Peer-review started: December 30, 2020
First decision: February 15, 2021
Revised: February 22, 2021
Accepted: March 11, 2021
Article in press: March 11, 2021
Published online: March 25, 2021
Processing time: 78 Days and 14.7 Hours
Low-molecular-weight dextran (LMWD) is considered a safe alternative to contrast media for blood displacement during optical coherence tomography (OCT) imaging.
To investigate whether the use of LMWD for OCT is protective against kidney injury in patients with advanced renal insufficiency.
In this retrospective cohort study, we identified 421 patients with advanced renal insufficiency (estimated glomerular filtration rate < 45 mL/min/1.73 m2) who underwent coronary angiography or percutaneous coronary intervention; 79 patients who used additional LMWD for OCT imaging (LMWD group) and 342 patients who used contrast medium exclusively (control group). We evaluated the differences between these two groups and performed a propensity score-matched subgroup comparison.
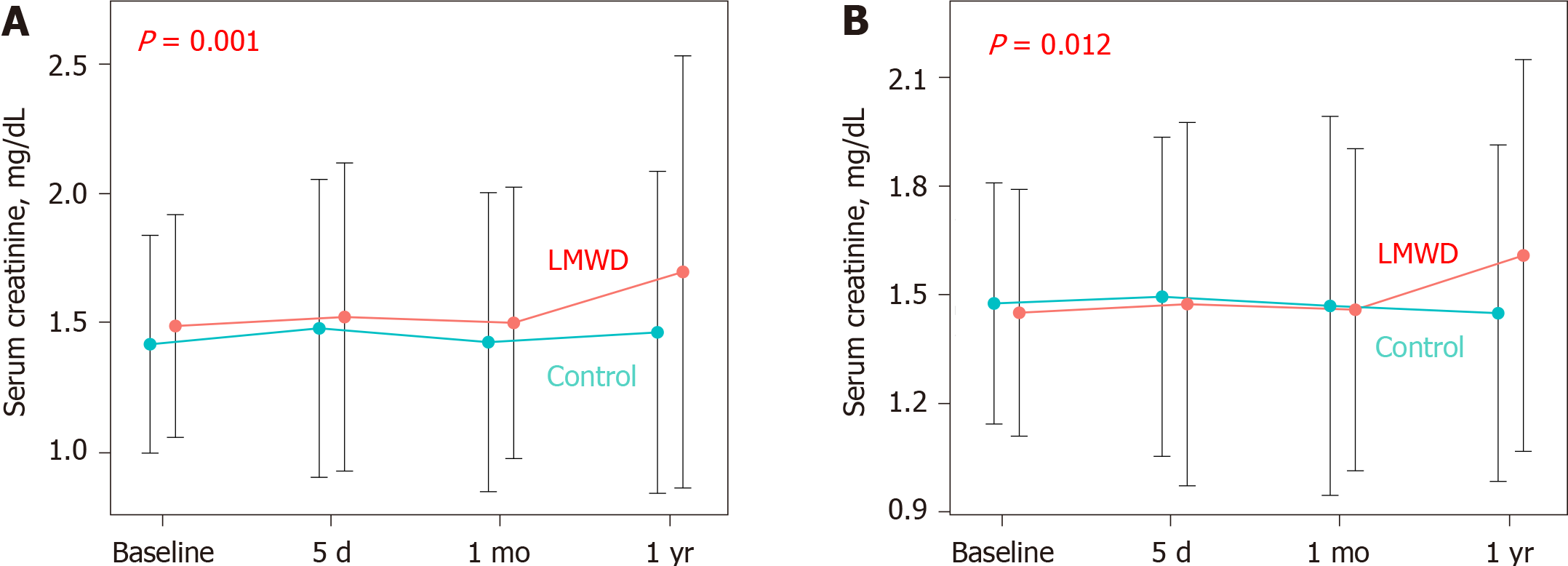
The median total volume of contrast medium was 133.0 mL in the control group vs 140.0 mL in the LMWD group. Although baseline renal function was not statistically different between these two groups, the LMWD group demonstrated a strong trend toward the progression of renal insufficiency as indicated by the greater change in serum creatinine level during the 1-year follow-up compared with the control group. Patients in the LMWD group experienced worsening renal function more frequently than patients in the control group. Propensity score matching adjusted for total contrast media volume consistently indicated a trend toward worsening renal function in the LMWD group at the 1-year follow-up. Delta serum creatinine at 1-year follow-up was significantly greater in the LMWD group than that in the control group [0.06 (-0.06, 0.29) vs -0.04 (-0.23, 0.08) mg/dL, P = 0.001], despite using similar contrast volume.
OCT using LMWD may not be protective against worsening renal function in patients with advanced renal insufficiency.
Core Tip: Low-molecular-weight dextran (LMWD) is considered a safe alternative to contrast during optical coherence tomography (OCT) imaging. We evaluated differences between patients who used additional LMWD for OCT (LMWD group) and those who used contrast exclusively (control group) and performed a propensity score-matched subgroup comparison. The LMWD group demonstrated a strong trend toward the progression of renal insufficiency during the 1-year follow-up. Propensity score-matched analysis indicated a trend toward worsening renal function in the LMWD group at the 1-year follow-up. Additional use of LMWD for OCT may not be protective against worsening renal function in patients with advanced renal insufficiency.
