Published online Mar 25, 2025. doi: 10.5527/wjn.v14.i1.101078
Revised: December 14, 2024
Accepted: January 7, 2025
Published online: March 25, 2025
Processing time: 138 Days and 5.4 Hours
Kidney dysfunction and reduced filtration capacity due to chronic kidney disease (CKD) lead to a shift in the body's acid-base balance, ultimately causing metabolic acidosis (MA). Sodium bicarbonate has been used as a supplement to alleviate the symptoms and reverse the acidosis, and it may even slow the progression of CKD. However, its safety profile and overall effectiveness are uncertain.
To conduct a systematic review and meta-analysis of clinical trials assessing sodium bicarbonate's safety and efficacy for treating CKD-induced MA.
Medline, Scopus, EMBASE, and Cochrane Central were systematically searched from inception until May 2024 to select all relevant randomized control trials (RCTs) and non-RCT (NRCTs) evaluating the effectiveness of sodium bicarbonate in correcting MA in end-stage renal disease patients. In addition, ClinicalTrials.gov, Medrxiv.org, and Google Scholar were searched for other literature. A random-effects meta-analysis was performed to derive mean differences (MD) and risk ratios (RR) with their 95%CI for continuous and dichotomous outcomes respectively.
Following a systematic search of the databases, 20 RCTs and 2 and NRCTs comprising 2932 patients were included in our study. The results revealed that sodium bicarbonate significantly increased serum bicarbonate in CKD patients (MD: 2.59, 95%CI: 0.95-4.22; P = 0.02; I2 = 95%). However, there was a non-significant increase in estimated glomerular filtration rate (eGFR) in patients on sodium bicarbonate therapy (MD: 0.93, 95%CI: -1.88-3.75; P = 0.52; I2 = 93%). Upon assessment of the safety profile of sodium bicarbonate, no significant association was found in the outcomes of death/prolonged hospitalization (RR: 1.05, 95%CI: 0.84-1.32; P = 0.66; I2 = 0%), or gastrointestinal disorders (RR: 1.64, 95%CI: 0.35-7.66; P = 0.53; I2 = 76%), or worsening edema (RR: 1.26, 95%CI: 0.94-1.68; P = 0.12; I2 = 37%) when compared to control.
Sodium bicarbonate therapy may halt worsening kidney function by correcting serum bicarbonate levels and treating MA. Although sodium bicarbonate does not significantly improve the eGFR, it may potentially prevent CKD progression while maintaining an overall favorable safety profile.
Core Tip: Sodium bicarbonate is a systemic alkalizer that increases serum bicarbonate, buffers hydrogen ions, and raises blood pH, thereby reversing the clinical manifestations of acidosis. It is often prescribed for the treatment of metabolic acidosis (MA) seen in severe renal disease. Studies have been conducted to assess the safety and efficacy of sodium bicarbonate as a pH equalizer. This meta-analysis aims to evaluate the safety profile and clinical efficacy of sodium bicarbonate in treating MA in patients with chronic kidney disease.
- Citation: Siddiqui AH, Batool F, Khan S, Rizvi SS, Usman S, Jawed H, Ali MH, Zehra T, Adil AR, Anwar M, Hanif A, Hassan SK, Noble MW, Moeed A, Surani S. Safety and efficacy of sodium bicarbonate for treating metabolic acidosis in chronic kidney disease: A systematic review and meta-analysis. World J Nephrol 2025; 14(1): 101078
- URL: https://www.wjgnet.com/2220-6124/full/v14/i1/101078.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i1.101078
Chronic kidney disease (CKD) refers to a progressive decline in kidney function over time. According to the Kidney Disease Improving Global Outcomes guidelines, CKD is characterized by reduced kidney function, as indicated by an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m²[1]. Although the etiology of CKD varies globally, diabetes and hypertension are known to be the leading causes of CKD worldwide[2].
By eliminating hydrogen ions in the urine and reabsorbing bicarbonate from the urine, the kidneys typically play a significant role in maintaining the pH balance of extracellular fluid[3]. If compromised, it may lead to a variety of unfavorable consequences, including hyperlipidemia, anemia, metabolic bone disease, and cardiovascular disease[4]. Metabolic acidosis (MA), defined as an increase in hydrogen ion concentration in the systemic circulation resulting in a serum HCO3 less than 24 mEq/L, is the most frequent complication among patients with CKD[5].
One of the main pathophysiological factors leading to the development of MA in CKD patients is a reduced ability to reabsorb filtered bicarbonate in conjunction with a compromised capacity to generate sufficient bicarbonate[6]. Acid retention because of this makes it difficult to balance the net endogenous acid load. It causes muscle atrophy, bone disease development or aggravation, hypoalbuminemia, elevated inflammation, CKD progression, protein malnutrition, changes in insulin, leptin, and growth hormone, and a higher death rate[7,8].
Several therapeutic options are available to counter MA, including alkali therapy, dietary changes, and hydration[9,10]. Of them, bicarbonate supplementation is the most important. It lowers the systemic acid load and neutralizes excess hydrogen ions to restore acid-base equilibrium. This can slow the progression of CKD and postpone the start of dialysis[11]. In contrast to other therapies like dietary adjustments, which may have a later onset of action, it helps safeguard bone health by avoiding acid-induced bone demineralization. This can be substantiated by a study where it was reported that correcting MA with oral sodium bicarbonate in patients with CKD stage III-V notably diminishes the progression from stage V CKD to dialysis and enhances patient survival, regardless of their initial renal function status[12]. While some articles highlight the benefits of sodium bicarbonate therapy, others do not show improvement in patient outcomes[13]. This discrepancy may be due to the adverse effects of sodium bicarbonate infusion for the treatment of MA, such as fluid overload, hypokalemia, hypocalcemia, alkalemia, and hypernatremia[14]. However, current evidence points to the potential benefits of sodium bicarbonate supplementation in CKD[9].
A meta-analysis was carried out to assess the advantages and safety of employing sodium bicarbonate supplementation to treat MA in patients with CKD. Compared to individual studies alone, this method offers a more thorough and trustworthy conclusion, which helps to improve comprehension of the overall effect of this therapy option. In addition to highlighting research gaps, this in-depth review supports evidence-based decision-making. It helps build well-informed healthcare policies, which improve patient care in managing CKD.
