INTRODUCTION
Point of care ultrasonography (POCUS) is a focused ultrasound examination performed by the clinician at the bedside to guide patient management[1]. Recent years have witnessed a swift uptake of POCUS in almost all the clinical specialties and several medical schools have started teaching this skill to their students. According to a 2020 survey, 57% of the responding United States medical schools (69 out of 122) integrated POCUS instruction into undergraduate medical curriculum[2]. Once confined to procedural guidance such as dialysis catheter placement, the scope of POCUS in nephrology has now greatly expanded to include a wide array of diagnostic applications ranging from kidney ultrasound to focused echocardiography[3,4]. Some nephrology fellowship programs have even incorporated detailed hemodynamic monitoring using advanced Doppler techniques into their curricula[5]. Figure 1 illustrates the sonographic applications that can be performed by nephrologists trained in POCUS. We have also seen in these past few years the emergence of Onco-Nephrology as a subspecialty within Nephrology[6,7]. This field focuses on management of kidney disorders in patients who have an active malignancy and are undergoing treatment for this. The breadth of kidney disorders seen and addressed in Onco-Nephrology practice includes acute kidney injury, hypertension, proteinuria, chronic kidney disease, fluid and electrolyte disorders to name a few. This article seeks to discuss the role and potential of POCUS in positively impacting the clinical practice of Onco-Nephrology by providing a few representative clinical scenarios.
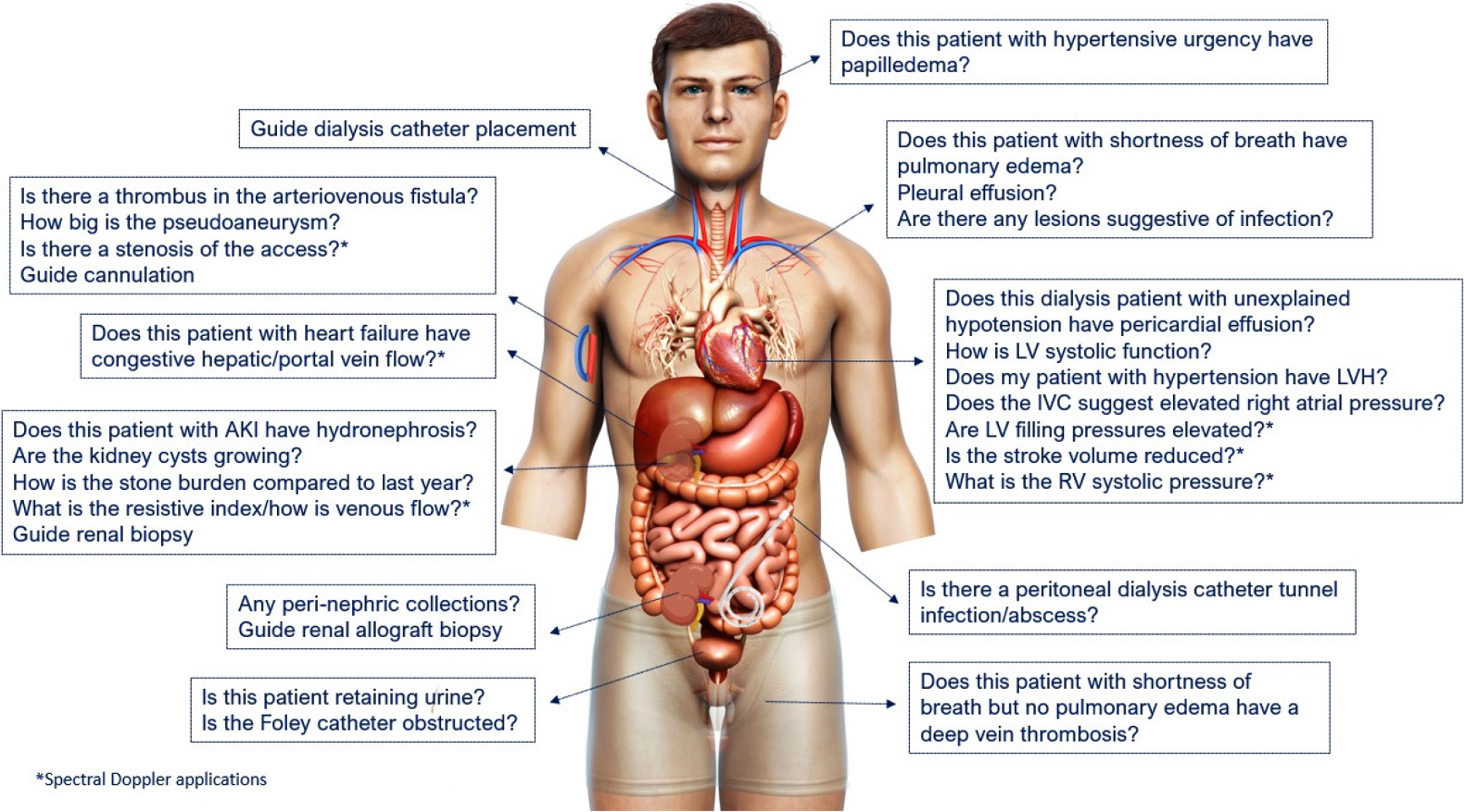
Figure 1 Scope of nephrology-related point of care ultrasonography.
