Published online Sep 25, 2025. doi: 10.5527/wjn.v14.i3.102667
Revised: March 9, 2025
Accepted: April 2, 2025
Published online: September 25, 2025
Processing time: 327 Days and 16.4 Hours
Sepsis-associated acute kidney injury is common in critically ill patients and is strongly associated with an increased risk of adverse outcomes and mortality. While early and appropriate antimicrobials for sepsis have been associated with an increased probability of survival, adequate dosing is a challenge in these patients. Critical illness with acute kidney injury is characterized by marked physiological derangements. This impacts the pharmacokinetic and pharmacodynamic profiles of antimicrobials, which complicates the predictability of drug disposition. Conventional antimicrobial dosing may not be optimal in this patient population, leading to under or overexposure to antimicrobials. This review summarizes an overview of the drug dosing considerations and relevant evidence of appropriate dosing strategies of common antimicrobials encountered in critically ill patients with acute kidney injury to optimize therapeutic efficacy and to reduce toxicity and adverse events.
Core Tip: Currently, there are few published reviews focusing on dosing considerations for critically ill patients with acute kidney injury across key antimicrobial categories, including antibiotics, antivirals, and antifungals. Recognizing how physiological changes affect drug-related pharmacokinetics and pharmacodynamics in this context can help guide dosing strategies to enhance clinical outcomes for these patients.
- Citation: Tee C, Ngai M, See KC. Antimicrobial dosing considerations in critically ill patients with acute kidney injury: A review. World J Nephrol 2025; 14(3): 102667
- URL: https://www.wjgnet.com/2220-6124/full/v14/i3/102667.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i3.102667
Critically ill patients frequently experience sepsis-associated acute kidney damage, which is highly correlated with increased risks of unfavorable outcomes and death. More than half of intensive care unit (ICU) patients have acute kidney injury (AKI), and of those, 25% would require kidney replacement therapy (KRT). This raises the risk of death, prolonged hospital and ICU stays, and secondary onset of chronic renal disease[1].
There is a higher chance of survival when antimicrobials are used at the appropriate time, choice, and dosage in septic shock[2,3]. According to Roberts et al[4], critically ill patients who failed to achieve optimal β-lactam concentrations had a lower probability of favorable clinical outcomes in the Defining Antibiotic Levels in Intensive Care Unit Patients study. Inadequate antibiotic dosing is a substantial independent risk factor for mortality that is frequently observed in critically ill patients[5]. Although the likelihood of survival has been linked to the use of antimicrobials in the early stages of sepsis, the optimal dosing in these individuals remains a challenge.
Critical illness with AKI is characterized by significant physiological derangements in pharmacokinetic (PK) and pharmacodynamic (PD) conditions.
PK is the study of how a drug is absorbed, distributed, metabolized, and eliminated by the body. Some common PK parameters include the volume of distribution (Vd) which quantifies the distribution of a drug throughout the body relative to the concentration of the drug in the blood or plasma and the extent of protein binding (PB) that the antimicrobial exhibits, as only free and unbound drug exerts antimicrobial effects. On the other hand, PD focuses on the relationship between the drug concentration at the site of infection and its effects of killing or inhibiting the growth of microorganisms. Dosing decisions have to be based on the variabilities in the Vd, PB, augmented renal clearance (ARC), residual renal function, and the initiation of KRT which further complicates the predictability of drug disposition[6]. In this patient population, standard or conventional antibiotic dosing may result in either under- or over-exposure. A higher risk of adverse medication responses, pathogen resistance, clinical treatment failure, and mortality can result from using antimicrobials at inappropriate dosages[7].
Currently, there are limited published reviews for clinicians, focusing on the antimicrobial dosing recommendations for critically ill patients with AKI and receiving KRT. This review aims to provide an overview of the drug dosing considerations and relevant evidence of appropriate dosing strategies of common antimicrobials (antibiotics, antifungals, and antivirals) in this context, to optimize therapeutic efficacy and reduce toxicity and adverse events. While our manuscript focuses on the PK and PD considerations in dosing strategies across various stages of kidney injury, we acknowledge the need to consider other clinical aspects such as liver dysfunction, extremes of weight, and drug-drug interactions, although further elaboration of these other areas is beyond the scope of this review.
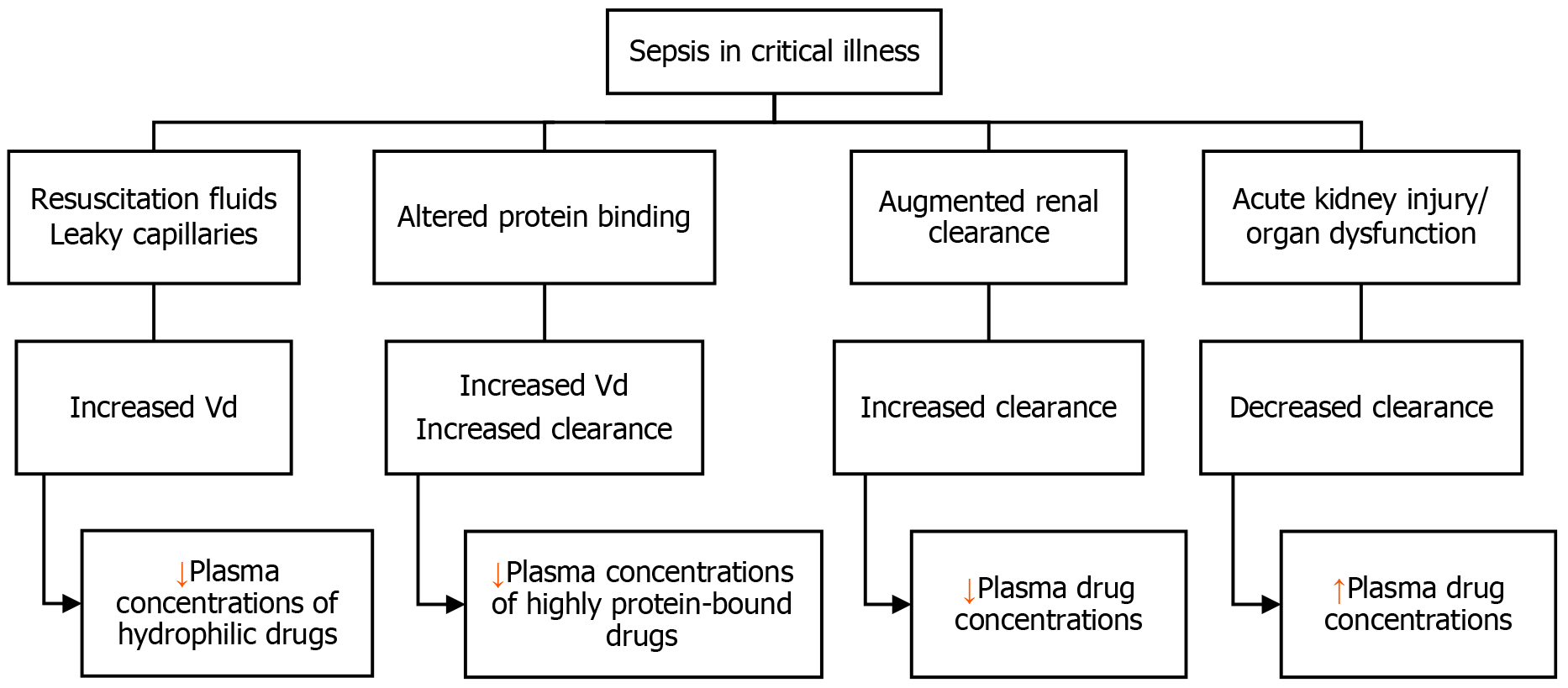
Antimicrobial PK is influenced by many patient-related factors. One of the most important pathophysiological changes seen in critically ill individuals with sepsis or septic shock is increased Vd. Considerable fluid shifts and variations in Vd are caused by aggressive fluid resuscitation and the "capillary leak syndrome", which results in third spacing. This phenomenon is likely to decrease the plasma concentrations of hydrophilic antimicrobials such as aminoglycosides, glycopeptides, lipopeptides, and β-lactams, particularly in the earlier phase of disease[4].
Therefore, to compensate for the larger Vd in critically ill patients with sepsis or septic shock, higher initial loading doses should be administered. This is especially true for hydrophilic and concentration-dependent antimicrobials like aminoglycoside antibiotics. For this patient population, higher starting loading doses of amikacin, colistin, gentamicin, and vancomycin are required to quickly reach therapeutic concentrations[8,9]. In general, loading doses should not be altered in patients with renal impairment or those receiving KRT[10]. In contrast, lipophilic antimicrobials (such as fluoroquinolones, macrolides, and lincosamides) are less affected by fluid shifts and variations in Vd since their higher intracellular partitioning and sequestration into adipose tissue compartments give them a larger Vd by nature[11].
Hypoalbuminemia is commonly encountered in the critically ill patient population. This predominantly impacts drugs that are highly bound to proteins, including various antifungal agents such as isavuconazole and echinocandins, as well as antibiotics such as cefazolin, ceftriaxone, ertapenem, daptomycin, flucloxacillin, and teicoplanin[12]. For highly protein-bound medicines, hypoalbuminemia leads to higher free drug concentrations, increased clearance, and greater Vd. For example, compared to patients who are not critically ill, ceftriaxone (95% bound to albumin) had a 90% larger Vd and a 100% higher clearance in critically ill patients[12,13]. To account for these PK changes, maintenance dosages for these antibiotics should be raised. This increase in dosing is particularly significant for antimicrobials which have time-dependent characteristics, such as β-lactams.