This meta-analysis was conducted in accordance with the PRISMA guidelines and conforms to the framework set by the Cochrane Collaboration.
Medical literature databases, including Medline, Scopus, EMBASE, and Cochrane Central, were searched from inception until May 2024 using a search strategy based on PICO (patient, intervention, control, and outcomes). Search terms and MeSH terms used were “sodium bicarbonate”; “metabolic acidosis”; “chronic kidney disease”; and CKD. Boolean operators (“AND”, and “OR”) were used to form a search string. No limitations or filters were applied. Gray literature was identified via ClinicalTrials.gov, Medrxiv.org, and Google Scholar. A further expanded search was done into the references of the included studies to find any missing literature. A detailed description of the search strategy has been summarized in Supplementary Table 1.
Two authors (Saad Khalid Hassan and Fizzah Batool) independently screened the results based on the title and abstract after duplicate removal was conducted on the EndNote Reference library (Version X7.5; Clarivate Analytics, Philadelphia, Pennsylvania). Any discrepancies were resolved through a discussion with a third author (Abdul Hannan Siddiqui). The inclusion criteria mandated to include: (1) Studies with adults over 18 years of age; (2) Patients having CKD with an eGFR of <15 to 60 mL/min per 1.73 m²; (3) Articles comparing sodium bicarbonate to a control; (4) Relevant primary or secondary outcomes; (5) Studies published in the English language; (6) Per oral intervention either as a powder or in capsule form; and (7) Original articles comprising of randomized controlled trials, non-randomized controlled trials or cohort studies. Meanwhile, letters, reviews, case reports, non-comparative studies, and studies with inadequate outcomes data were excluded.
Our primary outcomes of interest were: (1) Changes in eGFR (mL/min); (2) Incidence of death/prolonged hospitalization, and (3) Changes in serum HCO3 (mmol/L). The secondary outcomes of interest included: (1) Changes in edema; and (2) Gastrointestinal (GI) adverse effects.
The study design, study population, number of patients in each group, general patient characteristics (age and gender), sample size, comorbidities, follow-up period, primary and secondary endpoints were all extracted and verified onto an Excel sheet by two independent authors (Tatheer Zehra and Syed Shabbeer Rizvi). A third reviewer (Abdul Hannan Siddiqui) resolved discrepancies through consensus and discussion.
Using the Revised Cochrane risk-of-bias tool for randomized controlled trials (ROB 2), two authors (Saad Usman and Shayan Khan) independently evaluated the quality of the clinical trials. Studies were examined for missing data, selective reporting of results, randomization of participants to exposure, and creation of allocation sequences. The Risk of Bias in Non-randomized Studies of Interventions tool was used to evaluate the quality of non-randomized clinical trials. A third author (Abdul Hannan Siddiqui) was sought to resolve any disputes.
All relevant meta-analyses of this study were conducted using RevMan (version 5.4.1; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2020), while the meta-regression analyses were carried out using Comprehensive Meta-Analysis (Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Comprehensive Meta-Analysis Version 3. Englewood, NJ: Biostat; 2013). The risk ratios (RR) and their 95%CI were pooled by using a random-effects model for both the meta-analyses and meta-regression. For every outcome, a P value < 0.05 was taken to be statistically significant. Using the Higgins I2 test, heterogeneity was evaluated. The I2 values of 25%-50% were considered mild, 50%-75% moderate, and > 75% significant heterogeneity. Sensitivity analysis was used to examine differences in the significance of the outcomes in studies with a > 50% degree of heterogeneity. The meta-regression results were presented as coefficients (Coeff) and P values.
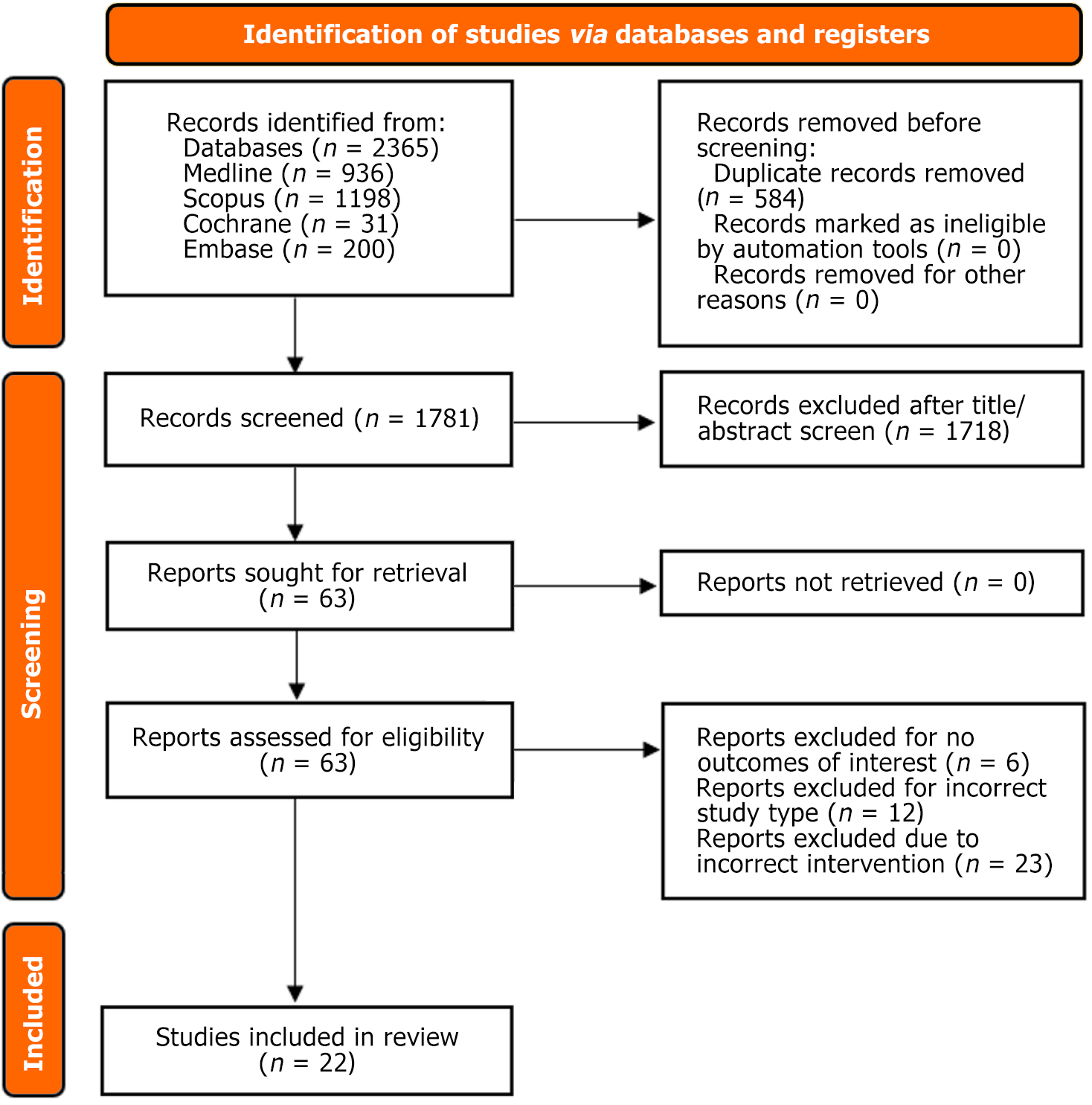
A total of 1397 studies were collected initially from all the databases. From them, 22 studies were included in our final analysis. They comprised 20 randomized control trials (RCTs)[15-34] and 2 clinical trials[35,36]. The sample size included 2932 patients (intervention: n = 1545; control: n = 1387), comprising 52.7% males. The overall mean age of patients was found to be 58 years. The average follow-up time was around 14.84 months. Most of these trials were conducted in the United States (Table 1 and Table 2). The PRISMA flow chart presents the detailed study selection process (Figure 1).