- Citation: Misawa T, Sugiyama T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, Nagamine T, Nogami K, Yasui Y, Usui E, Lee T, Yonetsu T, Sasano T, Kakuta T. Low-molecular-weight dextran for optical coherence tomography may not be protective against kidney injury in patients with renal insufficiency. World J Nephrol 2021; 10(2): 8-20
- URL: https://www.wjgnet.com/2220-6124/full/v10/i2/8.htm
- DOI: https://dx.doi.org/10.5527/wjn.v10.i2.8
The presence of renal insufficiency has been reported to be associated with the increased risk of cardiovascular events[1]. Contrast-induced acute kidney injury (AKI) is a contributing factor to poor outcomes after angiographic procedures[2-4]. The incidence of contrast-induced AKI increases sharply as renal function decreases[5]. Some patients may even experience a prolonged decrease in renal function late after the index procedure[6]. Moreover, the presence of prolonged renal insufficiency in the long-term was associated with the increased risk of cardiovascular events[7]. Renal insufficiency and the total contrast volume are widely known as risk factors for contrast-induced AKI[3,4]. Thus, reducing total contrast volume during coronary angiography and/or percutaneous coronary intervention (PCI) is an important priority to prevent contrast-induced worsening renal function, particularly in patients with advanced renal dysfunction.
Recently, intracoronary optical coherence tomography (OCT) has been widely used to assess coronary plaque characteristics and optimize PCI in patients with coronary artery disease[8,9]. OCT imaging requires blood displacement from the vessel lumen, and contrast media is the standard flushing agent, although the potential risk of contrast-induced AKI exists[2,3]. Previous studies have demonstrated the feasibility of low-molecular-weight dextran (LMWD) as a safe alternative to contrast media for blood displacement during OCT imaging. Previous studies reported that OCT using LMWD might decrease the required total amount of contrast without losing image quality[10,11]. On the other hand, LMWD-induced AKI has been repeatedly reported[12-15]. Therefore, the protective role of LMWD against kidney injury remains uncertain in patients with advanced renal insufficiency, particularly regarding the long-term influence.
In the present study, we sought to investigate whether the additional use of LMWD for OCT imaging is protective against kidney injury in patients with advanced renal insufficiency undergoing coronary angiography and/or PCI by using propensity score-matched subgroup analysis during 1-year follow-up.
This study was performed in compliance with our institutional ethics committee guidelines, and the study received its approval. All patients provided written informed consent before invasive coronary angiography or PCI for future data utilization.
In this retrospective cohort study, we identified 700 patients with advanced renal insufficiency [estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2] who underwent diagnostic coronary angiography or PCI between March 2016 and June 2019 at Tsuchiura Kyodo General Hospital. The study period was chosen because, after March 2016, the total volume of injected flushing agents, including contrast medium and LMWD, was accurately documented by the use of an automated power injector. The final decision to perform an OCT examination by LMWD was at the operator’s discretion. Patients receiving maintenance dialysis were excluded from the analysis. Patients with insufficient data regarding the use of contrast/LMWD or follow-up renal function were also excluded from the analysis. Thus, the final analysis included 421 patients with advanced renal insufficiency, including 342 patients with contrast medium exclusively (control group) and 79 patients receiving additional LMWD for OCT imaging (LMWD group) (Figure 1). We compared patients’ clinical characteristics and the factors associated with renal function between these two groups using propensity score-matched subgroup analysis. All patient data and procedural details were obtained from patients’ medical records.
Each patient underwent diagnostic coronary angiography via the radial artery with a 5-French system, and PCI with a 6-French or 7-French system. A low-osmolarity contrast medium (iopamidol; Fuji Pharma Co., Ltd., Tokyo, Japan) was injected using an automated power injector (ACIST CVi; ACIST Medical Systems Inc., Eden Prairie, MN, United States) at a rate of approximately 3.0-4.0 mL/s. All patients undergoing PCI were treated by coronary drug-eluting stent implantation. To determine the appropriate stent size and obtain optimal stent expansion, we used online quantitative coronary angiography and intracoronary imaging, including OCT and intravascular ultrasound findings. The type of stent was chosen at the operator’s discretion, and the interventionist determined the PCI strategy.
OCT images were acquired using frequency–domain OCT systems: Abbott OCT (ILUMIEN OPTIS; Abbott Vascular, Santa Clara, CA, United States) or Terumo optical frequency–domain imaging system (Lunawave; Terumo Corporation, Tokyo, Japan). The technique of OCT image acquisition has been described elsewhere[8]. OCT imaging pullbacks were performed automatically by the dedicated devices while injecting the flushing agent, which was either contrast medium or LMWD-40 (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan), at a flow rate of 3.0-4.5 mL/s via the guiding catheter using an automated power injector. Pullback speed was 18 mm/s with the Abbott OCT system and 20 mm/s with the Terumo optical frequency-domain imaging system.
Laboratory data were sampled before coronary angiography and/or PCI at the protocol-specified timing for evaluating renal function. Morning fasting blood samples on the day of the procedure were obtained in patients undergoing elective procedures. In patients with acute coronary syndrome (ACS) and those undergoing urgent procedures, blood samples were obtained at admission. We calculated eGFR using the equation modified for Japanese patients: eGFR (mL/min/1.73 m2) = 194 × (serum creatinine)-1.094 × (age)-0.287 × 0.739 (for women)[16]. Advanced renal insufficiency was defined as eGFR < 45 mL/min/1.73 m2[17].
Contrast-induced AKI was defined as a ≥ 0.3 mg/dL increase in serum creatinine level from baseline within 5 d after the procedure[18]. Worsening renal function in the long-term was defined as a ≥ 0.3 mg/dL increase in serum creatinine level from baseline to the 1-year follow-up[19]. We evaluated the incidence of contrast-induced AKI and worsening renal function, and serial changes in serum creatinine level within 5 d, and at 1 mo and 1-year post-procedure. We defined delta creatinine (ΔCre) as the difference between post- and pre-procedural serum creatinine levels. Risks for contrast-induced renal dysfunction were stratified by the Mehran risk score[20].