Organ-specific focused questions that can be answered by bedside ultrasonography. Those marked with asterisk (*) indicate advanced sonographic applications requiring a higher operator skill level/additional training. Figure reused from reference # 3 with kind permission of the American Society of Nephrology.
THE RATIONALE FOR POCUS ENHANCED PHYSICAL EXAMINATION
Unlike a consultative ultrasound study which is expected to image an entire area in question (e.g., abdominal ultrasound) with documentation of predefined measurements and parameters, POCUS is intended to answer focused questions that either narrow the differential diagnosis or provide a final diagnosis when interpreted in conjunction with history and physical examination by the treating physician. Moreover, it allows monitoring of a particular parameter in response to therapy without having to repeat the whole comprehensive study. For example, a nephrologist can follow a patient with uremic pericardial effusion in the outpatient dialysis unit with serial POCUS exams thereby avoiding repeated trips of the patient to the echocardiography laboratory. It is analogous to using a stethoscope (point of care device) to listen to heart and lung sounds, which is why some authors describe POCUS as a fifth pillar of bedside physical examination in addition to inspection, palpation, percussion, and auscultation[8]. This raises the question why we need an enhancement to physical examination in the first place and does POCUS have better diagnostic accuracy. The diagnostic performance of conventional physical examination is poor for several clinical questions that nephrologists deal with in day-to-day practice. For example, in a study including 50 patients with severely reduced left ventricular ejection fraction, the combined sensitivity of rales, edema, and elevated jugular venous pressure (JVP) was only 58% to detect an elevated pulmonary capillary wedge pressure of > 22 mmHg[9]. Similarly, in another study including 58 non-edematous patients with serum sodium less than 130 mEq/L, clinical assessment was able to accurately identify only 47% of hypovolemic and 48% of euvolemic patients[10]. Likewise, in a meta-analysis of 22 studies, pooled sensitivities of orthopnea, peripheral edema, JVP, third heart sound and rales were only 50%, 51%, 39%, 13% and 60% respectively to diagnose congestive heart failure[11]. Further, there is no conventional physical examination parameter to answer focused questions requiring visualization of internal anatomy such as the presence or absence of hydronephrosis, systemic venous congestion etc. POCUS aids in answering such questions at the bedside without having to wait for multiple consultative ultrasound studies and potentially avoiding unnecessary radiation. The diagnostic superiority of POCUS is well established in various clinical settings compared to conventional examination. For instance, in a study including 79 patients on hemodialysis, the sensitivity of lung crackles and peripheral edema was only 9% and 3% respectively to detect severe lung congestion found on lung POCUS[12]. In the context of critical illness, a study including 926 patients admitted to the intensive care unit found that 51% of those who had pulmonary edema on lung POCUS demonstrated normal auscultatory findings[13]. With respect to focused cardiac ultrasound, in a recent meta-analysis of 9 studies, the sensitivity of POCUS-assisted examination for diagnosing left ventricular dysfunction and valvular disease was found to be significantly higher compared to conventional assessment (84% vs 43%, and 71% vs 46% respectively)[14]. In addition, the utility of POCUS for rapid evaluation and management of patients with undifferentiated hypotension, chest trauma and possible pericardial tamponade is well-recognized[15]. All these studies highlight the need for enhancing our bedside examination with POCUS. Furthermore, there are emerging data suggesting that POCUS enhances patient satisfaction and shared diagnostic understanding between patients and clinicians[16,17]. Even in developing countries and low-resource settings where one might expect slow adoption of technological advances due to cost issues, POCUS has shown to favorably impact clinical care. In fact, POCUS might be more beneficial in these scenarios to facilitate timely and accurate diagnosis as patients often present with advanced disease. For example, in a Tanzanian cohort of 55 hospitalized patients, a change in management plan was supported by POCUS findings in 53% cases leading to earlier initiation of appropriate treatment[18]. Similar findings were observed in a study from Sri Lanka where POCUS utilization in critically ill patients facilitated early diagnosis and/or interventions[19].
Below are a few situations commonly encountered in Onco-Nephrology practice where POCUS enhanced physical examination can provide valuable information.