| Ref. | Study design | Study sample | Intervention/control name | Sample size | Follow-up duration | Dosage of sodium bicarbonate | ||
| Intervention | Control | Intervention | Control | Months | ||||
| Mathur et al[28] | RCT | Mild to moderate CKD with serum creatinine < 5 mg/dL | Sodium bicarbonate | Placebo | 20 | 20 | 3 | 1.2 mEq/kg |
| Ashurst et al[27] | RCT | Creatinine clearance 15 to 30 ml/min per 1.73 m2 and serum bicarbonate 16 to 20 mmol/L | Sodium bicarbonate | Standard care | 67 | 67 | 24 | 600 mg |
| Goraya et al[26] | RCT | eGFR, 15-29 ml/min per 1.73 m2 and PTCO2 level < 22 mm | Sodium bicarbonate | Fruits and vegetables | 35 | 36 | 12 | 1.0 mEq/kg |
| Abramowitz et al[35] | Clinical trial | eGFR 15-45 ml/min per 1.73 m2 and serum bicarbonate 20-24 mEq/L | Sodium bicarbonate | Placebo | 20 | 20 | 3 | 1.0 mEq/kg |
| Goraya et al[25] | RCT | eGFR 30-59 ml/min and metabolic acidosis characterized by plasma TCO2 > 22 but < 24 mmol/L | Sodium bicarbonate | Standard care | 36 | 36 | 36 | 0.3 mEq/kg |
| Jeong et al[36] | Clinical trial | eGFR < 15-30 mL/min per 1.73 m2 and CO2 less than 22 mEq/L | Sodium bicarbonate | Standard care | 40 | 40 | 12 | 1000 mg |
| Bellasi et al[29] | RCT | CKD-3b-4 with DM2 and serum bicarbonate levels below 24 mEql/L | Sodium bicarbonate | Standard care | 71 | 74 | 12 | 0.5 mmol/kg |
| Yan et al[30] | RCT | eGFR of 15-60 ml/min/1.73 m2, serum HCO3- level of 16-20 mmol/L | Sodium bicarbonate | Placebo | 42 | 42 | 4.25 | N/A |
| Kendrick et al[31] | RCT | eGFR 15-44 ml/min per 1.73 m2 with low serum bicarbonate levels (16-21 mEq/L) | Sodium bicarbonate | Control group | 10 | 10 | 6 | 650 mg |
| Di Iorio et al[22] | RCT | CKD stage 3-5, serum bicarbonate > 18 and < 24 mmol/L | Sodium bicarbonate | Standard care | 376 | 364 | 36 | N/A |
| Aigner et al[24] | RCT | CKD stage 3-4 with serum HCO3- ≤ 21 mmol/L | Sodium bicarbonate | Rescue group | 18 | 17 | 1 | 840 mg |
| Goraya et al[23] | RCT | eGFR 30-59 mL/min/1.73 m2 and plasma total CO2 > 22 mM but < 24 mM | Sodium bicarbonate | Standard care | 36 | 36 | 60 | 0.3 mEq/kg |
| Raphael et al[17] | RCT | eGFR 20-44 or 45-59 ml/min per 1.73 m2 and serum bicarbonate 20-28 meq/L | Sodium bicarbonate | Placebo | HD: 90; LD: 90 | 52 | 2.25 | 0.8 mEq/kg |
| Kittiskulnam et al[28] | RCT | eGFR 15-59 mL/min/1.73 m2, serum bicarbonate level < 22 mEq/L | Sodium bicarbonate | LD sodium bicarbonate | 21 | 21 | 4 | 300 mg |
| Melamed et al[19] | RCT | eGFR of 15-59 ml/min/1.73m2, and serum bicarbonate levels 20 to 26 mEq/L | Sodium bicarbonate | Placebo | 74 | 75 | 24 | 0.4 mEq/kg |
| Dubey et al[33] | RCT | CKD stages 3 and 4 and serum bicarbonate levels < 22 mEq/dL | Sodium bicarbonate | Standard care | 94 | 94 | 6 | 0.5 mEq/kg |
| Witham et al[20] | RCT | CKD stages 3 and 4, and serum bicarbonate levels < 22 mEq/dL | Sodium bicarbonate | Placebo | 152 | 148 | 24 | 500 mg |
| Alva et al[21] | RCT | eGFR of 15-30 mL/min/1.73 m2 and serum bicarbonate 10-20 mm/L | Sodium bicarbonate | Standard care | 33 | 34 | 9 | 600 mg |
| Gaggl et al[34] | RCT | eGFR between 60 and 15 mL/min per 1.73 m2 and a serum HCO3- of ≤ 21 mmol/L | Sodium bicarbonate | Rescue group | 24 | 23 | 2 | N/A |
| Bovée et al[16] | RCT | eGFR 15-30 ml/min/1.73m2 and plasma bicarbonate levels between 15.0 and 24.0 mmol/L | Sodium bicarbonate | Sodium chloride | 15 | 15 | 1 | 1000 mg |
| Mohebbi et al[32] | RCT | eGFR between 15 mL/min per 1.73 m² and 89 mL/min per 1.73 m² and serum bicarbonate of 22 mmol/L or less | Sodium bicarbonate | Placebo | 119 | 121 | 24 | 500 mg |
| Sorohan et al[15] | RCT | eGFR between 45 and 15 mL/min/1.73 m2 and serum bicarbonate between 10 and 22 mmol/L | Sodium bicarbonate | Sodium citrate | 62 | 62 | 12 | 600 mg |
| Ref. | Age (year) | Male | eGFR (mL/min) | Serum HCO3- (mmol/L) | Serum K+ (mmol/L) | Serum Na+ (mmol/L) | ||||||