Our institutional standard protocol for hydration was applied in all patients undergoing elective procedures and in selected patients with ACS. Intravenous normal saline (1.5 mL/kg/h) was administrated for at least 3 h before contrast exposure after blood sampling for baseline renal function, and was continued for at least 12 h after the procedure. After the literature review, this protocol was approved by the institutional ethics committee on the condition of close clinical monitoring for signs of intolerance in patients with heart failure[21].
The statistical analysis was performed using R version 3.6.2 (The R Project for Statistical Computing, Vienna, Austria). Categorical data are expressed as absolute frequencies and percentages and were compared using the χ2 test or Fisher’s exact test, as appropriate. Continuous variables are expressed as mean ± SD for normally distributed variables and median (25th-75th percentile) for non-normally distributed variables. Analyses were performed with the Mann-Whitney U test for non-normally distributed variables. Changes in serum creatinine (baseline, 5 d, 1 mo, and 1-year post-procedure) were evaluated using one-way repeated measures analysis of variance.
To reduce the effect of bias regarding exposure to LMWD in this observational study, we adjusted for significant differences in patients’ baseline characteristics between the control and LMWD groups using propensity score-matched subgroup analysis. We applied propensity score-matched subgroup analysis, adjusted for age, sex, indication (coronary angiography or PCI), baseline eGFR level, and total contrast volume. The propensity score-matched subgroup analysis adjusted for these confounders was performed with a 1:1 algorithm using nearest-neighbor matching with a caliper width of ± 0.10 and no replacement. The effect of the additional use of LMWD on renal function was assessed between these propensity score-matched subgroups. The relationship between worsening renal function (dependent variable), flushing agent, and other potential confounders was evaluated using multivariate logistic regression analysis (stepwise forward method) to assess whether LMWD use remained associated with worsening renal function. The associated variables in the univariate analyses (P < 0.10) were entered into the propensity score-matched models. We used the Hosmer–Lemeshow test to establish the goodness-of-fit of the model, and P > 0.05 indicated that the model provided a valid representation. Receiver operating characteristic (ROC) curves were analyzed to determine the optimal cut-off values for the confounding variables to predict worsening renal function. A two-sided P < 0.05 was considered statistically significant.
The final analysis included 342 patients in the control group and 79 patients in the LMWD group (Figure 1). Patients’ characteristics in these two groups are shown in Table 1. The baseline eGFR level and the distribution of GFR categories were not different between the two groups. A median volume of 133.0 mL of contrast medium was used in the control group. In the LMWD group, a median LMWD volume of 67.6 mL for OCT imaging plus 140.0 mL of contrast medium was used. No anaphylactic reactions occurred. Hemodialysis was required in one patient in the control group during the 1-year follow-up.
| Total (n = 421) | Control (n = 342) | LMWD (n = 79) | P value | |
| Sex | ||||
| Male | 281 (66.7) | 219 (64.0) | 62 (78.5) | 0.017 |
| Female | 140 (33.3) | 123 (36.0) | 17 (21.5) | |
| Age, yr | 75.0 [69.0, 80.0] | 76.0 [69.0, 80.0] | 74.0 [68.0, 79.0] | 0.178 |