CLINICAL SCENARIO 1: ACUTE KIDNEY INJURY IN CANCER
Acute kidney injury (AKI) is a frequent complication of either the underlying malignancy or its treatment and is an independent predictor of mortality in patients with cancer[20,21]. The incidence of AKI in a large Danish cohort of cancer patients was reported to be 17.5% at 1 year and 27% over the course of 5 years, which highlights the enormity of the problem[22]. Similarly, in a Chinese study, the incidence of AKI in hospitalized cancer patients was reported to be 7.5% (hospital acquired in 6% of the cases)[23]. The etiologies of AKI vary across solid organ and hematological malignancies as well as in patients undergoing stem cell transplantation. Hemodynamic AKI resulting from volume depletion is the predominant cause of AKI in patients with an underlying cancer[24] as they may develop nausea, vomiting or diarrhea as complication of cancer chemotherapy or due to the underlying cancer. Post renal obstructive etiology may be the driver of AKI in patients with genitourinary malignancies or locally invasive primary gynecological or gastrointestinal malignancies or metastatic disease[25]. Moreover, as a significant proportion of malignancies treated with radiotherapy are in the abdomen and pelvis, complications such as radiation-induced ureteral and urethral stenosis must be considered in the differential diagnosis of obstructive nephropathy in these patients[26]. Intrinsic renal injury may be mediated by nephrotoxic chemotherapy, paraproteins, glomerulopathies, contrast exposure, infiltration by the primary malignancy or progression of ischemic kidney injury[25].
POCUS considerations
Renal sonography is frequently ordered as part of the initial diagnostic algorithm to rule out obstructive etiology, which is potentially reversible if treated promptly. Bedside POCUS can easily identify hydronephrosis and bladder masses that may be causing urinary obstruction[27]. POCUS can also help delineate intrinsic processes such as infiltrative diseases which may be arising secondary to lymphoma for instance[28]. The kidney size tends to be preserved or larger than expected in these cases with alterations noted in cortical echogenicity. Determination of kidney size and cortical echogenicity while keeping in context the clinical picture can help understand if the renal impairment appears to be a chronic vs. acute process and the realistic probability of renal recovery which can then impact the future diagnostic and therapeutic considerations for these patients[29]. We previously proposed SECONDS checklist for systematic interpretation of renal POCUS, which is helpful for novice users[30]. It stands for Size (renal length and thickness), Echogenicity (cortical brightness), Collecting system (obstruction), Outline (smooth vs irregular), Notable lesions (such as cysts and stones), Doppler (to distinguish between hydronephrosis and vasculature) and Surroundings (peri-nephric collections). Figure 2 illustrates some of the pathologies seen on renal ultrasound in cancer patients. It is also important to evaluate urinary bladder by POCUS in any patient with AKI and/or oliguria to exclude etiologies such as obstructed Foley catheter or bladder outlet obstruction due to extrinsic compression. Moreover, automated bladder scanners cannot distinguish between pelvic ascites and urinary bladder, which may cause confusion in some cases where POCUS aids in correct diagnosis[31]. As hemodynamic AKI is the most frequent etiology of AKI in patients with cancer, the role of POCUS in this clinical scenario deserves a special mention and is discussed in more detail under volume management below.
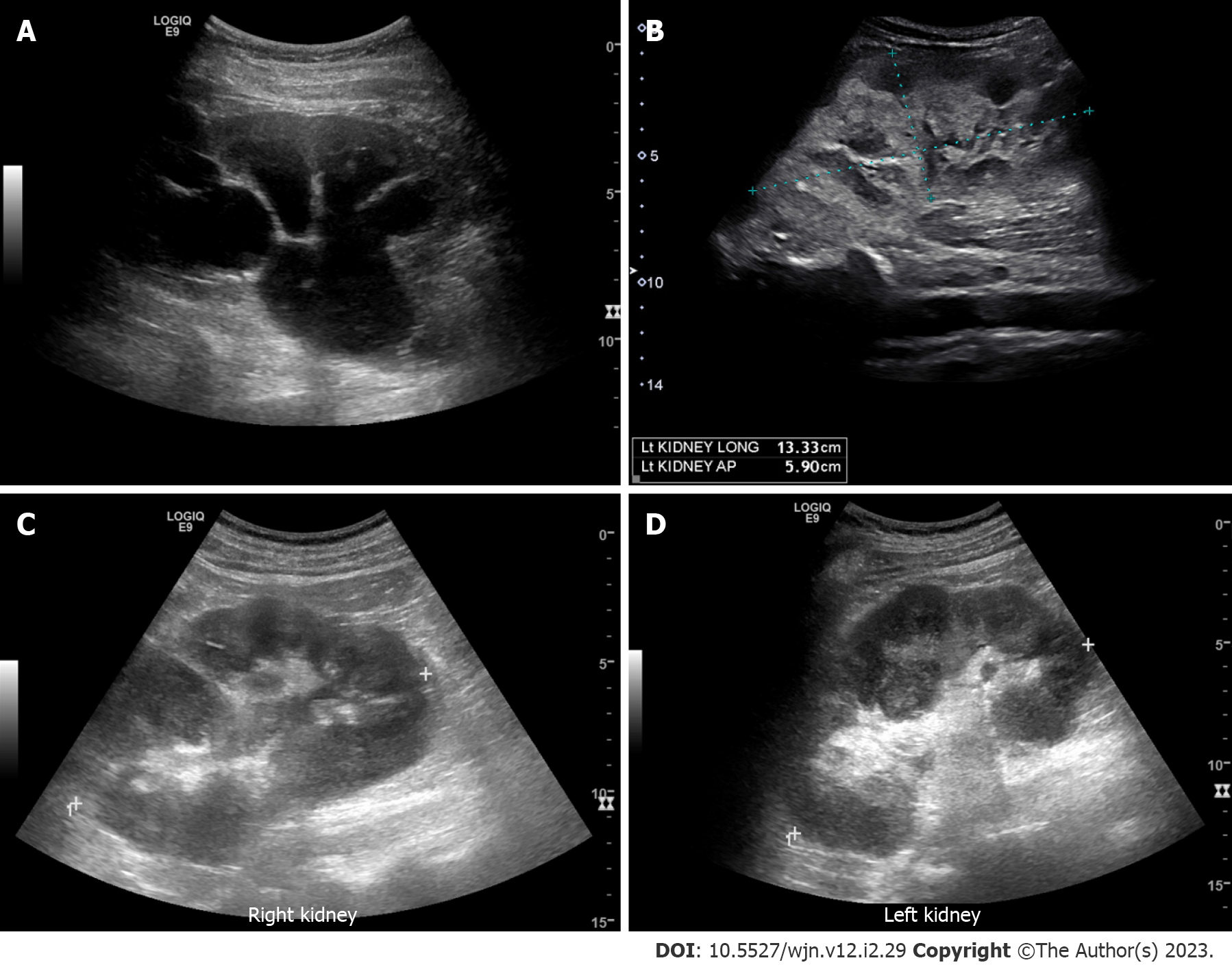
Figure 2 Renal ultrasound images demonstrating.
