Published online Sep 25, 2024. doi: 10.5527/wjn.v13.i3.99105
Revised: August 27, 2024
Accepted: August 30, 2024
Published online: September 25, 2024
Processing time: 67 Days and 12.9 Hours
Kidney disease is a severe complication of diabetes that often leads to end-stage renal disease. Early diagnosis is crucial for prevention or delay. However, the current diagnostic methods, with their limitations in detecting the disease in its early stages, underscore the urgency and importance of finding new solutions. miRNAs encapsulated inside urinary exosomes (UEs) have potential as early biomarkers for kidney diseases. The need for reference miRNAs for accurate interpretation currently limits their translational potential.
To identify consistently expressing reference miRNAs from UEs of controls and patients with type 2 diabetesmellitus (T2DM) and biopsy-confirmed kidney diseases.
miRNA profiling was performed on UEs from 31 human urine samples using a rigorous and unbiased method. The UEs were isolated from urine samples collected from healthy individuals (n = 6), patients with T2DM (n = 13), and T2DM patients who also had kidney diseases (including diabetic nephropathy, n = 5; membranous nephropathy, n = 5; and IgA nephropathy, n = 2) through diffe
Microarray data analysis identified 14 miRNAs that were consistently expressed in UEs from 31 human samples, representing various kidney conditions: diabetic controls, diabetic nephropathy, membrane nephropathy, IgA nephropathy, and healthy controls. Through in silico analysis, we determined that 10 of these miRNAs had significant potential to serve as reference genes in UEs.
We identified uniformly expressing UE miRNAs that could serve as reference genes kidney disease biomarkers.
Core Tip: Early prevention and detection of end-stage renal disease is currently inadequate. miRNAs found in urinary exosomes (UEs) may serve as early biomarkers for kidney diseases. However, the lack of reference miRNAs for normali
- Citation: Mishra DD, Maurya PK, Tiwari S. Reference gene panel for urinary exosome-based molecular diagnostics in patients with kidney disease. World J Nephrol 2024; 13(3): 99105
- URL: https://www.wjgnet.com/2220-6124/full/v13/i3/99105.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i3.99105
Kidney disease is a severe complication of diabetes[1], and presents clinical hallmarks of diabetic nephropathy. This progression includes an initial phase of glomerular hyperfiltration, increasing albuminuria, and hypertension, ultimately leading to a gradual decline in renal function and ending in end-stage renal disease (ESRD) after 5–15 years. Patients with ESRD are left with the only choice of renal replacement therapies, which is an economic burden and results in poor quality of life. However, timely diagnosis could prevent or, at least, delay this debilitating condition. The existing diagnostic methods in clinical use are limited in their ability to detect the disease at its early stage. However, the discovery of urinary exosomes (UEs), rich in possible biomarkers for kidney disease, offers a promising source for diagnosis, instilling hope for improved early detection and intervention.
Extracellular vesicles (EVs) are membrane vesicles released by almost every cell of the body into the extracellular fluid, such as blood, urine, semen, saliva, breast milk, tears, and bile[1-8]. In the urine, the EVs originate from the cells lining the urinary tract and the entire nephron lumen. The EVs, particularly the nanosized ones known as exosomes, and in the case of urine specifically referred to as UEs, are thought to reflect the content of their originating cell as they originate in the late endosomal compartment by the inward budding of multivesicular bodies. They play an essential role in mediating cellular communication, primarily by transferring their nucleic acid content (mRNA and miRNA)[9-11]. Thus, the EVs are of scientific interest for their diagnosis and therapeutic potential, including drug delivery and targeted therapy. UEs stand out for their high diagnostic value in kidney disease biomarker discovery, making them a promising area of research[2,12-15]. However, optimal normalization is a significant challenge.
