Published online Aug 9, 2018. doi: 10.5500/wjt.v8.i4.84
Peer-review started: March 1, 2018
First decision: March 29, 2018
Revised: April 17, 2018
Accepted: May 30, 2018
Article in press: May 30, 2018
Published online: August 9, 2018
Processing time: 161 Days and 17.5 Hours
Data from World Health Organization estimates that the hepatitis C virus (HCV) prevalence is 3% and approximately 71 million persons are infected worldwide. HCV infection is particularly frequent among patients affected by renal diseases and among those in dialysis treatment. In addition to produce a higher rate of any cause of death, HCV in renal patients and in renal transplanted patients produce a deterioration of liver disease and is a recognized cause of transplant glomerulopathy, new onset diabetes mellitus and lymphoproliferative disorders. Treatment of HCV infection with interferon alpha and/or ribavirin had a poor efficacy. The treatment was toxic, expensive and with limited efficacy. In the post-transplant period was also cause of severe humoral rejection. In this review we have highlighted the new direct antiviral agents that have revolutionized the treatment of HCV both in the general population and in the renal patients. Patients on dialysis or with low glomerular filtration rate were particularly resistant to the old therapies, while the direct antiviral agents allowed achieving a sustained viral response in 90%-100% of patients with a short period of treatment. This fact to date allows HCV patients to enter the waiting list for transplantation easier than before. These new agents may be also used in renal transplant patients HCV-positive without relevant clinical risks and achieving a sustained viral response in almost all patients. New drug appears in the pipeline with increased profile of efficacy and safety. These drugs are now the object of several phases II, III clinical trials.
Core tip: The prevalence of hepatitis C virus (HCV) infection is high in patients with end-stage renal disease and HCV has clinical challenges in patients who undergo kidney transplantation. Historically, interferon-based treatment options have been limited by low rates of efficacy and significant side effects, including risk of precipitating rejection. Direct acting antiviral (DAA) drugs revolutionized the treatment of HCV. In this review we highlighted the most recent studies and clinical trial with DAA in renal patients including patients waiting for transplantation and already transplanted. In these studies all-oral DAA therapy appears to be safe and effective for such patients.
- Citation: Salvadori M, Tsalouchos A. Hepatitis C and renal transplantation in era of new antiviral agents. World J Transplant 2018; 8(4): 84-96
- URL: https://www.wjgnet.com/2220-3230/full/v8/i4/84.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i4.84
The World Health Organization (WHO) estimates that the global prevalence of hepatitis C virus (HCV) infection averages 3%, and the incidence is 3-4 millions of new infections every year[1]. HCV prevalence is not similar worldwide and ranges from less than 0.1% in Northern Europe to 1%-5% in other countries, such as Eastern Europe and the Indian subcontinent[2], to 25% in Egypt[2]. HCV infection is considered to be an endemic disease in some country as Taiwan[3].
HCV prevalence is increasing annually and the October 2017 report from the WHO revealed that 71 million of people are infected worldwide. However, some population-based studies[4-6] have demonstrated that prevalence estimates based on blood donors, underestimate the true HCV prevalence in the general population.
HCV prevalence increases in patients with kidney diseases. HCV may cause chronic kidney disease (CKD) via some forms of glomerulonephritis (GN), primarily membranoproliferative GN (MPGN), which may be caused by mixed cryoglobulinemia that represents HCV/anti-HCV immune complex associated with rheumatoid factor and complement[7]. Epidemiological studies in the United States (NHANES III) and Taiwan have recently demonstrated the relationship between HCV infection and CKD[8,9].
HCV infection is a frequent consequence of CKD in stages 4-5. Blood transfusions and nosocomial transmission in dialysis units contribute to the much higher prevalence of HCV infection in CKD stage 5 than in the general population. Epidemiological studies documented that HCV infection is associated with a higher risk and shorter time to CKD despite the lower prevalence of many CKD risk factors (ERCHIVES Study)[10]. Another study[11] confirmed that HCV-positive patients exhibit 40% higher odds for renal insufficiency compared with HCV-negative patients after adjustment for age, race, gender, diabetes and hypertension. One retrospective study[12] did not confirm these findings, but the authors recognized the limitation of their study. A relevant longitudinal study including of 23820 adults aged 30-65 years old was performed in Taiwan. The study included 18541 anti-HCV serum-negative patients and 1095 anti-HCV serum-positive patients. The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (REVEAL)-HCV study is a large prospective community based cohort study in Taiwan, and long term diseases provide an excellent opportunity to investigate the natural history of chronic hepatitis C and long-term diseases associated with this chronic infection[13]. Lee et al[3] documented an association of HCV status and any cause of death. Lai et al[14] assessed the risk of developing end-stage renal disease (ESRD) in relation to HCV serostatus, HCV RNA level and HCV genotypes.
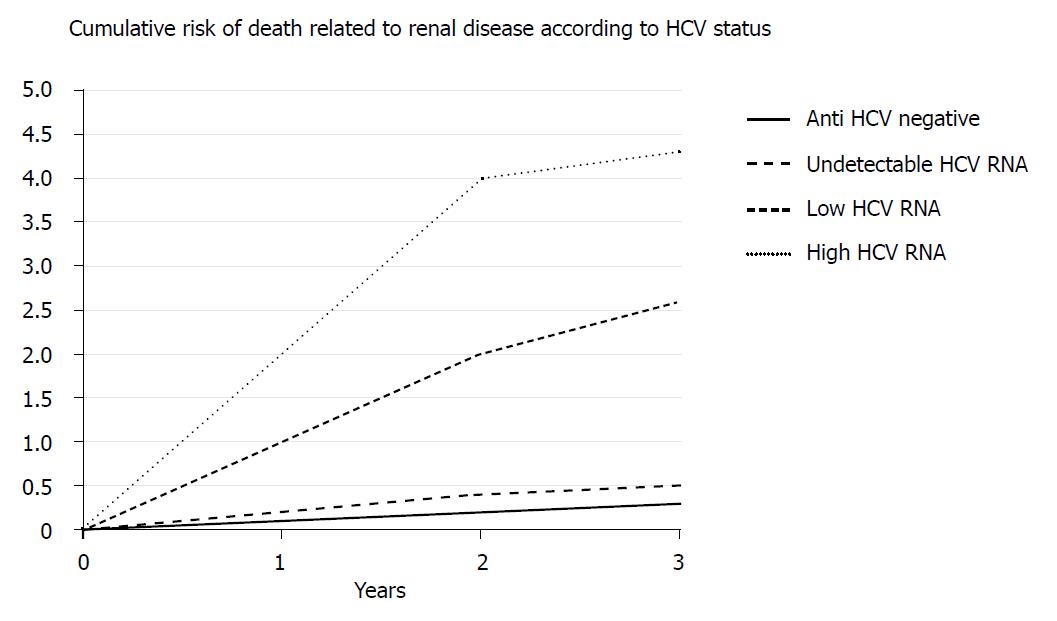
The Lai et al[14] study documented that chronic HCV infection is an independent risk factor for the development of ESRD. Participants with low and high HCV RNA levels exhibited a 2.6- and a 4.3-fold increased risk of developing ESRD, respectively, compared with participants who were not chronically HCV infected. Patients with HCV genotype 1 exhibit a higher risk of developing ESRD (Figure 1).
Survival of HCV-infected patients in ESRD is significantly lower in HCV-positive RNA-positive dialysis patients compared to HCV-positive RNA-positive kidney transplant recipients[15-17]. However, the persistence of HCV infection after renal transplantation is a true risk factor for graft and patient survival. The following complications primarily occur after renal transplantation in HCV-positive patients.
Immunosuppression facilitates HCV replication and accelerates liver disease to result in chronic hepatitis, fibrosing cholestatic hepatitis and rapidly progressive liver failure[18,19]. Therefore, preemptive treatment of HCV infection during dialysis is recommended.
HCV with associated cryoglobulinemia frequently causes MPGN even after renal transplantation[20,21]. Similarly, HCV may cause membranous nephropathy in renal transplant patients[20,22], and it may occur as a recurrent or de novo disease. A higher frequency of acute rejection was found in HCV-positive patients, but this association is controversial[23,24]. Acute, often humoral, rejection is frequent in the patients receiving interferon (IFN) therapy[25]. Treatment of patients prior to transplantation is necessary, especially when IFN therapy is used. An increased risk of transplant glomerulopathy, the glomerular phenotype of chronic rejection, is associated with HCV infection[26,27]. An increased risk of new onset diabetes mellitus is associated with HCV infection[28,29]. An increase in post-transplant lympho-proliferative disorders was described in HCV patients transplanted with different organs[30].
These findings clearly document the need to manage HCV. The need for treatment during the dialysis period prior to transplantation is also clear. The standard therapy until recently consisted of IFN ± ribavirin administration, but the results were poor with this treatment. IFN was toxic, expensive and exhibited limited efficacy in the pre-transplant period. IFN treatment in the post-transplant period was also dangerous because it caused acute humoral rejections. HCV treatment may be divided in two periods: (1) IFN-based therapies; and (2) Direct acting antiviral (DAA) therapies.
The first drug used for the treatment of HCV-positive patients with ESRD or transplantation was the recombinant alpha interferon (IFNα) eventually in combination with ribavirin, but the results in terms of sustained viral response (SVR) were poor.
Recombinant IFNα was first used as a monotherapy for chronic hepatitis C, but the drug only produced a modest SVR, several side effects were reported, and treatment was expensive and generated severe acute rejection when used after transplantation[25,31-35]. Fabrizi et al[36] performed a meta-analysis and concluded that the efficacy and safety of IFN-based therapies in renal transplant recipients were not satisfactory. The combination of IFNα with ribavirin increased the response rate, but induced the hemolysis as a new dose-dependent side effect[37]. This treatment was the standard of care until 1998. The introduction of pegylated IFNα increased the response rate by an additional 10%[38] and this treatment remained the standard of care until 2011.
The use of antiviral therapy was recommended for HCV patients in renal transplant candidates prior to transplantation because it was safer, effective and sustainable[1]. Several studies[39-44] confirmed these effects, including the Fabrizi et al[45] meta-analysis. One large randomized controlled trial recently demonstrated the greater efficacy and safety of combination antiviral therapy (pegylated IFN plus low-dose ribavirin, 200 mg daily) versus monotherapy (pegylated IFN alone) for HCV in a hemodialysis population[46]. The rates of sustained viral response were approximately 70%, and most dialysis patients tolerated the dual therapy well with appropriate patient monitoring.
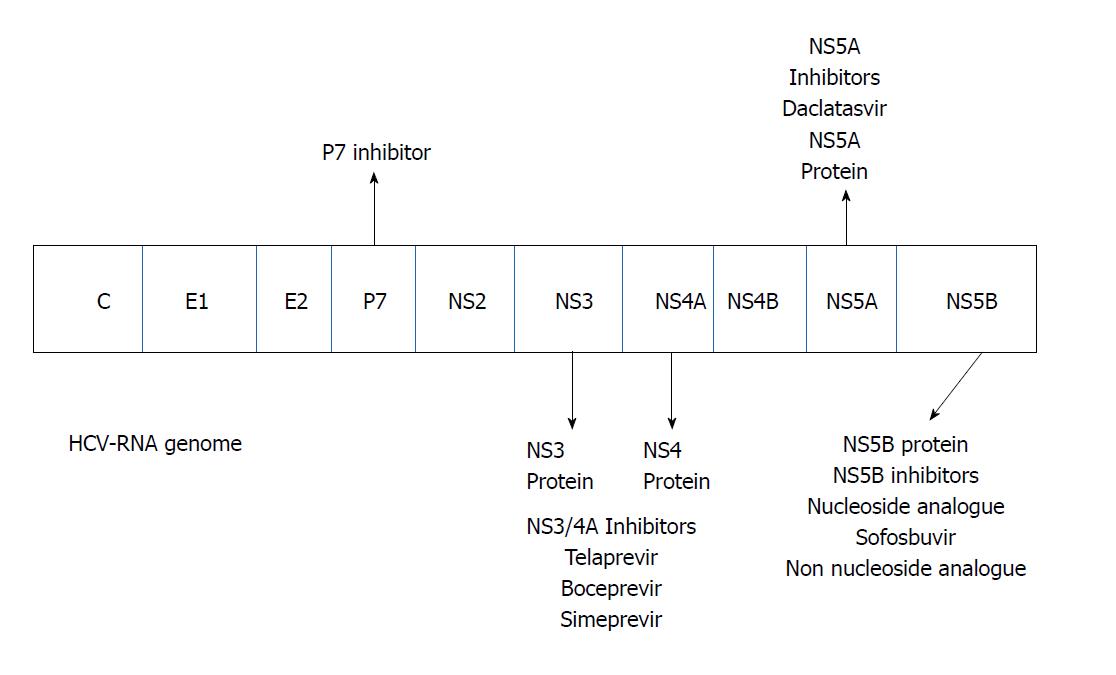
Accumulating evidence and knowledge of the mechanism of action of HCV and the viral proteins involved in its replication during the 2000s allowed for the development of specific drugs for direct antiviral treatment (Figure 2). To date the DAAs may be divided in four classes according the mechanism of action (Table 1)
| The four classes of DAAs | Mechanism of action | Drugs (targeted genotypes in brackets) |
| NS3/4A PIs (PIs) | Block a viral enzyme (protease) that enables the HCV to survive and replicate in host cells | Glecaprevir (1-6) Paritaprevir (1, 4) Voxilaprevir (1-6) Grazoprevir (1, 3, 4) |
| Nucleoside and nucleotide NS5B polymerase inhibitors | Target the HCV to stop it from making copies of itself in the liver. So doing block the virus from multiplying | Sofosbuvir (1-4) |
| NS5A inhibitors | Block a virus protein, NS5A, that HCV needs to reproduce and for various stages of infection | Ombitasvir (1, 4) Pibrentasvir (1-6) Daclatasvir (3) Elbasvir (1, 4) Ledipasvir (1) Ombitasvir (1) Velpatasvir (1-6) |
| Non-nucleoside NS5B polymerase inhibitors | Stop HCV from reproducing by inserting themselves into the virus so that other pieces of the HCV cannot attach to it | Dasabuvir (1) |
The first stage of this therapeutic revolution was the therapeutic introduction of protease inhibitors (PIs). The first generation of DAAs was represented by boceprevir and telaprevir, which inhibited NS3/4A protease activity. These drugs are inhibitors and substrates of the cytochrome (CYP) 3A4 isoenzyme in the liver and the intestinal P-glycoprotein (Pgp) transporter. However, these drugs may develop viral resistance. Therefore, these DAAs must be combined with pegylated IFN and ribavirin. No dose adjustment is necessary for patients with hepatic or renal impairment[47-50].
New drugs target the 3 non-structural proteins of the NS3 serine protease. These serine PIs include simeprevir, paritaprevir and asunaprevir. Simeprevir is an inhibitor of gut cytochrome 3A4 and organic anion-transporting peptide 1B1/3 (OATP1B1/3), and treatment may produce indirect hyperbilirubinemia. Paritaprevir acts on the same cytochromes as simeprevir. These agents are better tolerated than boceprevir and telaprevir, but the antiviral activity is primarily limited to the HCV genotype I. These drugs remain subject to viral resistance and are used in combination with other antiviral drugs. No dose adjustments are necessary in patients with renal impairment[51].
Another group of DAAs are inhibitors of NS5A, such as daclatasvir, ledispasvir and ombitasvir. These drugs inhibit the NS5A protein that controls phosphorilation/hyperphosphorilation and plays a vital role in HCV viral replication. These drugs also exhibit a low barrier of resistance and must be used in combination in combination with others antiviral[51]. No dose adjustments are necessary in patients with CKD.
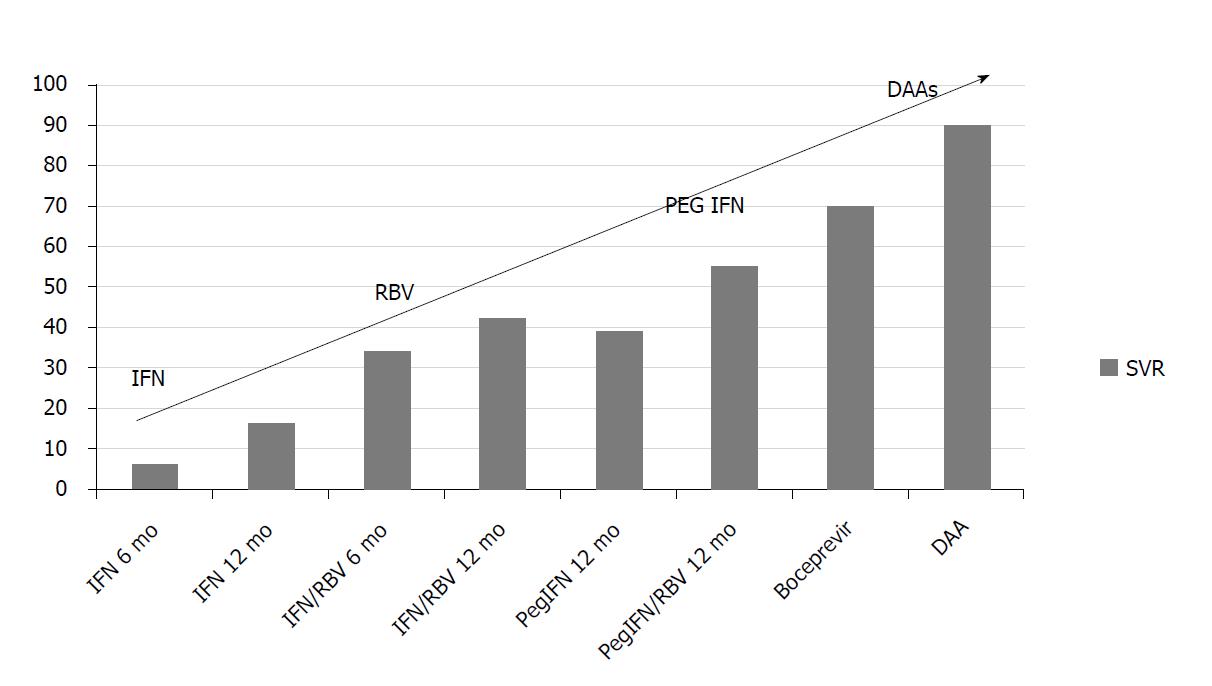
The newest DAAs include the NS5B inhibitors. These agents are divided into two classes: Nucleoside and non-nucleoside inhibitors. Non-nucleoside inhibitors are less potent, produce viral resistance and are less frequently used[51]. The most important nucleoside NS5B inhibitor is sofosbuvir, which was recently approved for use in combination with other DAAs. Sofosbuvir targets HCV RNA synthesis at the catalytic site of the NS5B enzyme. Incorporation into the new RNA by the polymerase leads to premature chain termination. Numerous IFN-free regimens are in phase 2 and phase 3 clinical trials and these combination regimens attained SVR in 90%-95% of patients[52-54]. Two publications summarize these drugs[55,56]. Figure 3 illustrates SVR improvement over time with different therapies for HCV genotype 1. These data refer to the general population.
The prevalence of HCV infection in dialysis patients and patients on waiting lists for renal transplantation is high, between 6% and 40% and varies geographically[57,58]. In the Dialysis Outcomes Practice Patterns Study (DOPPS) the seroprevalence of HCV infection varies from 20% to 50% according the length on dialysis[59].
Patients with kidney disease are difficult to treat because they present with a high rate of co-morbid conditions, such as hypertension, diabetes mellitus and cardiovascular disease. Co-morbidities facilitate several adverse effects. Few data exist on the pharmacokinetics of DAAs in patients with reduced glomerular filtration rate (GFR). Drug-drug interactions between DAAs and drugs used for lipid-lowering and cardiovascular disease were reported[60]. Table 2 lists the currently available approved DAA-based regimens for the treatment of HCV in patients with renal failure based on HCV genotype[61].
| Genotype 1a | Genotype 4 |
| Ledipasvir + sofosbuvir Paritaprevir + ritonavir + ombitasvir + dasabuvir Sofosbuvir+ simeprevir ± ribavirin | Ledipasvir + sofosbuvir Paritaprevir + ritonavir + ombitasvir + dasabuvir + ribavirin Sofosbuvir + ribavirin + pegIFN Sofosbuvir + simeprevir + ribavirin |
| Genotype 1b | Genotype 5 |
| Ledipasvir + sofosbuvir Paritaprevir + ritonavir + ombitasvir + dasabuvir Sofosbuvir + simeprevir | Sofosbuvir + ribavirin PegIFN + ribavirin |
| Genotype 2 | Genotype 6 |
| Sofosbuvir + ribavirin | Ledipasvir + sofosbuvir Sofosbuvir + ribavirin + pegIFN |
| Genotype 3 | Pangenotype |
| Sofosbuvir + ribavirin Sofosbuvir + ribavirin + pegIFN | Glecaprevir + pibrentasvir Sofosbuvir + velatapasvir |
The first-wave DAAs (e.g., boceprevir and telaprevir) exhibited poor efficacy and few patients were treated with these agents. SVR was less than 70% and combination with IFN and ribavirin was mandatory because of viral resistance. Pockros et al[62] demonstrated that the combination of ombitasvir, paritaprevir and ritonavir produced SVR in 90% of patients with genotype 1 and stage 4/5 CKD. The regimen was well tolerated, and only the addition of ribavirin produced anemia (Study RUBY I NCT02207088). A more recent study[63] treated 104 patients with CKD and HCV genotypes 1, 2, 3, 4, 5 or 6 with the combination of the NS3/4A protease inhibitor glecaprevir and the NS5A inhibitor pibrentasvir for 3 mo. SVR was obtained in 98% of patients with few adverse events primarily consisting of pruritus, fatigue and nausea (NCT 02651194).
The C-SURFER study (NCT02092350) is a phase 3 study of the administration of NS3/4A protease inhibitor granzoprevir (100 mg) and the NS5A inhibitor elbasvir (50 mg) to 111 patients for 12 wk. The control group received placebo. SVR was obtained in 94.3% of patients, and only 4% of patients reported adverse events, which consisted of headache, nausea and fatigue[64,65]. The recent approval of the first pangenotypic NS5B inhibitor, sofosbuvir, revolutionized the treatment of HCV infection.
Sofosbuvir is a uridine nucleotide analog that inhibits hepatitis C RNA-dependent RNA polymerase and it is effective in all hepatitis C genotypes. Phase II and phase III studies reported that genotype I patients who received sofosbuvir in combination with other DAAs achieved a sustained virological response rate greater than 90%. Different drug associations with sofosbuvir are suggested based on the HCV genotype[66]. Several studies demonstrated the efficacy and safety of these associations[67,68]. Some of these associations are principally useful in particular conditions. For example the association of sofosbuvir and velapatasvir revealed to be efficient in the case of HCV genotype 1, 2 and 3[69] and as rescue therapy in patients who developed viral resistance[53]. Many of these studies were performed in the context of the HCV-TARGET study.
HCV-TARGET is an observational longitudinal survey of patients affected by HCV different genotypes with different levels of renal function. The study is performed at academic and community medical centers in North America and Europe. The study evaluates the efficacy and safety of antiviral regimens, including sofosbuvir, in 1893 patients (NCT01474811).
Sofosbuvir use was restricted to patients with an eGFR > 30 mL/min, and a few studies investigated the use of sofosbuvir in patients with ESRD[70-73]. Recently, the combination of sofosbuvir plus simeprevir was administered to 17 patients with ESRD. The SVR was 100% after 12 wk treatment. Few patients reported minor or mild adverse events[70].
Rostaing et al[74] recently reviewed the treatment of HCV infection in kidney transplant candidates with poor renal function or on dialysis. Saxena et al[75] reported the efficacy of sofosbuvir in association with ribavirin in 73 patients with an eGFR < 45 mL/min, and SVR was achieved in 83% of patients. However, these patients exhibited higher rates of anemia and deterioration of renal function regardless of the use of ribavirin. Because of this fact and because of pharmacokinetic studies, sofosbuvir should be administered with extreme caution to patients with reduced GFR. Indeed, the use of sofosbuvir in patients with renal impairment causes an increase in serum levels of sofosbuvir and an increase of the AUC of 171%. Desnoyer et al[76] performed a pharmacokinetic study in hemodialysis patients receiving two different doses of sofosbuvir and demonstrated that sofosbuvir did not accumulate in either regimen. Beinhardt et al[77] treated 25 patients (10 on dialysis and 15 had received renal or combined liver-renal transplantation with sofosbuvir in association with other DAAs. SVR was obtained in 96% of patients after 12 and 24 wk of treatment, but the treatment response was slower in hemodialysis patients[77]. Alternative treatments for patients with ESRD were reported recently from Japan, where the combined use of daclatasvir plus asuneprevir in genotype I dialysis patients achieved a very high SVR rate[78-80].
We highlighted that the treatment of HCV renal transplant patients in the IFNα era was dangerous, poorly effective and frequently produced acute humoral rejection. Several recent studies demonstrated that the HCV infection eradication was feasible in renal transplant patients using DDAs, with few treatment-related side effects. However, these studies are recent, and the first guidelines for the use of DDAs in renal transplant patients were published at the end of 2017.
Colombo et al[81] performed a recent phase 2, open-label clinical trial to evaluate the safety and efficacy of the combination of ledipasvir and sofosbuvir in 5 European centers in 114 renal transplant patients infected with chronic genotype 1 or 4 HCV (NCT 02251717). The authors obtained SVR in 100% of patients after 12 wk of treatment. The eGFR remained stable, and adverse events were common (64%) and included headache, asthenia and fatigue. In one center the association of amiodarone and sofosbuvir probably caused a bradyarrhithmia and the patient interrupted the treatment[82]. The authors concluded that treatment with ledipasvir-sofosbuvir for 12 wk was well tolerated and achieved SVR in 12 wk with an acceptable safety profile. Sawinski et al[83] treated 20 renal transplant patients with HCV infection with a sofosbuvir-based therapy. SVR was obtained in all patients at 12 wk. Renal function remained stable, and no rejection occurred. However, 45% of patients required a dose reduction of the calcineurin inhibitor while receiving treatment. Saxena et al[84] reported the efficacy of DDAs therapy in 443 patients who received kidney (60) or liver transplant (347) or combined liver-kidney transplantation (36). The study was performed in the context of the vast HCV-TARGET study. Most patients had HCV genotype I. Patients were treated with sofosbuvir/ledipasvir ± ribavirin (85%), sofosbuvir plus daclatasvir ± ribavirin (9%) and ombitasvir/paritaprevir plus dasabuvir ± ribavirin (6%). SVR was achieved in 95.9% of patients after 12 wk of treatment. Six episodes of acute rejection occurred during HCV treatment. The authors concluded that different combinations of DAAs were effective and safe in kidney and/or liver transplant patients. Ribavirin did not influence SVR, and graft rejections were rare. Kamar et al[85] demonstrated the efficacy and safety of sofosbuvir-based antiviral therapy for HCV infection after renal transplantation in 25 patients. HCV RNA was not detectable in any patient 12 wk after completing DAA therapy. Treatment was well tolerated without graft rejections or reductions in renal function. Kamar did not observe any drug interaction with calcineurin inhibitors. These data differ from the findings of most studies. Hussein et al[86] reported the successful treatment of HCV genotype 4 in 3 renal transplant patients using the combination of sofosbuvir and ribavirin. Fernández et al[87] recently published data of the HepaC, which is a Spanish registry of 103 patients treated with DAAs after kidney transplantation. Most patients received a combination of sofosbuvir/ledipasvir or sofosbuvir/daclatasvir. The SVR at 12 wk was 98%. Three episodes of acute humoral rejection occurred, but there were no statistically significant differences in serum creatinine, eGFR or proteinuria before and after treatment. Most patients required immunosuppression dose adjustment, and 36% of patients, mostly cirrhotic, experienced renal dysfunction during antiviral treatment. The authors concluded that a close follow-up is required during treatment because of adjustments in immunosuppression therapy.
The phase 3, open-label, single-arm MAGELLAN-2 study evaluated a 12-wk course of the combination of the pangenotypic NS3/4A inhibitor glecaprevir and the pangenotypic NS5A inhibitor pibrentasvir in liver or renal transplant patients with chronic HCV genotype 1-6. Previous studies demonstrated that all these drugs exhibited a high barrier to resistance, sufficient potency against common NS3 and NS5A polymorphisms and synergistic antiviral activity. The study involved 80 liver transplant patients and 20 kidney transplant patients. The study demonstrated that the treatment with this combination for 12 weeks achieved a 99% SVR in patients with HCV genotypes 1-6. The treatment was well tolerated with few adverse events and confirmed the results obtained by Gane et al[63] in patients with ESRD. This new association represents an important alternative in treatment HCV patients after transplantation[88].
The American Association for the Study of Liver Diseases (AASLD) published the following HCV guidelines for kidney transplant patients in 2017[89] (Table 3): (1) The recommended drug association for the treatment of naïve and experienced kidney transplant patients with a genotype 1 or 4 infection: Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 wk. An alternative is a daily fixed-dose combination of ledipasvir (90 mg) and sofosbuvir (400 mg) for 12 wk; and (2) The recommended association for the treatment of naïve and experienced kidney transplant patients with HCV genotypes 2, 3, 5 and 6: Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 wk. An alternative is daily daclatasvir (60 mg) plus sofosbuvir (400 mg) and a low initial dose of ribavirin for 12 wk.
| Recommended | Duration | Rating |
| Recommended regimens listed by evidence level and alphabetically for treatment-naive and experienced kidney transplant patients with genotype 1 or 4 infection, with or without compensated cirrhosis | ||
| Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 wk | I, A1 |
| IIa, C2 | ||
| Daily fixed dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) | 12 wk | I, A |
| Recommended and alternative regimens for treatment-naïve and experienced kidney transplant patients with genotype 2, 3, 4, 5 or 6 infection, with or without compensated cirrhosis | ||
| Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 wk | I, A3 |
| IIa, C4 | ||
| Alternative | ||
| Daily daclatasvir (60 mg) plus sofosbuvir (400 mg) plus low initial dose of ribavirin (600 mg; increased as tolerated) | 12 wk | II, A |
The most important factor to be considered in the treatment of HCV-infected renal transplant patients with DAAs is the possible interactions between DAAs and immunosuppressants. Kwo et al[90] recently reviewed this issue and found the following results: Sofosbuvir may be administered to transplant patients without any expected interaction with calcineurin inhibitors. A recent report[91] demonstrated no interaction with mycophenolate mofetil, prednisone or azathioprine. The combination of sofosbuvir and ledipasvir did not reveal any significant interaction with calcineurine inhibitors. No data on possible interactions with sirolimus or everolimus are available. The NS34A protease inhibitor simeprevir did not interact with tacrolimus (TAC), but recent pharmacokinetic studies demonstrated a 5.81-fold increase in the simeprevir AUC levels when administered with cyclosporine (CsA). Therefore, simeprevir should not be administered with CsA. A pharmacokinetic analysis was performed in patients receiving the combination of paritaprevir, ombitasvir and dasabuvir with TAC[92]. There was a 57-fold increase in the TAC AUC, and modeling suggested 0.5 mg of TAC every 7 d with strict monitoring of the TAC levels. A 5.8-fold increase in the CsA AUC was similarly observed, and CsA should be reduced to 1/5. No interaction data are available for paritaprevir, ombitasvir and dasabuvir with sirolimus and everolimus, and the co administration is not recommended. The NS5A inhibitor daclatasvir does not affect the CsA or TAC levels and no dose adjustment is required. The combination of elbasvir and grazoprevir produced a 15-fold increase in the grazoprevir AUC when administered with CsA, and this association is not recommended[89]. The combination of glecaprevir and pibrentasvir with CsA produced a 5-fold increase in the glecaprevir AUC when high doses of CsA were used. This same drug combination with TAC produced a 1.45-fold increase in the TAC AUC, and careful monitoring of the TAC levels is required[89]. Fernández-Ruiz et al[93] recently examined eGFR and 24-h proteinuria in 49 renal transplant patients who received sofosbuvir and ledipasvir for 12 mo after treatment. The TAC levels were higher at 12 mo compared to the end of treatment, and the eGFR was significantly decreased. The authors suggested adjusting immunosuppressants when DAAs are administered. Drug monitoring should also be performed after the end of the HCV treatment as well as monitoring of the renal function.
In the pre DAAs era, because of organ shortage, several transplant centers transplanted kidneys from HCV positive donors into HCV positive recipients. The issue was controversial. One of the largest reports using such strategy is the study of Morales et al[94]. In this study 162 HCV positive recipients received a kidney from HCV positive donors and were compared with 306 HCV positive recipients who received kidney from HCV negative donors. The 5 and 10 year patients survival was similar as well as the 5 and 10 years graft survival. The outcomes of the liver disease were also similar in both groups and the Cox regression analysis could not identify the donor’s HCV serology as a significant risk factor. These data strongly suggest the use of kidneys from HCV positive donors in HCV positive recipients. Accordingly, the Kidney Disease Improving Global Outcomes (Kdigo)[1] recommended that transplantation of kidneys from HCV RNA positive donors should be directed to the HCV positive recipients. In United States, currently patients with untreated hepatitis C, who accept organ from HCV positive donors, may have a shorter time on transplant waiting list, while in other continents as Europe the positions differ according the different national programs. As afore mentioned direct-acting antiviral has revolutionized the treatment of hepatitis C infection also with implications for the use of HCV vermeil donors. Two recent papers reported the safety of transplanting kidneys from HCV positive donors to HCV positive recipients using DAAs[95,96]. The recommendation is to initiate early post-transplantation a pan-genotype therapy. A sustained SVR was near 100% and the DAA treatment after surgery was 125 d. Looking forward, the American Society of Transplantation (AST) held a consensus conference on the use of HCV viremic donors in solid organ transplantation[97].
The consensus conclusions established that: The term “HCV viremic donors” should be adopted; The provision of DAA to allow transplantation of HCV viremic donors into negative recipients is justified; The transplantation of organs from HCV viremic donors into HCV-negative recipients should be conducted only under monitored protocols and studies; There is a need for well-designed clinical trials of adequate power with conclusive findings to justify payer coverage of DAAs medications.
In this context the trial Exploring Renal Transplants Using Hepatitis C Infected Donors for HCV-negative Recipients (EXPANDER 1)[98] was started at the Johns Hopkins University. If the donor had genotype 1, the treatment included Grazoprevir and Elbasvir started immediately after transplantation and continued for 12 weeks. If the donor had genotype 1 with resistance variants, ribavirin was added. If the donor had genotype 2 or 3, sofosbuvir will be added. The data of this pilot study has been presented at the American Transplant Congress (ATC) 2017. Eight patients have been treated. After treatment no recipient had HCV-RNA detected and no graft failure was observed[99].
There has been a revolution in the treatment of chronic hepatitis C. Several oral regimens combining direct-acting antivirals (DAAs) from different families (NS5B nucleotide inhibitors, NS5B non-nucleoside inhibitors, NS5A replication complex inhibitors and NS3/4A PIs) have been developed. These regimens result in an increase in sustained virological response (SVR) rates to above 90% and reduce the duration of treatment to 12 wk or less. As of 2017 several regimens will be approved with additive potencies, without cross-resistance and with a good safety profile. Remaining issues will include increasing screening and access to care so that HCV may become the first chronic viral infection eradicated worldwide.
The efficacy and safety of these new DAAs are primarily important in the field of renal diseases of patients affected by ESRD and of patients in dialysis waiting for a renal transplant and in patients already transplanted, but with HCV infection. The problem of HCV infection was particularly relevant in uremic patients in the pre-DAAs era and HCV was difficult to be eradicated. The main studies in this field are cited in Table 4. Table 5 and Table 6 shows the recommendations for treating HCV in patients with renal impairment given from the American Association for the study of liver disease (AASLD)[90] and the European Association for the Study of the Liver (EASL)[60]. The access to transplantation to dialysis patients was allowed, but complications after transplantation were frequent and treatment was not possible after transplantation.
| Ref. | Title | Journal | Year |
| [62] | Efficacy of direct-acting antiviral combination for patients with HCV genotype 1 infection and severe renal impairment or end-stage renal disease | Gastroenterology | 2016 |
| [63] | Glecaprevir and Pibrentasvir in patients with HCV and severe renal impairment | N Engl J Med | 2017 |
| [64] | Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with HCV genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): A combination phase 3 study | Lancet | 2015 |
| [65] | Elbasvir plus grazoprevir in patients with HCV infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomized, double-blind, placebo-controlled trial | Lancet Gastroenterol Hepatol | 2017 |
| [70] | Use of sofosbuvir-based direct-acting antiviral therapy for HCV infection in patients with severe renal insufficiency | Infect Dis | 2015 |
| [71] | Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of hepatitis C in patients with end stage renal disease | J Hepatol | 2015 |
| [72] | Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR < 30 mL/min | Liver Int | 2016 |
| [74] | Use of direct-acting agents for HCV-positive kidney transplant candidates and kidney transplant recipients | Transpl Int | 2016 |
| [75] | Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function | Liver Int | 2016 |
| Recommended | Rating | Genotype | Duration |
| Recommendations for patients with CKD stage 1, 2 or 3 | |||
| No dose adjustment is required when using (1) Daclatasvir (60 mg) (2) Daily fixed-dose combination of elbasvir (50 mg)/grazopevir (100 mg) (3) Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) (4) Fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) (5) Fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) (6) Simeprevir (150 mg) (7) Fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) (8) Sofosbuvir (400 mg) | I, A | ||
| Recommendations for patients with CKD stage 4 or 5 (eGFR < 30 mL/min or ESRD | |||
| Daily fixed-dose combination of elbasvir (50 mg)/grazoprevir (100 mg) | I, B | 1a, 1b, 4 | 12 wk |
| Daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) | I, B | 1, 2, 3, 4, 5, 6 | 8 to 16 wk |
| Hemodialysis patients, particularly those who are suitable candidates for renal transplantation, should be considered for antiviral therapy (B1) |
| Hemodialysis patients should receive an IFN-free, if possible ribavirin-free regimen, for 12 wk in patients without cirrhosis, for 24 wk in patients with cirrhosis (B1) |
| Simeprevir, daclatasvir, and the combination of ritonavir-boosted paritaprevir, ombitasvir and dasabuvir are cleared by hepatic metabolism and can be used in patients with severe renal disease (A1) |
| Sofosbuvir should not be administered to patients with an eGFR < 30 mL/min per 1.73 m2 or with end-stage renal disease until more data is available (B2) |
DAAs are able to eradicate HCV in dialysis patients with a short course therapy obtaining a SVR close to 100%. Additionally, DAA-treatment is successful even after transplantation. Particular attention must be devolved to the interference between DAAs and calcineurin inhibitors. Either an increase of CsA or TAC AUC or an increase of DAA AUC is possible and monitoring is essential even after long time after transplantation
| 1. | 2008 KDIGO Introduction. Kidney Int. 2008;73:S6-S9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 658] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 3. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ; R. E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 4. | Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology. 1997;25:1490-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Guadagnino V, Stroffolini T, Rapicetta M, Costantino A, Kondili LA, Menniti-Ippolito F, Caroleo B, Costa C, Griffo G, Loiacono L. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology. 1997;26:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1934] [Cited by in RCA: 1901] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 7. | Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud-Ratti V, Yamamoto AM, Camproux AC, Hausfater P, Musset L. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 366] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Huang JF, Chuang WL, Dai CY, Ho CK, Hwang SJ, Chen SC, Lin ZY, Wang LY, Chang WY, Yu ML. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link? J Intern Med. 2006;260:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Tsui JI, Vittinghoff E, Shlipak MG, O’Hare AM. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Butt AA, Wang X, Fried LF. HCV infection and the incidence of CKD. Am J Kidney Dis. 2011;57:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Dalrymple LS, Koepsell T, Sampson J, Louie T, Dominitz JA, Young B, Kestenbaum B. Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol. 2007;2:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Moe SM, Pampalone AJ, Ofner S, Rosenman M, Teal E, Hui SL. Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis. 2008;51:885-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587-4593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Lai TS, Lee MH, Yang HI, You SL, Lu SN, Wang LY, Yuan Y, L’Italien G, Chien KL, Chen CJ; REVEAL-HCV Study Group. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology. 2017;66:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Knoll GA, Tankersley MR, Lee JY, Julian BA, Curtis JJ. The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis. 1997;29:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Pereira BJ, Natov SN, Bouthot BA, Murthy BV, Ruthazer R, Schmid CH, Levey AS. Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. The New England Organ Bank Hepatitis C Study Group. Kidney Int. 1998;53:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR. Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant. 2005;5:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Toth CM, Pascual M, Chung RT, Graeme-Cook F, Dienstag JL, Bhan AK, Cosimi AB. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation. 1998;66:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Muñoz De Bustillo E, Ibarrola C, Colina F, Castellano G, Fuertes A, Andrés A, Aguado JM, Rodicio JL, Morales JM. Fibrosing cholestatic hepatitis in hepatitis C virus-infected renal transplant recipients. J Am Soc Nephrol. 1998;9:1109-1113. [PubMed] |
| 20. | Cruzado JM, Carrera M, Torras J, Grinyó JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Hammoud H, Haem J, Laurent B, Alamartine E, Diab N, Defilippis JP, Berthoux P, Berthoux F. Glomerular disease during HCV infection in renal transplantation. Nephrol Dial Transplant. 1996;11 Suppl 4:54-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Morales JM, Pascual-Capdevila J, Campistol JM, Fernandez-Zatarain G, Muñoz MA, Andres A, Praga M, Martinez MA, Usera G, Fuertes A. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63:1634-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Vosnides GG. Hepatitis C in renal transplantation. Kidney Int. 1997;52:843-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Morales JM, Campistol JM, Andrés A, Rodicio JL. Hepatitis C virus and renal transplantation. Curr Opin Nephrol Hypertens. 1998;7:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Baid S, Tolkoff-Rubin N, Saidman S, Chung R, Williams WW, Auchincloss H, Colvin RB, Delmonico FL, Cosimi AB, Pascual M. Acute humoral rejection in hepatitis C-infected renal transplant recipients receiving antiviral therapy. Am J Transplant. 2003;3:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Baid-Agrawal S, Farris AB 3rd, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, Collins AB, Frei U, Colvin RB. Overlapping pathways to transplant glomerulopathy: chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int. 2011;80:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7:2124-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Morales JM, Aguado JM. Hepatitis C and renal transplantation. Curr Opin Organ Transplant. 2012;17:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Burra P, Buda A, Livi U, Rigotti P, Zanus G, Calabrese F, Caforio A, Menin C, Canova D, Farinati F. Occurrence of post-transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol. 2006;18:1065-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Ozgür O, Boyacioğlu S, Telatar H, Haberal M. Recombinant alpha-interferon in renal allograft recipients with chronic hepatitis C. Nephrol Dial Transplant. 1995;10:2104-2106. [PubMed] |
| 32. | Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation. 1995;59:1426-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 146] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Morales JM, Campistol JM. Transplantation in the patient with hepatitis C. J Am Soc Nephrol. 2000;11:1343-1353. [PubMed] |
| 34. | Heim MH. 25 years of interferon-based treatment of chronic hepatitis C: an epoch coming to an end. Nat Rev Immunol. 2013;13:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Wéclawiack H, Kamar N, Mehrenberger M, Guilbeau-Frugier C, Modesto A, Izopet J, Ribes D, Sallusto F, Rostaing L. Alpha-interferon therapy for chronic hepatitis C may induce acute allograft rejection in kidney transplant patients with failed allografts. Nephrol Dial Transplant. 2008;23:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Fabrizi F, Penatti A, Messa P, Martin P. Treatment of hepatitis C after kidney transplant: a pooled analysis of observational studies. J Med Virol. 2014;86:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Tang S, Cheng IK, Leung VK, Kuok UI, Tang AW, Wing Ho Y, Neng Lai K, Mao Chan T. Successful treatment of hepatitis C after kidney transplantation with combined interferon alpha-2b and ribavirin. J Hepatol. 2003;39:875-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Pageaux GP, Hilleret MN, Garrigues V, Bismuth M, Audin-Mamlouk H, Zarski JP, Mourad G. Pegylated interferon-alpha-based treatment for chronic hepatitis C in renal transplant recipients: an open pilot study. Transpl Int. 2009;22:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Kamar N, Toupance O, Buchler M, Sandres-Saune K, Izopet J, Durand D, Rostaing L. Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol. 2003;14:2092-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Rendina M, Schena A, Castellaneta NM, Losito F, Amoruso AC, Stallone G, Schena FP, Di Leo A, Francavilla A. The treatment of chronic hepatitis C with peginterferon alfa-2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Hakim W, Sheikh S, Inayat I, Caldwell C, Smith D, Lorber M, Friedman A, Jain D, Bia M, Formica R. HCV response in patients with end stage renal disease treated with combination pegylated interferon alpha-2a and ribavirin. J Clin Gastroenterol. 2009;43:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Deltenre P, Moreno C, Tran A, Ollivier I, Provôt F, Stanke F, Lazrek M, Castel H, Canva V, Louvet A. Anti-viral therapy in haemodialysed HCV patients: efficacy, tolerance and treatment strategy. Aliment Pharmacol Ther. 2011;34:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK, Chen SI. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med. 2013;159:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Tseng PL, Chen TC, Chien YS, Hung CH, Yen YH, Chang KC, Tsai MC, Lin MT, Lee CT, Shen CH. Efficacy and safety of pegylated interferon alfa-2b and ribavirin combination therapy versus pegylated interferon monotherapy in hemodialysis patients: a comparison of 2 sequentially treated cohorts. Am J Kidney Dis. 2013;62:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Fabrizi F, Dixit V, Messa P, Martin P. Antiviral therapy (pegylated interferon and ribavirin) of hepatitis C in dialysis patients: meta-analysis of clinical studies. J Viral Hepat. 2014;21:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Liu CH, Liu CJ, Huang CF, Lin JW, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK. Peginterferon alfa-2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 2 receiving haemodialysis: a randomised trial. Gut. 2015;64:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Dumortier J, Guillaud O, Gagnieu MC, Janbon B, Juillard L, Morelon E, Leroy V. Anti-viral triple therapy with telaprevir in haemodialysed HCV patients: is it feasible? J Clin Virol. 2013;56:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Lawitz EJ, Membreno FE. Response-guided therapy in patients with genotype 1 hepatitis C virus: current status and future prospects. J Gastroenterol Hepatol. 2014;29:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | de Kanter CT, den Hollander JG, Verweij-van Wissen CP, Burger DM. Telaprevir pharmacokinetics in a hepatitis C virus infected patient on haemodialysis. J Clin Virol. 2014;60:431-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Mehawej M, Rostaing L, Alric L, Del Bello A, Izopet J, Kamar N. Boceprevir-Based Triple Antiviral Therapy for Chronic Hepatitis C Virus Infection in Kidney-Transplant Candidates. J Transplant. 2015;2015:159795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 998] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 52. | Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 888] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 53. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 658] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 54. | Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 55. | Fabrizi F, Martin P, Messa P. New treatment for hepatitis C in chronic kidney disease, dialysis, and transplant. Kidney Int. 2016;89:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Fabrizi F, Messa P, Martin P. Update to hepatitis C review. Kidney Int. 2014;85:1238-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Saab S, Martin P, Brezina M, Gitnick G, Yee HF Jr. Serum alanine aminotransferase in hepatitis c screening of patients on hemodialysis. Am J Kidney Dis. 2001;37:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Schneeberger PM, Keur I, van Loon AM, Mortier D, de Coul KO, van Haperen AV, Sanna R, van Der Heijden TG, van Den Hoven H, van Hamersvelt HW. The prevalence and incidence of hepatitis C virus infections among dialysis patients in the Netherlands: a nationwide prospective study. J Infect Dis. 2000;182:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 60. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 911] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 61. | Sawinski D, Bloom RD. Novel Hepatitis C Treatment and the Impact on Kidney Transplantation. Transplantation. 2015;99:2458-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, Bernstein DE, Cohen DE, Shulman NS, Wang D. Efficacy of Direct-Acting Antiviral Combination for Patients With Hepatitis C Virus Genotype 1 Infection and Severe Renal Impairment or End-Stage Renal Disease. Gastroenterology. 2016;150:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 63. | Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017;377:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 64. | Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 534] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 65. | Bruchfeld A, Roth D, Martin P, Nelson DR, Pol S, Londoño MC, Monsour H Jr, Silva M, Hwang P, Arduino JM, Robertson M, Nguyen BY, Wahl J, Barr E, Greaves W. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2017;2:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 66. | Noell BC, Besur SV, deLemos AS. Changing the face of hepatitis C management - the design and development of sofosbuvir. Drug Des Devel Ther. 2015;9:2367-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, Lim JK, Darling J, Pockros P, Galati JS. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | German P, Mathias A, Brainard D, Kearney BP. Clinical Pharmacokinetics and Pharmacodynamics of Ledipasvir/Sofosbuvir, a Fixed-Dose Combination Tablet for the Treatment of Hepatitis C. Clin Pharmacokinet. 2016;55:1337-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, McNally J, Brainard DM, Han L, Doehle B. Sofosbuvir Plus Velpatasvir Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Hundemer GL, Sise ME, Wisocky J, Ufere N, Friedman LS, Corey KE, Chung RT. Use of sofosbuvir-based direct-acting antiviral therapy for hepatitis C viral infection in patients with severe renal insufficiency. Infect Dis (Lond). 2015;47:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Bhamidimarri KR, Czul F, Peyton A, Levy C, Hernandez M, Jeffers L, Roth D, Schiff E, O’Brien C, Martin P. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 72. | Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR < 30 ml/min. Liver Int. 2016;36:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 73. | Perumpail RB, Wong RJ, Ha LD, Pham EA, Wang U, Luong H, Kumari R, Daugherty TJ, Higgins JP, Younossi ZM. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Rostaing L, Alric L, Kamar N. Use of direct-acting agents for hepatitis C virus-positive kidney transplant candidates and kidney transplant recipients. Transpl Int. 2016;29:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, Liapakis A, Nelson DR, Fried MW, Terrault NA; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 76. | Desnoyer A, Pospai D, Lê MP, Gervais A, Heurgué-Berlot A, Laradi A, Harent S, Pinto A, Salmon D, Hillaire S. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 77. | Beinhardt S, Al Zoairy R, Ferenci P, Kozbial K, Freissmuth C, Stern R, Stättermayer AF, Stauber R, Strasser M, Zoller H. DAA-based antiviral treatment of patients with chronic hepatitis C in the pre- and postkidney transplantation setting. Transpl Int. 2016;29:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Miyazaki R, Miyagi K. Effect and Safety of Daclatasvir-Asunaprevir Combination Therapy for Chronic Hepatitis C Virus Genotype 1b -Infected Patients on Hemodialysis. Ther Apher Dial. 2016;20:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Sato K, Yamazaki Y, Ohyama T, Kobayashi T, Horiguchi N, Kakizaki S, Kusano M, Yamada M. Combination therapy with daclatasvir and asunaprevir for dialysis patients infected with hepatitis C virus. World J Clin Cases. 2016;4:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Suda G, Kudo M, Nagasaka A, Furuya K, Yamamoto Y, Kobayashi T, Shinada K, Tateyama M, Konno J, Tsukuda Y. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2016;51:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 81. | Colombo M, Aghemo A, Liu H, Zhang J, Dvory-Sobol H, Hyland R, Yun C, Massetto B, Brainard DM, McHutchison JG. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Ann Intern Med. 2017;166:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 82. | Fontaine H, Lazarus A, Pol S, Pecriaux C, Bagate F, Sultanik P, Boueyre E, Corouge M, Mallet V, Vallet-Pichard A. Bradyarrhythmias Associated with Sofosbuvir Treatment. N Engl J Med. 2015;373:1886-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Sawinski D, Kaur N, Ajeti A, Trofe-Clark J, Lim M, Bleicher M, Goral S, Forde KA, Bloom RD. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplant. 2016;16:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 84. | Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, Sulkowski MS, O’Leary JG, Koraishy F, Galati JS, Kuo AA, Vainorius M, Akushevich L, Nelson DR, Fried MW, Terrault N, Reddy KR. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 85. | Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, Esposito L, Del Bello A, Métivier S, Barange K. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection After Kidney Transplantation. Am J Transplant. 2016;16:1474-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 86. | Hussein NR, Saleem ZS. Successful Treatment of Hepatitis C Virus Genotype 4 in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplant. 2016;16:2237-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Fernández I, Muñoz-Gómez R, Pascasio JM, Baliellas C, Polanco N, Esforzado N, Arias A, Prieto M, Castells L, Cuervas-Mons V. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol. 2017;66:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 88. | Reau N, Kwo PY, Rhee S, Brown RS Jr, Agarwal K, Angus P, Gane ED, Kao JH, Mantry PS, Reddy KR, Tran TT, Hu JB, Gulati N, Krishnan P, Dumas EO, Shulman NS, Trinh R, Forns X. MAGELLAN-2: safety and efficacy of glecaprevir/pibrentasvir in liver or renal transplant adults with chronic hepatitis C genotype 1–6 infection. J Hepatol. 2017;66:Supplement 1: S90-S91. [DOI] [Full Text] |
| 89. | American Association for the Study of Liver Diseases. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Available from: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_September_21_2017_h.pdf. |
| 90. | Kwo PY, Badshah MB. New hepatitis C virus therapies: drug classes and metabolism, drug interactions relevant in the transplant settings, drug options in decompensated cirrhosis, and drug options in end-stage renal disease. Curr Opin Organ Transplant. 2015;20:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Charlton M, Gane E, Manns MP, Brown RS Jr, Curry MP, Kwo PY, Fontana RJ, Gilroy R, Teperman L, Muir AJ, McHutchison JG, Symonds WT, Brainard D, Kirby B, Dvory-Sobol H, Denning J, Arterburn S, Samuel D, Forns X, Terrault NA. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 92. | Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R Jr, Gordon F, Levitsky J, Terrault NA, Burton JR Jr, Xie W, Setze C, Badri P, Pilot-Matias T, Vilchez RA, Forns X. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 93. | Fernández-Ruiz M, Polanco N, García-Santiago A, Muñoz R, Hernández AM, González E, Mercado VR, Fernández I, Aguado JM, Praga M. Impact of anti-HCV direct antiviral agents on graft function and immunosuppressive drug levels in kidney transplant recipients: a call to attention in the mid-term follow-up in a single-center cohort study. Transpl Int. 2018;31:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Morales JM, Campistol JM, Domínguez-Gil B, Andrés A, Esforzado N, Oppenheimer F, Castellano G, Fuertes A, Bruguera M, Praga M. Long-term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients. Am J Transplant. 2010;10:2453-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 95. | Sawinski D, Wyatt CM, Locke JE. Expanding the use of hepatitis C-viremic kidney donors. Kidney Int. 2017;92:1031-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Bhamidimarri KR, Ladino M, Pedraza F, Guerra G, Mattiazzi A, Chen L, Ciancio G, Kupin W, Martin P, Burke G. Transplantation of kidneys from hepatitis C-positive donors into hepatitis C virus-infected recipients followed by early initiation of direct acting antiviral therapy: a single-center retrospective study. Transpl Int. 2017;30:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 97. | Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J, Friedman J, Goldberg D, Hall S, Ison M. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17:2790-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 98. | Johns Hopkins University. Exploring Renal Transplants Using Hepatitis C Infected Donors for HCV-negative Recipients (EXPANDER-1). Available from: https://clinicaltrials.gov/ct2/show/NCT02781649. |
| 99. | Durand C, Brown D, Wesson R, Bhair N, Naqvi F, Ostrander D, Bowring M, Massie A, Rasmussen S, Sugarman J. EXPANDER-1: Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients. Am J Transplant. 2017;17:Abstract number 2. |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cholongitas E, Cheungpasitporn W, Larrubia JR, Zhu X S- Editor: Ji FF L- Editor: A E- Editor: Tan WW