A: Severe hydronephrosis (branching anechoic area); B: Enlarged kidney with hyperechoic cortex in a patient with myeloma; C and D: Bilateral renal involvement with lymphoma. Note irregular outline and heterogenous parenchyma.
CLINICAL SCENARIO 2: VOLUME ASSESSMENT AND MANAGEMENT
Patients with a diagnosis of cancer are often administered intravenous fluids around chemotherapy with the hope of mitigating the risk of AKI, which can lead to iatrogenic fluid overload if the volume status is not objectively assessed. Further, the volume status in these patients is often tenuous, complicated by increased losses through vomiting and diarrhea as well as third spacing due to hypoalbuminemia. Additionally, certain types of chemotherapies may cause cardiac dysfunction predisposing to volume overload. An important reason for Onco-Nephrology consultation on the inpatient Nephrology service is volume assessment and management in patients undergoing stem cell transplantation (SCT) where volume overload occurs frequently. Allogeneic SCT is a well-established treatment for various hematological malignancies as well as a few nonmalignant disorders[32]. Fluid overload in these patients significantly impacts mortality and is associated with poorer survival[33]. As such, it is imperative that we use objective bedside tools such as POCUS to assess hemodynamic status and guide therapy.
POCUS considerations
Multiorgan POCUS in these cases allows accurate volume assessment. We call this the Pump, Pipes and Leaks approach. The pump denotes focused cardiac ultrasound, pipes represent inferior vena cava (IVC) ultrasound and systemic venous Doppler, and the leaks indicate assessment of the extravascular lung and abdominal fluid[29] (Figure 3). This way, the whole hemodynamic circuit is assessed instead of relying on isolated parameters such as lung or IVC ultrasound, which are error prone. For example, B-lines on lung ultrasound (vertical artifacts signifying interlobular septal thickening) can be seen in cardiogenic pulmonary edema or an infectious process or even fibrosis. In addition to paying attention to parameters such as irregular pleural line suggestive of local pathology, assessment of left ventricular diastolic function using Doppler aids in proper diagnosis. Similarly, IVC is not reliable to assess right atrial pressure in mechanically ventilated patients. Moreover, it can be chronically dilated in patients with pulmonary hypertension and may not provide meaningful information when interpreted in isolation with respect to guiding therapy. Doppler assessment of systemic venous congestion (VExUS) aids in the management of such patients[34-36]. Detailed discussion of VExUS grading to quantify systemic venous congestion is beyond the scope of this manuscript and is concisely illustrated in Figure 4. On the other hand, IVC can be small despite elevated right atrial pressure in intra-abdominal hypertension. Furthermore, a small collapsible IVC can be seen both in euvolemia and hypovolemia and cannot be used in isolation to distinguish between these two conditions. Bedside assessment of stroke volume helps in this situation as it is expected to be low in hypovolemia. Therefore, a multiparametric POCUS approach is the key to appropriate diagnosis and management of volume disorders and these findings must be interpreted in the right clinical context. As most of this information can be obtained by consultative imaging, some might question the need for clinician-performed POCUS. There are two important justifications for this: (1) Hemodynamics are dynamic. For example, a patient with a normal echocardiogram few days ago might have a completely different hemodynamic picture now. Moreover, it is not prudent to obtain a formal echocardiogram daily to monitor selected hemodynamic parameters in response to treatment when POCUS can accomplish the same during daily rounds; and (2) POCUS reduces fragmentation of care. For instance, to assess the pump, pipes and leaks, multiple consultative studies must be obtained – echocardiography performed by the cardiology department, a chest radiograph (lung ultrasound is typically not performed by the ultrasound department), an abdominal sonogram (to look for ascites), a right upper quadrant Doppler (for hepatic and portal vein Doppler [a part of VExUS]) and a Doppler renal ultrasound (for renal venous congestion). Conversely, a POCUS-trained physician with knowledge of the patient’s clinical history/course can perform a focused assessment answering all the key questions in less than 15 min and tailor therapy accordingly.