Albumin excretion over 24 hours (usually g/day or µg/min) is a classical normalization method for urine-based biomarkers. Still, it has a limitation of total dependency on the urine volume, which is generally unstable and changes governed by body size, hydration status, and other factors[16,17]. Normalization using creatinine is the standard method; however, the creatinine excretion rate varies in certain conditions, such as precariousness in acute kidney injury[18-20]. The sizing and counting of the number of UEs in whole urine samples have also been recommended but require sophis
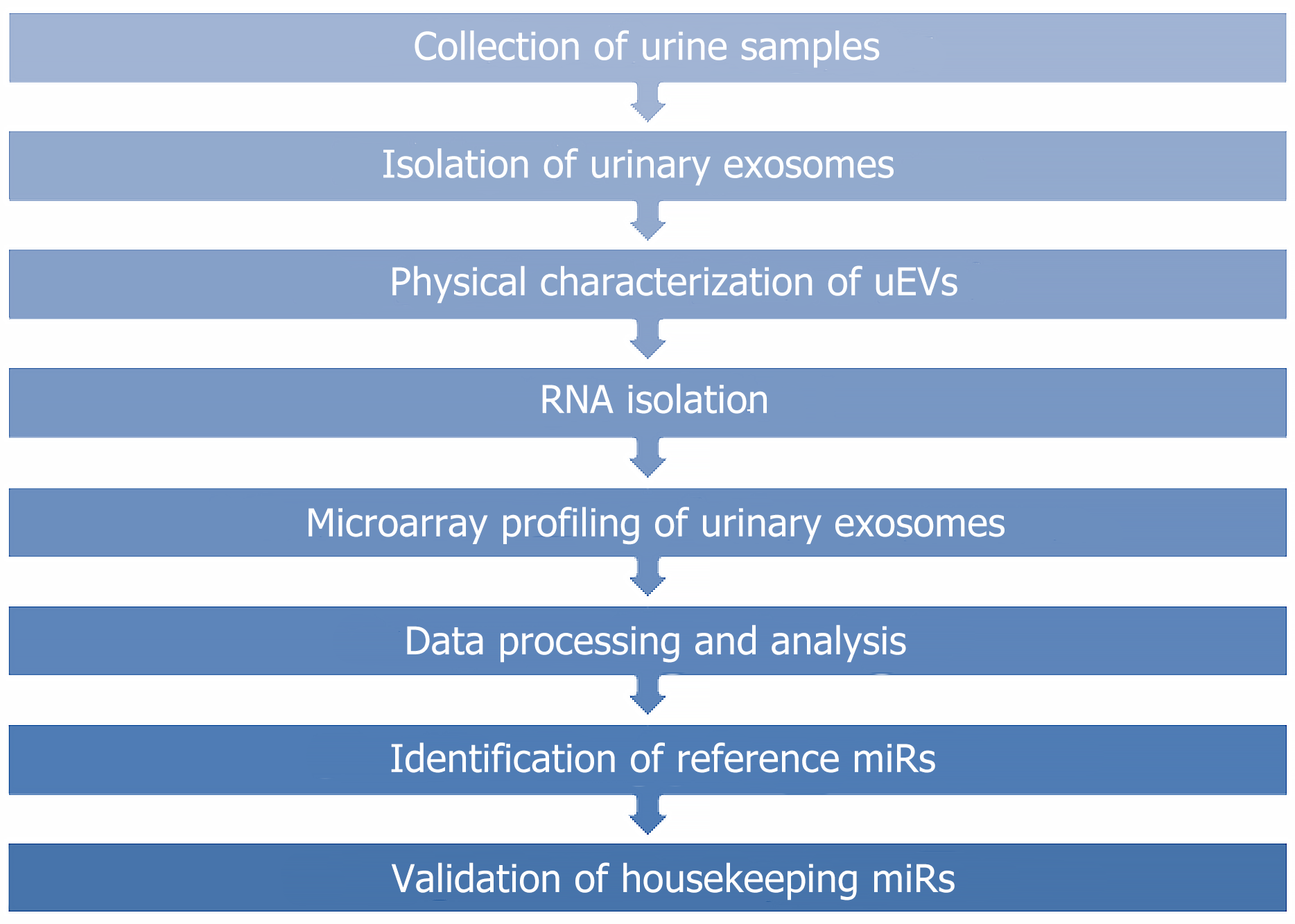
The current study focused on identifying the reference miRNAs in UEs. The study was approved by the Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences (Ref No. 2021-194-PhD-EXP-41). The flow chart shows step-by-step methods and analysis (Figure 1).
Urine samples (first morning) were collected from patients with diabetes with or without kidney disease (n = 12 or 13/group). The subjects had one of the following biopsy-proven kidney disease subtypes; diabetic nephropathy (n = 5), membranous nephropathy (n = 5), or IgA nephropathy (n = 2). Urine samples were collected from kidney disease patients at the Department of Nephrology, Sanjay Gandhi Post Graduate Institute of Medical Sciences-Lucknow, and Christian Medical College-Vellore, India. Urine samples were also collected from six healthy subjects. Urine samples were collected from all the subjects after obtaining written informed consent. These samples underwent low-speed centrifugation (1000 × g for 10 min at 4°C) to eliminate cellular debris then stored at 80°C with the addition of a protease inhibitors cocktail (1.67 mL of 100 mmol/L NaN3, 2.5 mL of 11.5 mmol/L 4-[2-aminoethyl] benzenesulfonyl fluoride, and 50 l of 1 mmol/L leupeptin). Table 1 shows the number of urine samples collected from three groups: T2DM patients without kidney disease; T2DM patients with kidney disease; and healthy subjects without T2DM or kidney disease.
| Groups | Kidney conditions | Group | Urine samples |
| Group 1 | T2DM patients without kidney disease | Diabetic control | 13 |
| Group 2 | T2DM patients with kidney disease | Diabetic nephropathy | 5 |
| Membranous nephropathy | 5 | ||
| IgA nephropathy | 2 | ||
| Group 3 | Healthy subjects without T2DM or kidney disease | Healthy control | 6 |
UEs were isolated from a 10-mL aliquot of the diluted urine as previously described[23], using high-speed centrifugation following an initial low-speed spin to remove whole cells and debris.
