Published online Jan 16, 2019. doi: 10.5500/wjt.v9.i1.14
Peer-review started: November 5, 2018
First decision: December 6, 2018
Revised: December 11, 2018
Accepted: January 5, 2019
Article in press: January 6, 2019
Published online: January 16, 2019
Processing time: 74 Days and 6.4 Hours
Longstanding research describes the mechanisms whereby the restoration of blood flow and reoxygenation (reperfusion) aggravates the ischaemic injury caused by a period of anoxia to a donor liver. This phenomenon, called ischaemia-reperfusion injury (IRI), leads to parenchymal cell death, microcirculatory failure, and inflammatory immune response. Clinically, IRI is the main factor responsible for the occurrence of posttransplant graft dysfunction and ischaemic-type biliary lesions. While extended criteria donor livers are more vulnerable to IRI, their utilisation is required to address the shortfall in donor organs. Thus, the mitigation of IRI should drive the setting of a new benchmark for marginal organ preservation. Herein, strategies incorporating different modalities of machine perfusion of the liver to alleviate IRI are discussed in conjunction with advantages and disadvantages of individual protocols. Techniques leading to reperfusion of the liver during machine perfusion (in situ normothermic regional perfusion and ex situ normothermic machine perfusion) may mitigate IRI by shortening the ischaemic period of the organs. This benefit potentially escalates from the minimum level, obtained following just partial alleviation of the ischaemic period, to the maximum level, which can be potentially achieved with ischaemia-free organ transplantation. Techniques that do not lead to reperfusion of the liver during machine perfusion (hypothermic, subnormothermic, and controlled-oxygenated rewarming) optimise mitochondrial oxidative function and replenish cellular energy stores, thereby lowering reactive oxygen species production as well as the activation of downstream inflammatory pathways during reperfusion. Further mechanistic insights into IRI may guide the development of donor-specific protocols of machine perfusion on the basis of the limitations of individual categories of extended criteria donor organs.
Core tip: Hepatic ischaemia-reperfusion injury (IRI) is the main culprit of post-transplantation graft dysfunction and ischaemic-type biliary lesions. Despite the increased demand, extended-criteria donor livers are more vulnerable to IRI, thereby presenting inferior postoperative outcomes. Hence, the mitigation of IRI should drive the setting of a new benchmark for extended-criteria donor organ preservation. Machine perfusion of the liver has the potential to mitigate IRI via a shortening of the ischaemic period of the livers or the reconditioning of their bioenergetic status. Interventions to further alleviate IRI, such as pharmacological or nonpharmacological metabolic modulation of donor organs, may amplify this effect.
- Citation: Boteon YL, Afford SC. Machine perfusion of the liver: Which is the best technique to mitigate ischaemia-reperfusion injury? World J Transplantation 2019; 9(1): 14-20
- URL: https://www.wjgnet.com/2220-3230/full/v9/i1/14.htm
- DOI: https://dx.doi.org/10.5500/wjt.v9.i1.14
Ischaemia-reperfusion injury (IRI) is the phenomenon whereby the hypoxic damage imposed on an organ is aggravated during the reestablishment of the blood flow along with reoxygenation[1]. This biphasic detrimental process affects donor livers during liver transplantation (LT) and is the main responsible factor for the occurrence of graft dysfunction (primary nonfunction and delayed graft function) after the procedure[2,3]. Additionally, IRI is associated with the occurrence of ischaemic-type biliary lesions (ITBL) posttransplantation, which, in turn, leads to high rates of graft loss and retransplantation[4,5]. During ischaemia, the absence of oxygen interrupts the shuttling of electrons through the mitochondria electron transport chain (ETC), as oxygen is the terminal electron acceptor during cellular respiration. The affected ETC interrupts the transfer of protons (H+) across the inner mitochondrial membrane, thereby hampering the generation of the proton motive force required for oxidative phosphorylation and adenosine triphosphate (ATP) synthesis. The cellular ATP stores are then rapidly consumed and the process of anaerobic glycolysis is commenced in order to produce energy to the cells using the glycogen stores and the glucose available in the surrounding fluid. Activation of the former metabolic pathway results in lactate accumulation with local tissue acidosis as well as failure of the Na+/K+-ATPase pump with depolarisation of the cell membrane and influx of Ca2+/Na+ to the cytosol of the endothelial and Kupffer cells, leading to cell swelling. Additionally, the presence of vasoconstrictive substances such as endothelin and thromboxane-A2 not balanced by the vasodilatory nitric oxide (NO) can cause endothelial cell dysfunction with vasoconstriction and microcirculatory failure[2]. On reperfusion, when the blood flow is re-established, the damage caused by the ischaemic period is aggravated by the reoxygenation. This is initiated by the mitochondrial release of reactive oxygen species (ROS) due to an inhibited ETC causing the activation of Kupffer cells, which in turn will release proinflammatory cytokines, such as tumour necrosis factor-alpha/interleukin 1-beta, recruiting neutrophils and inducing the expression of adhesion molecules on sinusoidal endothelial cells. Activated neutrophils produce more ROS, perpetuating the inflammatory response that ultimately results in tissue damage and the initiation of cell death programs such as necrosis, apoptosis, or autophagy[2,3,6].