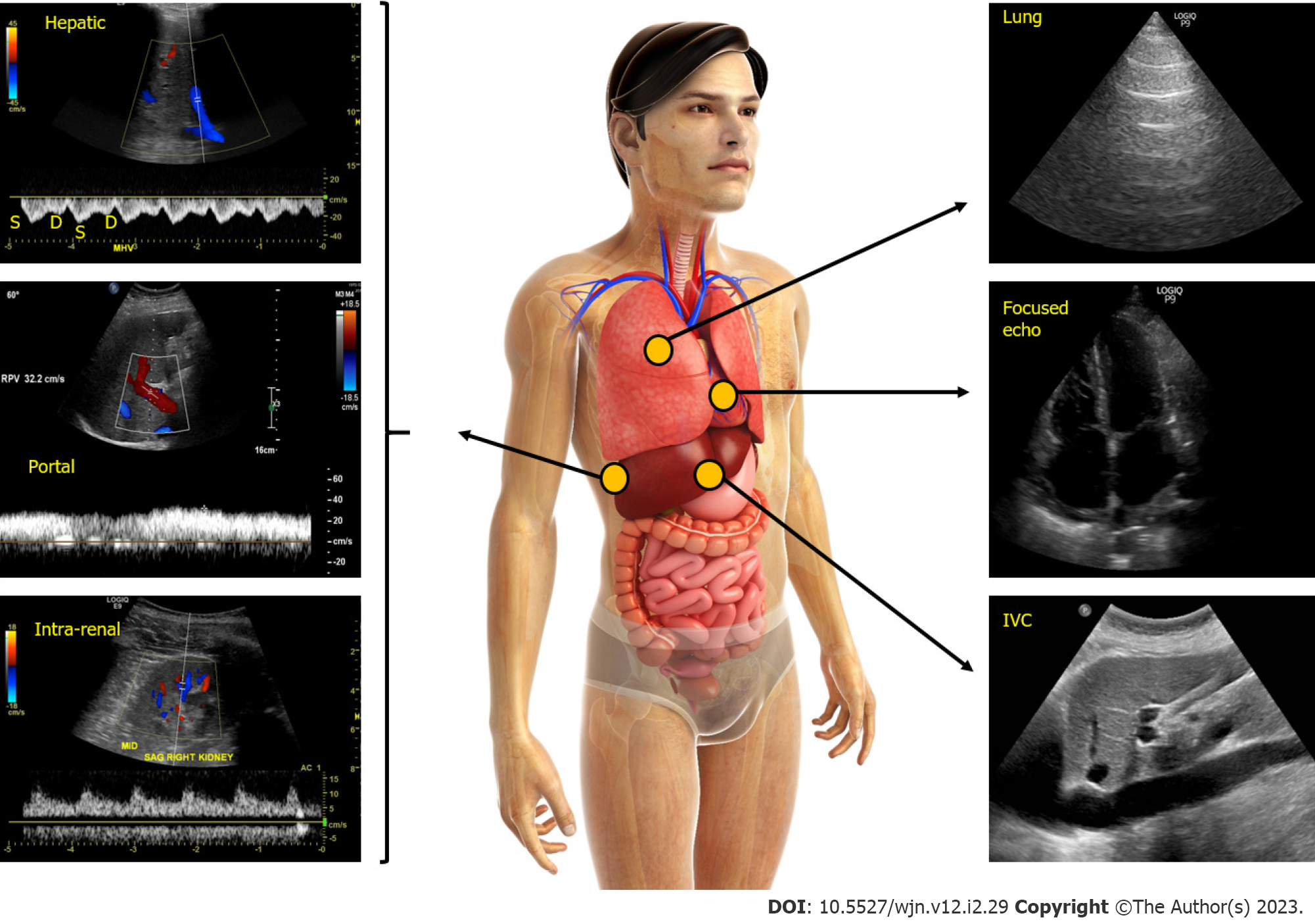
Figure 3 Figure illustrating the integration of multi-point sonographic assessment including focused cardiac, lung and venous Doppler ultrasound.
Normal waveforms are shown. IVC: Inferior vena cava. Adapted from corresponding author’s prior open access publication.