While AKI is thought to affect 20%-50% of ICU patients, ARC may affect up to 65% of patients[14]. The enhanced cardiac output and enhanced circulation through the kidneys due to vigorous fluid resuscitation therapy and vasopressor needs result in ARC, which is commonly characterized as a creatinine clearance (CrCl) ≥ 130 mL/minute/1.73 m2[15]. Young patients (< 55 years old), sepsis, trauma, burns, and hematologic malignancies are common risk factors related to this phenomenon[16]. ARC can significantly reduce serum antimicrobial levels and overall drug exposure, hence limiting the probability of PK/PD goal attainment, especially for bacterial strains with high minimum inhibitory concentration (MIC) values. This is more relevant for antibiotics with significant renal elimination such as β-lactams, carbapenems, and glycopeptides[11,17].
Figure 1 summarizes the physiological derangements in critical illness and the effects on antimicrobial PKs.
Reduced glomerular filtration and compromised tubular secretion and reabsorption are linked to sepsis-induced AKI. This may result in a longer half-life, decreased clearance of hydrophilic antibiotics (such as β-lactams and aminoglycosides), and possible toxicity from increased antibiotic plasma concentrations and metabolite accumulations. When AKI is present and/or when KRT is required, adjusting dosage regimens might be difficult[18].
Dosing adjustments of antimicrobials with a wide therapeutic index (i.e., β-lactams) may not be required in the first 48 hours for patients who present with AKI in the ICU and should be deferred until > 48 hours later when sustained renal injury can be better characterized. More than half of the patients with AKI at admission in a study by Crass and colleagues recovered in less than 48 hours[19]. Compared to non-adjusted doses, changing antibiotic dosages based on estimated glomerular filtration rate (GFR) was linked to increased rates of treatment failure and mortality in a trial involving 126 critically ill patients with renal impairment[20]. In a recent prospective, multicenter observational trial, delayed β-lactam antibiotic adjustments reduced in-hospital mortality as compared to patients with earlier adjustments in those admitted to the critical care unit with sepsis and AKI and started on antipseudomonal β-lactam therapy[21].
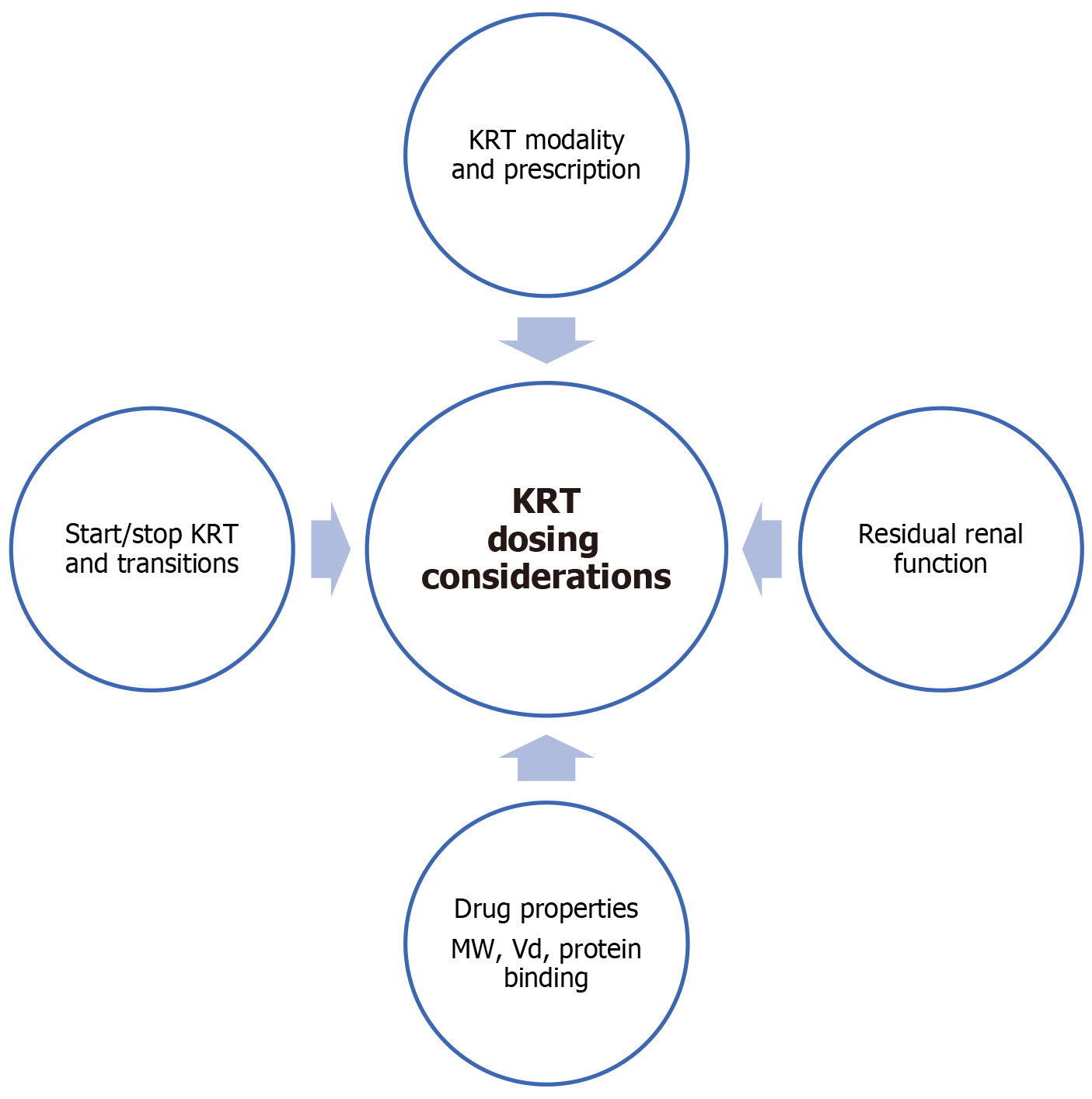
Prolonged intermittent kidney replacement treatment (PIKRT), intermittent conventional hemodialysis (IHD), and continuous KRT (CKRT) are KRT modalities utilized in the ICU context. For improved hemodynamic stability and fluid balance, CKRT is most commonly used in critically ill patients[1,12]. KRT-adjusted antimicrobial dosing is highly challenging and heterogeneous owing to variation in drug disposition due to patient and pathophysiologic factors, drug physicochemical qualities, and KRT prescription[12].
One of the most important aspects of modifying antimicrobial dosage is effluent flow rates. The impact of three different effluent flow rates (20, 30, and 40 mL/kg/hour) on meeting cefepime PK/PD targets was assessed in a population PK analysis, where higher effluent flow rates were associated with a reduced likelihood of reaching the drug target concentrations[7,22].
Antibiotic elimination is also influenced by the length of the KRT session. When compared to IHD, continuous or protracted approaches (CKRT or PIKRT) can result in increased antibiotic removal. If a comparable blood flow rate is applied in both continuous and prolonged intermittent KRT, the extent of drug removal is greatest in CKRT, followed by PIKRT then IHD (CKRT > PIKRT > IHD)[23].
Technological issues can disrupt dialysis (such as clotting of venous access devices) and change residual renal function when patients' clinical conditions improve or deteriorate, varying the drug exposure in patients with CKRT over time. When hemodynamic instability resolves before renal function, patients undergoing either PIKRT or CKRT may subsequently be switched to standard IHD[12].
Therapeutic drug monitoring (TDM) assessments should be taken into consideration at these transition periods to guide dosing when available. The relationship between medication administration and the beginning/ending of dialysis and changes in dialysis modes is important in guiding dosing strategies and interpretation of drug levels.
Residual renal function of the patients might have a considerable impact on antimicrobial clearance. There is a paucity of studies evaluating how antimicrobial exposure in patients undergoing KRT is affected by residual renal function. In order to achieve adequate PK/PD targets, patients with preserved diuresis (> 500 mL/day) should receive longer infusions for time-dependent antibiotics and higher dosages for concentration-dependent antibiotics, according to two studies on linezolid and meropenem[12,24].
The main pharmacological parameters for predicting drug removal by dialysis are molecular weight (MW), PB rate, and the Vd[25]. In general, when dosing antimicrobials in patients on CKRT, dosing recommendations are based on a reduced clearance, considering CrCl between 10 and 30 mL/minute[26].
The MW of drugs is inversely related to their diffusion capacity in dialysis. The majority of antibiotics have MWs less than 1000 Da, which confers significant KRT clearance[12]. The pore size of CKRT membranes is larger than that of IHD filter membranes, allowing for the efficient removal of larger molecules. Drugs with mostly hydrophilic renal elimination, low PB, and a decreased Vd are thought to be relevant candidates for CKRT clearance, which is a measure of how permeable the CKRT cartridge membrane is to the drug. Additionally, medications with a higher MW can be removed when using biosynthetic membranes, which have wider pores than standard cartridges such as OxirisTM (Baxter, Meyzieu, France)[27].
Nonetheless, there are certain exceptions. For instance, among the β-lactams, ceftriaxone, and oxacillin have considerable biliary clearance, while among the fluoroquinolones, despite their lipophilicity, levofloxacin, and ciprofloxacin have renal clearance[27,28].
Due to the possibility of a continuous redistribution of the drug from the tissues to the blood, antibiotics with low Vd (< 1 L/kg), which includes β-lactams, aminoglycosides, and daptomycin as examples, will be more affected by removal during CKRT than those with high Vd (> 2 L/kg) such as fluoroquinolones and macrolides[29].
Highly protein bound drugs are expected to have minimal clearance via KRT as only the free fractions of drugs are removed. In critically ill patients who have wide variability in PB, this can further increase KRT-related clearance and possibly reduce antibiotic exposure[12].
Figure 2 summarizes selected drug dosing considerations in KRT.