Donor organs with steatosis, organs that have been exposed to prolonged preservation times, organs from elderly donors, or organs from donation after circulatory death (DCD) are all more vulnerable to IRI and therefore are referred to as marginal or extended criteria donor (ECD) organs[7]. The defining parameters of ECD organs can vary slightly amongst centres[8], although, consistently, ECD-LT is associated with high rates of graft dysfunction and lower patient and graft survival posttransplantation[9-11]. Despite inferior outcomes, the utilisation of ECD livers is required to tackle the shortfall of donor organs for transplantation. Whilst transplant surgeons do not have control over these donor features, they can consider alternatives to better preserve or even recondition ECD livers. The wider utilisation of ECD livers has exceeded the preservation capacities of traditional static cold storage (SCS), and machine perfusion (MP) of the liver is considered to be a possible alternative preservation method. The use of this technique may offer several advantages in comparison with SCS, including superior organ preservation, limiting ischaemia; the assessment of organ function prior to transplantation; and the possibility of improving or repairing highly vulnerable organs[12]. Nevertheless, benefits may vary between different modalities of MP (Table 1); therefore, those protocols are frequently seen as divergent or even competitive at this time. Herein, the advantages and limitations of each individual technique in relation to the possibility of IRI mitigation are briefly discussed in an attempt to identify which is the best technique of MP of the liver.
| Advantage | Disadvantage | |
| Machine perfusion of the liver (All modalities) | Continuous circulation-improved preservation of the microcirculation; Nutrients and oxygen delivery for cellular metabolism; Removal of metabolic waste products; Delivery of cytoprotective agents and/or metabolic-modulating agents | Costly procedure; Requires specialised team |