| Body mass index, kg/m2 | 24.0 [21.1, 26.2] | 23.9 [21.1, 26.0] | 24.4 [21.3, 26.6] | 0.273 |
| Procedure | ||||
| Coronary angiography | 279 (66.3) | 238 (69.6) | 41 (51.9) | 0.004 |
| PCI | 142 (33.7) | 104 (30.4) | 38 (48.1) | |
| Diagnosis | ||||
| Stable CAD | 350 (83.1) | 288 (84.2) | 62 (78.5) | 0.243 |
| Acute coronary syndrome | 71 (16.9) | 54 (15.8) | 17 (21.5) | |
| Prior myocardial infarction | 155 (36.8) | 106 (31.0) | 49 (62.0) | < 0.001 |
| Prior PCI | 209 (49.6) | 148 (43.3) | 61 (77.2) | < 0.001 |
| Prior CABG | 22 (5.2) | 20 (5.8) | 2 (2.5) | 0.397 |
| Hypertension | 266 (63.2) | 210 (61.4) | 56 (70.9) | 0.122 |
| Dyslipidemia | 178 (42.3) | 140 (40.9) | 38 (48.1) | 0.257 |
| Diabetes mellitus | 218 (51.8) | 172 (50.3) | 46 (58.2) | 0.214 |
| Stroke | 21 (5.0) | 20 (5.8) | 1 (1.3) | 0.147 |
| Current smoking | 56 (13.3) | 35 (10.2) | 21 (26.6) | < 0.001 |
| Serum creatinine, mg/dL | 1.33 [1.22, 1.56] | 1.33 [1.20, 1.52] | 1.36 [1.27, 1.62] | 0.057 |
| eGFR, mL/min/1.73 m2 | 38.4 [32.9, 42.3] | 38.5 [32.6, 42.4] | 36.9 [33.1, 41.8] | 0.368 |
| GFR category | ||||
| 3b (30 ≤ eGFR < 45) | 352 (83.6) | 284 (83.0) | 68 (86.1) | 0.783 |
| 4 (15 ≤ eGFR < 30) | 64 (15.2) | 54 (15.8) | 10 (12.6) | |
| 5 (eGFR < 15) | 5 (1.2) | 4 (1.2) | 1 (1.3) | |
| Hemoglobin A1c, % | 6.3 [5.8, 6.9] | 6.3 [5.7, 7.0] | 6.4 [6.0, 6.9] | 0.109 |
| Low-density lipoprotein cholesterol, mg/dL | 86 [71, 107] | 87 [72, 108] | 82 [70, 103] | 0.301 |
| Hemoglobin, g/dL | 11.8 [10.5, 13.4] | 11.8 [10.5, 13.4] | 12.1 [10.9, 13.5] | 0.375 |
| C-reactive protein, mg/dL | 0.14 [0.05, 0.42] | 0.13 [0.05, 0.40] | 0.15 [0.06, 0.53] | 0.180 |
| NT-proBNP, pg/mL | 864.5 [279.8, 3471.3] | 991.0 [288.3, 3700.0] | 633.0 [177.3, 1775.5] | 0.099 |
| LVEF, % | 57 [44, 66] | 58 [44, 66] | 52 [43, 63] | 0.140 |
| Mehran risk score | 8 [6, 11] | 8 [6, 11] | 8 [7, 11] | 0.710 |
| Catheterization procedure | ||||
| Total agent volume, mL | 150.0 [103.0, 226.0] | 133.0 [92.0, 192.3] | 207.0 [167.5, 271.8] | < 0.001 |
| Total contrast volume, mL | 135.0 [95.0, 193.0] | 133.0 [92.0, 192.3] | 140.0 [102.0, 195.0] | 0.618 |
| LMWD volume, mL | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | 67.6 [43.3, 86.0] | < 0.001 |
| OCT | 79 (40.6) | 92 (26.9) | 79 (100.0) | < 0.001 |
| Renal function post-procedure | ||||
| ΔCre within 5 d, mg/dL | −0.01 [-0.11, 0.13] | 0.00 [-0.10, 0.14] | −0.03 [-0.14, 0.12] | 0.414 |
| ΔCre at 1 mo, mg/dL | −0.01 [-0.15, 0.11] | -0.01 [-0.16, 0.11] | -0.01 [-0.13, 0.09] | 0.686 |
| ΔCre at 1 yr, mg/dL | 0.01 [-0.13, 0.18] | 0.00 [-0.14, 0.16] | 0.07 [-0.04, 0.34] | 0.004 |
| Acute kidney injury | 51 (12.1) | 42 (12.3) | 9 (11.4) | 1.000 |
| Worsening renal function (ΔCre ≥ 0.3 mg/dL/1 yr) | 80 (19.0) | 58 (17.0) | 22 (27.8) | 0.039 |
The LMWD group demonstrated a strong trend toward the progression of renal insufficiency as indicated by the greater change in serum creatinine levels during the 1-year follow-up compared with the control group (Table 1, Figure 2A). Contrast-induced AKI occurred in 9 patients (11.4%) in the LMWD group and 42 patients (12.3%) in the control group (P = 1.000). However, patients in the LMWD group experienced worsening renal function more frequently than patients in the control group (Table 1).
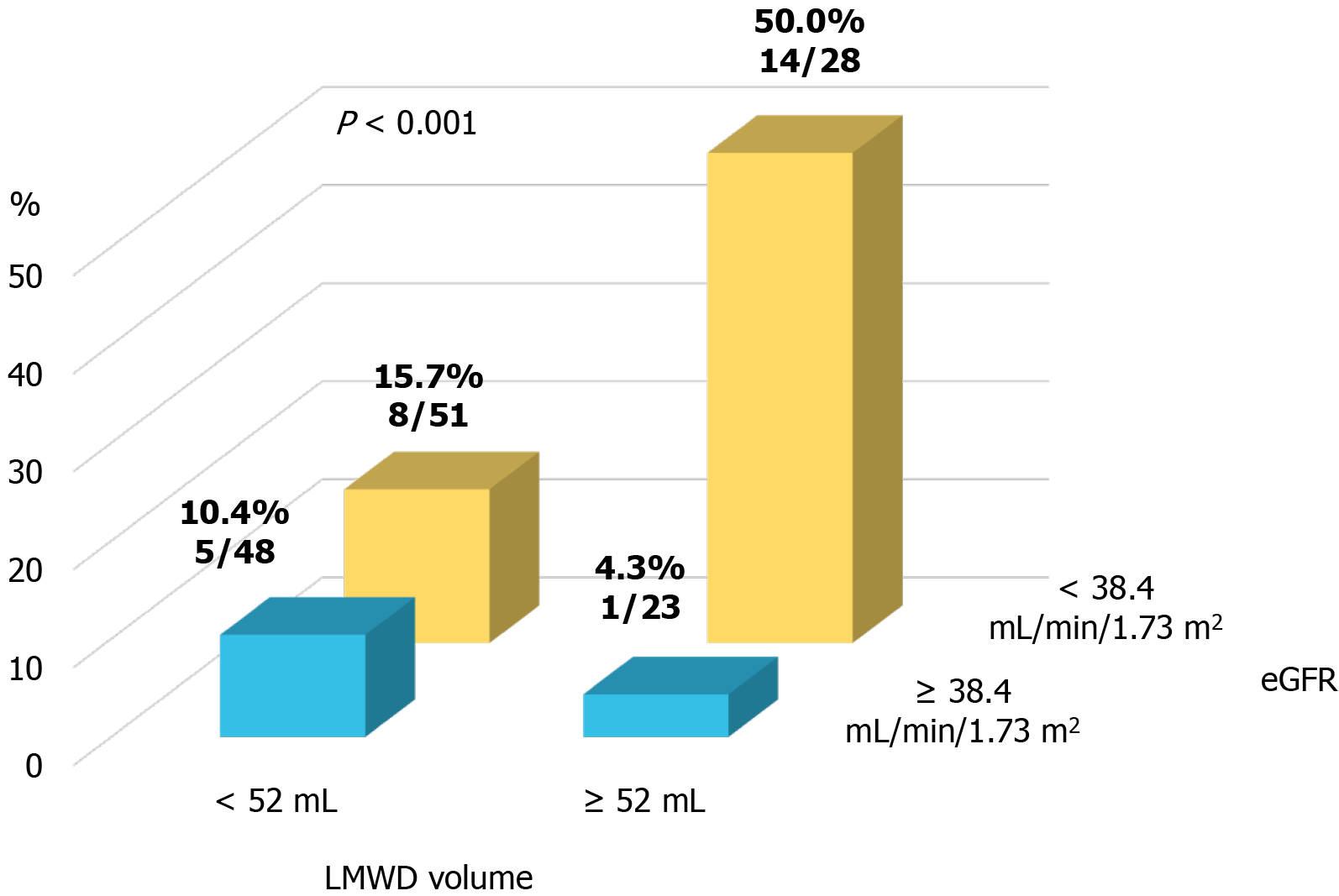