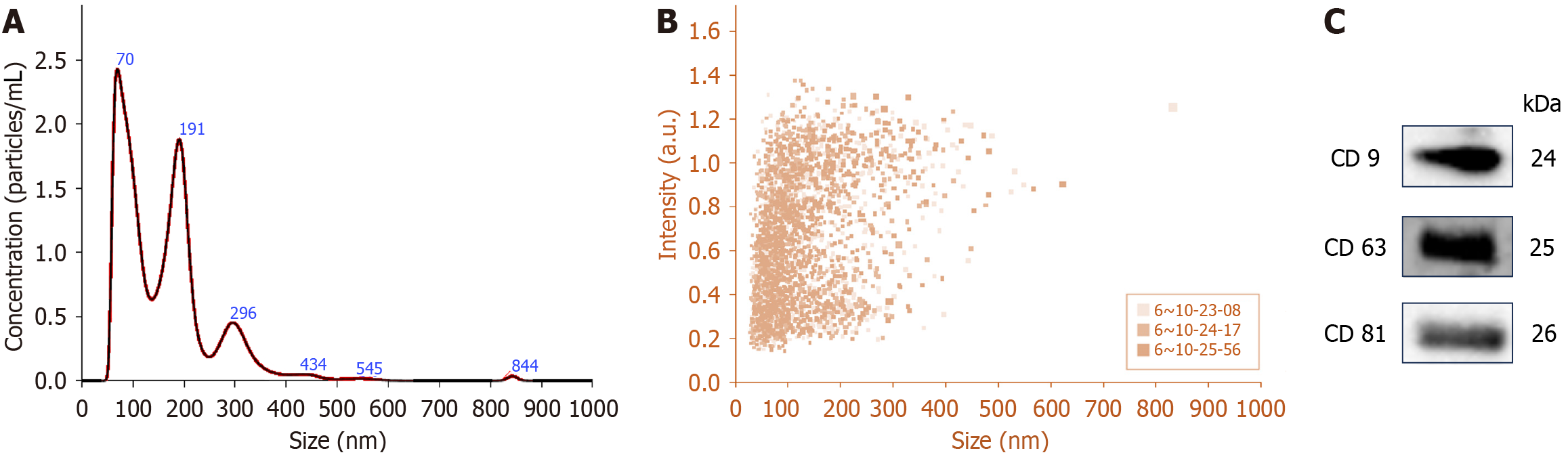
After isolation, UEs were physically characterized by nanoparticle tracking analysis (NTA) with the NanoSight NS300 (Malvern Instruments Ltd., UK) at the Central Analytical Research Facility of the Indian Institute of Toxicological Research, Lucknow. Additionally, exosomal proteins from urine samples were analyzed via immunoblotting with antibodies against exosome-specific markers (CD9, CD63 and CD81) from Abcam (Cambridge, MA, USA). All images were captured using the ChemiDoc Imaging System (Universal Hood III, Bio-Rad, Hercules, CA, USA).
Total RNA was extracted from the UE pellet using the miRNeasy Mini kit (Qiagen, Valencia, CA, USA). The con
Microarray analysis was conducted using the Affymetrix Human miRNA 4.0 Array (Affymetrix-GeneChip™ miRNA 4.0 Array; Thermo Fisher Scientific, Waltham, MA, USA). Biotin-labelled RNA was created using the Affymetrix® FlashTagTM Biotin HSR RNA Labelling Kit (Thermo Fisher Scientific). Approximately 130 ng of total RNA underwent poly-A tailing at the 3' end, followed by the attachment of a biotin-labeled 3DNA molecule via DNA ligase. The biotin-labeled RNA samples were hybridized to GeneChip miRNA 4.0 arrays in an Affymetrix® Oven 455 (Thermo Fisher Scientific) at 48°C for 18 h with 60 rpm rotation. After hybridization, the miRNA 4.0 arrays were washed and stained using the GeneChip® Hybridisation, Wash, and Stain Kit (Thermo Fisher) in Fluidics Station 450 (Thermo Fisher Scientific). The hybridized targets on the arrays were stained with streptavidin–phycoerythrin from the kit and detected using 3000 7G scanner (Thermo Fisher Scientific). Transcriptome Analysis Console software was used for data analysis.
