Published online Feb 24, 2018. doi: 10.5500/wjt.v8.i1.23
Peer-review started: November 7, 2017
First decision: November 20, 2017
Revised: January 13, 2018
Accepted: February 4, 2018
Article in press: February 5, 2018
Published online: February 24, 2018
Processing time: 109 Days and 22 Hours
To validate intracellular cytokine production functional assay as means of cell-mediated immunity monitoring of post-transplant patients with opportunistic infection (OI).
Intracellular cytokine-producing CD4+ and CD8+ T-cell monitoring was carried out in 30 liver transplant (LTr) and 31 kidney transplant (KTr) recipients from 2010 to 2012. Patients were assessed in our Department of Immunology at the Clinical University ‘Hospital Virgen de la Arrixaca-IMIB’ in Murcia, Spain for one year following transplantation. FACS Canto II flow cytometer was employed to quantify the intracellular production of IL-17, IFNγ and IL-10 cytokines on stimulated CD4+CD69+ and CD8+CD69+ T cells and BD FACS DIVA v.6 software was used to analysed the data. Statistical analysis was carried out using SPSS 22.0.
LTr with OI had significantly lower % of CD8+CD69+IFNγ+ T cells at 60 (7.95 ± 0.77 vs 26.25 ± 2.09, P < 0.001), 90 (7.47 ± 1.05 vs 30.34 ± 3.52, P < 0.001) and 180 (15.31 ± 3.24 vs 24.59 ± 3.28, P = 0.01) d post-transplantation. Higher % of CD4+CD69+IL-10+ as well as CD4+CD69+IL-17+ T cells were yet reported at 30 (14.06 ± 1.65 vs 6.09 ± 0.53, P = 0.0007 and 4.23 ± 0.56 vs 0.81 ± 0.14, P = 0.005; respectively), 60 (11.46 ± 1.42 vs 4.54 ± 0.91, P = 0.001 and 4.21 ± 0.59 vs 1.43 ± 0.42, P = 0.03; respectively) and 90 d (16.85 ± 1.60 vs 4.07 ± 0.63, P < 0.001 and 3.97 ± 0.43 vs 0.96 ± 0.17, P = 0.001). Yet, KTr with OI had significantly lower percentage of CD4+CD69+IFNγ+ at 30 (11.80 ± 1.59 vs 20.64 ± 3.26, P = 0.035), 60 (11.19 ± 1.35 vs 15.85 ± 1.58, P = 0.02), 90 (11.37 ± 1.42 vs 22.99 ± 4.12, P = 0.028) and 180 (13.63 ± 2.21 vs 21.93 ± 3.88, P = 0.008) d post-transplantation as opposed to CD4+CD69+IL-10+ and CD8+CD69+IL-10+ T cells which percentages were higher at 30 (25.21 ± 2.74 vs 8.54 ± 1.64, P < 0.001 and 22.37 ± 1.35 vs 17.18 ± 3.54, P = 0.032; respectively), 90 (16.85 ± 1.60 vs 4.07 ± 0.63, P < 0.001 and 23.06 ± 2.89 vs 10.19 ± 1.98, P = 0.002) and 180 (21.81 ± 1.72 vs 6.07 ± 0.98, P < 0.001 and 19.68 ± 2.27 vs 10.59 ± 3.17, P = 0.016) d post-transplantation. The auROC curve model determined the most accurate cut-off values to stratify LTr and KTr at high risk of OI and Cox Regression model confirmed these biomarkers as the most significant risk factors to opportunistic infection.
Post-transplant percentages of T-cell subsets differed significantly amongst infected- and non-infected-LTr and -KTr and yet this imbalance was found to contribute towards a worst clinical outcome.
Core tip: The aim of this research was to validate predictive biomarkers for the occurrence of post-transplant opportunistic infection in both liver and kidney recipients. The imbalance in the percentage of cytokine-producing cultured CD4+CD69+ and CD8+CD69+ T cells was shown to be the most significant recipient risk factor to develop opportunistic infection.