| Techniques leading to reperfusion of the liver during machine perfusion (In situ normothermic regional perfusion; Ex situ normothermic machine perfusion) | Support organ full metabolism; Assessment of organ viability Assessment of hepatocellular injury; Potential to extend the period of organ storage; Possibility to shorten the ischaemic period of the livers | Persuade reperfusion on the machine; Risk of organ injury in case of organ failure or unrecognised problems with cannulation of the vessels; Require the use of an oxygen carrier in the perfusate |
| Techniques that do not lead to reperfusion of the liver during machine perfusion (Hypothermic oxygenated machine perfusion; dual-vessel hypothermic oxygenated perfusion; subnormothermic machine perfusion; controlled oxygenated rewarming) | Assessment of hepatocellular injury; Enhancement of mitochondrial function and replenishment of cellular energy stores; Lower rates of intra-hepatic biliary complications post-transplantation; Does not require oxygen carriers in the perfusate | Limited metabolic rate of the organs does not favour assessment of organ viability; Definition of the biomarkers to individualise perfusion times and assess responses to treatment in real-time is still pending |
| Ischaemia-free organ transplantation | Potential to abolish completely ischaemia-reperfusion injury | Limited application to donation after brainstem death thus far; Challenging procedure; Logistically challenging in a multivisceral retrieval setting; Just a single case reported |
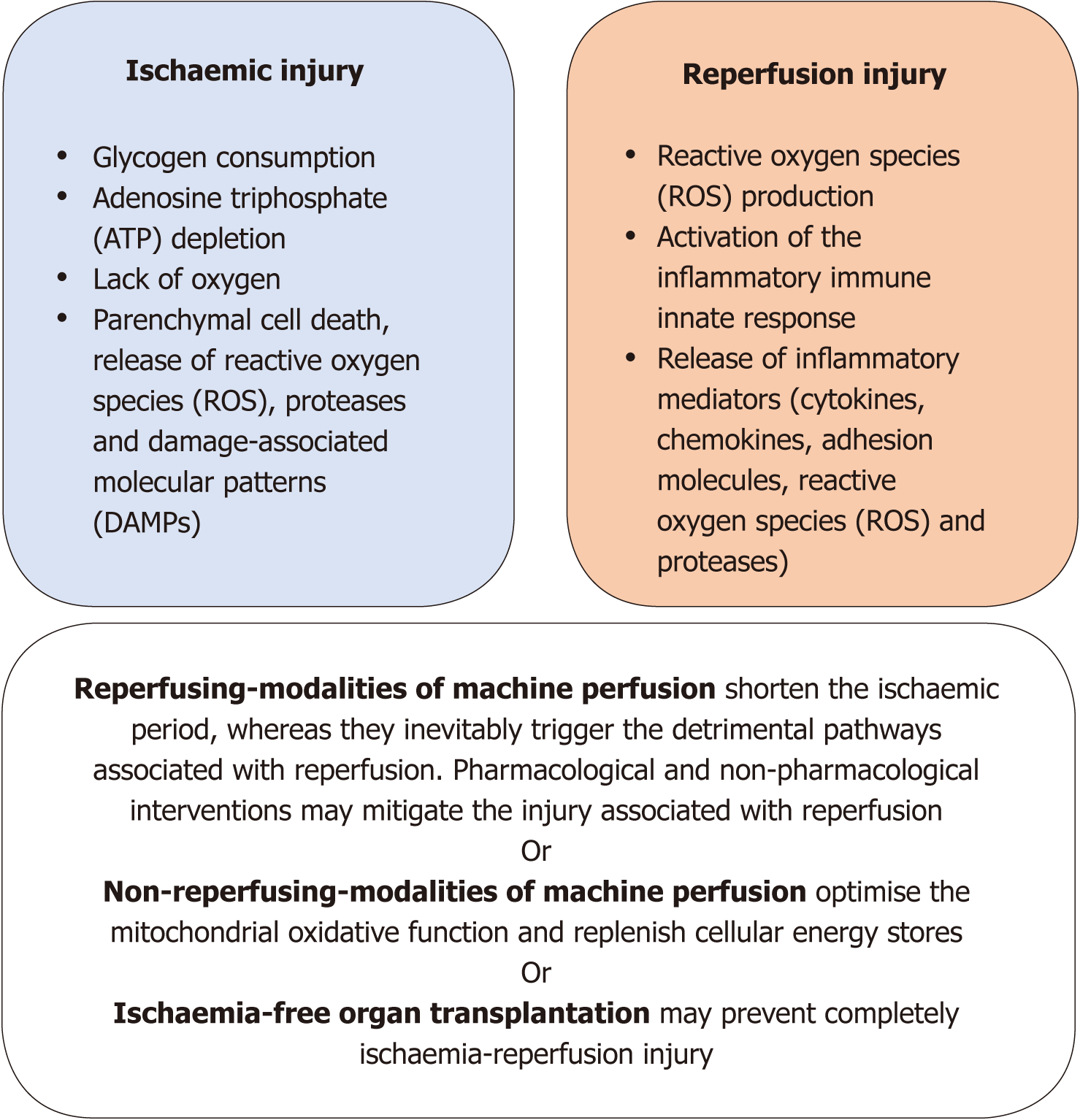
Considering its clinical significance, the mitigation of IRI should drive the setting of a new benchmark for ECD organ preservation. In accordance, the approach to this question might take into consideration how the different modalities of MP address IRI (Figure 1). For study purposes, these different modalities were categorised on the basis of either the occurrence of reperfusion of the liver during MP or not.
The common feature of this group of MP techniques is the abbreviation of the hypoxic period via reperfusion of the organ within physiological temperatures to support cellular metabolic function during preservation. This approach avoids further depletion of ATP stores and the accumulation of metabolic waste products, although experimental models have suggested that, even without the presence of leukocytes and platelets in the circuit, reperfusion during NMP induces oxidative tissue injury and the activation of the inflammatory immune response[13,14].
Ex situ normothermic MP (NMP) can be employed as a preservation method, fully replacing SCS; hence, it has the potential to limit the hypoxic injury to the minimum period required for organ preparation and the setting of the machine. Additionally, the presence of a constant flow of fluids in the vessels during organ preservation is advocated to improve the expression of vasoprotective endothelial genes alleviating the microcirculatory failure associated with IRI[15]. The benefits of this technique were recently shown in the largest clinical trial to date that compared this modality of NMP and SCS[16]. Nasralla et al[16] reported the results of transplantation of 121 donor livers following preservation NMP. The authors found a 50% decrease in the release of aspartate transaminase (AST) in the recipient within the first seven postoperative days in comparison with grafts that had SCS[16]. Nevertheless, the former study did not show superiority of NMP in terms of the occurrence of ITBL. This finding suggests that the limitation of hypoxic injury per se is not enough to prevent ITBL formation; thus, the etiopathogenesis of these lesions should rely also on the reperfusion injury, which is supported by an in vitro study[4]. The strongest evidence supporting the advantages of limiting IRI is the newly developed ischaemia-free organ transplantation (IFOT) technique[17], described by He et al[17], whereby complete elimination of hypoxia via continuous NMP was shown to prevent postreperfusion syndrome and vasoplegia after revascularisation of a severely steatotic donor liver. Moreover, NMP can also be performed after a period of SCS in an end-ischaemic approach. Whilst end-ischaemic NMP is logistically less challenging, it restrains the NMP’s ability to shorten the time of hypoxic injury to the organs. Finally, NMP may take advantage of the nearly physiological environment to assess the function of the organ prior to transplantation and to offer therapeutic approaches, such as cytoprotective and/or metabolic-modulating agents, for the treatment of IRI during NMP. This option is still underexplored thus far, although experiments involving pharmacological modulation of the lipid metabolism during NMP exemplify the benefits of this approach[18].
In situ normothermic regional perfusion (NRP) re-establishes the delivery of oxygen to the organs following asystole in DCD donors and, thus, limits the injury associated with a longer warm ischaemia period. Additionally, NRP may have a preconditioning effect, which could revert the detrimental mechanisms of warm injury[19,20]. While animal experiments involving dogs revealed that NRP is able to negate endothelial cell damage in livers harvested after 20 min of cardiac arrest[21], studies providing an in-depth analysis of the mechanistic effects of the procedure on the metabolism of human donor organs remain lacking.
This category encompasses the hypothermic and subnormothermic techniques of MP as well as controlled oxygenated rewarming. All of them share as a common feature the absence of organ reperfusion, as perfusate temperatures do not exceed 20 ºC. Within this category, the vast majority of mechanistic studies were performed so far on hypothermic oxygenated perfusion (HOPE) by the Zurich group. It has been proposed that the delivery of oxygen at hypothermic temperatures enhances the mitochondrial oxidative function, replenishing the cellular ATP stores prior to reperfusion[14]. This hypothesis is sustained by experimental studies that found a decrease in the expression of markers indicating oxidative tissue damage and the activation of Kupffer cells and leukocytes. In addition, these studies reported a lower release in the perfusate of markers of mitochondrial injury, damage-associated molecular patterns, and cytokines in livers after reperfusion following the HOPE procedure[13,14,22]. The Groningen group has been working on dual-vessel (hepatic artery and portal vein) hypothermic oxygenated perfusion (D-HOPE) and reported similar mechanistic findings to those obtained using HOPE[23,24].
Subnormothermic MP (SMP) is performed usually at around 20 ºC with active oxygenation of the perfusion fluid. Transplant animal models suggest that SMP can positively impact mitochondrial function, increase organs’ ATP stores, decrease the release of markers of tissue injury (e.g., transaminases and cytokines), and improve graft function postoperatively[25,26]. Defenders of this technique advocate that the increase in the organ’s metabolic rate that occurs as a result of the increase in temperature (from 10 ºC to 20 ºC) is sufficient for viability testing[25]. Minor et al[27] proposed a variant of the SMP technique called the controlled oxygenated rewarming (COR) method. In a reperfusion porcine model using ex situ NMP, as compared with hypothermic MP or SMP alone, COR was found to increase cellular ATP stores and decrease the release of lipid peroxides and markers of hepatocellular injury (AST and ALT) in the perfusate after reperfusion[27]. During NMP, organs that had undergone COR exhibited increased bile production, lower vascular resistance, and decreased expression of proinflammatory genes (e.g., intercellular adhesion molecule 1, toll-like receptor 4, and tumour necrosis factor alpha)[27].
Contemporary scientific evidence supports the concept that techniques of MP of the liver leading to organ reperfusion may mitigate IRI by shortening the ischaemic period. This benefit escalates from the minimum level, obtained following just partial alleviation of ischaemic injury during end-ischaemic NMP, to the maximum level, which can be potentially achieved with IFOT. However, these modalities of MP inevitably lead to ROS production, oxidative injury, and activation of the inflammatory immune response, with some degree of cell damage occurring during reperfusion[13]. Whilst this former detrimental phenomenon may not affect organs with enough metabolic reserve to overcome this injury, it can be a decisive factor when considering high-risk organs with limited metabolic reserve[28,29]. Consequently, most of the evidence accumulated thus far supports the advantages of NMP over SCS regarding organ preservation and viability assessment, although the resuscitative capacity of NMP per se is still unclear.
Mounting data suggest that techniques of MP of the liver that do not lead to organ reperfusion are able to mitigate IRI by way of optimisation of the mitochondrial oxidative function and replenishment of the cellular ATP stores during MP. The enhanced mitochondrial oxidative function decreases ROS production as well as the subsequent activation of downstream inflammatory pathways during reperfusion[30]. These mechanistic effects were shown to have a positive impact on the recovery of the metabolic function of discarded human donor livers submitted to NMP for viability assessment following the use of hypothermic oxygenated techniques of MP[29]. Conversely, the lower metabolic rate of the organs during hypothermic MP does not favour their functional assessment prior to transplantation. Arguably, strategies to evaluate mitochondrial metabolism and the energetic recovery of the organs, in real time, may warrant further promising studies be performed on this subject[22].
Despite the complex interaction between cells and signal molecules during IRI, future investigations determining the susceptibility of each individual cell population of the liver to the different periods of liver IRI (i.e., warm ischaemia, cold ischaemia, and reperfusion) might help with driving the allocation of donor organs to specific MP techniques. Thus far, existing evidence associates warm ischaemia mainly with Kupffer-cell-mediated hepatocellular injury, whereas cold ischaemia damages primarily sinusoidal endothelial cells[2,31]. Cholangiocytes have been reported to be less vulnerable to anoxia than hepatocytes; however, during reperfusion, they produce higher amounts of ROS, leading to cell death[4]. If exposure of the organ to an ischaemic period is unavoidable, the careful consideration of strategies to alleviate the local immune activation during reperfusion is desirable, such as employing preceding short periods of non-normothermic perfusions as a therapeutic approach or incorporating the delivery of pharmacological agents during NMP[29].
To conclude, whilst all techniques of MP of the liver have the potential to mitigate IRI, they offer different benefits and present diverse limitations. Therefore, there is no solid evidence yet to suggest the superiority of one technique over the others. A better mechanistic understanding of the intricate pathways of IRI may guide the development of personalised protocols of MP for groups of ECD organs, such as DCD livers, steatotic livers, or organs with prolonged cold ischaemia times.
This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We are extremely grateful to the Research Staff from the Centre for Liver and Gastrointestinal Research, whose continued support provides resources and intellectual input that is shaping our thoughts and future strategies for the continuing development of our research. We are also extremely grateful to all members of the Queen Elizabeth University Hospital Liver Transplant and Hepatobiliary Surgical Unit who are actively involved in the Birmingham machine perfusion projects, trials and organ procurement. YLB is funded by the Welcome Trust. We would like to thank the Liver Charities-University Hospitals Birmingham, Queen Elizabeth Hospital for their support to many projects involving machine perfusion.
| 1. | Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 458] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 505] [Article Influence: 38.8] [Reference Citation Analysis (9)] |
| 3. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 709] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 4. | Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2622] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 7. | Routh D, Naidu S, Sharma S, Ranjan P, Godara R. Changing pattern of donor selection criteria in deceased donor liver transplant: a review of literature. J Clin Exp Hepatol. 2013;3:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Silberhumer GR, Rahmel A, Karam V, Gonen M, Gyoeri G, Kern B, Adam R, Muehlbacher F, Rogiers X, Burroughs AK, Berlakovich GA. The difficulty in defining extended donor criteria for liver grafts: the Eurotransplant experience. Transpl Int. 2013;26:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 809] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1524] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 11. | Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, Baimakhanov Z, Piros L, Eguchi S. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. 2016;10:841-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Boteon YL, Afford SC, Mergental H. Pushing the Limits: Machine Preservation of the Liver as a Tool to Recondition High-Risk Grafts. Curr Transplant Rep. 2018;5:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Schlegel A, Kron P, Graf R, Dutkowski P, Clavien PA. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Selten J, Schlegel A, de Jonge J, Dutkowski P. Hypo- and normothermic perfusion of the liver: Which way to go? Best Pract Res Clin Gastroenterol. 2017;31:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Yuan X, Theruvath AJ, Ge X, Floerchinger B, Jurisch A, García-Cardeña G, Tullius SG. Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives. Transpl Int. 2010;23:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 907] [Article Influence: 113.4] [Reference Citation Analysis (2)] |
| 17. | He X, Guo Z, Zhao Q, Ju W, Wang D, Wu L, Yang L, Ji F, Tang Y, Zhang Z, Huang S, Wang L, Zhu Z, Liu K, Zhu Y, Gao Y, Xiong W, Han M, Liao B, Chen M, Ma Y, Zhu X, Huang W, Cai C, Guan X, Li XC, Huang J. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am J Transplant. 2018;18:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Boteon YL, Boteon APCS, Attard J, Mergental H, Mirza DF, Bhogal RH, Afford SC. Ex situ machine perfusion as a tool to recondition steatotic donor livers: Troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am J Transplant. 2018;18:2384-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Miñambres E, Suberviola B, Dominguez-Gil B, Rodrigo E, Ruiz-San Millan JC, Rodríguez-San Juan JC, Ballesteros MA. Improving the Outcomes of Organs Obtained From Controlled Donation After Circulatory Death Donors Using Abdominal Normothermic Regional Perfusion. Am J Transplant. 2017;17:2165-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 20. | Oniscu GC, Randle LV, Muiesan P, Butler AJ, Currie IS, Perera MT, Forsythe JL, Watson CJ. In situ normothermic regional perfusion for controlled donation after circulatory death--the United Kingdom experience. Am J Transplant. 2014;14:2846-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 21. | García-Valdecasas JC, Tabet J, Valero R, Taurá P, Rull R, García F, Montserrat E, González FX, Ordi J, Beltran J, López-Boado MA, Deulofeu R, Angás J, Cifuentes A, Visa J. Liver conditioning after cardiac arrest: the use of normothermic recirculation in an experimental animal model. Transpl Int. 1998;11:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Schlegel A, Muller X, Dutkowski P. Hypothermic Machine Preservation of the Liver: State of the Art. Curr Transplant Rep. 2018;5:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | van Rijn R, Karimian N, Matton APM, Burlage LC, Westerkamp AC, van den Berg AP, de Kleine RHJ, de Boer MT, Lisman T, Porte RJ. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Karangwa SA, Dutkowski P, Fontes P, Friend PJ, Guarrera JV, Markmann JF, Mergental H, Minor T, Quintini C, Selzner M, Uygun K, Watson CJ, Porte RJ. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am J Transplant. 2016;16:2932-2942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Berendsen TA, Bruinsma BG, Lee J, D'Andrea V, Liu Q, Izamis ML, Uygun K, Yarmush ML. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res. 2012;1:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, Soltys K, Shiva S, Paranjpe S, Sadowsky D, Barclay D, Zamora R, Stolz D, Demetris A, Michalopoulos G, Marsh JW. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15:381-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Minor T, Efferz P, Fox M, Wohlschlaeger J, Lüer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Watson CJE, Kosmoliaptsis V, Pley C, Randle L, Fear C, Crick K, Gimson AE, Allison M, Upponi S, Brais R, Jochmans I, Butler AJ. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 29. | Boteon YL, Laing RW, Schlegel A, Wallace L, Smith A, Attard J, Bhogal RH, Neil DAH, Hübscher S, Perera MTPR, Mirza DF, Afford SC, Mergental H. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018;24:1699-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Schlegel A, Kron P, Graf R, Clavien PA, Dutkowski P. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. 2014;260:931-937; discussion 937-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Jawad R, D'souza M, Selenius LA, Lundgren MW, Danielsson O, Nowak G, Björnstedt M, Isaksson B. Morphological alterations and redox changes associated with hepatic warm ischemia-reperfusion injury. World J Hepatol. 2017;9:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Chedid MF, Chello M, Cheungpasitporn W, Uhlmann D, Zhang R S- Editor: Cui LJ L- Editor: A E- Editor: Bian YN