The raw expression profile data were obtained as CEL files and were further processed using Affymetrix® Transcriptome Analysis Console software (version 4.0, Thermo Fisher Scientific). Background correction, quantile normalization, and data normalization with the expression console were done. After normalization of the microarray data, the expression of miRNA derived from UEs was analyzed by evaluating mean and SD instead of consistency among all 31 expression profiles categorized into three groups, as shown in Table 1. Mean and SD were evaluated for selecting homogeneously expressing miRNAs using one way ANOVA in SPSS software (IBM Corp. Version 20.0. Armonk, NY, USA). The absolute deviation of miRNA expression among groups was selected using the range value. The range value of 0.8 was chosen to filter out the genes having significant, consistent expression in all 31 urine samples. The clusters (http://biit.cs.ut.ee/clustvis/) software was utilized to draw the clustering heatmap of these identified common signatures[24].
The functional enrichment analysis of the evenly expressing miRs was performed using the miRNA Enrichment Analysis and Annotation Tool (miEAA 2.0; https://www.ccb.uni-saarland.de/mieaa2), and a miRNA-centric network visual analytics platform (miRNet 2.0; https://www.mirnet.ca). The miEAA 2. 0 provided the annotation of miRNAs in various categories such as related pathway, disease, chromosomal location, site of expression, miRNA–transcription factor (TF) interactions, family, and PubMed annotation[25]. The miRNet 2.0 generated an allied network of centric miRNA with TFs, target genes (from experimentally validated miRTarBase v8.0 database), associated disease and epigenetic modifiers[26]. For both tools, false discovery rate < 0.05 was considered significant for selecting annotation features.
GraphPad Prism 8 was used to prepare the graph by calculating significant mean values from tabular data by using one-way ANOVA.
The demographic details of subjects with five different kidney conditions i.e., diabetic controls, diabetic nephropathy, membranous nephropathy, IgA nephropathy, and healthy controls (Table 2), from whom, urine samples were collected for isolation of UEs. A total of 31 urine samples were collected, of which 13 were from diabetic controls, five each from diabetic nephropathy or membranous nephropathy, two from IgA nephropathy, and six from healthy controls. Patients with membranous nephropathy had highest mean age, while lowest duration of diabetes. Patients with membranous nephropathy and healthy controls were overweight with average BMI > 29.9.
| Serial number | Variable | Diabetic control | Diabetic nephropathy | Membranous nephropathy | IgA nephropathy | Healthy control | P value |
| 1 | Sample size (n) | 13 | 05 | 05 | 02 | 06 | NA |
| 2 | Age (yr), mean ± SD | 45.11 ± 7.65 | 47.11 ± 12 | 51 ± 3.8 | 49 ± 1.41 | 46 ± 10.79 | 0.04 |
| 3 | Gender (male/female) | 8/5 | 3/2 | 4/1 | 2/0 | 3/3 | NA |
| 4 | Duration of DM (yr), mean ± SD | 10± 6.08 | 6 ± 5 | 2.4 ± 2 | 5.12 ± 6.9 | NA | 0.06 |
| 5 | BMI (kg/m2), mean ± SD | 24 ± 3.64 | 24 ± 3.64 | 26.6 ± 6 | 23 ± 5.23 | 26 ± 7.04 | 0.09 |
| 6 | Medical condition | DM or DM & HTN both | DM or DM & HTN both | DM or HTN or DM & HTN both | IgA nephropathy | NA | NA |
The isolated UEs were physically characterized using NTA. Figure 2A presents NTA plots depicting the average concentration (particles/mL) and size of vesicles isolated from human urine samples, with peak sizes at 70 nm and 191 nm corresponding to exosomes. In Figure 2B, the concentration distribution clearly shows that the small vesicles (below 200 nm) were more abundant in our preparation compared to moderate or large particles. Additionally, vesicles ranging between 30 and 150 nm in size had the highest intensity. A total of 40 µg of UE protein showed the presence of exosome-specific marker proteins, CD9, CD63 and CD81, using western blotting (Figure 2C).