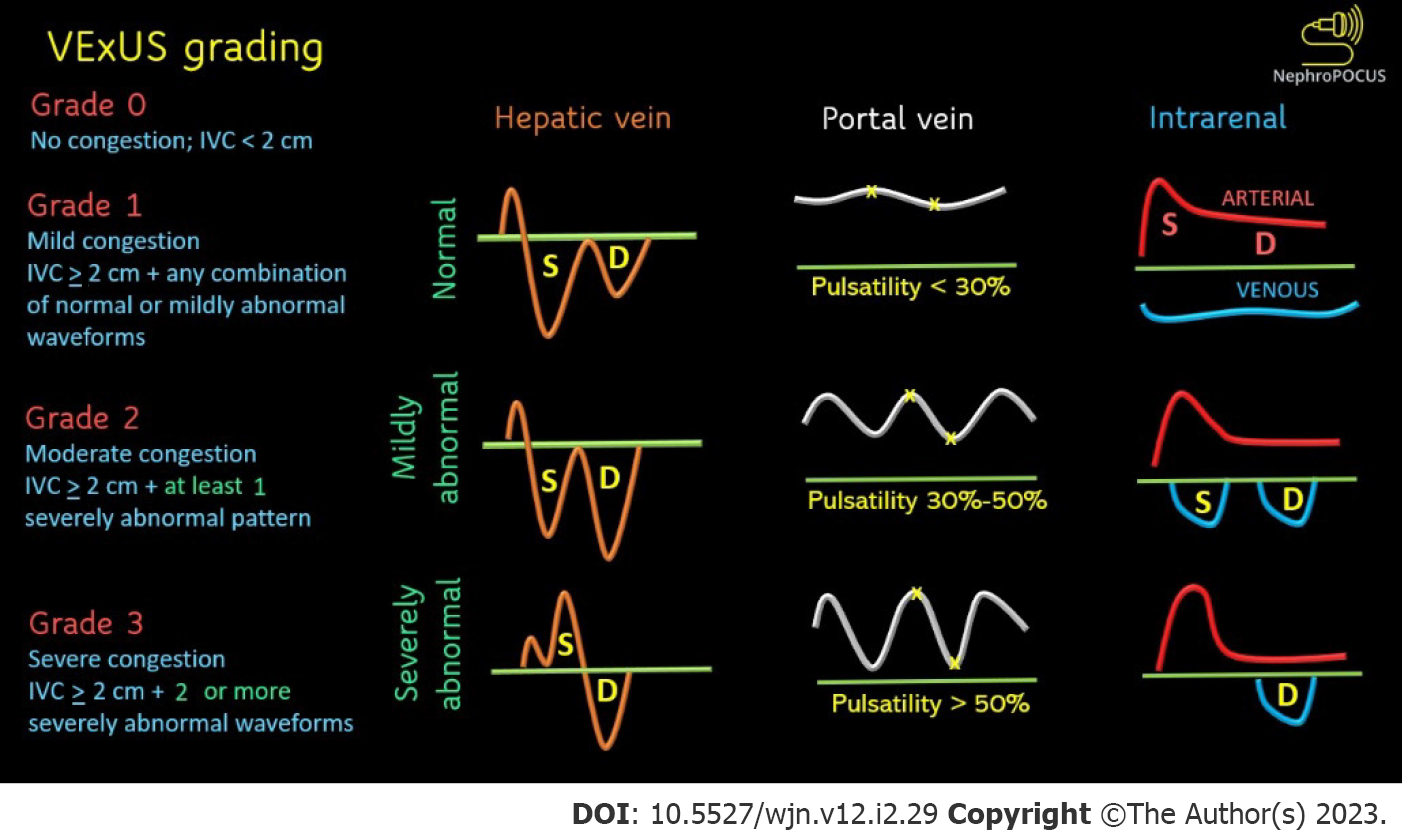
Figure 4 Venous excess ultrasound grading.
When the diameter of inferior vena cava is > 2 cm, three grades of congestion are defined based on the severity of abnormalities on hepatic, portal, and renal parenchymal venous Doppler. Hepatic vein Doppler is considered mildly abnormal when the systolic (S) wave is smaller than the diastolic (D) wave, but still below the baseline; it is considered severely abnormal when the S-wave is reversed. Portal vein Doppler is considered mildly abnormal when the pulsatility is 30% to 50%, and severely abnormal when it is ≥ 50%. Asterisks represent points of pulsatility measurement. Renal parenchymal vein Doppler is mildly abnormal when it is pulsatile with distinct S and D components, and severely abnormal when it is monophasic with D-only pattern. Adapted from NephroPOCUS.com with permission.
CLINICAL SCENARIO 3: EFFUSIONS
Pleural effusion secondary to an underlying malignancy is seen in about 15% of cancers. Metastatic lung (in males) and breast cancer (in females) account for 50%-65% of all cases of malignant pleural effusion. Patients presenting with pleural effusion will require additional imaging for diagnosis and planning of therapeutic interventions. Bedside POCUS is increasingly being utilized for guidance for thoracentesis[37]. Pericardial effusions are noted in 5%-20% patients with an underlying malignancy and significantly impacts the survival and prognosis in these cases[38,39]. Pericardial involvement may result from direct extension of the tumor into the pericardial cavity or hematogenous spread. Opportunistic infections in patients undergoing cancer chemotherapy as well as deranged liver, kidney or cardiac function arising as a result of the underlying cancer or cancer chemotherapy and radiation (like anthracyclines, docetaxel, busulfan, tyrosine kinase inhibitors, arsenic trioxide which can affect the myocardium) may play a role as well in causing pericardial effusion. Majority of the pericardial effusions associated with malignancies are moderate to large in size with pericardial tamponade being noted in one third of the patients with malignant pericardial effusion with poorer outcomes reported in these patients[39]. In addition, ascites is a frequent accompaniment of gastrointestinal and metastatic malignancy.