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Mathur et al[28] | 37.5 ± 17 | 43.5 ± 10.5 | 12 (60) | 13 (65) | - | - | 19.49 ± 5.51 | 19.35 ± 3.74 | - | - | - | - |
| Ashurst et al[27] | 54.78 ± 2.56 | 54.77 ± 2.34 | 35 (52) | 34 (51) | - | - | 19.8 ± 2.2 | 19.9 ± 1.5 | - | - | - | - |
| Goraya et al[26] | 54.2 ± 5.3 | 53.9 ± 6.9 | 18 (51) | 20 (56) | - | - | - | - | 4.1 ± 0.2 | 4.1 ± 0.2 | 139.9 ± 1.2 | 139.8 ± 1.3 |
| Abramowitz et al[35] | 63.1 ± 10.8 | - | 8 (40) | - | 32.9 ± 8.9 | - | 23.0 ± 2.4 | - | 4.6 ± 0.4 | - | - | - |
| Goraya et al[25] | 53.6 ± 5.3 | 53.9 ± 4.8 | 16 (44) | 16 (44) | - | - | - | - | 4.24 ± 0.12 | 4.29 ± 0.14 | - | - |
| Jeong et al[36] | 53.3 ± 13.5 | 55.8 ± 12.7 | 21 (52.5) | 26 (57.5) | 16.7 ± 6.1 | 17.7 ± 6.4 | 5.25 ± 0.71 | 5.44 ± 0.63 | - | - | ||
| Bellasi et al[29] | 64.9 ± 11.8 | 66.0 ± 12.9 | 47 (66) | 36 (48) | - | - | 21.2 ± 1.9 | 21.6 ± 2.0 | - | - | - | - |
| Yan et al[30] | 53.10 ± 6.11 | 53.10 ± 8.66 | 25 (59.5) | 24 (57.1) | 19.09 ± 2.26 | 18.43 ± 1.89 | 16.49 ± 1.22 | 16.14 ± 1.40 | - | - | - | - |
| Kendrick et al[31] | 59 ± 12 | - | 10 (50) | - | 25 ± 8 | 24 ± 8 | 19.3 ± 2.9 | 19.7 ± 2.3 | - | - | - | - |
| Di Iorio et al[22] | 67.6 ± 15.1 | 68.1 ± 14.7 | 234 (62.2) | 224 (61.5) | 33.4 ± 12.4 | 36.9 ± 10.8 | 21.7 ± 2.6 | 21.4 ± 2.1 | 4.9 ± 0.5 | 4.9 ± 0.6 | 139 ± 2.9 | 139 ± 2.8 |
| Aigner et al[24] | 59.94 ± 12.46 | 53.12 ± 17.74 | 12 (66.7) | 11 (52.9) | 23.64 ± 5.87 | 27.45 ± 10.05 | 18.78 ± 1.59 | 19.28 ± 1.55 | 4.83 ± 0.47 | 4.94 ± 0.61 | 140 ± 3.03 | 139 ± 2.6 |
| Goraya et al[23] | 53.6 ± 5.3 | 53.9 ± 4.8 | 16 (44.4) | 16 (44.4) | 39.6 ± 6.6 | 39.5 ± 6.9 | - | - | - | - | - | - |
| Raphael et al[17] | HD: 67 ± 11 LD: 66 ± 14 | 66 ± 11 | HD: 61 (68) LD: 69 (60) | 39 (75) | HD: 36 ± 10 LD: 37 ± 10 | 35 ± 9 | HD: 24 ± 2 LD: 24 ± 2 | 24 ± 2 | 4.4 ± 0.4 | 4.5 ± 0.5 | - | - |
| Kittiskulnam et al[28] | 61.2 ± 9.8 | 61.2 ± 9.8 | 12 (57.1) | 12 (57.1) | 32.4 ± 14.1 | - | 21.0 ± 2.1 | - | - | - | - | - |
| Melamed et al[19] | 60.3 ± 14.1 | 61.6 ± 10.9 | 36 (49) | 33 (44) | 36.4 ± 11.4 | 36.2 ± 11.1 | 24.0 ± 2.2 | 24.1 ± 2.6 | 4.5 ± 0.5 | 4.6 ± 0.5 | - | - |
| Dubey et al[33] | 50.12 ± 11.6 | 50.30 ± 11.4 | 68 (72.3) | 66 (70.2) | 29.2 (27-31.3) | 31.5 (29.3-33.8) | 18.1 (17.7-18.6) | 18.1 (17.6-18.6) | - | - | - | - |
| Witham et al[20] | 73.9 ± 7.6 | 74.0 ± 6.6 | 110 (72.4) | 104 (70.3) | 19.7 ± 6.5 | 18.2 ± 6.4 | 20.6 ± 2.6 | 20.1 ± 2.5 | 4.9 ± 0.5 | 4.9 ± 0.5 | - | - |
| Alva et al[21] | - | - | 23 (69.7) | 25 (73.53) | 22.39 ± 4.08 | 21.21 ± 4.37 | 16.62 ± 3.05 | 16.84 ± 2.17 | - | - | - | - |
| Gaggl et al[34] | 58 ± 12.8 | 56 ± 16.5 | 15 (62) | 14 (61) | 25.33 ± 7.5 | 27 ± 9.7 | 18.8 ± 1.8 | 19.2 ± 1.5 | - | - | - | - |
| Bovée et al[16] | 61 ± 17 | 61 ± 14 | 11 (73) | 14 (93) | 21 ± 6 | 22 ± 3 | 20.8 ± 3.9 | 21.8 ± 2.9 | 5.1 ± 0.7 | 5.0 ± 0.6 | - | - |
| Mohebbi et al[32] | 55.7 ± 13.2 | 55.3 ± 13.8 | 82 (69) | 85 (70) | 48.2 ± 16.3 | 47.7 ± 15.8 | 21.3 ± 2.6 | 21.0 ± 2.7 | - | - | - | - |
| Sorohan et al[15] | 57.37 ± 10.25 | 57.95 ± 10.22 | 35 (56.5) | 35 (56.5) | 23.68 ± 9.05 | 24.93 ± 6.70 | 17.16 ± 1.62 | 17.53 ± 2.05 | 5.00 ± 0.34 | 4.88 ± 0.44 | 138.71 ± 1.41 | 138.44 ± 2.61 |
Upon our assessment of the studies included, most RCTs were classified as having an overall "low" risk of bias. Studies by Witham et al[20], Alva et al[21] and Aigner et al[24] did show some concerns, while studies done by Sorohan et al[15], Yan et al[30], and Kendrick et al[31] were classified as having an overall "high" risk of bias (Supplementary Figure 1). The non-randomized studies of the effects of interventions were classified as having an overall "low to moderate" risk of bias owing to their robust methodology (Supplementary Figure 2).