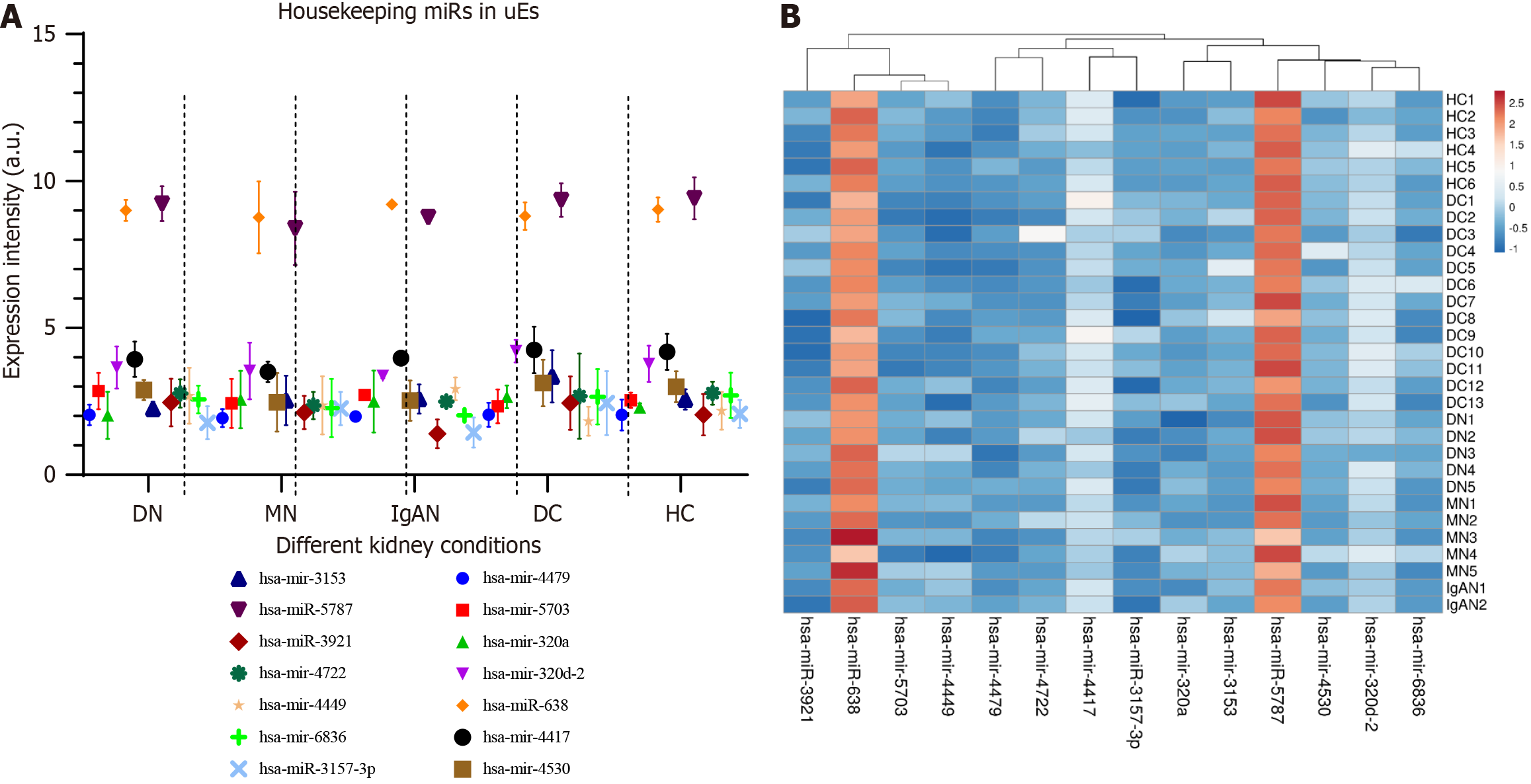
A total of 14 miRNAs showed consistent expression across all groups, both healthy and diseased (Table 3). Figure 3A illustrates the uniform expression of these 14 miRNAs (considered housekeeping genes) across the three groups, while Figure 3B displays a heatmap of these miRNAs.
| ID | Transcript ID | Healthy control | Diabetic control | Diabetic nephropathy | Membranous nephropathy | IgA nephropathy |
| 20536748 | has-mir-4479 | 2.036667 | 2.024615 | 2.04 | 1.93 | 1.985 |
| 20537225 | hsa-mir-5703 | 2.536667 | 2.173077 | 2.85 | 2.43 | 2.715 |
| 20534637 | hsa-mir-320a | 2.295 | 2.558462 | 2.026 | 2.562 | 2.495 |
| 20535940 | hsa-mir-320d-2 | 3.778333 | 4.185385 | 3.65 | 3.536 | 3.37 |
| 20504391 | hsa-miR-638 | 9.026667 | 8.643077 | 9 | 8.762 | 9.205 |
| 20536665 | hsa-mir-4417 | 4.183333 | 4.378462 | 3.932 | 3.504 | 3.97 |
| 20536810 | hsa-mir-4530 | 2.996667 | 2.891538 | 2.886 | 2.468 | 2.525 |
| 20536248 | hsa-mir-3153 | 2.561667 | 3.033846 | 2.242 | 2.53 | 2.58 |
| 20522537 | hsa-miR-5787 | 9.41 | 9.218462 | 9.228 | 8.386 | 8.77 |
| 20518446 | hsa-miR-3921 | 2.045 | 2.076923 | 2.46 | 2.12 | 1.395 |
| 20536956 | hsa-mir-4722 | 2.78 | 2.642308 | 2.768 | 2.358 | 2.49 |
| 20536702 | hsa-mir-4449 | 2.178333 | 1.791538 | 2.69 | 2.364 | 2.925 |
| 20537581 | hsa-mir-6836 | 2.701667 | 2.528462 | 2.566 | 2.272 | 2.025 |
| 20515578 | hsa-miR-3157-3p | 2.071667 | 2.483846 | 1.772 | 2.256 | 1.44 |
The 14 identified housekeeping miRNAs were further analyzed and annotated using the miEAA 2.0 server. This explored their links to diseases, associations with TFs, interactions with mRNAs or genes, and their sites of expression. Among the 14 reference miRNAs, seven showed associations with various diseases according to the Human MicroRNA Disease Database, and 13 were found to interact with different TFs based on data from TransmiR (a transcription factor–miRNA regulation database) during enrichment and annotation analysis.
