during the break-in period. Int Urol Nephrol. 2018;50:541-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Jin H, Fang W, Wang L, Zang X, Deng Y, Wu G, Li Y, Chen X, Wang N, Jiang G, Guo Z, Wang X, Qi Y, Lv S, Ni Z. A Randomized Controlled Trial Comparing Automated Peritoneal Dialysis and Hemodialysis for Urgent-Start Dialysis in ESRD. Kidney Int Rep. 2024;9:2627-2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Jain D, Haddad DB, Goel N. Choice of dialysis modality prior to kidney transplantation: Does it matter? World J Nephrol. 2019;8:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Joshi VD. Quality of life in end stage renal disease patients. World J Nephrol. 2014;3:308-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 16. | Teitelbaum I. Peritoneal Dialysis. N Engl J Med. 2021;385:1786-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 17. | Mushi L, Marschall P, Fleßa S. The cost of dialysis in low and middle-income countries: a systematic review. BMC Health Serv Res. 2015;15:506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Méndez-Durán A, Ignorosa-Luna MH, Pérez-Aguilar G, Rivera-Rodríguez FJ, López-Ocaña LR. The Instituto Mexicano del Seguro Social gives dialysis therapies to 426 students without cost. Rev Med Inst Mex Seguro Soc. 2018;56:163-166. [PubMed] |
| 19. | Stewart F, Kistler K, Du Y, Singh RR, Dean BB, Kong SX. Exploring kidney dialysis costs in the United States: a scoping review. J Med Econ. 2024;27:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Karopadi AN, Mason G, Rettore E, Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28:2553-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Okpechi IG, Jha V, Cho Y, Ye F, Ijezie CI, Jindal K, Klarenbach S, Makusidi MA, Okpechi-Samuel US, Okwuonu C, Shah N, Thompson S, Tonelli M, Johnson DW, Bello AK. The case for increased peritoneal dialysis utilization in low- and lower-middle-income countries. Nephrology (Carlton). 2022;27:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Flythe JE, Watnick S. Dialysis for Chronic Kidney Failure: A Review. JAMA. 2024;332:1559-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/