The impact of critical illness, AKI, and KRT on drug dosing is also dependent on the pathogen eradication characteristics of antimicrobials. On the basis of their kill or inhibition characteristics, antimicrobials are broadly described as either concentration- or time-dependent, or a combination (concentration- and time-dependent antimicrobial) to reflect their modes of bacterial, fungal, or viral killing[11].
The maximal plasma concentration (Cmax)/MIC ratio is linked to optimal killing for concentration-dependent antibiotics, such as aminoglycosides. Therefore, for aminoglycosides, the likelihood of achieving the target Cmax/MIC is increased while limiting toxicity with suggestions of using high doses at extended intervals[8,12].
In time-dependent antimicrobials, prolonging the duration of effective drug exposure leads to greater antimicrobial killing. The cumulative percentage of time by which the drug concentration exceeds the MIC (%fT > MIC) best describes their activity (e.g., β-lactam antibiotics)[11]. Continuous or prolonged β-lactam infusions have been advocated to enhance the attainment of PK/PD targets. In a recent trial, Sember and colleagues showed that patients receiving CKRT may achieve sufficient PK/PD targets with a dosing regimen of an extended 4-hour infusion of ceftazidime and cefepime at effluent rates of 20-30 mL/kg/hour[30].
In the context of both concentration- and time-dependent kill characteristics, the ratio of 24-hour area under the concentration time curve to MIC (AUC24/MIC) best describes their antimicrobial activity (e.g., fluoroquinolones and glycopeptides)[11].
Optimal antimicrobial dosing is also dependent on the local resistance epidemiology, which can affect the rates of PK/PD target attainment in these patients. In critically ill patients with the rising prevalence of drug resistance, MICs of pathogens are reported to be 2-4 times higher than those in non-critically ill patients[31].
Drug-specific physicochemical properties should be integrated into dosing considerations. Hydrophilic medications, such as β-lactams, aminoglycosides, and vancomycin, have significant renal elimination, low Vd, and are not readily distributed to the tissues. AKI or KRT may significantly affect the clearance of hydrophilic antibiotics. Lipophilic drugs like macrolides, quinolones, echinocandins, posaconazole, and voriconazole show large apparent Vd and predominant hepatic elimination and are less likely to be affected by RRT because a low proportion of the drug is present in the bloodstream from where clearance occurs[8,12].
β-lactams are the most common antimicrobial class used in critically ill patients. They are first-line recommendations for frequently encountered infections, such as bacteremia, pneumonia, intra-abdominal infections, endocarditis, and urinary tract infections[32].
β-lactam antibiotics are generally hydrophilic, with low Vd and significant renal elimination, which warrants graded dosing adjustments according to CrCl corresponding to different stages of AKI. Although there is variation within this group, most β-lactams exhibit moderate (30%-70%) to low (less than 30%) levels of PB. In critically ill patients, heterogeneity in β-lactam PK is significant and could impact treatment outcomes[33].
The time-dependent bactericidal activity of β-lactams confers %T > MIC as the optimal PK/PD parameter for efficacy[34]. In general, evidence suggests that maintenance of drug concentrations at 4-8 times the MIC for at least 40%-70% of the dosing interval is associated with maximum efficacy. Compared to 40%-70% fT > MIC in non-ICU patients, it may be beneficial to aim for a free drug concentration in ICU patients that is 100% above the minimal inhibitory concentration (100% fT > MIC) and concentrations that are four times the MIC (100% fT > 4 MIC)[32]. Attaining the intended PK/PD objectives is linked to enhanced survival, decreased clinical failure rates, and successful microbiological outcomes[35]. Higher drug levels also correlate with improved tissue penetration and bioavailability of the drug at the infection sites.
Although AKI potentially exposes β-lactams to overdosing, therapeutic failures in this context have been reported, which may be related to subtherapeutic drug levels. Recommendations for dose modifications in differential stages of renal impairment for both older β-lactams and novel agents are generally based on evidence from patients with chronic kidney disease and may not be applicable in the dynamic clearance changes seen in critically ill patients. It is recommended to administer a loading dose regardless of CrCl, and dose adjustments should only be made after the first 24 to 48 hours of therapy due to the increased Vd in critically ill patients, the potential for acute renal dysfunction to resolve within the first few days after treatment, the high therapeutic index, and the limited risk of neurotoxicity with short exposure to high doses[19,36].
Prolonged infusions of β-lactam antibiotics have been advocated over standard infusions to reduce mortality or increase clinical cure among severely ill adult patients, particularly those with gram-negative infections[37]. Extending the β-lactam infusion duration results in increased drug exposure and the ability to reach higher PK/PD targets, as demonstrated by Monte Carlo simulations. This could potentially lead to better patient outcomes during severe infections. When utilizing an extended or continuous infusion, it is also important to consider the drug's stability data[38].
β-lactam TDM is not routinely recommended and while TDM-guided continuous infusions were more likely to achieve PD targets, no improvements in clinical outcomes have been established[39,40]. TDM could be considered in the critically ill patient population as they are at marked risk for PK/PD changes. In these situations, multidisciplinary collaboration between the intensivist, pharmacist, infectious disease specialist, and microbiologist is recommended[17].
Using higher doses of β-lactams to improve efficacy and decrease bacterial resistance can also raise the risk of drug-associated adverse effects. Neurotoxicity is associated with higher plasma trough concentrations and has been documented in up to 10%-15% of critically sick patients receiving a β-lactam medication[6,41]. Among the β-lactams under discussion, cefepime, imipenem, and aztreonam have the highest relative pro-convulsive activity. Although nephrotoxicity is uncommon, it can manifest as AKI, nephropathy from hemolytic anemia, and acute interstitial nephritis (AIN). AIN is more common in cephalosporins and penicillins. While piperacillin has the highest prevalence of drug-induced AKI, ceftriaxone and piperacillin are more commonly linked to hemolytic anemia[41]. Unfortunately, therapeutic values associated with toxicity are not well established for all β-lactams.
Aiming for a target of 100% fT > 4 × MIC can put patients with pathogens that have higher MICs at increased risk of toxicity. For instance, in targeting 100% fT > 4 × MIC for a pathogen with a MIC of 8 mg/L while treating with cefepime, the goal trough concentration would be approximately 32 mg/L. This trough level exceeds 20 mg/L, potentially increasing the risk of drug-related toxicities[4]. Current literature evaluating the incidence of adverse effects while on aggressive dosing regimens in the context of CKRT is limited and warrants further characterization.
In recent years, four innovative β-lactam/β-lactamase inhibitor (BL/BLI) combinations (ceftolozane-tazobactam, ceftazidime-avibactam, imipenem-relebactam and meropenem-vaborbactam) and a new siderophore cephalosporin (cefiderocol) have been approved for the treatment of certain drug-resistant gram-negative infections[42].
New BL/BLI combinations and cefiderocol share similar characteristics with older β-lactams, including high therapeutic indexes, heterogeneous inoculum effect, hydrophilicity, small MWs, significant renal clearance, low protein-binding (except for cefiderocol) suggesting effective elimination in AKI and via KRT. Hence, strategies of loading doses and prolonged infusions have also been advocated for the new BL/BLI combinations, especially if patients are also on CKRT[17,43].
Aminoglycosides are commonly prescribed as an adjunct to empirical therapy regimens in septic ICU patients, especially in the contexts of suspected resistant gram-negative infections like Pseudomonas aeruginosa and Acinetobacter baumannii, and Extended-Spectrum Beta–Lactamase Enterobacterales[44].
They have low Vd (0.3 to 0.4 L/kg), low PB capacity, and significant renal clearance with minimal intracellular penetration, all of which are consistent with their hydrophilic nature. The significant renal elimination confers drug clearance proportional to CrCl changes. These characteristics make them vulnerable to PK alterations in critically ill patients and enhance clearance through CKRT[45]. While decreased renal clearance may be a factor in high trough concentrations, an increase in Vd in critically ill patients may result in insufficient peak drug concentrations[46].
Aminoglycosides demonstrate concentration-dependent bactericidal effects and have a notable post-antibiotic effect (PAE), which can inhibit bacterial regrowth for extended periods when drug levels drop below the MIC[47]. As aminoglycosides have a narrow therapeutic index and may cause nephrotoxicity and ototoxicity, TDM is frequently utilized to achieve these goals while reducing toxicity by treatment individualization[48].
Once-daily dosing strategies are preferred to obtain high maximal concentrations, where the optimal antibacterial activity is achieved when the peak is eight to ten times greater than the pathogen's MIC in the context of systemic gram-negative infections. Apart from optimizing efficacy, this approach also trends towards reduced renal toxicity and an extended PAE, potentially lowering the risks of resistance emergence[18].
A wide range of Cmax and drug half-life (T1/2) have been observed for aminoglycosides during AKI and CKRT, hence dosing regimens should be individualized[49]. Amikacin dosing in CKRT was strategized in an extended-interval high loading dose of amikacin (25 mg/kg every 48 hours) combined with TDM[50]. More conservative recommendations include IV amikacin at a dose of 15-25 mg/kg every 48 hours, along with TDM adjustments to achieve PK/PD objectives that are contextualized to the infection sites and the microorganisms' susceptibility[51]. Gentamicin dosing in CKRT was suggested to be 3-5 mg/kg every 24-48 hours based on therapeutic targets and combined with TDM[52]. A combination of 7 mg/kg gentamicin every 24 hours with a high dose of 40 mL/kg/hour CKRT dose was also suggested as an initiating regimen in a population PK model study[53].
Vancomycin is a glycopeptide antibiotic often chosen for targeted or empirical treatment of various Gram-positive bacterial infections, particularly Methicillin-resistant Staphylococcus aureus (MRSA). It is a hydrophilic drug with a log P of 3.1, exhibits moderate PB of approximately 50%, and has an average MW of 1450 Da[54]. As the kidneys are primarily responsible for vancomycin elimination, its dosing is attenuated in accordance with renal function[55]. Additionally, substantial drug removal with CKRT can also be anticipated.