The predicted tissue-specific expression of these 14 reference miRNAs indicated that 12 of them were expressed in kidney tissue, as illustrated in the heatmap (Figure 4A). Additionally, the Word cloud analysis confirmed the kidney as the primary site of expression for these housekeeping miRNAs (Figure 4B).
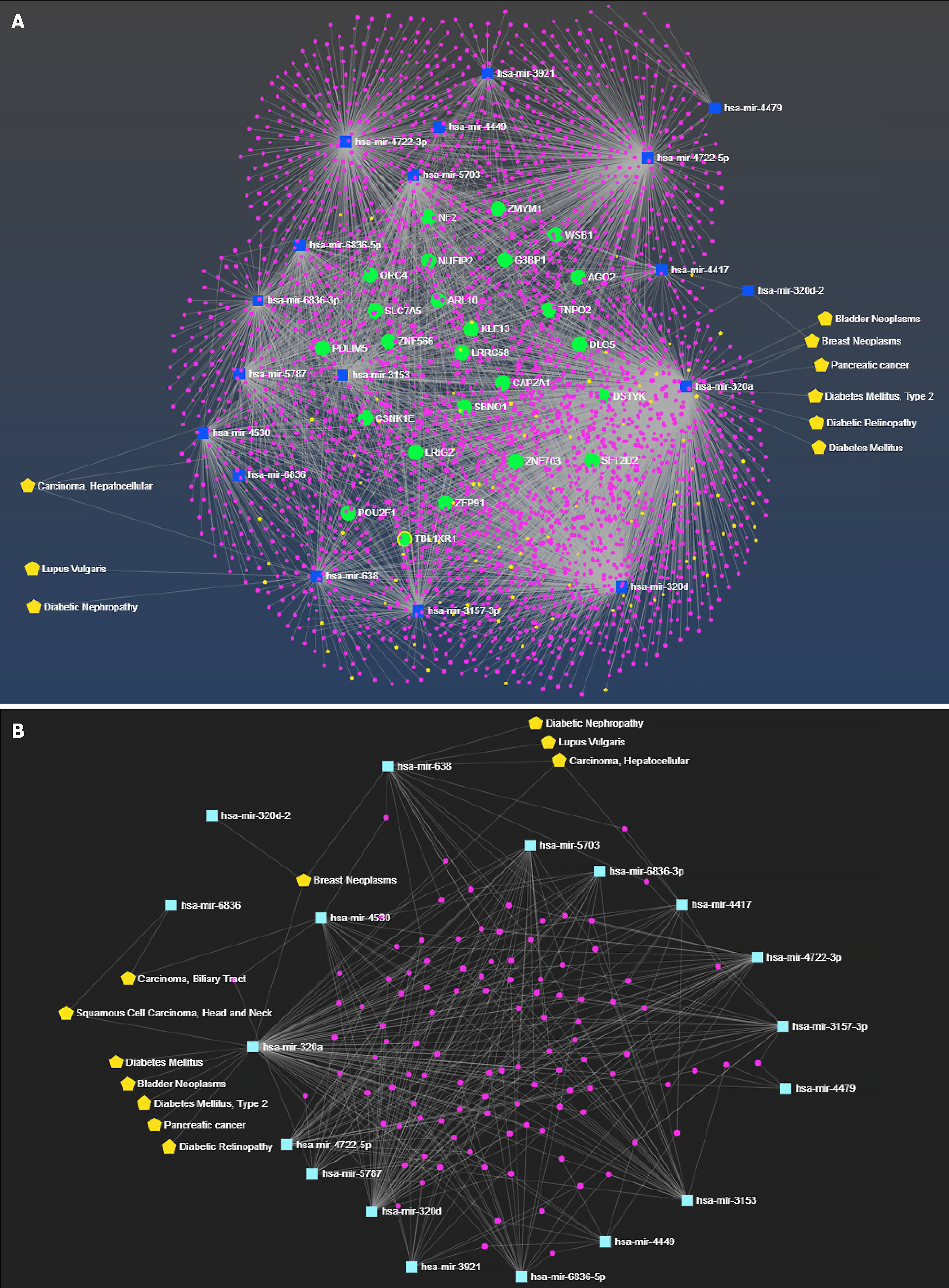
The fully annotated miRNA-centric network has been formed with the 14 housekeeping miRNAs with enriched features such as interacting miRNA, mRNA/protein, and their corresponding disease annotation (with a total of 3633 nodes and 5105 edges) (Figure 5A). The constructed functional network contained 18 miRNAs (13 native and five enriched), their interacting 3513 gene partners, and 102 disease annotations corresponding to the miRNAs and their interactions.