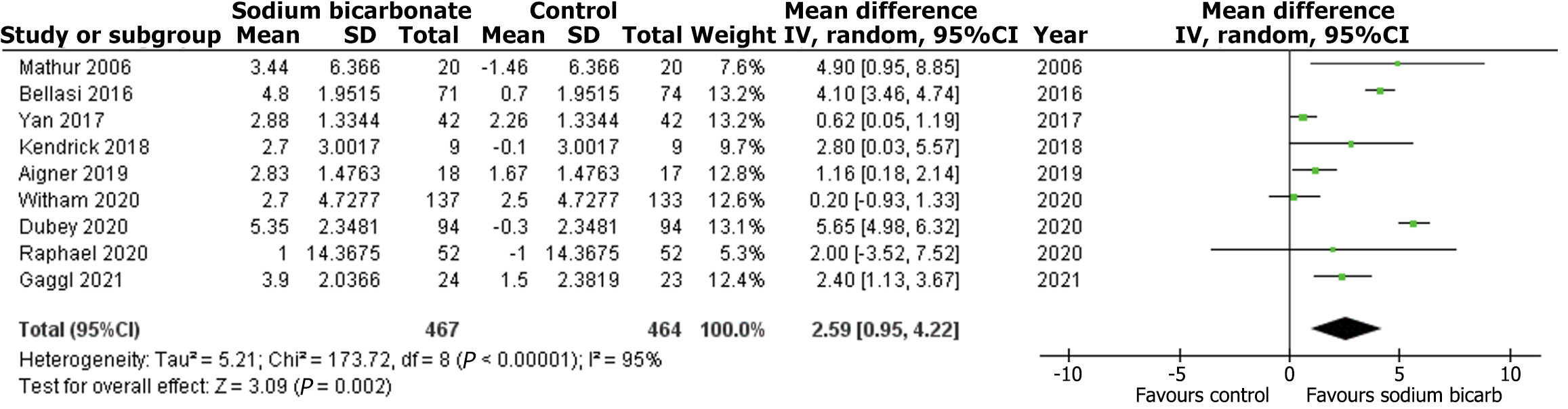
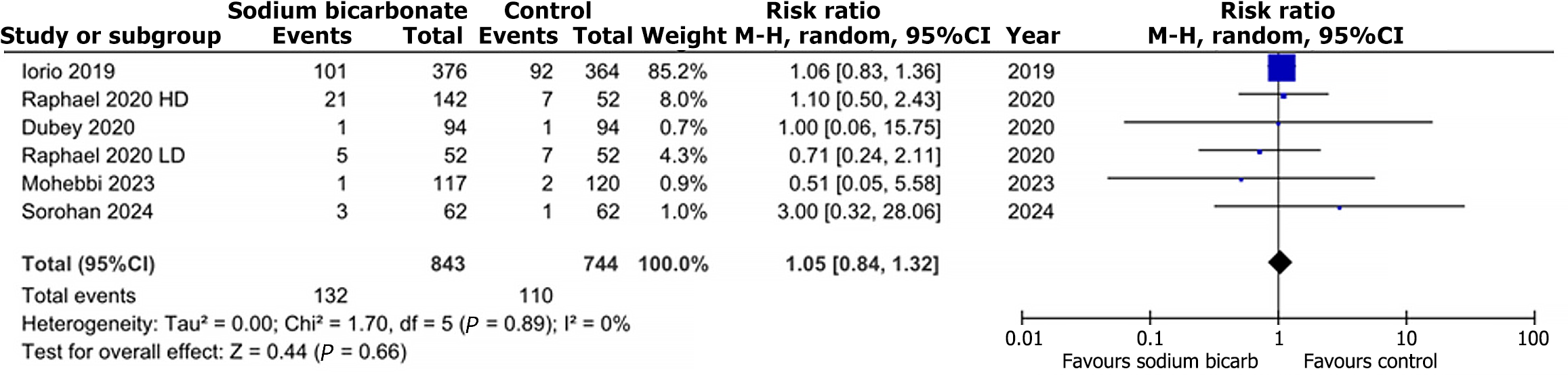
Out of the 22 articles, nine studies reported data on change in eGFR, nine trials reported data on change in serum bicarbonate, and five trials reported the risk of death/prolonged hospitalization. Compared with the comparison group, the change in eGFR from baseline to trial completion was statistically insignificant in the intervention group (MD: 0.93, 95%CI: -1.88-3.75; P = 0.52; I2 = 93%) (Figure 2). Regarding the change in serum bicarbonate concentration, the analysis showed a statistically significant difference between the two groups (MD: 2.59, 95%CI: 0.95-4.22; P = 0.002; I2 = 95%) (Figure 3). The random effects analysis showed a statistically insignificant risk for death/prolonged hospitalization with the intervention compared to the comparison group (RR: 1.05, 95%CI: 0.84-1.32; P = 0.66; I2 = 0%) (Figure 4).