Vancomycin has a narrow therapeutic index and is commonly associated with adverse drug effects related to nephrotoxicity and ototoxicity and requires TDM to guide dosing. Nephrotoxicity remains the most severe vancomycin adverse effect and is associated with increased mortality, hospital length of stay and costs. It also correlates with vancomycin troughs > 20 μg/mL and AUC/MIC > 550-600 μg·hour/mL[56,57].
In recent years, vancomycin TDM recommendations have undergone a paradigm shift. The traditional trough-only TDM monitoring approach with a target trough concentration of 15-20 μg/mL is no longer recommended in patients with severe MRSA infections due to the lack of efficacy and higher nephrotoxicity occurrence. In contrast, there is advocacy for targeting the AUC/MIC target range of 400-600 μg·hour/mL (assuming MIC values are ≤ 1 μg/mL) to achieve both adequate clinical efficacy and safety[58].
In terms of dosing strategies in CKRT, a therapeutic target of 400-600 AUC/MIC with a loading dose of 20-25 mg/kg followed by a maintenance dose of 7.5-10 mg/kg every 12 hours with CKRT effluent rates of 20-25 mL/kg/hour have been suggested[58]. In another study, vancomycin doses of ≥ 15 mg/kg/day are needed to achieve early therapeutic targets in patients on high-intensity CKRT[59]. Higher doses (total dose ≥ 2.75 g/day) of vancomycin than commonly cited regimens of 1.5 g/day were required in CKRT patients with a vancomycin MIC ≥ 1 mg/L[60]. Due to inconsistent dosing recommendations in the literature, it is recommended to adjust the individual dose according to TDM.
Daptomycin is the first of the cyclic lipopeptide class of antibiotic lactams; it is active against a wide range of Gram-positive bacteria, including MRSA and vancomycin-resistant Enterococci; hence, it is usually used for the treatment of critically ill patients with bacteremia, endocarditis, or complicated skin and soft-tissue infections[61].
Daptomycin is a hydrophilic drug with a small Vd (0.1 L/kg) and a high PB rate (90%-93%), with more than 50% renal elimination[62]. It exhibits concentration-dependent bactericidal activity, and its AUC/MIC ratio is the most relevant PK/PD index of efficacy[63]. Daptomycin at 8-10 mg/kg every 24 hours in patients receiving CKRT achieved a higher peak and maximizes daptomycin’s concentration-dependent activity. Creatine phosphokinase levels have to be monitored due to possible adverse skeletal muscle effects with high-dose daptomycin[49,61].
Fluoroquinolones are an important group of antibiotics for both community-acquired and hospital-acquired infections; however, increasing bacterial resistance to quinolones is an ongoing challenge. Fluoroquinolones are lipophilic, with low to moderate PB and have a high Vd, which confers extensive tissue distribution and attains good extracellular and intracellular concentrations[64]. Ciprofloxacin has 50%-70% renal elimination while levofloxacin is excreted largely as an unchanged drug in the urine, both drugs are expected to have significant elimination via CKRT[65].
Fluoroquinolones exhibit concentration-dependent bactericidal activity, and the most relevant PK/PD index predicting their clinical efficacy is the AUC24/MIC ratio. The optimal target attainment for ciprofloxacin was suggested to be AUC24/MIC > 125, or > 100 for the unbound (free) drug concentration (ƒAUC24/MIC)[64]. Hence, maximal doses have been advocated when fluoroquinolones are used in the critically ill population i.e., levofloxacin 750 mg every 24 hours; ciprofloxacin 400 mg every 8 hours[11]. Although there has been a rise in reports of seizures associated with fluoroquinolones, there is no established toxicity threshold[66].
Acyclovir is used in the treatment of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections. Acyclovir (MW 225 Da) is poorly absorbed, with a bioavailability of 10% to 20% in patients with normal renal function. Its bioavailability is known to decrease with higher doses; hence, IV acyclovir is often preferred in more severe, disseminated infections. Acyclovir is about 15% bound to plasma protein, Vd 0.8 L/kg and widely distributed to tissues and fluids including the cerebrospinal fluid[67]. Acyclovir is converted to acyclovir monophosphate by viral enzymes and then to the active form by cellular enzymes. Elimination is largely dependent on renal function, with 62% to 91% of the unchanged drug and metabolites excreted by kidneys (urine). Elimination half-life is also prolonged in patients with impaired renal function, from approximately 2.5 hours in normal renal function to 20 hours in ESKD.
Acyclovir may result in AKI, in most instances this is reversible upon dose reduction or discontinuation. Nevertheless, it is prudent to ensure appropriate dosing in patients with impaired renal functions. For patients treated for central nervous system (CNS) related infections from HSV or VZV, the usual dosing in normal renal function is IV 10 mg/kg every 8 hours. Dose reduction is needed when CrCl < 50 mL/minute/1.73 m2 (adjusted for BSA). Based on PK parameters, acyclovir is highly dialyzable, with up to 60% reduction in drug concentrations after a 6-hour hemodialysis session; hence, it should be administered post-dialysis. In CKRT, dosing of IV 5 to 10 mg/kg/dose every 12 to 24 hours is recommended based on high-flux dialyzers and effluent flow rates of 20 to 25 mL/kg/hour[68]. In patients with ARC, drug dosing follows the maximum dosing based on the indication, with close monitoring for adverse events, including AKI and neurotoxicity.
Ganciclovir is primarily used in the treatment of cytomegalovirus (CMV) infections. Ganciclovir has a small MW of 255 Da, minimal PB of 1%-2%, and a Vd of 0.7 L/kg with widespread distribution to the tissues and CSF. Drug elimination half-life is prolonged in renal impairment from 3.5 hours in normal renal function to 10.7 hours (CrCl < 25 mL/minute). Ganciclovir is predominantly (80%-99%) excreted unchanged in the urine. Based on PK parameters, elimination and clearance is largely dependent on renal function and dosing adjustment is required in impaired renal function. In the treatment of CMV infections, IV ganciclovir is given 5 mg/kg/dose every 12 hours. The manufacturer recommends dose reduction when CrCl < 70 mL/minute. Krens et al's PK study[69] suggests using higher than manufacturer-recommended ganciclovir doses in critically ill patients with renal impairment [chronic kidney disease epidemiology collaboration (CKD-EPI) eGFR < 50 mL/minute/1.73 m2] to achieve minimum ganciclovir target trough concentrations of > 1.5 mg/L. However, the benefits of optimizing dosing by utilizing higher doses to prevent drug resistance and treatment failure need to be weighed against the potential drug-related toxicities such as renal injury and myelosuppression. For patients on low-flux hemodialysis, 50%-60% of the drug is dialyzed, hence IV ganciclovir should be administered after dialysis. In patients on CKRT, a dose of IV 2.5 mg/kg/dose every 24 hours appears to be adequate for anuric critically ill patients[70]. Increased serum creatinine has been reported in elderly patients and transplant patients on concomitant nephrotoxic drugs; hence, close monitoring of renal function is recommended.
Foscarnet is also used in the treatment of CMV infections, especially in situations when ganciclovir cannot be used. Foscarnet has a MW of 300 Da, low PB of 14%-17% and a Vd of approximately 0.5 L/kg[71]. Foscarnet does not undergo significant metabolism and is excreted by the kidneys (28% as unchanged in the urine). The elimination half-life is 3-4 hours, and the terminal half-life extends to approximately 88 hours due to bone deposition. Drug clearance is reduced, and plasma half-life is prolonged in patients with altered renal function: CrCl of 10-24 mL/minute correlates to clearance of 0.43 mL/minute/kg and a plasma half-life of about 25.3 hours, compared to a CrCl of 50-80 mL/minute, which corresponds to a clearance of about 1.33 mL/minute/kg and a plasma half-life of about 3.35 hours. For the treatment of CMV infection, the recommended dose in normal renal function is IV 60 mg/kg/dose every 8 hours or 90 mg/kg/dose every 12 hours. For both induction and maintenance dosing, a reduction in dose is required for CrCl < 1.4 mL/minute/kg. However, for CrCl < 0.4 mL/minute/kg, the use of foscarnet is not recommended by the manufacturer. There is currently limited data to guide dosing of foscarnet in hemodialysis and CKRT. Given its small molecular size, low PB, low Vd, and complete urinary excretion, foscarnet is expected to be readily removed via dialysis. Up to 38% of foscarnet is removed during a 2.5-hour hemodialysis session with a high-flux membrane[72]. Doses of 45 to 60 mg/kg/dose post-hemodialysis (3 times/week) for the treatment of CMV infection have been recommended[72,73]. In a case report by Wieruszewski et al[74], IV foscarnet dose of 30 mg/kg every 12 hours was given to a patient during continuous veno-venous hemofiltration (CVVH) (hemofiltration rate of 3000 mL/hour). This dosing was derived based on extrapolation from the manufacturer’s recommendation in renal impairment.
Oseltamivir has been used in the treatment and prophylaxis of influenza. Oseltamivir, a prodrug, is rapidly metabolized to the active form, oseltamivir carboxylate (OC). OC inhibits influenza virus neuraminidase enzymes and is essential for the release of progeny virions from infected host cells. OC (MW 284.4 Da) has negligible PB at approximately 3%, and a mean Vd of 23 L[75]. Oseltamivir is primarily (> 99%) eliminated by conversion to OC, in the urine. The elimination half-life of OC is 6-10 hours. For the treatment of influenza A, oseltamivir dosing in patients with normal renal function is 75 mg twice daily. Renal dosing adjustment is required for patients with altered kidney function (CrCl < 60 mL/minute)[76].