With 3633 nodes and 5105 edges, the above network was complex for analysis of the enrichment of housekeeping miRNAs. Therefore, a subnetwork or module from the above network containing 18 enriched housekeeping miRNAs, their common interactors, and corresponding diseases was extracted from the complex network. The complete function annotation of miRNA is shown in Figure 5B.
The analysis of the above-selected module showed the involvement of only two housekeeping miRNAs, hsa-mir-638 and hsa-mir-320a, in kidney-related diseases. hsa-mir-638 was associated with diabetic nephropathy, lupus vulgaris, breast neoplasms, and hepatocellular carcinoma; whereas hsa-mir-320a was found in diabetic retinopathy, T2DM, bladder neoplasms, breast neoplasms, squamous cell carcinoma, and pancreatic cancer.
The translational potential of UEs as a rich source of kidney disease biomarkers is widely recognized. However, this potential of urine EVs has been limited due to the absence of optimal reference genes (miRNAs) for accurate data norma
We identified 14 potential reference miRNAs that were expressed uniformly in the microarray profile of 31 human urinary EV samples from subjects with T2DM with or without kidney disease and healthy controls. The genes with minimal dispersion in their fold expression, from the mean value obtained from all 31 samples, were considered uniformly expressed genes. The shortlisted genes with SD < 0.8 were taken as uniformly expressed genes. A high throughput unbiased approach has not been done to identify reference/housekeeping miRNA in UEs for kidney disease studies. Only a handful of studies attempted to find potential reference genes from a targeted or shortlisted set of genes[22,30]. Our thorough approach ensured the reliability of our findings.
Out of the 14 potential reference genes identified in our study, we conducted a comprehensive in silico analysis. We found that renal expression or interactions were not found for miR-4417 and miR-320d-2. Also, the functional enrichment and annotation suggested the association of miR-320a and miR-638 with diabetic nephropathy, T2DM and diabetic retinopathy (Figure 2B). Therefore, we omitted miR-4417, miR-320d-2, miR-320a and miR-638 from our list of potential reference gene for kidney disease UE data normalization. The in silico analysis of the remaining 10 miRNAs showed an association of miR-4530 miR-6836 with various cancers such as biliary tract carcinoma, breast carcinoma, and head and neck squamous cell carcinoma. For the other eight miRNAs, no information was available for the pathways or disease association. All these miRNAs were reported to have a strong expression in kidney tissues. It would be interesting to determine a role for these novel miRNAs by wet laboratory experiments; however, this was beyond the scope of our present study. We did not find any published report for any of these 10 reference miRNAs identified in our study.
Our study, while comprehensive, was not without limitations. We acknowledge that our sample size was limited and we did not test in different cohorts. Moreover, validating the findings in samples from more than one time point of the study through longitudinal studies would have strengthened the findings.
The existing strategies for normalization of UE genes by RT-PCR include miRNAs (such as miR-16) reference genes for disease controls or non-disease controls[22]. However, whether these genes remain stably expressed in different kidney diseases has not been studied. In our study we included different kidney disease types, and used biopsy-proven disease samples, which ensured the endogenous expression of the reported reference genes, at least in major types of kidney disease. Several studies focusing on kidney disease have used spike-in controls for RT-PCR data normalization. These controls could be a better way to address any technical issues with the reactions; however, amplification bias, which is assumed to be gene specific, cannot be addressed by spike-in normalization[31].
Overall, we identified 10 miRNAs that were consistently expressed in different kidney diseases, and had high expression levels in kidney tissue. These miRNAs showed similar expression levels in UEs from patients with T2DM, regardless of whether they had kidney disease, as well as in healthy individuals. Therefore, these miRNAs could serve as ideal reference genes for analyzing and normalizing data on UE miRNAs by RT-PCR in studies related to kidney disease. After confirmation in a more extensive set of samples, the multiple gene panels reported here could provide a more accurate interpretation and be preferred to a single reference gene for better accuracy.