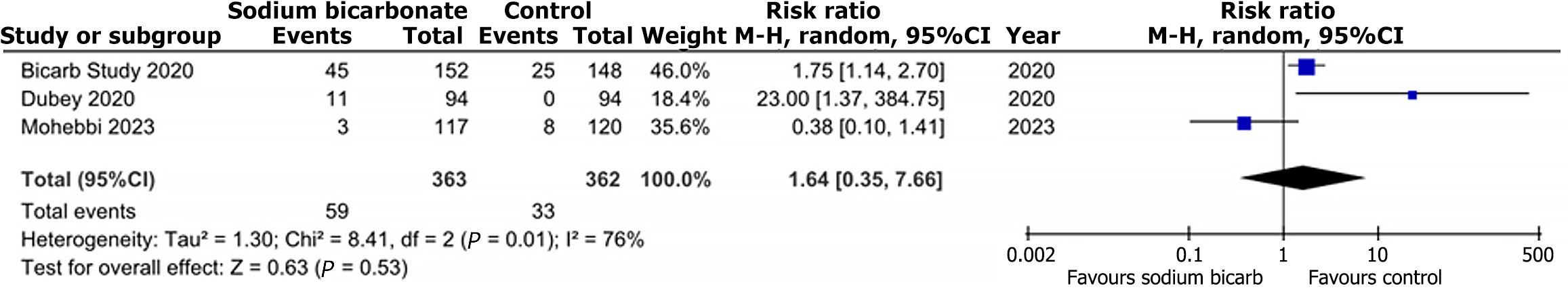
Compared to the comparison group, treatment with sodium bicarbonate did not significantly increase the risk of GI disorders, as reported by three trials. (RR: 1.64, 95%CI: 0.35-7.66; P = 0.53; I2 = 76%) (Figure 5). Similarly, outcomes data from three trials did not show any significant difference in the risk of worsening edema (RR: 1.26, 95%CI: 0.94-1.68; P = 0.12; I2 = 37%) between the two groups (Figure 6).
A sensitivity analysis was performed for all outcomes except for death/prolonged hospitalization and worsening edema due to the low heterogeneity observed in data. For the change in eGFR outcome, removing Witham et al[20] and Goraya et al[23] significantly reduced heterogeneity (I2 = 93% to I2 = 34%, P = 0.48 to P = 0.21). However, the results were statistically nonsignificant (Supplementary Figure 3). Change in serum bicarbonate saw a great reduction in heterogeneity when Witham et al[20] and Dubey et al[33] were removed (I2 = 95% to I2 = 38%, P = 0.03 to P = 0.01) (Supplementary Figure 4), while removing Mohebbi et al[32] in GI disorders showed a smaller decrease in heterogeneity (I2 = 76% to I2 = 72%, P = 0.52 to P = 0.26) (Supplementary Figure 5). While the overall status of change in eGFR and GI disorders is still insignificant, change in serum bicarbonate remains significant even after the decrease in heterogeneity.
We assessed age as a probable covariate having an impact on effect sizes for all outcomes and found that there was no statistically significant association between them except for change in eGFR (Coeff: -0.1337, P = 0.0417) (Supplementary Figure 6). The estimates of the effect of death/prolonged hospitalization were also assessed (Coeff: -0.0093, P = 0.8549) (Supplementary Figure 7), along with serum bicarbonate (Coeff: -0.0552, P = 0.2761) (Supplementary Figure 8), (Supp
When evaluating the male gender as a possible covariate impacting the estimates of effect, it was statistically insignificantly associated with death/prolonged hospitalization (Coeff: -0.0308, P = 0.4425) (Supplementary Figure 9). However, it’s association with change in eGFR (Coeff: -0.1006, P = 0.0121) (Supplementary Figure 10) and serum HCO3 (Coeff: -0.1501, P = 0.0063) (Supplementary Figure 11) was statistically significant (Supplementary Table 2-Supplementary Table 4).
When baseline serum bicarbonate was assessed as a covariate impacting the estimates of effect, it was statistically insignificantly associated with a change in eGFR (Coeff: 0.0379, P = 0.2837) (Supplementary Figure 12), death/prolonged hospitalization (Coeff: -0.0724, P = 0.5733) (Supplementary Figure 13) and serum HCO3 (Coeff: -0.2468, P = 0.3313) (Supplementary Figure 14), (Supplementary Table 2-Supplementary Table 4).
This systematic review and meta-analysis studying the effects of sodium bicarbonate therapy on MA in CKD included 20 RCTs and 2 clinical trials (n = 2932 patients). Primary outcome analysis unveils that sodium bicarbonate was the reason for a substantial elevation in serum bicarbonate levels among CKD patients. Conversely, no significant correlation was observed between the administration of sodium bicarbonate and the occurrences of death or prolonged hospitalization in the study. Similarly, while assessing our secondary outcomes, treatment with sodium bicarbonate, compared to a comparison group, did not lead to a notable rise in the likelihood of GI disorders. Likewise, no significant change was noted in the risk of worsening edema between the two groups.
A recently conducted meta-analysis[37] shows that sodium bicarbonate has a decreasing effect on the decline of eGFR. The sodium bicarbonate group had fewer hospitalizations. Additionally, a second meta-analysis published in 2020[38] included fifteen trials, compared with placebo or no study medication, and yielded similar results. Another study[39] also showed findings consistent with our results. However, edema was found to be a significant side effect of sodium bicarbonate supplementation.
Meanwhile, our analysis showed no significant progression in the worsening of edema. Our observation is based on the latest and larger clinical trials incorporated, which did not identify edema as a significant adverse effect. This inclusion accounts for the observed distinction.