As OC has a low MW, minimal PB, and predominantly undergoes renal clearance, it is likely that dialysis would contribute significantly to drug clearance. Eyler et al[75] suggest dosing oseltamivir 75 mg once a day for critically ill patients requiring continuous veno-venous hemodialysis (CVVHD) with effluent flow rates used in the study (3300 ± 919 mL/hour). More aggressive dosing can be considered when CKRT effluent rates are higher. For patients on hemodialysis, oseltamivir is given 30 mg once and then 30 mg after every hemodialysis session for at least 5 days (oseltamivir is approximately 50% dialyzable). There is currently no available clinical or PK data on the optimal dosing of oseltamivir in ARC.
Remdesivir is a nucleotide analog prodrug with activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Remdesivir undergoes metabolism to form the active nucleoside triphosphate metabolite (GS-443902), which is incorporated into the SARS-CoV-2 RNA viral chains, preventing its replication. The nucleoside core metabolite (GS-441524) is the predominant remdesivir derivative detected in the urine. Approximately 10% of the parent remdesivir is eliminated in the urine as metabolite[77,78]. Remdesivir has a MW of 602.6 Da and is highly protein-bound (88%-93.6%).
Intravenous remdesivir formulations contain sulfobutylether-beta-cyclodextrin, an excipient that accumulates in patients with renal dysfunction. In the initial prescribing information for IV remdesivir, administration is not recommended in patients with an estimated GFR (eGFR) less than 30 mL/minute/1.73 m2 due to the presence of cyclodextrin which may accumulate in kidney dysfunction and worsen kidney or hepatic outcomes. Despite earlier concerns about its cyclodextrin vehicle, studies have reported the safe use of remdesivir in patients with AKI and chronic kidney disease without worsening kidney function or other adverse effects[79-81]. Since July 2023, the Food and Drug Administration approved the use of remdesivir for the treatment of coronavirus disease 2019 in patients with all stages of renal impairment, including patients on dialysis.
There is currently limited information regarding the optimal dosing of remdesivir in CKRT. Based on the physiochemical (hydrophilic) and PK properties (MW, PB) of remdesivir, it is potentially cleared via CKRT, and likely more so in high-intensity dialysis[82]. Standard dosing of remdesivir 200 mg IV loading dose, followed by 100 mg daily is recommended for all stages of renal impairment, including patients receiving dialysis and CKRT. Remdesivir may be administered regardless of the timing of dialysis sessions.
Echinocandins (anidulafungin, caspofungin, and micafungin) are widely used in the treatment of invasive candidiasis. Anidulafungin, caspofungin, and micafungin are highly bound to plasma albumin (99%, 97%, and 99%, respectively), and minimally (approximately 1%) excreted unchanged via the urinary tract. With a MW > 500 Da, and highly protein bound, echinocandins are poorly dialyzed via hemodialysis.
Another potential mechanism for drug removal in CKRT is drug adsorption to the dialysis filter membrane. The extent of drug absorption is dependent on drug properties, filter membrane material, and surface area. Various studies have reported that the drug concentrations of echinocandins may be reduced by filter adsorption[83-85]. However, the clinical significance of these findings remains unclear. In patients with renal insufficiency, hemodialysis, or CKRT, dosing adjustments for echinocandins are not required.
Another class of antifungals used in the critical care setting is the azoles, which include fluconazole, voriconazole, and isavuconazole[86].
Fluconazole (MW 306 Da) is well absorbed and has good oral bioavailability. It has a Vd of 0.6 L/kg and is widely distributed throughout the body. Plasma PB is low at 11%-12%. Drug clearance is largely dependent on renal function, with up to 80% of the drug excreted unchanged via the kidneys. The elimination half-life is approximately 30 hours and is prolonged in patients with impaired renal function[87]. Dose reduction is necessary in patients with impaired renal function. In patients with CrCl ≤ 50 mL/minute, it is recommended to reduce the maintenance dose by 50%. With low MW, low PB, and significant renal clearance, fluconazole is significantly dialyzable and should be administered after hemodialysis. There are 2 suggested dosing strategies: Either 100% of the maintenance dose given 3 times a week post hemodialysis, or 50% of the maintenance dose given daily[68,87]. The initial or loading dose remains the same as that for patients with normal renal function. Due to extensive drug clearance during CKRT, especially in anuric patients where tubular reabsorption is absent, higher than normal renal function doses have been recommended. In patients on CKRT (high-flux dialyzers and effluent flow rates of 20-25 mL/kg/hour), for the recommended dose of 400 mg once daily, a higher dose of 800 mg loading dose, followed by maintenance doses of 800 mg/day in 1-2 divided doses has been recommended[88]. Similarly, in patients with ARC, a higher than standard dosing may be required[89].
Voriconazole has a MW of 349 Da, PB of 58% and is extensively distributed to the tissues with a Vd of 4.6 L/kg. Less than 2% of the drug is excreted unchanged by the kidneys. No dosing adjustments are required for both the oral and IV formulations of voriconazole in patients with kidney dysfunction. However, the IV formulation contains the vehicle sulfobutyl ether beta-cyclodextrin sodium (SBECD) which accumulates in patients with renal impairment, and may lead to kidney injury. Hence, the manufacturer recommends avoiding the use of IV voriconazole in patients with renal impairment (CrCl < 50 mL/minute), and to use oral voriconazole preferentially if clinically appropriate. However, IV voriconazole has been used safely in patients with renal impairment[90,91]. Both voriconazole and SBECD are dialyzable. A 4-hour hemodialysis session does not remove voriconazole significantly to require dose adjustment[92]. In patients with ARC, TDM may be warranted[89].
Isavuconazole is a novel extended-spectrum azole antifungal with activity against yeasts, molds, and dimorphic fungi. It has a favorable safety profile (lack of QTc interval prolongation), more predictable PKs, and fewer drug-drug interactions[93]. Isavuconazole (MW 437 Da) is highly protein bound > 99% and extensively distributed to most tissues with a Vd 450 L. It is minimally (< 1%) excreted unchanged in the urine. As isavuconazole is highly protein-bound, it does not undergo significant clearance during hemodialysis or CKRT. No dosing adjustment is needed in all stages of kidney dysfunction. Lower plasma concentration of isavuconazole has been reported in critically ill patients receiving CKRT; however, this clinical significance is unclear[94,95]. For the treatment of invasive aspergillosis, a standard dose of isavuconazole 200 mg every 8 hours for 6 doses followed by a maintenance dose of 200 mg once daily can be safely administered to patients regardless of renal function. Of note, the IV formulation of isavuconazole does not contain cyclodextrin (as in IV voriconazole), which may accumulate in kidney dysfunction and potentially cause nephrotoxicity.
Amphotericin B remains one of the therapies for the treatment of severe and invasive fungal infections. Amphotericin B deoxycholate is highly bound to plasma protein (90%). It is widely distributed throughout the body, with a Vd of 4 L/kg. CNS penetration is poor (inflamed or noninflamed meninges). Less than 5% of the drug is excreted as the biologically active form in the urine. As such, dosing adjustment is not necessary for all stages of renal impairment, including hemodialysis and CKRT[96]. For patients with ARC, no adjustment is required as well.
Lipid-based amphotericin B formulations (e.g., liposomal amphotericin B) were introduced to improve the safety profile of conventional amphotericin B deoxycholate, in particular infusion-related reactions and risks of nephrotoxicity. Liposomal amphotericin B is highly protein-bound and lipophilic. As renal clearance accounts for approximately 5% of total clearance[97], it is unlikely to be dialyzed. Similarly, no dosing adjustment is required for patients with renal impairment and on dialysis.
This review highlights the complexity of challenges clinicians face when dosing antimicrobials in critically ill patients with various degrees of AKI. Some authors based the GFR calculations on the CKD-EPI equation, which was established in non-ICU patients; hence, may be of limited applicability in the critically ill patient population. We generally do not use GFR for dosing guidance, as most antimicrobial dosing guidelines are based on CrCl threshold values.
When dosing antimicrobials with a paucity of published CKRT dosing recommendations, we extrapolate dosing strategies based on the drug's physicochemical properties and the estimated extent of drug clearance in AKI/CKRT. Drugs with low Vd (< 1 L/kg) and lower PB (< 50%) would be expected to have more significant clearance via CKRT.
Optimal drug dosing strategies is crucial to favorable clinical outcomes. In general, we tend to dose most antimicrobials aggressively, at the higher end of the dosing range. This is especially for drugs that exhibit a wide therapeutic index, i.e., when dosing β-lactams, we advocate using loading doses and extended infusions to maximize its PK/PD in critically ill patients). Despite the increasing interest in the use of β-lactam TDM in recent years, we do not routinely adopt this practice due to substantial costs and logistical considerations in our local context.