A propensity score-matched cohort adjusted for age, sex, indication (coronary angiography or PCI), baseline eGFR level, and total contrast volume yielded 150 (75 patients each in the control and LMWD groups) well-matched patients regarding potential confounding baseline variables for the clinical and procedural characteristics. A comparison of renal function after the procedure between the control and LMWD groups in the propensity score-matched cohort is shown in Table 2. The baseline eGFR level and the distribution of GFR categories were not different between the two groups. Moreover, no patients had GFR category 5 in the matched cohort. The incidence of contrast-induced AKI was not significantly different between the two groups. In contrast, the LMWD group demonstrated a strong trend toward the progression of renal insufficiency as indicated by the greater change in serum creatinine level during the 1-year follow-up compared with the control group (Table 2, Figure 2B). Although the prevalence of worsening renal function was not statistically different (25.3% vs 12.0%, P = 0.059), ΔCre at 1-year follow-up was significantly greater in the LMWD group than that in the Control group [0.06 (-0.06, 0.29) vs -0.04 (-0.23, 0.08) mg/dL, P = 0.001], despite using similar contrast volume. ROC curve analyses demonstrated that the optimal cut-off values for LMWD volume and baseline eGFR level to predict worsening renal function were 52.0 mL for LMWD volume [area under the curve (AUC): 0.614; 95% confidence interval (CI), 0.504-0.724] and 38.4 mL/min/1.73 m2 for baseline eGFR level (AUC: 0.654; 95%CI: 0.555-0.753). Multivariable logistic regression analysis revealed that the factors independently associated with worsening renal function were greater LMWD volume (≥ 52.0 mL) [odds ratio (OR): 2.83, P = 0.019] and lower baseline eGFR level (< 38.4 mL/min/1.73 m2) (OR: 4.27, P = 0.004) (Table 3). The Hosmer–Lemeshow test provided a P value of 0.993 in the matched cohort, which indicated a proper goodness-of-fit for this model. Moreover, greater LMWD volume particularly identified patients with a high risk for worsening renal function in worse baseline renal function (Figure 3).
| Total (n = 150) | Control (n = 75) | LMWD (n = 75) | P value | |
| Sex | ||||
| Male | 119 (79.3) | 61 (81.3) | 58 (77.3) | 0.687 |
| Female | 31 (20.7) | 14 (18.7) | 17 (22.7) | |
| Age, yr | 75.0 [67.5, 80.0] | 75.0 [69.0, 80.0] | 74.0 [67.0, 79.5] | 0.643 |
| Body mass index, kg/m2 | 23.7 [21.0, 25.9] | 23.0 [20.8, 25.4] | 24.5 [21.3, 26.8] | 0.030 |
| Procedure | ||||
| Coronary angiography | 84 (56.0) | 43 (57.3) | 41 (54.7) | 0.869 |
| PCI | 66 (44.0) | 32 (42.7) | 34 (45.3) | |
| Diagnosis | ||||
| Stable CAD | 121 (80.7) | 61 (81.3) | 60 (80.0) | 1.000 |
| Acute coronary syndrome | 29 (19.3) | 14 (18.7) | 15 (20.0) | |
| Prior myocardial infarction | 77 (51.3) | 30 (40.0) | 47 (62.7) | 0.009 |
| Prior PCI | 93 (62.0) | 34 (45.3) | 59 (78.7) | < 0.001 |
| Prior CABG | 4 (2.7) | 2 (2.7) | 2 (2.7) | 1.000 |
| Hypertension | 98 (65.3) | 45 (60.0) | 53 (70.7) | 0.230 |
| Dyslipidemia | 63 (42.0) | 28 (37.3) | 35 (46.7) | 0.321 |
| Diabetes mellitus | 81 (54.0) | 37 (49.3) | 44 (58.7) | 0.326 |
| Stroke | 5 (3.3) | 4 (5.3) | 1 (1.3) | 0.367 |
| Current smoking | 29 (19.3) | 9 (12.0) | 20 (26.7) | 0.037 |
| Serum creatinine, mg/dL | 1.36 [1.25, 1.63] | 1.37 [1.26, 1.71] | 1.35 [1.25, 1.61] | 0.612 |
| eGFR, mL/min/1.73 m2 | 37.6 [32.8, 42.0] | 38.0 [31.0, 42.2] | 37.0 [33.2, 41.8] | 0.949 |
| GFR category | ||||
| 3b (30 ≤ eGFR < 45) | 125 (83.3) | 59 (78.7) | 66 (88.0) | 0.189 |
| 4 (15 ≤ eGFR < 30) | 25 (16.7) | 16 (21.3) | 9 (12.0) | |
| 5 (eGFR < 15) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hemoglobin A1c, % | 6.3 [5.9, 6.9] | 6.1 [5.7, 6.9] | 6.5 [6.0, 7.0] | 0.058 |
| Low-density lipoprotein cholesterol, mg/dL | 87 [70, 108] | 88 [72, 114] | 81 [70, 103] | 0.155 |
| Hemoglobin, g/dL | 11.8 [10.5, 13.4] | 11.5 [10.3, 13.5] | 12.1 [10.9, 13.3] | 0.230 |
| C-reactive protein, mg/dL | 0.14 [0.05, 0.46] | 0.12 [0.05, 0.35] | 0.15 [0.06, 0.53] | 0.320 |
| NT-proBNP, pg/mL | 835.5 [244.8, 3265.3] | 1560.0 [270.0, 4268.5] | 632.0 [175.0, 1679.5] | 0.076 |
| LVEF, % | 53 [43, 64] | 56 [44, 64] | 52 [43, 63] | 0.697 |
| Mehran risk score | 8 [7, 11] | 8 [7, 11] | 8 [7, 11] | 0.548 |
| Catheterization procedure | ||||
| Total agent volume, mL | 174.1 [120.0, 244.8] | 130.0 [88.0, 196.5] | 209.7 [170.1, 271.8] | < 0.001 |
| Total contrast volume, mL | 138.0 [97.5, 200.0] | 130.0 [88.0, 196.5] | 142.0 [104.5, 200.0] | 0.408 |
| LMWD volume, mL | 7.6 [0.0, 68.2] | 0.0 [0.0, 0.0] | 68.5 [43.9, 86.0] | < 0.001 |
| OCT | 95 (63.3) | 20 (26.7) | 75 (100.0) | < 0.001 |
| Renal function post-procedure | ||||
| ΔCre within 5 d, mg/dL | -0.03 [-0.15, 0.13] | -0.04 [-0.15, 0.14] | -0.03 [-0.14, 0.10] | 0.848 |
| ΔCre at 1 mo, mg/dL | -0.03 [-0.17, 0.09] | -0.06 [-0.23, 0.10] | -0.02 [-0.14, 0.08] | 0.276 |
| ΔCre at 1 yr, mg/dL | 0.01 [-0.14, 0.19] | -0.04 [-0.23, 0.08] | 0.06 [-0.06, 0.29] | 0.001 |
| Acute kidney injury | 14 (9.3) | 7 (9.3) | 7 (9.3) | 1.000 |
| Worsening renal function (ΔCre ≥ 0.3 mg/dL/1 yr) | 28 (18.7) | 9 (12.0) | 19 (25.3) | 0.059 |
| Variable | Univariable | Multivariable | ||||||
| OR | 95%CI lower limit | 95%CI upper limit | P value | OR | 95%CI lower limit | 95%CI upper limit | P value | |
| Female | 1.72 | 0.68 | 4.39 | 0.256 | ||||
| Age | 0.96 | 0.92 | 0.99 | 0.036 | Not selected | |||
| Body mass index, kg/m2 | 1.02 | 0.92 | 1.13 | 0.759 | ||||
| PCI | 1.61 | 0.70 | 3.67 | 0.260 | ||||
| Acute coronary syndrome | 1.92 | 0.75 | 4.95 | 0.175 | ||||
| Prior myocardial infarction | 1.33 | 0.58 | 3.05 | 0.496 | ||||
| Prior PCI | 1.68 | 0.69 | 4.11 | 0.258 | ||||
| Hypertension | 0.95 | 0.40 | 2.23 | 0.897 | ||||
| Diabetes mellitus | 2.04 | 0.86 | 4.87 | 0.107 | ||||
| Stroke | 1.09 | 0.12 | 10.20 | 0.938 | ||||
| Current smoking | 1.17 | 0.43 | 3.22 | 0.756 | ||||
| eGFR < 38.4 mL/min/1.73 m2 | 4.18 | 1.58 | 11.00 | 0.004 | 4.27 | 1.59 | 11.40 | 0.004 |
| LVEF, % | 0.98 | 0.95 | 1.01 | 0.220 | ||||
| Mehran risk score | 1.07 | 0.98 | 1.17 | 0.127 | ||||
| Total agent volume, mL | 1.00 | 0.99 | 1.01 | 0.533 | ||||
| Total contrast volume, mL | 0.99 | 0.99 | 1.01 | 0.776 | ||||
| LMWD volume ≥ 52 mL | 2.76 | 1.19 | 6.37 | 0.018 | 2.83 | 1.18 | 6.76 | 0.019 |
The main findings of the present study are: (1) The additional use of LMWD for OCT was identified in 18.8% (79/421) of the study patients with advanced renal insufficiency; (2) The LMWD group demonstrated a strong trend toward the progression of renal insufficiency as indicated by the greater change in serum creatinine level during the 1-year follow-up compared with the control group; (3) After propensity score matching, the LMWD group demonstrated a significant trend in the progression of renal insufficiency at the 1-year follow-up; and (4) Multivariable logistic regression analysis revealed that greater LMWD volume and lower baseline eGFR level were independently associated with worsening renal function in the propensity score-matched cohort. To the best of our knowledge, this is the first study to demonstrate that the additional use of LMWD for OCT imaging may not be protective against worsening renal function in patients with advanced renal insufficiency, particularly with respect to the long-term influence.
Various studies have reported that renal insufficiency is independently associated with cardiovascular events and mortality[22-24]. Notably, patients with eGFR < 45 mL/min/1.73 m2 were associated with a 1.49-fold increase in the risk of cardiovascular events compared with patients with eGFR > 75 mL/min/1.73 m2, which resulted in a substantial reduction in life expectancy for patients with eGFR < 45 mL/min/1.73
OCT enables clear visualization of culprit and non-culprit plaque morphologies[8,9] and PCI optimization[28,29]. LMWD is considered a safe alternative to contrast media for blood displacement during OCT imaging. Moreover, a recent study demonstrated that the changes in serum creatinine level just after the procedure did not differ between the patients treated with OCT guidance and those with intravascular ultrasound guidance, which included mainly normal and mild renal insufficiency[30]. However, these previous studies enrolled a relatively small number of patients, and sample sizes were too small to draw definitive recommendations for the safety of additional use of LMWD for OCT in patients with advanced renal insufficiency. In contrast, our study involved a much larger number of patients with advanced renal insufficiency receiving LMWD additionally as a substitute flushing agent. In daily clinical practice, the additional use of LMWD for OCT has been preferentially performed in patients with advanced renal insufficiency to reduce the total contrast volume and prevent contrast-induced AKI. However, the safety of LMWD over contrast medium against kidney injury, particularly over the long-term, has not been fully studied. To our knowledge, our study is the first report examining the possibility of LMWD-related kidney injury when LMWD is used for OCT combined with contrast medium in patients with advanced renal insufficiency during a 1-year follow-up.
In the previous studies that reported the association between LMWD and AKI[12-15], the total LMWD volume was much greater than that used for OCT because LMWD was used mainly to correct hypovolemia as a substitute for plasma concentrate. In contrast, in our study, the LMWD group showed a strong trend toward worsening renal function at the 1-year follow-up, after propensity score-matched comparisons, even though LMWD volume for OCT was a median of 68.5 (43.9, 86.0) mL. The pathogenesis of contrast-induced AKI involves several mechanisms, such as nephrotoxic effects on tubular epithelial cells, tubular obstruction, decreased renal perfusion, and renal vasoconstriction[4]. Osmotic nephrosis characterized by vacuolization and swelling of proximal tubular cells is induced by many substrates such as contrast medium and LMWD[15]. LMWD is also associated with hyperoncotic kidney injury[13]. Excretion of LMWD particles could be reduced, particularly in the presence of renal insufficiency. In previous studies, approximately 70% of LMWD was excreted by the kidneys within 24 h, while the remaining LMWD was excreted over several days[14,31]. Administration of LMWD accompanied by a certain amount of contrast medium could potentially cause prolonged renal insufficiency in the long-term.
The present study indicated that the additional use of LMWD in patients with advanced renal insufficiency showed a strong trend toward renal impairment at the 1-year follow-up. Our results also suggested that greater LMWD volume may be a contributing factor to the progression of long-term renal dysfunction in patients with advanced renal insufficiency. Excessive use of LMWD in place of contrast medium may not effectively decrease the prevalence of renal impairment after OCT imaging.
Our results, including the propensity score-matched comparison, suggest that using LMWD for OCT in patients with advanced renal insufficiency should be avoided, or at least care should be exercised to reduce the amount of LMWD. The present study also suggested the importance of long-term follow-up of renal function after LMWD use for OCT imaging. Since our study is of hypothesis generating nature, further studies are needed to test this hypothesis.
First, this was a retrospective study from a single center with a relatively small sample size and, thus, has inherent limitations. Although we used propensity score matching to adjust for differences in patients’ baseline clinical characteristics, including renal function, the final decision regarding the choice of flushing agents for OCT was at the operator’s discretion, and selection bias cannot be canceled. Second, because we collected data from our institutional OCT registry, some patients with ACS underwent pre- and post-PCI OCT examinations for the culprit lesion, and some underwent OCT examination for the non-culprit lesion before or after culprit lesion assessment/ treatment for the clinical research. This nature of the study cohort might have led to an increase in contrast medium and/or LMWD volume. Third, we did not evaluate the influence of medical therapy and clinical status post-procedure on the incidence of contrast-induced AKI and worsening renal function, which could have affected our results. Although this study evaluated the renal function only within 5 d, and at 1 mo and 1-year post-procedure, the mid-term effect of LMWD on renal function was not fully studied. Fourth, the prevalence and severity of proteinuria and the cause of renal insufficiency were not assessed, and both could be closely related to the progression of renal dysfunction. Finally, we did not assess clinical outcomes or perform extensive subgroup analyses because of the relatively small number of events.
In this retrospective study, we observed the greater extent of the progression of renal dysfunction and the higher prevalence of worsening renal function, particularly at the 1-year follow-up, in patients with advanced renal insufficiency who underwent OCT imaging using LMWD. These findings provide a novel insight regarding LMWD use by interventionalists who are involved in OCT examinations. Further large, prospective studies are warranted.
Low-molecular-weight dextran (LMWD) is considered a safe alternative to contrast media for blood displacement during optical coherence tomography (OCT) imaging. On the other hand, LMWD-induced acute kidney injury has been repeatedly reported.
The protective role of LMWD against kidney injury remains uncertain in patients with advanced renal insufficiency, particularly regarding the long-term influence.
To investigate whether the use of LMWD for OCT is protective against kidney injury in patients with advanced renal insufficiency.
In this retrospective cohort study, we identified 421 patients with advanced renal insufficiency (estimated glomerular filtration rate < 45 mL/min/1.73 m2) who underwent coronary angiography or percutaneous coronary intervention; 79 patients who used additional LMWD for OCT imaging (LMWD group) and 342 patients who used contrast medium exclusively (control group). We evaluated the differences between these two groups and performed a propensity score-matched subgroup comparison.
Although baseline renal function was not statistically different between these two groups, the LMWD group demonstrated a strong trend toward the progression of renal insufficiency as indicated by the greater change in serum creatinine level during the 1-year follow-up compared with the control group. Patients in the LMWD group experienced worsening renal function more frequently than patients in the control group. Propensity score matching adjusted for total contrast media volume consistently indicated a trend toward worsening renal function in the LMWD group at the 1-year follow-up. Delta serum creatinine at 1-year follow-up was significantly greater in the LMWD group than that in the control group [0.06 (-0.06, 0.29) vs -0.04 (-0.23, 0.08) mg/dL, P = 0.001], despite using similar contrast volume.
Additional use of LMWD for OCT may not be protective against worsening renal function in patients with advanced renal insufficiency.
Since our study is of hypothesis generating nature, further large, prospective studies are warranted.
| 1. | Parikh NI, Hwang SJ, Larson MG, Levy D, Fox CS. Chronic kidney disease as a predictor of cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2008;102:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 514] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 3. | Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1226] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 4. | Mehran R, Dangas GD, Weisbord SD. Contrast-Associated Acute Kidney Injury. N Engl J Med. 2019;380:2146-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 411] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 5. | McCullough PA, Choi JP, Feghali GA, Schussler JM, Stoler RM, Vallabahn RC, Mehta A. Contrast-Induced Acute Kidney Injury. J Am Coll Cardiol. 2016;68:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 6. | Wang F, Peng C, Zhang G, Zhao Q, Xuan C, Wei M, Wang N. Delayed kidney injury following coronary angiography. Exp Ther Med. 2016;12:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Turin TC, James MT, Jun M, Tonelli M, Coresh J, Manns BJ, Hemmelgarn BR. Short-term change in eGFR and risk of cardiovascular events. J Am Heart Assoc. 2014;3:e000997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW; Expert's OCT Review Document. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 657] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 9. | Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang IK, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PW, Di Mario C; Expert's OCT Review Document. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513-2520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Ozaki Y, Kitabata H, Tsujioka H, Hosokawa S, Kashiwagi M, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Takarada S, Kubo T, Kimura K, Tanaka A, Hirata K, Mizukoshi M, Imanishi T, Akasaka T. Comparison of contrast media and low-molecular-weight dextran for frequency-domain optical coherence tomography. Circ J. 2012;76:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Frick K, Michael TT, Alomar M, Mohammed A, Rangan BV, Abdullah S, Grodin J, Hastings JL, Banerjee S, Brilakis ES. Low molecular weight dextran provides similar optical coherence tomography coronary imaging compared to radiographic contrast media. Catheter Cardiovasc Interv. 2014;84:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Feest TG. Low molecular weight dextran: a continuing cause of acute renal failure. Br Med J. 1976;2:1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Moran M, Kapsner C. Acute renal failure associated with elevated plasma oncotic pressure. N Engl J Med. 1987;317:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Zwaveling JH, Meulenbelt J, van Xanten NH, Hené RJ. Renal failure associated with the use of dextran-40. Neth J Med. 1989;35:321-326. [PubMed] |
| 15. | Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Japan nephrology society. [Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012]. Nihon Jinzo Gakkai Shi. 2012;54:1034-1191. [PubMed] |
| 17. | Farrington K, Covic A, Nistor I, Aucella F, Clyne N, De Vos L, Findlay A, Fouque D, Grodzicki T, Iyasere O, Jager KJ, Joosten H, Macias JF, Mooney A, Nagler E, Nitsch D, Taal M, Tattersall J, Stryckers M, van Asselt D, Van den Noortgate N, van der Veer S, van Biesen W. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrol Dial Transplant. 2017;32:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Mehran R, Faggioni M, Chandrasekhar J, Angiolillo DJ, Bertolet B, Jobe RL, Al-Joundi B, Brar S, Dangas G, Batchelor W, Prasad A, Gurm HS, Tumlin J, Stone GW. Effect of a Contrast Modulation System on Contrast Media Use and the Rate of Acute Kidney Injury After Coronary Angiography. JACC Cardiovasc Interv. 2018;11:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG. Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int. 2000;57:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 711] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 21. | Balemans CE, Reichert LJ, van Schelven BI, van den Brand JA, Wetzels JF. Epidemiology of contrast material-induced nephropathy in the era of hydration. Radiology. 2012;263:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Freeman RV, Mehta RH, Al Badr W, Cooper JV, Kline-Rogers E, Eagle KA. Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol. 2003;41:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 2002;106:2207-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Sørensen CR, Brendorp B, Rask-Madsen C, Køber L, Kjøller E, Torp-Pedersen C. The prognostic importance of creatinine clearance after acute myocardial infarction. Eur Heart J. 2002;23:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1380] [Cited by in RCA: 1413] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 26. | Turin TC, Tonelli M, Manns BJ, Ravani P, Ahmed SB, Hemmelgarn BR. Chronic kidney disease and life expectancy. Nephrol Dial Transplant. 2012;27:3182-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, Harms HJ, Osborne MT, Bravo P, Andrikopolou E, Divakaran S, Bibbo CF, Hainer J, Skali H, Taqueti V, Steigner M, Dorbala S, Charytan DM, Prabhu SD, Blankstein R, Deo RC, Solomon SD, Di Carli MF. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation. 2020;141:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, Morel O, Lefrançois Y, Descotes-Genon V, Silvain J, Braik N, Chopard R, Chatot M, Ecarnot F, Tauzin H, Van Belle E, Belle L, Schiele F. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation. 2016;134:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 29. | Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, Shite J, Fusazaki T, Otake H, Kozuma K, Ioji T, Kaneda H, Serikawa T, Kataoka T, Okada H, Akasaka T; OPINION Investigators. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38:3139-3147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 30. | Kurogi K, Ishii M, Sakamoto K, Komaki S, Kusaka H, Yamamoto N, Takashio S, Arima Y, Yamamoto E, Kaikita K, Tsujita K. Optical Coherence Tomography-Guided Percutaneous Coronary Intervention With Low-Molecular-Weight Dextran-Effect on Renal Function. Circ J. 2020;84:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Nearman HS, Herman ML. Toxic effects of colloids in the intensive care unit. Crit Care Clin. 1991;7:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sureshkumar K S-Editor: Fan JR L-Editor: A P-Editor: Wang LL