The current meta-analysis stands crucial in support of the 2024 Kidney Disease Improving Global Outcomes guidelines[1], which suggests prescribing bicarbonate supplementation to inhibit the progression of MA to levels of serum bicarbonate at or below 18 mmol/L and is also consistent with the results of previous meta-analysis. However, this study includes recent and more trials with 20 RCTs and addresses various outcomes. We reported the implications of sodium bicarbonate therapy on GI disorders, a relatively less explored domain.
The therapeutic use of sodium bicarbonate in patients with CKD is attributed to its several potential mechanisms of action in mitigating MA. In CKD, the functioning nephrons undergo hypertrophy to excessively generate NH3 to neutralize the excess H+ ions through the formation of NH4+[40]. This surplus production of NH3/NH4+ activates the complement system and leads to tubulointerstitial inflammation and fibrosis, which advances the decline in GFR[41]. However, maintaining normal serum HCO3- levels (24-26 mmol/L) through dietary alkali and sodium bicarbonate supplementation helps lower interstitial ammonium levels, thereby minimizing CKD progression by mitigating MA[41].
The recently published meta-analysis[37] included the studies of the age group < 65 years; meanwhile, this systematic review does not impose any such threshold for age as an inclusion or exclusion criterion. Despite the similarities in the outcomes of this meta-analysis and the aforementioned study, studies with an age limit of > 65 years as an inclusion criterion, when analyzed individually, exhibited insignificant treatment effects, with the risk of developing adverse effects on the rise. Therefore, it is suggested that more treatment options should be explored for elderly patients with CKD and MA.
To the best of our knowledge, this meta-analysis is the first study to report the GI adverse effects of sodium bicarbonate therapy in CKD patients with MA as a secondary outcome. The basis of the inclusion of GI effects as a secondary outcome stems from the fact that bicarbonate reacts with the hydrogen chloride found in the gastric lumen, leading to the formation of carbon dioxide gas, causing GI adverse effects like diarrhea, vomiting, nausea, and epigastric pain. Three of our included studies reported GI adverse effects; the study by Sorohan et al[15] analyzed these effects in sodium bicarbonate and sodium citrate therapy groups. While the comparative results were insignificant, the cumulated outcome leading to treatment withdrawal was statistically significant, with a higher relative ratio in the sodium bicarbonate group. Sodium bicarbonate therapy may be associated with edema; however, we analyzed insignificant associations with little to no requirement of initiating diuretics therapy. Further research may be warranted to explore these effects more comprehensively and to identify strategies for mitigating these adverse reactions in CKD patients.
Our study has some limitations. Firstly, we set no threshold for the maximum age of participants, which is important since it is observed that sodium bicarbonate is minimally effective on elderly patients, yet trials with patients over 65 years of age are included, which may affect the scope of our study. Second, adverse GI effects were outside the primary or secondary outcomes of many studies, raising concerns about the generalizability of the findings. Third, for some outcomes, high heterogeneity existed due to the inclusion of some studies, which, upon their removal, caused a dramatic reduction in the heterogeneity. This may be due to variations in therapeutic dosage, modalities, treatment duration, and baseline kidney function.
Additionally, most of the trials included in our meta-analysis predominantly featured male participants, with significantly fewer female patients. This gender imbalance could affect the generalizability of our findings, as the results may not fully represent female populations. Gender differences in physiology, disease progression, and treatment response could lead to varying outcomes between males and females, potentially limiting the applicability of our conclusions across both sexes. The treatment may be associated with high blood pressure; however, we did not explore this comorbidity as an outcome. The intake of vegetables and fruits as alkali therapeutic agents proves invaluable in treating MA in CKD patients. However, since this meta-analysis aimed to study the outcomes of supplementation only, we were unable to include the dietary interventions alongside the supplementation therapy.
Finally, analysis for the outcome of worsening edema failed to reach sufficient statistical power to detect significant differences between the treatment and the comparison groups. The trend showed an increased risk of worsening edema in the sodium bicarbonate group (RR: 1.26, 95%CI: 0.94-1.68), however the result was statistically non-significant. This may be attributed to the relatively small sample size (n = 471) available for this outcome. Hence, more patient data is needed to definitively assess sodium bicarbonate and whether it plays a significant role in the worsening of edema.
Based on the studies we evaluated in this meta-analysis; sodium bicarbonate therapy may halt worsening kidney function by correcting serum bicarbonate levels and treating MA. Although sodium bicarbonate does not significantly improve the eGFR, it may potentially prevent CKD progression while maintaining an overall favorable safety profile. This analysis also revealed that sodium bicarbonate did not have a statistically significant association with adverse effects like death/prolonged hospitalization, GI adverse events or worsening edema. Larger and more stringent randomized clinical trials are strongly suggested to establish the benefits and risks of sodium bicarbonate in CKD patients with MA.