Most CKRT drug dosing recommendations assume the ultrafiltration and dialysis flow rates of 1-2 L/hour. The mode and characteristics of CKRT highly influence drug disposition and should be considered when dosing and monitoring antimicrobials. We adjust antimicrobial dosing to accommodate for differences in ultrafiltration and dialysis flow rates, residual renal function, severity of infections, and CKRT interruptions in relation to drug administration timing and dosing. A summary of the PK/PD profiles and recommended CKRT dosing strategies of common antimicrobials encountered in the critically ill patient population can be found in Tables 1, 2 and 3 for reference[98-100].
| Antibiotics | PK/PD index | PK profile | Renal clearance (%) | Suggested CKRT dosing |
| β-lactam (prolonged infusion strategies should be used with drug/brand specific stability data) | ||||
| Aztreonam | %T > MIC | MW: 435[100]; PB: 56%; Vd: 0.15-0.18 L/kg | 60-70 | 2 g every 8 hours |
| Cefepime | %T > MIC | MW: 481; PB: 20%; Vd: 0.3 L/kg | 85 | 2 g every 8-12 hours |
| Ceftazidime | %T > MIC | MW: 547; PB < 10%; Vd: 0.28-0.4 L/kg | 60-85 | 2 g every 8 hours |
| Imipenem/cilastatin | %T > MIC | MW: 317/380; PB: 20/40%; Vd: 0.22-0.24 L/kg | 20-70/60 | 500 mg every 6-8 hours |
| Meropenem | %T > MIC | MW: 383; PB: 2%; Vd: 0.35 L/kg | 70 | 1-2 g every 8 hours |
| Piperacillin-tazobactam | %T > MIC | MW: 518/300; PB: 26-33/31%-32%; Vd: 0.24/0.40 L/kg | 75-90/65 | 4.5 g every 8 hours |
| Novel agents BL/BLI (prolonged infusion strategies should be used with drug/brand specific stability data) | ||||
| Cefiderocol | %T > MIC | MW: 752; PB: 40%-60%; Vd: 18 L | 90 | 1.5 g every 12 hours to 2 g every 8 hours[17] |
| Ceftazidime-avibactam | %T > MIC | MW: 637/265; PB: < 10%; Vd: 14-17 L | 80-90 | 1.25 g every 8 hours |
| Ceftolozane-tazobactam | %T > MIC | MW: 765/322; PB: 16%-21%; Vd: 13-18 L | 66 | 1.5 g every 8 hours, 1.5-3 g every 8 hours[17] |
| Imipenem-relebactam | %T > MIC | MW: 317/366; PB: 20%; Vd: 24 L | 63 | 1.25 g single dose, followed by 0.75 g every 6 hours |
| Meropenem-vaborbactam | %T > MIC | MW: 437/297; PB: 2%; Vd: 19 L | 40-60 | 2 g every 8 hours |
| Aminoglycosides (aminoglycosides dosing recommendations are in the context of systemic gram-negative infections) | ||||
| Amikacin | Cmax/MIC | MW: 586; PB: 0%-11%; Vd: 0.22-0.5 L/kg | 95 | 15-25 mg/kg every 48 hours with TDM; 25 mg/kg every 48 hours combined with TDM[50] |
| Gentamicin | Cmax/MIC | MW: 478; PB: < 30%; Vd: 0.36 L/kg | 95 | 3-5 mg/kg every 24-48 hours with TDM; 7 mg/kg every 24 hours with high dose of 40 mL/kg/hour CKRT dose[53] |
| Glycopeptides | ||||
| Vancomycin | AUC24/MIC | MW: 1448; PB: 55%; Vd: 0.47-1.1 L/kg | 90-100 | Load 20-25 mg/kg followed by 7.5-10 mg/kg every 12 hours with AUC monitoring[58] |
| Fluoroquinolones | ||||
| Ciprofloxacin | AUC24/MIC | MW: 331; PB: 20-40%; Vd: 2.5 L/kg | 50-70 | 400 mg IV every 8-12 hours |
| Levofloxacin | AUC24/MIC | MW: 361; PB: 24-38; Vd: 1.1-1.5 L/kg | 67-87 | 750 mg IV once followed by 750 mg IV every 24 hours with effluent flow rates > 20 mL/kg/hour[11] |
| Lipopeptides | ||||
| Daptomycin | AUC24/MIC | MW: 1620; PB: 90%-93%; Vd: 0.1-0.13 L/kg | 78 | 6 mg/kg every 24 hours; 8-10 mg/kg every 24 hours[49,61] |
| Antivirals | PK/PD index | PK profile | Renal clearance (%) | Suggested CKRT dosing |
| Acyclovir | No data | MW: 225; PB: 15%; Vd: 0.8 L/kg | 62-91 | 5-10 mg/kg/dose IV every 12-24 hours (high-flux dialyzers and effluent flow rates of 20-25 mL/kg/hour) |
| Ganciclovir | No data | MW: 255; PB: 1%-2%; Vd: 0.7 L/kg | 80-99 | 2.5 mg/kg/dose IV every 24 (induction dose) |
| Foscarnet | No data | MW: 300; PB: 14%-17%; Vd: 0.5 L/kg | 28 as unchanged in the urine | 30 mg/kg IV every 12 hour (CVVH; hemofiltration rate of 3000 mL/hour)[74] |
| Oseltamivir[75] | No data | Oseltamivir carboxylate; MW: 284.4; PB: 3%; Vd: 23 L | > 99 | 75 mg once a day (CVVHD; effluent flow rates used in the study (3300 ± 919 mL/hour) |
| Remdesivir | No data | MW: 602.6; PB: 88%-93.6%; Vd: Low tissue distribution | Remdesivir: 10; GS-441524: 49 | 200 mg IV loading dose, followed by 100 mg daily |
| Antifungals | PK/PD index | PK profile | Renal clearance (%) | Suggested CKRT dosing |
| Echinocandins[99] | ||||
| Anidulafungin | AUC24/MIC | MW: 1140.3; PB: > 99%; Vd: 30-50 L | < 1 | No dosage adjustment necessary (poorly dialyzed) |
| Caspofungin | AUC24/MIC | MW: 1213.42; PB: 97%; Vd: 9.7 L | Approximately 1% of total dose as unchanged drug | No dosage adjustment necessary (poorly dialyzed) |
| Micafungin | AUC24/MIC | MW: 1292.26; PB: > 99%; Vd: 0.39 L/kg | < 1 | No dosage adjustment necessary (poorly dialyzed) |
| Azoles | ||||
| Fluconazole | AUC24/MIC | MW: 306; PB: 11%-12%; Vd: 0.6 L/kg | 80% | For recommended dose of 400 mg once daily, to give 800 mg loading dose, followed by maintenance doses of 800 mg/day in 1 to 2 divided doses |
| Voriconazole | AUC24/MIC | MW: 349; PB: 58%; Vd: 4.6 L/kg | < 2 | No dosing adjustment necessary |
| Isavuconazole | AUC24/MIC | MW: 437; PB: > 99%; Vd (IV): 450 L | < 1 | No dosing adjustment necessary |
| Polyenes | ||||
| Amphotericin B deoxycholate | Cmax/MIC | MW: 924; PB: 90%; Vd: 4 L/kg | < 5 | No dosage adjustment necessary (unlikely dialyzed) |
Future directions to consider would include the exploration of point-of-care TDM of β-lactams to improve turnaround times for drug level results, to aid clinicians in optimizing therapy in a timely manner, especially in the ICU setting. There is also a need to consider validating dosing and titration algorithms in the TDM of β-lactams. This can be supported by relevant research evaluating the correlation between drug levels to expected clinical outcomes and toxicity effects. Most CKRT drug dosing recommendations assume the ultrafiltration and dialysis flow rates of 1-2 L/hour and may not adequately reflect the CKRT settings in practice. Differential dosing strategies in various CKRT modes (continuous veno-venous hemodiafiltration, CVVHD, CVVH) and sustained low-efficiency dialysis can be validated in future clinical studies.
Conventional antimicrobial dosing regimens may not be optimal for critically ill patients with sepsis and septic shock, who commonly present with AKI and are eventually initiated on KRT. Considerations include the altered physiology in critical illness which impacts the PK/PD profiles of common antimicrobials, the complex interplay with pathogen-specific factors, and drug clearance trends in AKI and KRT which would influence dosing requirements in this patient population. Strategies of loading doses, prolonged infusions for time-dependent antimicrobials i.e., β-lactams, coupled with evidence-based TDM in the ICU can be used to assess the adequacy of dosing regimens and optimize clinical outcomes.