The work was carried out under the Center of Advanced Research Excellence (CARE) funded by Indian Council of Medical Research to ST (Coord/7 (1)/CAREKD/ 2018/NCD-II, No. 5/4/7-12/13/NCD-II). The authors wish to acknowledge Dr. Mansi Bhardwaj a (SGPGIMS) for their technical help with the analysis; Prof. Narayan Prasad (Department of Nephrology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow), and Prof. Santosh Varughese (Department of Nephrology, Christian Medical College, Vellore) for subject recruitment.
| 1. | Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience. 2015;65:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 952] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 2. | Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368-13373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1494] [Cited by in RCA: 1726] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 3. | Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1055] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 4. | Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M, Hladik F. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42:7290-7304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 5. | Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R, Vered M. Human saliva-derived exosomes: comparing methods of isolation. J Histochem Cytochem. 2015;63:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Grigor’eva AE, Tamkovich SN, Eremina AV, Tupikin AE, Kabilov MR, Chernykh VV, Vlassov VV, Laktionov PP, Ryabchikova EI. Exosomes in tears of healthy individuals: Isolation, identification, and characterization. Biochem Moscow Suppl Ser B. 2016;10:165-172. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Yoon SB, Chang JH. Extracellular vesicles in bile: a game changer in the diagnosis of indeterminate biliary stenoses? Hepatobiliary Surg Nutr. 2017;6:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 8. | Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1613] [Cited by in RCA: 1832] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 9. | van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 10. | Hu Q, Su H, Li J, Lyon C, Tang W, Wan M, Hu TY. Clinical applications of exosome membrane proteins. Precis Clin Med. 2020;3:54-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 11. | Perocheau D, Touramanidou L, Gurung S, Gissen P, Baruteau J. Clinical applications for exosomes: Are we there yet? Br J Pharmacol. 2021;178:2375-2392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006;5:1760-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006;7:R80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 522] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 14. | Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS. Urine Exosomes: An Emerging Trove of Biomarkers. Adv Clin Chem. 2017;78:103-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Street JM, Koritzinsky EH, Glispie DM, Yuen PST. Urine Exosome Isolation and Characterization. Methods Mol Biol. 2017;1641:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Gunasekaran PM, Luther JM, Byrd JB. For what factors should we normalize urinary extracellular mRNA biomarkers? Biomol Detect Quantif. 2019;17:100090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Hermida RC, Calvo C, Ayala DE, López JE. Decrease in urinary albumin excretion associated with the normalization of nocturnal blood pressure in hypertensive subjects. Hypertension. 2005;46:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Wagner BD, Accurso FJ, Laguna TA. The applicability of urinary creatinine as a method of specimen normalization in the cystic fibrosis population. J Cyst Fibros. 2010;9:212-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Adedeji AO, Pourmohamad T, Chen Y, Burkey J, Betts CJ, Bickerton SJ, Sonee M, McDuffie JE. Investigating the Value of Urine Volume, Creatinine, and Cystatin C for Urinary Biomarkers Normalization for Drug Development Studies. Int J Toxicol. 2019;38:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J, Bath LE, Webb DJ, Gregory CD, Bailey MA, Dear JW. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol. 2013;591:5833-5842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Lange T, Stracke S, Rettig R, Lendeckel U, Kuhn J, Schlüter R, Rippe V, Endlich K, Endlich N. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS One. 2017;12:e0183435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, Tiwari S. Wilm's tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One. 2013;8:e60177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566-W570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1771] [Cited by in RCA: 2487] [Article Influence: 226.1] [Reference Citation Analysis (0)] |
| 25. | Kern F, Fehlmann T, Solomon J, Schwed L, Grammes N, Backes C, Van Keuren-Jensen K, Craig DW, Meese E, Keller A. miEAA 2.0: integrating multi-species microRNA enrichment analysis and workflow management systems. Nucleic Acids Res. 2020;48:W521-W528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 26. | Chang L, Zhou G, Soufan O, Xia J. miRNet 2.0: network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020;48:W244-W251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 627] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 27. | Sonoda H, Lee BR, Park KH, Nihalani D, Yoon JH, Ikeda M, Kwon SH. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci Rep. 2019;9:4692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Solé C, Moliné T, Vidal M, Ordi-Ros J, Cortés-Hernández J. An Exosomal Urinary miRNA Signature for Early Diagnosis of Renal Fibrosis in Lupus Nephritis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Huebner AR, Somparn P, Benjachat T, Leelahavanichkul A, Avihingsanon Y, Fenton RA, Pisitkun T. Exosomes in urine biomarker discovery. Adv Exp Med Biol. 2015;845:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Singh AD, Koyyada R, Samal R, Alvi SB, Patnam S, Rengan AK, Mudigonda SS, Maitra S, Manda SV. Establishment of Optimal Housekeeping Genes for Urinary Extracellular Vesicle Based Biomarker Development: A Step Towards Non-Invasive Diagnostics. 2021. [DOI] [Full Text] |
| 31. | Tung PY, Blischak JD, Hsiao CJ, Knowles DA, Burnett JE, Pritchard JK, Gilad Y. Batch effects and the effective design of single-cell gene expression studies. Sci Rep. 2017;7:39921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/