POCUS considerations
The diagnostic superiority of POCUS to detect multiple effusions is well-established. For example, lung POCUS is more sensitive than physical examination or chest radiography for the detection of small pleural effusions and can detect as small as 3-5 cc of fluid in the pleural space[40-42]. In addition to visualization of pleural effusion, POCUS can help identify loculations in the fluid, thickening and nodularity of the diaphragm, findings which are relatively specific for the diagnosis of malignant pleural effusion. Ultrasound guided pleural biopsies may also be undertaken. The diagnostic accuracy of ultrasound is comparable to computed tomography (CT) in these cases while avoiding the radiation exposure associated with CT imaging. POCUS has also shown to be highly accurate for detecting pericardial effusions of any size and can detect tamponade physiology prior to that of physical examination or vital signs[43]. Therefore, POCUS-performing physician can seek timely consultations prior to clinical decompensation of the patient. Of note, the classic Beck classic triad (jugular venous distension, hypotension, and muffled heart sounds) is a late finding and is neither sensitive nor specific for tamponade[44,45]. With regard to ascites, ultrasound is substantially better than physical examination and can detect as little as 100 cc of peritoneal fluid. In an interesting study from 1982 comparing the diagnostic accuracy of physical examination with that of ultrasound for ascites, overall accuracy of physical examination maneuvers was only 58%[46]. POCUS guidance for paracentesis is essentially a standard procedure in developed countries and has shown to be associated with lower rates of bleeding, decreased hospital length of stay, and cost savings compared to the traditional landmark-based technique[47]. Recently, Nauka et al[48] have proposed a FASC protocol (Focused Assessment with Sonography in Cancer), a simple six-point assessment technique to assess multiple effusions in cancer patients that can be easily used by physicians with limited training. Figure 5 illustrates the sonographic appearance of various effusions.
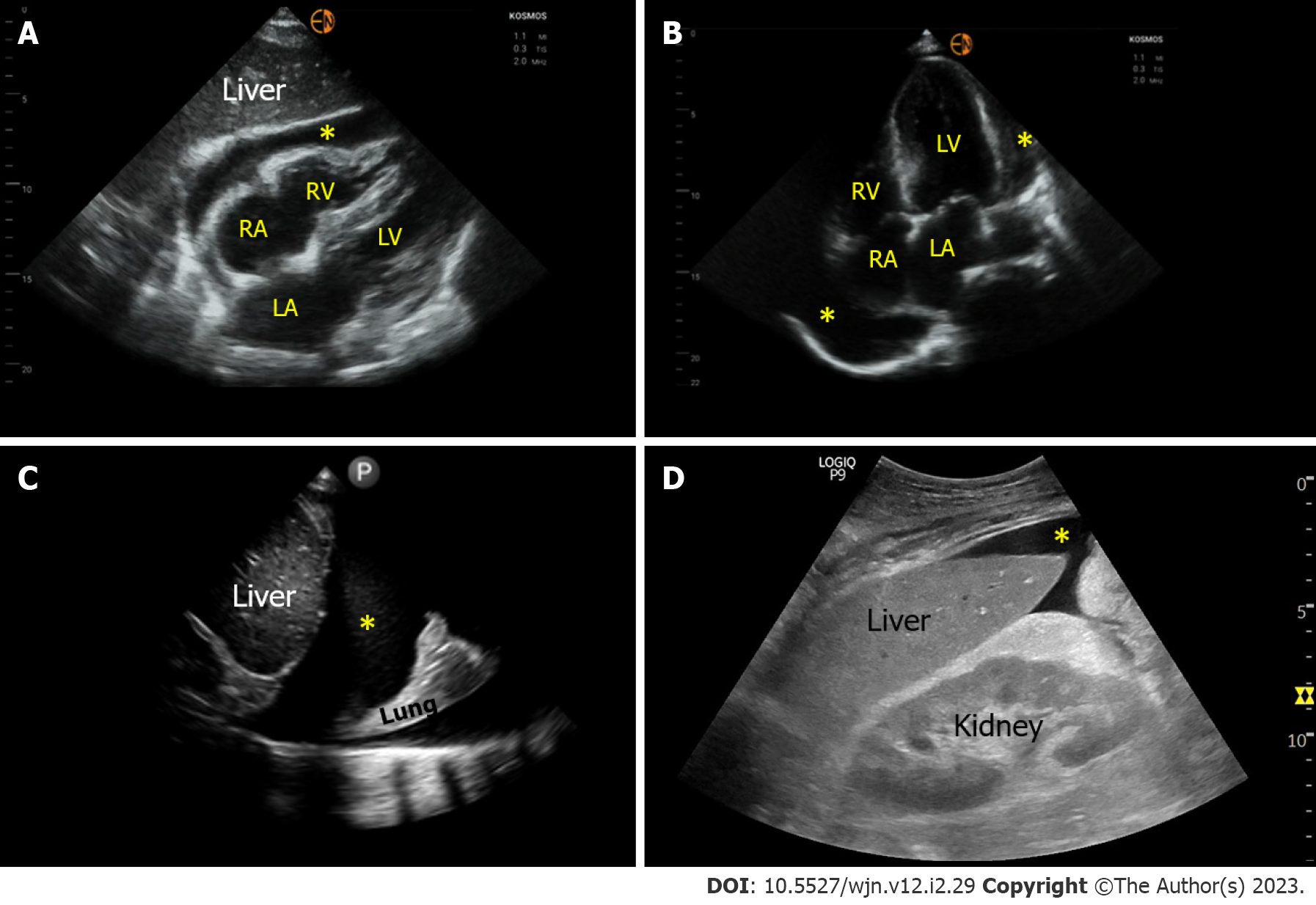
Figure 5 Sonographic images demonstrating various effusions.