| 1. | Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2063] [Cited by in RCA: 1992] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 2. | Kollef MH, Shorr AF, Bassetti M, Timsit JF, Micek ST, Michelson AP, Garnacho-Montero J. Timing of antibiotic therapy in the ICU. Crit Care. 2021;25:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4783] [Cited by in RCA: 4084] [Article Influence: 204.2] [Reference Citation Analysis (0)] |
| 4. | Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J; DALI Study. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 846] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 5. | Lewis SJ, Mueller BA. Antibiotic Dosing in Patients With Acute Kidney Injury: "Enough But Not Too Much". J Intensive Care Med. 2016;31:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Jang SM, Lewis SJ, Rhie SJ. Optimal antipseudomonal ꞵ-lactam drug dosing recommendations in critically-ill Asian patients receiving CRRT. J Crit Care. 2022;72:154172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Al-Shaer MH, Maguigan K, Ashton J, Venugopalan V, Droege ME, Philpott CD, Droege CA, Healy DP, Mueller EW, Peloquin CA. Applying Cefepime Population Pharmacokinetics to Critically Ill Patients Receiving Continuous Renal Replacement Therapy. Antimicrob Agents Chemother. 2022;66:e0161121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Roger C, Nucci B, Louart B, Friggeri A, Knani H, Evrard A, Lavigne JP, Allaouchiche B, Lefrant JY, Roberts JA, Muller L. Impact of 30 mg/kg amikacin and 8 mg/kg gentamicin on serum concentrations in critically ill patients with severe sepsis. J Antimicrob Chemother. 2016;71:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Cristallini S, Hites M, Kabtouri H, Roberts JA, Beumier M, Cotton F, Lipman J, Jacobs F, Vincent JL, Creteur J, Taccone FS. New Regimen for Continuous Infusion of Vancomycin in Critically Ill Patients. Antimicrob Agents Chemother. 2016;60:4750-4756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Roger C. Understanding antimicrobial pharmacokinetics in critically ill patients to optimize antimicrobial therapy: A narrative review. J Intensive Med. 2024;4:287-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 11. | Póvoa P, Moniz P, Pereira JG, Coelho L. Optimizing Antimicrobial Drug Dosing in Critically Ill Patients. Microorganisms. 2021;9:1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Kanji S, Roger C, Taccone FS, Muller L. Practical considerations for individualizing drug dosing in critically ill adults receiving renal replacement therapy. Pharmacotherapy. 2023;43:1194-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother. 2001;47:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, Boots RJ, Lipman J. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 2014;42:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Chen IH, Nicolau DP. Augmented Renal Clearance and How to Augment Antibiotic Dosing. Antibiotics (Basel). 2020;9:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís MÁ. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin Pharmacokinet. 2018;57:1107-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 17. | Barbier F, Hraiech S, Kernéis S, Veluppillai N, Pajot O, Poissy J, Roux D, Zahar JR; French Intensive Care Society. Rationale and evidence for the use of new beta-lactam/beta-lactamase inhibitor combinations and cefiderocol in critically ill patients. Ann Intensive Care. 2023;13:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 18. | Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc. 2015;16:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Crass RL, Rodvold KA, Mueller BA, Pai MP. Renal Dosing of Antibiotics: Are We Jumping the Gun? Clin Infect Dis. 2019;68:1596-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Camargo MS, Mistro S, Oliveira MG, Passos LCS. Association between increased mortality rate and antibiotic dose adjustment in intensive care unit patients with renal impairment. Eur J Clin Pharmacol. 2019;75:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 21. | Aldardeer NF, Alshreef MM, Alharbi EA, Aljabri AK, Aljawadi MH, Almangour TA, Alobaili S, Alarifi MI, Alomari A, Alhammad AM. Early Versus Late Antipseudomonal β-Lactam Antibiotic Dose Adjustment in Critically Ill Sepsis Patients With Acute Kidney Injury: A Prospective Observational Cohort Study. Open Forum Infect Dis. 2024;11:ofae059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Chaijamorn W, Shaw AR, Lewis SJ, Mueller BA. Ex vivo Ceftolozane/Tazobactam Clearance during Continuous Renal Replacement Therapy. Blood Purif. 2017;44:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Hoff BM, Maker JH, Dager WE, Heintz BH. Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann Pharmacother. 2020;54:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Jamal JA, Roberts DM, Udy AA, Mat-Nor MB, Mohamad-Nor FS, Wallis SC, Lipman J, Roberts JA. Pharmacokinetics of piperacillin in critically ill patients receiving continuous venovenous haemofiltration: A randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;46:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Pehlivanli A, Yanik Yalçin T, Yeşiler Fİ, Şahintürk H, Kurt Azap Ö, Zeyneloğlu P, Başgut B. Antimicrobial dosing recommendations during continuous renal replacement therapy: different databases, different doses. J Chemother. 2024;36:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46:997-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Corona A, Veronese A, Santini S, Cattaneo D. "CATCH" Study: Correct Antibiotic Therapy in Continuous Hemofiltration in the Critically Ill in Continuous Renal Replacement Therapy: A Prospective Observational Study. Antibiotics (Basel). 2022;11:1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 28. | Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, Lipman J, Bellomo R; RENAL Replacement Therapy Study Investigators. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Bugge JF. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol Scand. 2001;45:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Sember AM, LoFaso ME, Lewis SJ. An optimal extended-infusion dosing of cefepime and ceftazidime in critically ill patients with continuous renal replacement therapy. J Crit Care. 2022;69:154011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1158] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 32. | Maguigan KL, Al-Shaer MH, Peloquin CA. Beta-Lactams Dosing in Critically Ill Patients with Gram-Negative Bacterial Infections: A PK/PD Approach. Antibiotics (Basel). 2021;10:1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva JA, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA; Infection Section of European Society of Intensive Care Medicine (ESICM); Pharmacokinetic/pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID); Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT); Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC). Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper(). Intensive Care Med. 2020;46:1127-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 782] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 34. | Dilworth TJ, Schulz LT, Micek ST, Kollef MH, Rose WE. β-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Weighing the Challenges and Opportunities to Assess Clinical Value. Crit Care Explor. 2022;4:e0726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Al-Shaer MH, Rubido E, Cherabuddi K, Venugopalan V, Klinker K, Peloquin C. Early therapeutic monitoring of β-lactams and associated therapy outcomes in critically ill patients. J Antimicrob Chemother. 2020;75:3644-3651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Gatti M, Pea F. Antimicrobial Dose Reduction in Continuous Renal Replacement Therapy: Myth or Real Need? A Practical Approach for Guiding Dose Optimization of Novel Antibiotics. Clin Pharmacokinet. 2021;60:1271-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Hong LT, Downes KJ, FakhriRavari A, Abdul-Mutakabbir JC, Kuti JL, Jorgensen S, Young DC, Alshaer MH, Bassetti M, Bonomo RA, Gilchrist M, Jang SM, Lodise T, Roberts JA, Tängdén T, Zuppa A, Scheetz MH. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: Endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2023;43:740-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 38. | Pilmis B, Petitjean G, Lesprit P, Lafaurie M, El Helali N, Le Monnier A; on behalf the ATB PK/PD study group. Continuous infusion of ceftolozane/tazobactam is associated with a higher probability of target attainment in patients infected with Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2019;38:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Ewoldt TMJ, Abdulla A, Rietdijk WJR, Muller AE, de Winter BCM, Hunfeld NGM, Purmer IM, van Vliet P, Wils EJ, Haringman J, Draisma A, Rijpstra TA, Karakus A, Gommers D, Endeman H, Koch BCP. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: a multicentre randomised clinical trial. Intensive Care Med. 2022;48:1760-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 40. | Hagel S, Bach F, Brenner T, Bracht H, Brinkmann A, Annecke T, Hohn A, Weigand M, Michels G, Kluge S, Nierhaus A, Jarczak D, König C, Weismann D, Frey O, Witzke D, Müller C, Bauer M, Kiehntopf M, Neugebauer S, Lehmann T, Roberts JA, Pletz MW; TARGET Trial Investigators. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: a randomized controlled trial. Intensive Care Med. 2022;48:311-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 41. | Roger C, Louart B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms. 2021;9:1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Ferous S, Anastassopoulou C, Pitiriga V, Vrioni G, Tsakris A. Antimicrobial and Diagnostic Stewardship of the Novel β-Lactam/β-Lactamase Inhibitors for Infections Due to Carbapenem-Resistant Enterobacterales Species and Pseudomonas aeruginosa. Antibiotics (Basel). 2024;13:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 43. | Gorham J, Taccone FS, Hites M. Drug Regimens of Novel Antibiotics in Critically Ill Patients with Varying Renal Functions: A Rapid Review. Antibiotics (Basel). 2022;11:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Roger C, Louart B, Elotmani L, Barton G, Escobar L, Koulenti D, Lipman J, Leone M, Muller L, Boutin C, Amour J, Banakh I, Cousson J, Bourenne J, Constantin JM, Albanese J, Roberts JA, Lefrant JY; Azurea Network. An international survey on aminoglycoside practices in critically ill patients: the AMINO III study. Ann Intensive Care. 2021;11:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Krueger CK, Bruno JJ, Tverdek FP, Hernandez M, Abudayyeh A. Aminoglycoside Pharmacokinetics in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Ann Pharmacother. 2023;57:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Brasseur A, Hites M, Roisin S, Cotton F, Vincent JL, De Backer D, Jacobs F, Taccone FS. A high-dose aminoglycoside regimen combined with renal replacement therapy for the treatment of MDR pathogens: a proof-of-concept study. J Antimicrob Chemother. 2016;71:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 828] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 48. | Duong A, Simard C, Wang YL, Williamson D, Marsot A. Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling? Antibiotics (Basel). 2021;10:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Li L, Li X, Xia Y, Chu Y, Zhong H, Li J, Liang P, Bu Y, Zhao R, Liao Y, Yang P, Lu X, Jiang S. Recommendation of Antimicrobial Dosing Optimization During Continuous Renal Replacement Therapy. Front Pharmacol. 2020;11:786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 50. | Roger C, Wallis SC, Muller L, Saissi G, Lipman J, Lefrant JY, Roberts JA. Influence of Renal Replacement Modalities on Amikacin Population Pharmacokinetics in Critically Ill Patients on Continuous Renal Replacement Therapy. Antimicrob Agents Chemother. 2016;60:4901-4909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Wolters Kluwer. Amikacin. Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. [cited 5 October 2024]. Available from: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrug. |