| 1. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117-S314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2033] [Article Influence: 1016.5] [Reference Citation Analysis (0)] |
| 2. | Hoogeveen EK. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022;2:433-442. [DOI] [Full Text] |
| 3. | Ogobuiro I, Tuma F. Physiology, Renal. 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 4. | Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35:329-344, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 5. | Burger M, Schaller DJ. Metabolic Acidosis. 2023 Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 6. | Mohammad Tinawi. Pathophysiology, Evaluation and Management of Metabolic Acidosis. Arch Clin Biomed Res. 2021;85-109. |
| 7. | Kuczera P, Ciaston-Mogilska D, Oslizlo B, Hycki A, Wiecek A, Adamczak M. The Prevalence of Metabolic Acidosis in Patients with Different Stages of Chronic Kidney Disease: Single-Centre Study. Kidney Blood Press Res. 2020;45:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Kraut JA, Madias NE. Adverse Effects of the Metabolic Acidosis of Chronic Kidney Disease. Adv Chronic Kidney Dis. 2017;24:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Chen W, Abramowitz MK. Treatment of metabolic acidosis in patients with CKD. Am J Kidney Dis. 2014;63:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Kim SM, Jung JY. Nutritional management in patients with chronic kidney disease. Korean J Intern Med. 2020;35:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Łoniewski I, Wesson DE. Bicarbonate therapy for prevention of chronic kidney disease progression. Kidney Int. 2014;85:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Cheng YL, Huang SC, Ho MY, Li YR, Yen CL, Chen KH, Sun WC, Fan PY, Chen JS, Lin C, Hsiao CC. Effect of sodium bicarbonate on cardiovascular outcome and mortality in patients with advanced chronic kidney disease. Front Pharmacol. 2023;14:1146668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Raphael KL. Metabolic Acidosis in CKD: Pathogenesis, Adverse Effects, and Treatment Effects. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Jung B, Huguet H, Molinari N, Jaber S. Sodium bicarbonate for the treatment of severe metabolic acidosis with moderate or severe acute kidney injury in the critically ill: protocol for a randomised clinical trial (BICARICU-2). BMJ Open. 2023;13:e073487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Sorohan BM, Obrișcă B, Jurubiță R, Lupușoru G, Achim C, Andronesi A, Frățilă G, Berechet A, Micu G, Ismail G. Sodium citrate versus sodium bicarbonate for metabolic acidosis in patients with chronic kidney disease: A randomized controlled trial. Medicine (Baltimore). 2024;103:e37475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Bovée DM, Roksnoer LCW, van Kooten C, Rotmans JI, Vogt L, de Borst MH, Zietse R, Danser AHJ, Hoorn EJ. Effect of sodium bicarbonate supplementation on the renin-angiotensin system in patients with chronic kidney disease and acidosis: a randomized clinical trial. J Nephrol. 2021;34:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Raphael KL, Isakova T, Ix JH, Raj DS, Wolf M, Fried LF, Gassman JJ, Kendrick C, Larive B, Flessner MF, Mendley SR, Hostetter TH, Block GA, Li P, Middleton JP, Sprague SM, Wesson DE, Cheung AK. A Randomized Trial Comparing the Safety, Adherence, and Pharmacodynamics Profiles of Two Doses of Sodium Bicarbonate in CKD: the BASE Pilot Trial. J Am Soc Nephrol. 2020;31:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Kittiskulnam P, Srijaruneruang S, Chulakadabba A, Thokanit NS, Praditpornsilpa K, Tungsanga K, Eiam-Ong S. Impact of Serum Bicarbonate Levels on Muscle Mass and Kidney Function in Pre-Dialysis Chronic Kidney Disease Patients. Am J Nephrol. 2020;51:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Melamed ML, Horwitz EJ, Dobre MA, Abramowitz MK, Zhang L, Lo Y, Mitch WE, Hostetter TH. Effects of Sodium Bicarbonate in CKD Stages 3 and 4: A Randomized, Placebo-Controlled, Multicenter Clinical Trial. Am J Kidney Dis. 2020;75:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Witham MD, Band M, Chong H, Donnan PT, Hampson G, Hu MK, Littleford R, Lamb E, Kalra PA, Kennedy G, McNamee P, Plews D, Rauchhaus P, Soiza RL, Sumukadas D, Warwick G, Avenell A. Sodium bicarbonate to improve physical function in patients over 60 years with advanced chronic kidney disease: the BiCARB RCT. Health Technol Assess. 2020;24:1-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Alva S, Divyashree M, Kamath J, Prakash PS, Prakash KS. A Study on Effect of Bicarbonate Supplementation on the Progression of Chronic Kidney Disease. Indian J Nephrol. 2020;30:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, Ceccarelli M, Di Lullo L, Capolongo G, Di Iorio M, Guastaferro P, Capasso G; UBI Study Group. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. 2019;32:989-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Goraya N, Munoz-Maldonado Y, Simoni J, Wesson DE. Fruit and Vegetable Treatment of Chronic Kidney Disease-Related Metabolic Acidosis Reduces Cardiovascular Risk Better than Sodium Bicarbonate. Am J Nephrol. 2019;49:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Aigner C, Cejka D, Sliber C, Fraunschiel M, Sunder-Plassmann G, Gaggl M. Oral Sodium Bicarbonate Supplementation Does Not Affect Serum Calcification Propensity in Patients with Chronic Kidney Disease and Chronic Metabolic Acidosis. Kidney Blood Press Res. 2019;44:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 26. | Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 27. | de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 565] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 28. | Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006;28:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M, Di Lullo L, Guastaferro P, Di Iorio B; UBI study investigators. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016;17:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Yan W, Wang L, Huang T, Xu G. Treatment for non-thyroidal illness syndrome in advanced chronic kidney disease: a single-blind controlled study. J Nephrol. 2017;30:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A, Chonchol M. Effect of Treatment of Metabolic Acidosis on Vascular Endothelial Function in Patients with CKD: A Pilot Randomized Cross-Over Study. Clin J Am Soc Nephrol. 2018;13:1463-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Mohebbi N, Ritter A, Wiegand A, Graf N, Dahdal S, Sidler D, Arampatzis S, Hadaya K, Mueller TF, Wagner CA, Wüthrich RP. Sodium bicarbonate for kidney transplant recipients with metabolic acidosis in Switzerland: a multicentre, randomised, single-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant. 2020;35:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Gaggl M, Repitz A, Riesenhuber S, Aigner C, Sliber C, Fraunschiel M, Cejka D, Sunder-Plassmann G. Effect of Oral Sodium Bicarbonate Treatment on 24-Hour Ambulatory Blood Pressure Measurements in Patients With Chronic Kidney Disease and Metabolic Acidosis. Front Med (Lausanne). 2021;8:711034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013;8:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Jeong J, Kwon SK, Kim HY. Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press. 2014;12:80-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Yang TY, Lin HM, Wang HY, Chuang MH, Hsieh CC, Tsai KT, Chen JY. Sodium Bicarbonate Treatment and Clinical Outcomes in Chronic Kidney Disease with Metabolic Acidosis: A Meta-Analysis. Clin J Am Soc Nephrol. 2024;19:959-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Hultin S, Hood C, Campbell KL, Toussaint ND, Johnson DW, Badve SV. A Systematic Review and Meta-Analysis on Effects of Bicarbonate Therapy on Kidney Outcomes. Kidney Int Rep. 2021;6:695-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Navaneethan SD, Shao J, Buysse J, Bushinsky DA. Effects of Treatment of Metabolic Acidosis in CKD: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2019;14:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 40. | Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol. 2007;293:F1238-F1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Zha Y, Qian Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients. 2017;9:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |