©The Author(s) 2025.
World J Nephrol. Sep 25, 2025; 14(3): 107571
Published online Sep 25, 2025. doi: 10.5527/wjn.v14.i3.107571
Published online Sep 25, 2025. doi: 10.5527/wjn.v14.i3.107571
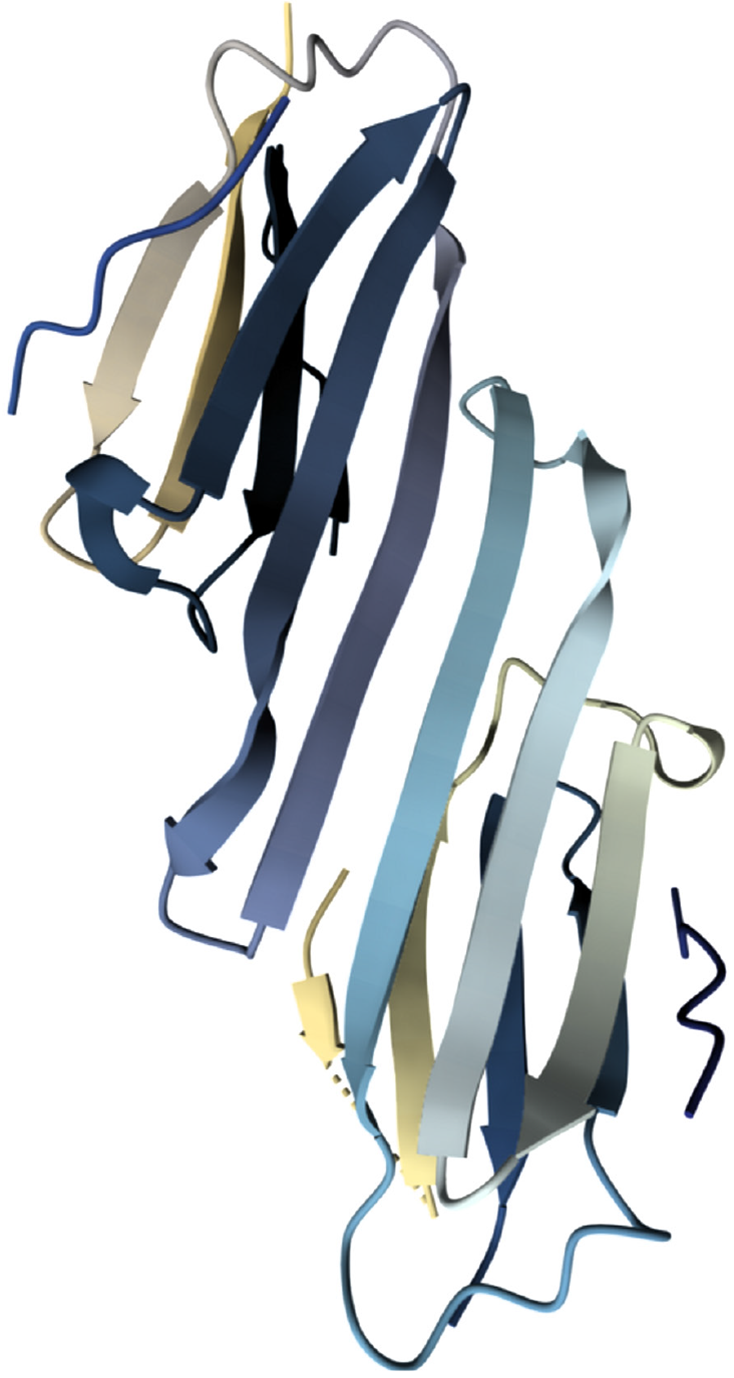
Figure 1 Molecular structure of heat shock protein 27[62].
Heat shock protein 27 (HSP27) possesses three key structural regions: A less structured N-terminal domain with motifs like WDPF crucial for large oligomer assembly and chaperone function, a conserved α-crystallin domain (ACD) that forms β-sandwich dimers essential for building higher-order structures and chaperone activity, and a flexible C-terminal domain containing an IXI/V-like motif that interacts with the ACD to aid oligomerization and regulate chaperone activity. These dynamic structural features, including the ACD's role in preventing amyloid fibrillation by binding misfolded proteins, enable HSP27 to shift between various oligomeric states. This adaptability allows it to effectively bind a wide range of client proteins and prevent their aggregation, especially under stress conditions, with its function further modulated by factors like phosphorylation. This figure was adapted with Mol* (MIT-licensed) from the Structure page for PDB ID 4MJH (CC0 1.0) on the RCSB Protein Data Bank (https://www.rcsb.org/structure/4MJH). Citation: Hochberg GK, Ecroyd H, Liu C, Cox D, Cascio D, Sawaya MR, Collier MP, Stroud J, Carver JA, Baldwin AJ, Robinson CV, Eisenberg DS, Benesch JL, Laganowsky A. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc Natl Acad Sci U S A 2014; 111: E1562-E1570. Copyright ©National Academy of Sciences 2014. Published by PNAS (Supplementary material).
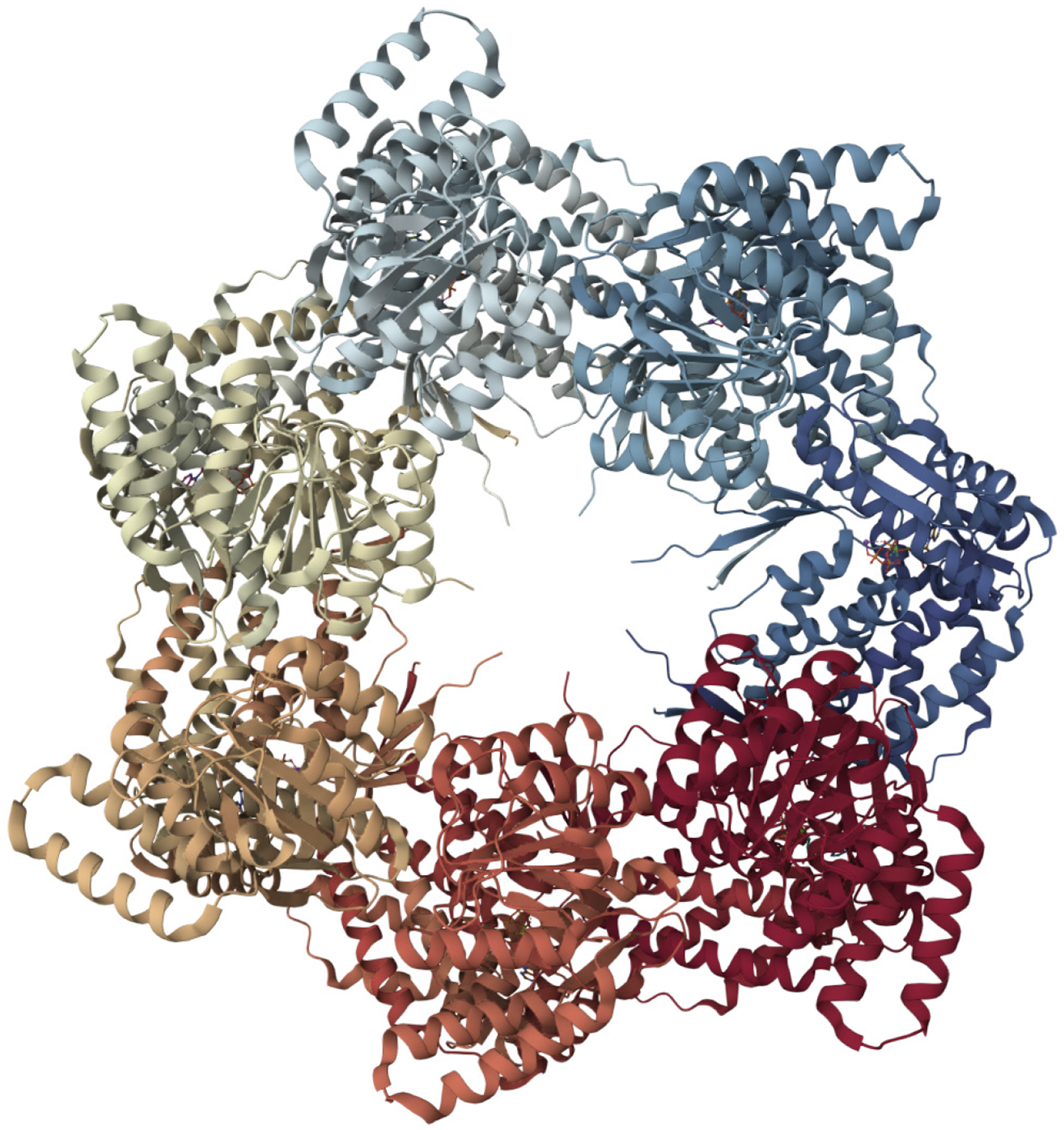
Figure 2 Molecular structure of heat shock protein 60[82].
Each heat shock protein 60 (HSP60) monomer features three key domains: The apical domain for binding substrates and the co-chaperone HSP10, an intermediate domain acting as a hinge, and an equatorial domain responsible for adenosine triphosphate (ATP) binding and inter-ring contacts. Notably, upon ATP binding, mitochondrial HSP60's apical domains adopt an asymmetric "up/down" configuration, a crucial feature for simultaneously recruiting HSP10 and engaging client proteins, thereby coordinating the folding process. This entire ATP-dependent mechanism involves significant conformational shifts that regulate the folding chamber, enabling HSP60 to efficiently fold proteins within the mitochondria. This figure was adapted with Mol* (MIT-licensed) from the Structure page for PDB ID 8G7M (CC0 1.0) on the RCSB Protein Data Bank (https://www.rcsb.org/structure/8G7M). Citation: Braxton JR, Shao H, Tse E, Gestwicki JE, Southworth DR. Asymmetric apical domain states of mitochondrial Hsp60 coordinate substrate engagement and chaperonin assembly. Nat Struct Mol Biol 2024; 31: 1848-1858. Copyright ©The Author(s) 2024. Published by Nature Pub. Group (Supplementary material).
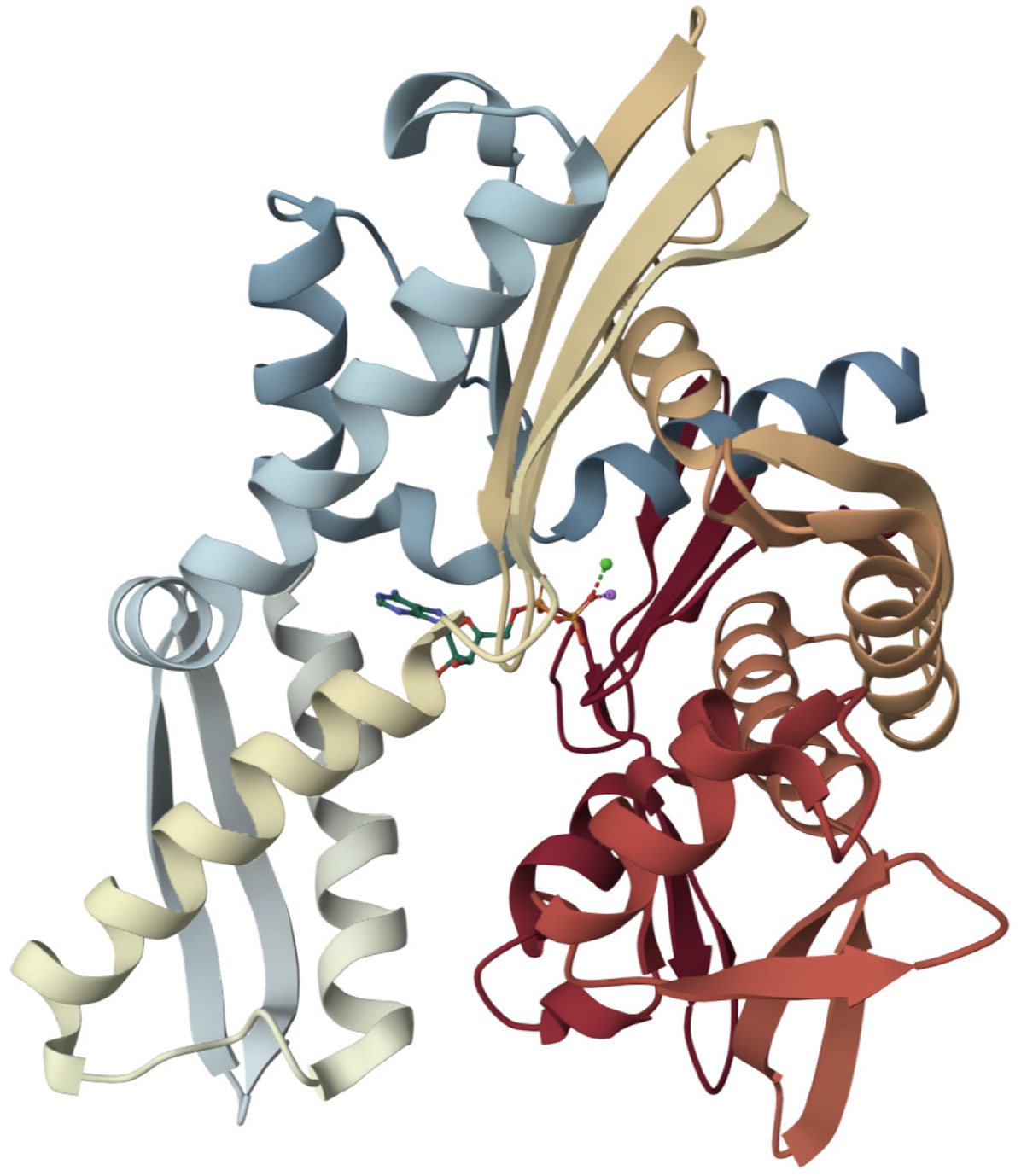
Figure 3 Molecular structure of heat shock protein 70[113].
Heat shock protein 70 (HSP70) is a molecular chaperone with two main domains: An N-terminal nucleotide-binding domain (NBD) (approximately 44 kDa) and a C-terminal substrate-binding domain (SBD) (approximately 25-30 kDa), connected by a flexible, conserved linker that facilitates allosteric communication. The NBD consists of two lobes creating a deep cleft for adenosine triphosphate (ATP) binding and hydrolysis, exhibiting a structural fold similar to actin. The SBD is characterized by a β-sandwich subdomain that forms a peptide-binding groove and an α-helical lid that covers the bound substrate. The crucial chaperone function of HSP70 relies on ATP-dependent conformational changes: ATP binding to the NBD leads to an open SBD lid and low substrate affinity, while ATP hydrolysis promotes lid closure and high-affinity substrate binding, enabling HSP70 to act as a molecular machine in protein folding. This figure was adapted with Mol* (MIT-licensed) from the Structure page for PDB ID 1S3X (CC0 1.0) on the RCSB Protein Data Bank (https://www.rcsb.org/structure/1S3X). Citation: Sriram M, Osipiuk J, Freeman B, Morimoto R, Joachimiak A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure 1997; 5: 403-414 Copyright ©2002 Elsevier Science Ltd. Published by Elsevier Inc (Supplementary material).
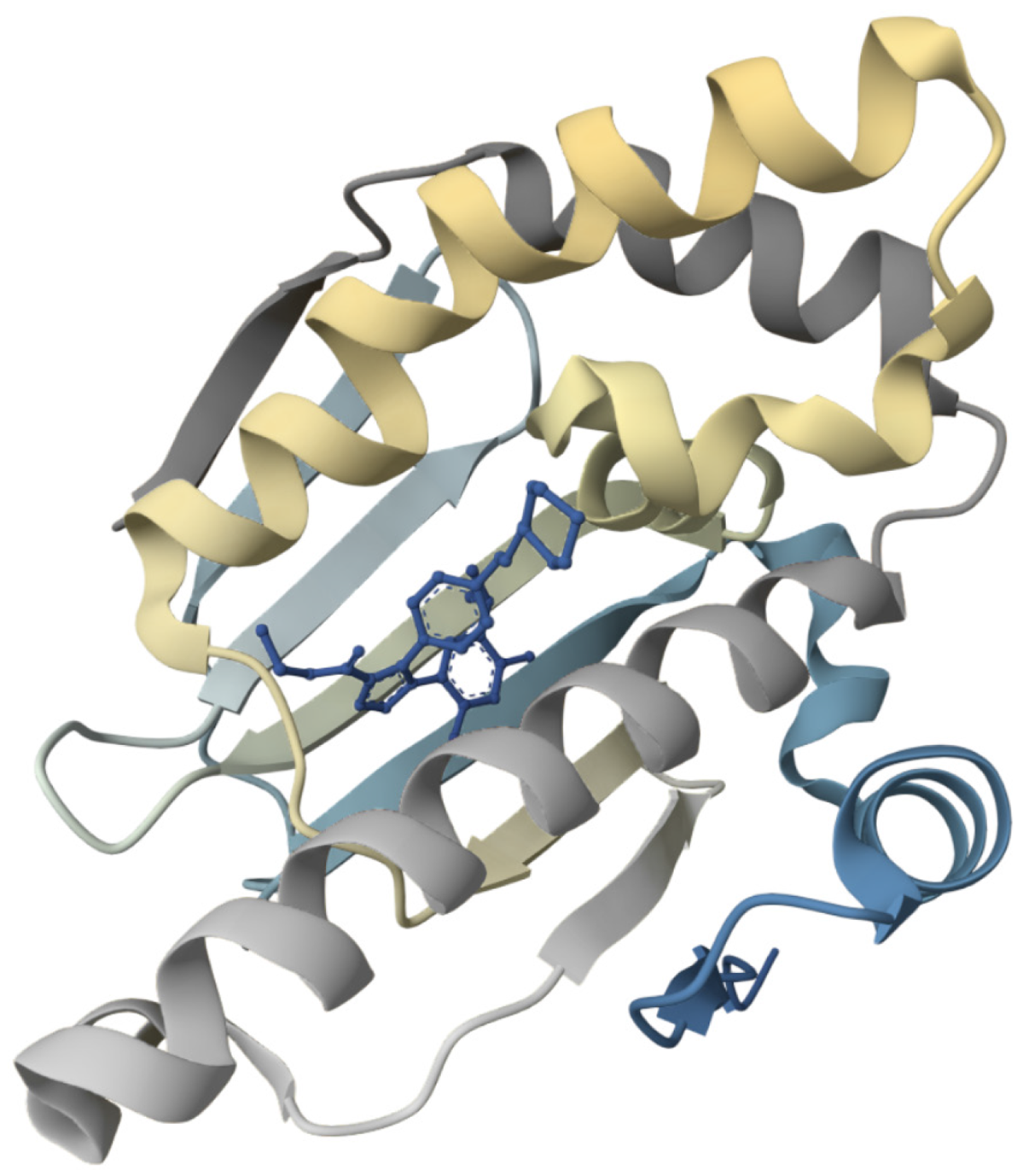
Figure 4 Molecular structure of heat shock protein 90[135].
Heat shock protein 90 functions as a homodimer, with each monomer comprising three key domains: An N-terminal domain (NTD) that binds adenosine triphosphate (ATP), a middle domain involved in client protein and co-chaperone interactions, and a C-terminal domain primarily responsible for dimerization. The NTD can form a 'molecular clamp', and ATP binding to the NTDs induces their transient dimerization, driving significant conformational changes between open and closed states, a process crucial for client protein activation and the overall chaperone cycle. This dynamic, ATP-dependent mechanism, regulated by co-chaperones, enables HSP90's flexible multi-domain architecture to interact with a wide range of client proteins, facilitating their proper folding, maturation, and stabilization. This figure was adapted with Mol* (MIT-licensed) from the Structure page for PDB ID 8AGI (CC0 1.0) on the RCSB Protein Data Bank (https://www.rcsb.org/structure/8AGI). Citation: Tassone G, Mazzorana M, Mangani S, Petricci E, Cini E, Giannini G, Pozzi C, Maramai S. Structural Characterization of Human Heat Shock Protein 90 N-Terminal Domain and Its Variants K112R and K112A in Complex with a Potent 1,2,3-Triazole-Based Inhibitor. Int J Mol Sci 2022; 23: 9458. ©2022 by the authors. Published by MDPI (Supplementary material).
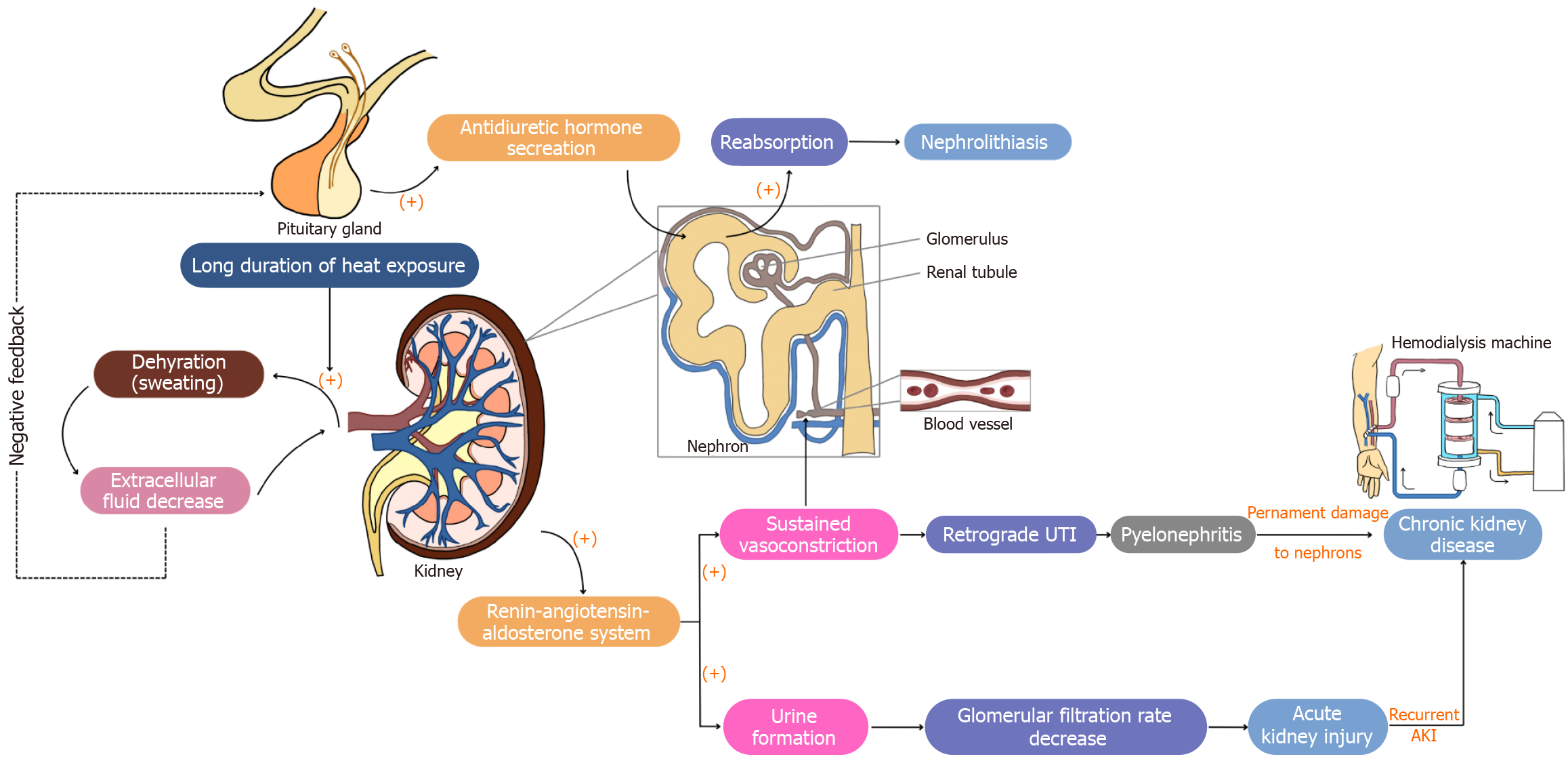
Figure 5 Non-inflammatory pathways of renal function in heat shock conditions.
Non-inflammatory pathways of heat-induced renal dysfunction are primarily driven by fluid loss and circulatory changes. Dehydration triggers the release of antidiuretic hormone, increasing water reabsorption and concentrating urine, which can lead to nephrolithiasis. The renin-angiotensin-aldosterone system is activated, causing vasoconstriction that can reduce renal blood flow and glomerular filtration rate, potentially leading to acute kidney injury. Reduced urine output increases the risk of urinary tract infections and pyelonephritis. Based on a summary of the findings from references. (+): Activate; (-): Inhibit.
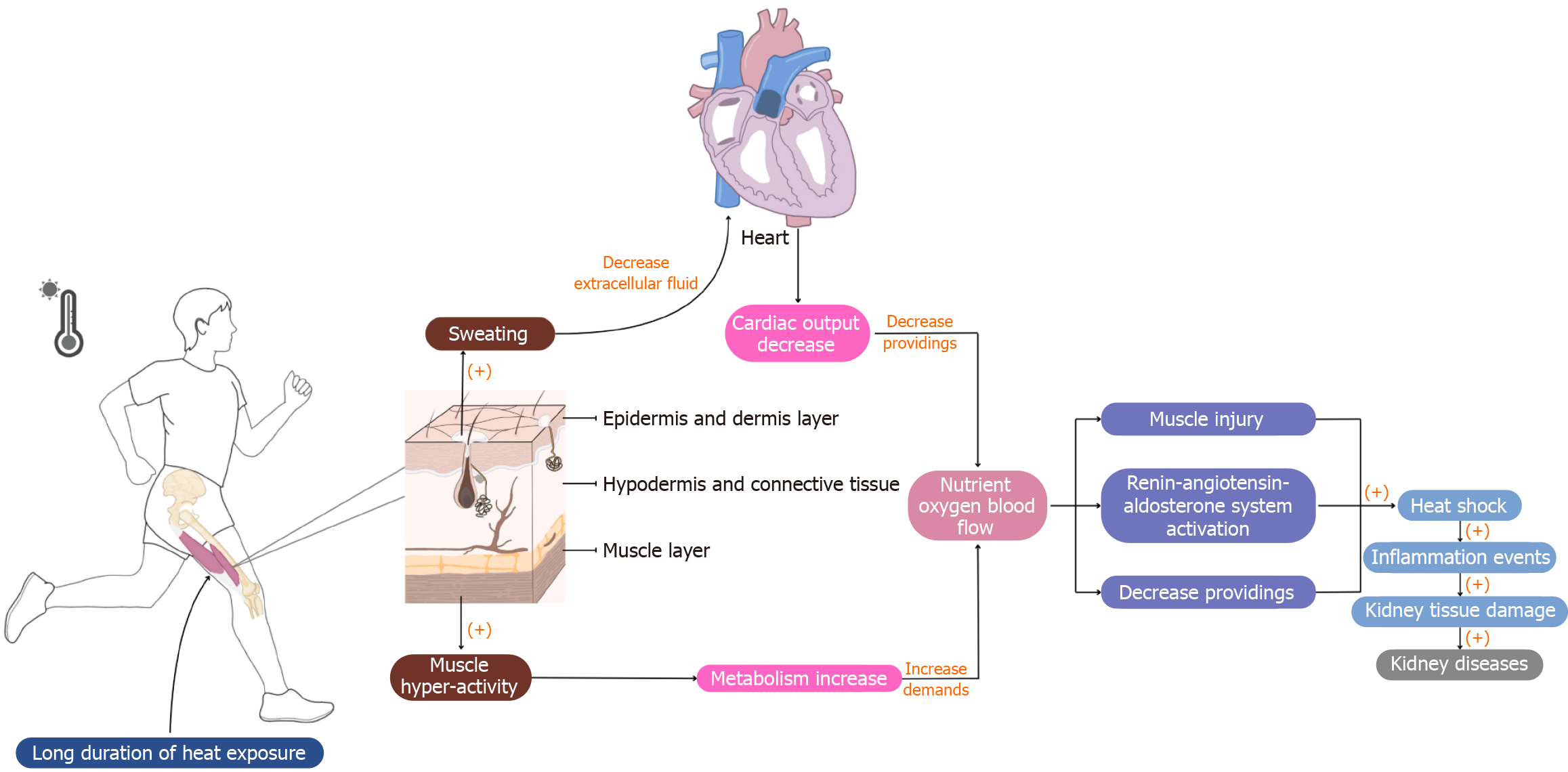
Figure 6 Inflammatory pathways of renal function in heat shock conditions.
Heat stress-induced muscle injury, hypoxia, and metabolic stress trigger a systemic inflammatory response, releasing pro-inflammatory cytokines that can directly impair renal function. Nonsteroidal anti-inflammatory drugs used for pain management can exacerbate renal dysfunction by inhibiting prostaglandin synthesis, which is crucial for maintaining renal blood flow. Heat shock proteins, though protective, may also act as damage-associated molecular patterns (DAMPs), amplifying inflammation. Based on a summary of the findings from references. (+): Activate; (-): Inhibit.
- Citation: Tran TTT, Tran KV, Nguyen TD, Pham NTT, Nguyen TH. Role of heat shock proteins in renal function and adaptation to heat stress: Implications for global warming. World J Nephrol 2025; 14(3): 107571
- URL: https://www.wjgnet.com/2220-6124/full/v14/i3/107571.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i3.107571