| 52. | Wolters Kluwer. Gentamicin. Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. [cited 5 October 2024]. Available from: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrug. |
| 53. | He S, Cheng Z, Xie F. Population Pharmacokinetics and Dosing Optimization of Gentamicin in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Drug Des Devel Ther. 2022;16:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Ghasemiyeh P, Vazin A, Zand F, Haem E, Karimzadeh I, Azadi A, Masjedi M, Sabetian G, Nikandish R, Mohammadi-Samani S. Pharmacokinetic assessment of vancomycin in critically ill patients and nephrotoxicity prediction using individualized pharmacokinetic parameters. Front Pharmacol. 2022;13:912202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Aljutayli A, Marsot A, Nekka F. An Update on Population Pharmacokinetic Analyses of Vancomycin, Part I: In Adults. Clin Pharmacokinet. 2020;59:671-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 56. | Alshehri N, Ahmed AE, Yenugadhati N, Javad S, Al Sulaiman K, M Al-Dorzi H, Aljerasiy M, Badri M. Vancomycin in ICU Patients with Gram-Positive Infections: Initial Trough Levels and Mortality. Ther Clin Risk Manag. 2020;16:979-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Ishigo T, Matsumoto K, Yoshida H, Tanaka H, Ibe Y, Fujii S, Fukudo M, Fujihara H, Yamaguchi F, Ebihara F, Maruyama T, Hamada Y, Samura M, Nagumoi F, Komatsu T, Tomizawa A, Takuma A, Chiba H, Nishi Y, Enoki Y, Taguchi K, Suzuki A. Relationship between nephrotoxicity and area under the concentration-time curve of vancomycin in critically ill patients: a multicenter retrospective study. Microbiol Spectr. 2024;12:e0373923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 58. | Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro B. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2020;71:1361-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 59. | Srour N, Lopez C, Succar L, Nguyen P. Vancomycin dosing in high-intensity continuous renal replacement therapy: A retrospective cohort study. Pharmacotherapy. 2023;43:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Charoensareerat T, Chaijamorn W, Boonpeng A, Srisawat N, Pummangura C, Pattharachayakul S. Optimal vancomycin dosing regimens for critically ill patients with acute kidney injury during continuous renal replacement therapy: A Monte Carlo simulation study. J Crit Care. 2019;54:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Grégoire N, Marchand S, Ferrandière M, Lasocki S, Seguin P, Vourc'h M, Barbaz M, Gaillard T, Launey Y, Asehnoune K, Couet W, Mimoz O. Population pharmacokinetics of daptomycin in critically ill patients with various degrees of renal impairment. J Antimicrob Chemother. 2019;74:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Woodworth JR, Nyhart EH Jr, Brier GL, Wolny JD, Black HR. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob Agents Chemother. 1992;36:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Wu J, Zheng X, Zhang L, Wang J, Lv Y, Xi Y, Wu D. Population pharmacokinetics of intravenous daptomycin in critically ill patients: implications for selection of dosage regimens. Front Pharmacol. 2024;15:1378872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 64. | Koch BCP, Muller AE, Hunfeld NGM, de Winter BCM, Ewoldt TMJ, Abdulla A, Endeman H. Therapeutic Drug Monitoring of Antibiotics in Critically Ill Patients: Current Practice and Future Perspectives With a Focus on Clinical Outcome. Ther Drug Monit. 2022;44:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Roehr AC, Frey OR, Koeberer A, Fuchs T, Roberts JA, Brinkmann A. Anti-infective drugs during continuous hemodialysis - using the bench to learn what to do at the bedside. Int J Artif Organs. 2015;38:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Chui CS, Chan EW, Wong AY, Root A, Douglas IJ, Wong IC. Association between oral fluoroquinolones and seizures: A self-controlled case series study. Neurology. 2016;86:1708-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Wolters Kluwer. Acyclovir. Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. [cited 5 October 2024]. Available from: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrug. |
| 68. | Heintz BH, Matzke GR, Dager WE. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy. 2009;29:562-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 69. | Krens SD, Hodiamont CJ, Juffermans NP, Mathôt RAA, van Hest RM. Population Pharmacokinetics of Ganciclovir in Critically Ill Patients. Ther Drug Monit. 2020;42:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Horvatits T, Kitzberger R, Drolz A, Zauner C, Jäger W, Böhmdorfer M, Kraff S, Fritsch A, Thalhammer F, Fuhrmann V, Schenk P. Pharmacokinetics of ganciclovir during continuous venovenous hemodiafiltration in critically ill patients. Antimicrob Agents Chemother. 2014;58:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Food and Drug Administration. Foscavir (Foscarnet) Package insert. Clinigen Healthcare Ltd. 2020. [cited 5 October 2024]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020068Orig1s025lbl.pdf. |
| 72. | Aweeka FT, Jacobson MA, Martin-Munley S, Hedman A, Schoenfeld P, Omachi R, Tsunoda S, Gambertoglio JG. Effect of renal disease and hemodialysis on foscarnet pharmacokinetics and dosing recommendations. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Jayasekara D, Aweeka FT, Rodriguez R, Kalayjian RC, Humphreys MH, Gambertoglio JG. Antiviral therapy for HIV patients with renal insufficiency. J Acquir Immune Defic Syndr. 1999;21:384-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Wieruszewski PM, Vijayvargiya P, Wilhelm MP, Razonable RR. Cytomegaloviraemia clearance with foscarnet during renal replacement therapy. J Antimicrob Chemother. 2018;73:548-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Eyler RF, Heung M, Pleva M, Sowinski KM, Park PK, Napolitano LM, Mueller BA. Pharmacokinetics of oseltamivir and oseltamivir carboxylate in critically ill patients receiving continuous venovenous hemodialysis and/or extracorporeal membrane oxygenation. Pharmacotherapy. 2012;32:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Wolters Kluwer. Oseltamivir. Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. [cited 5 October 2024]. Available from: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrug. |
| 77. | Gilead Sciences, Inc. Veklury (Remdesivir). Package insert. 2024. [cited 5 October 2024]. Available from: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. |
| 78. | Deb S, Reeves AA, Hopefl R, Bejusca R. ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential. Pharmaceuticals (Basel). 2021;14:655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 79. | Thakare S, Gandhi C, Modi T, Bose S, Deb S, Saxena N, Katyal A, Patil A, Patil S, Pajai A, Bajpai D, Jamale T. Safety of Remdesivir in Patients With Acute Kidney Injury or CKD. Kidney Int Rep. 2021;6:206-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 80. | Cheng M, Fowler R, Murthy S, Pinto R, Sheehan NL, Tseng A. Remdesivir in Patients With Severe Kidney Dysfunction: A Secondary Analysis of the CATCO Randomized Trial. JAMA Netw Open. 2022;5:e2229236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Seethapathy R, Wang Q, Zhao S, Strohbehn IA, Long JD, Dinulos JE, Harden D, Kadiyala VB, Moreno D, Sise ME. Effect of remdesivir on adverse kidney outcomes in hospitalized patients with COVID-19 and impaired kidney function. PLoS One. 2023;18:e0279765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 82. | Chaijamorn W, Rungkitwattanakul D, Nuchtavorn N, Charoensareerat T, Pattharachayakul S, Sirikun W, Srisawat N. Antiviral Dosing Modification for Coronavirus Disease 2019-Infected Patients Receiving Extracorporeal Therapy. Crit Care Explor. 2020;2:e0242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | González de Molina F, Martínez-Alberici Mde L, Ferrer R. Treatment with echinocandins during continuous renal replacement therapy. Crit Care. 2014;18:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Aguilar G, Ferriols R, Navarro D, Belda FJ. Echinocandin Dosing in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Curr Fungal Infect Rep. 2017;11:1-4. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Baud FJ, Jullien V, Secrétan PH, Houzé P, Lamhaut L. Are we correctly treating invasive candidiasis under continuous renal replacement therapy with echinocandins? Preliminary in vitro assessment. Anaesth Crit Care Pain Med. 2021;40:100640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Chatelon J, Cortegiani A, Hammad E, Cassir N, Leone M. Choosing the Right Antifungal Agent in ICU Patients. Adv Ther. 2019;36:3308-3320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Cousin L, Berre ML, Launay-Vacher V, Izzedine H, Deray G. Dosing guidelines for fluconazole in patients with renal failure. Nephrol Dial Transplant. 2003;18:2227-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Bergner R, Hoffmann M, Riedel KD, Mikus G, Henrich DM, Haefeli WE, Uppenkamp M, Walter-Sack I. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): need for a high daily dose of 800 mg. Nephrol Dial Transplant. 2006;21:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent JL. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care. 2016;20:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | Neofytos D, Lombardi LR, Shields RK, Ostrander D, Warren L, Nguyen MH, Thompson CB, Marr KA. Administration of voriconazole in patients with renal dysfunction. Clin Infect Dis. 2012;54:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Kim SH, Kwon JC, Park C, Han S, Yim DS, Choi JK, Cho SY, Lee HJ, Park SH, Choi SM, Choi JH, Yoo JH, Lee DG, Lee JW. Therapeutic drug monitoring and safety of intravenous voriconazole formulated with sulfobutylether β-cyclodextrin in haematological patients with renal impairment. Mycoses. 2016;59:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Pfizer. Vfend (Voricoanzole). Package insert. 2024. [cited 3 August 2024]. Available from: https://labeling.pfizer.com/showlabeling.aspx?id=618. |
| 93. | Lewis JS 2nd, Wiederhold NP, Hakki M, Thompson GR 3rd. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob Agents Chemother. 2022;66:e0017722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 94. | Martín-Cerezuela M, Maya Gallegos C, Marqués-Miñana MR, Broch Porcar MJ, Cruz-Sánchez A, Mateo-Pardo JC, Peris Ribera JE, Gimeno R, Castellanos-Ortega Á, Poveda Andrés JL, Ramírez Galleymore P. Isavuconazole Pharmacokinetics in Critically Ill Patients: Relationship with Clinical Effectiveness and Patient Safety. Antibiotics (Basel). 2024;13:706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 95. | Zurl C, Waller M, Schwameis F, Muhr T, Bauer N, Zollner-Schwetz I, Valentin T, Meinitzer A, Ullrich E, Wunsch S, Hoenigl M, Grinschgl Y, Prattes J, Oulhaj A, Krause R. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J Fungi (Basel). 2020;6:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Wolters Kluwer. Amphotericin B. Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. [cited 5 October 2024]. Available from: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrug. |
| 97. | Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 98. | Wolters Kluwer. UpToDate. 2024. [cited 5 October 2024]. Available from: https://www.uptodate.com. |
| 99. | Antimicrobial Therapy, Inc. The Sanford Guide to Antimicrobial Therapy. 2024. [cited 5 October 2024]. Available from: https://web.sanfordguide.com/. |
| 100. | National Center for Biotechnology Information. PubChem Compound Summary for CID 5742832, Aztreonam. Mar 26, 2025 [cited 3 October 2024]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Azactam. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/