A and B: Pericardial effusion (*) as seen on subxiphoid and apical 4-chamber cardiac views respectively; C: Right pleural effusion (*) seen from the lateral scanning window; D: Ascites (*) seen from the right upper quadrant. RA: Right atrium; LA: Left atrium; RV: Right ventricle; LV: Left ventricle.
CLINICAL SCENARIO 4: HYPONATREMIA
Hyponatremia is a very common electrolyte abnormality and may be noted in up to 50% of cancer patients[49]. It negatively impacts prognosis in these patients and may be reflective of advanced underlying disease, chemotherapy toxicity and new or progressive liver or cardiac involvement[50]. Syndrome of inappropriate antidiuresis and volume depletion are the most common etiologies for hyponatremia that complicates an underlying malignancy[50,51]. The traditional diagnostic workup for hyponatremia begins with measurement of plasma and urine osmolality, urine sodium concentration and assessment of volume status[52]. Unfortunately, physical examination has been reported to have poor sensitivity and specificity in this setting, underscoring the void in our bedside assessment[10].
POCUS considerations
A bedside focused ultrasound examination using proven diagnostic parameters can provide objective assessment of volume status, which would make a case for its incorporation in the initial diagnostic algorithm of hyponatremia[53]. The Pump, Pipes and Leaks approach mentioned above works well in this setting. For example, a small collapsing IVC with low stroke volume suggests hypovolemia whereas the same IVC with normal stroke volume suggests euvolemia. A plethoric IVC is more in favor of hypervolemia though Doppler parameters such as VExUS and transvalvular flow assessment are needed in patients with chronically dilated IVC. Several case reports have been published thus far demonstrating the utility of POCUS in the evaluation and management of hyponatremia as we furnished in our prior publication[53].
SUMMARY AND FUTURE DIRECTIONS
Current evidence clearly indicates that POCUS is superior to that of conventional physical examination in terms of diagnostic accuracy and thereby enhances physicians’ confidence in clinical decision making. Future studies must aim to investigate how to better integrate this diagnostic tool in day-to-day Onco-nephrology practice to positively impact measurable outcomes. While one cannot expect mortality benefit just by incorporating a diagnostic modality, outcomes such as duration of hospitalization, time to appropriate diagnosis and treatment, effective decongestion at hospital discharge, recovery of hemodynamic AKI, improvement in patient-reported quality of life, patient and family members’ understanding of the diagnosis are all important practical outcomes that POCUS can impact. On a note of caution, POCUS is operator dependent like anything else in medicine (history taking, physical examination, communication with patients) and proper training is the key to avoid unintentional patient harm. With the availability of low-cost ultraportable ultrasound equipment, POCUS is being increasingly utilized by physicians with limited or no training. It is particularly problematic when the user overestimates their skills and/or capabilities of the equipment (e.g., a novice user with limited understanding of Doppler principles assesses stroke volume using suboptimal image obtained by a low-quality handheld ultrasound device resulting in false conclusions and subsequent patient mismanagement). The burden of regulating and overseeing its use falls on the individual institutions till there are uniform guidelines put forth by professional societies for POCUS training and competency assessment. As a matter of fact, Emergency Care Research Institute has listed the increased adoption of POCUS outpacing institutional safeguards as one of the top health technology hazards[54]. One cannot expect to master physical examination by attending a half- or a one-day workshop and the same applies to POCUS; longitudinal training with emphasis on image acquisition, interpretation and clinical integration is the key to achieving competency and avoiding untoward consequences. As POCUS expertise among nephrologists is sparse at this time, collaboration with experts from various POCUS-performing specialties (e.g., emergency medicine, critical care) is vital for establishment of robust POCUS training programs with quality assurance measures in place.
CONCLUSION
POCUS is a valuable adjunct to physical examination in patients with cancer and renal dysfunction or fluid/electrolyte disorders. It provides better diagnostic accuracy than conventional physical examination. Proper training is the key to effectively integrate this diagnostic tool into routine clinical practice.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Munasinghe B, Sri Lanka; Vlachopanos G, Greece S-Editor: Liu JH L-Editor: A P-Editor: Liu JH