- Citation: Boix F, Llorente S, Eguía J, Gonzalez-Martinez G, Alfaro R, Galián JA, Campillo JA, Moya-Quiles MR, Minguela A, Pons JA, Muro M. In vitro intracellular IFNγ, IL-17 and IL-10 producing T cells correlates with the occurrence of post-transplant opportunistic infection in liver and kidney recipients. World J Transplant 2018; 8(1): 23-37
- URL: https://www.wjgnet.com/2220-3230/full/v8/i1/23.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i1.23
Despite the continuous improvement in the clinical management of solid organ transplant recipients (SOTr), opportunistic infection (OI) remains one of the leading causes of morbidity and mortality in this population[1]. Although current immunosuppressive regimens aim to prevent allograft acute rejection (AR)[2], clinicians still rely exclusively on therapeutic drug monitoring (TDM) of immunosuppression therapy (pharmacokinetics) to determine the immunological status of SOTr[3]. Indeed, the risk of an inadequate immunosuppression due to chronic exposure has been claimed to be one of the main reason of poor long-term outcomes[4,5]; hence there must be a balance to prevent not only AR but also reducing immunosuppression-related comorbidities, such as OI. Tailoring immunosuppressive regimens could potentially reduce the risk of life-threatening conditions, amongst other side effects, resulting in an improvement in the wellbeing of SOTr. Despite the aforementioned, TDM appears to be insufficient (intra- and inter-individual pharmacokinetic variability) in the provision of fulfilling information as to the real immunosuppressive status of SOTr[6].
In recent years new strategies, such as monitoring of cell-mediated immunity (CMI), have been seen to provide more accurate information with respect of the management of post-transplant SOTr. As such, CMI has been proposed as an alternative and reliable strategy in the search for predictive biomarkers of AR[7-10] and OI[11-14] amongst other clinical conditions.
The knowledge of T lymphocytes in host defense against infection has improved significantly over time. There is clear evidence that, upon pathogen derived-antigen contact, naïve T CD4+ (TH0) cells activate and differentiate into different functional subsets characterised by their cytokine secretion patterns (TH1, TH2, TH9, TH17, Tregs)[15]. Furthermore, when stimulated by microbial products through pattern recognition receptors (PRRs), antigen presenting cells (APCs) acquire the capacity to activate naïve T cells and differentiate into effector T cells that mediate adaptive immune responses. APCs stimulated with pathogens such as Bordetella pertussis, Klebsiella pneumoniae, and Mycobacterium tuberculosis produce a significant amount of IL-23, resulting in the development of TH17 cells, showing that this subset acts against both extracellular and intracellular infections[16,17]. Evidence has shown that TH17 cells are also required for host defense against fungal infection[18]. The classically established TH1/TH2 paradigm yet describes the role of these two T lymphocyte subsets in host defense against infections. TH1 cells are essential in the elimination of intracellular pathogens such as Leishmania and Mycobacteria[19], whereas TH2 secreted IL-10 cytokine cells has emerged as a key immunoregulator during infection with viruses, bacteria, fungi, protozoa, and helminths[20].
We therefore hypothesised, that CMI could be used to tackle T cell differentiation as a therapeutic target, providing thorough understanding of the adaptive immune response against pathogens after SOT. Hence, the aim of this uni-centre study was to prospectively monitor T helper lymphocyte cytokine responses against overall OI in a cohort of liver and kidney transplant recipients. As such, CMI could aid clinicians in the provision of better prophylaxis therapies, potentially reducing the occurrence of post-transplant OI.
From 2010 to 2012, 61 consecutive adult patients; of whom 30 patients diagnosed with end-stage liver disease underwent LT and 31 patients diagnosed with end-stage renal failure underwent KT, alongside 16 healthy control (HC) volunteers were recruited from the Immunology Service of the Clinical University Hospital ‘Virgen de la Arrixaca’, Murcia (Spain) for a prospective uni-centre study. Peripheral venous blood samples were obtained from individual participants for laboratory testing at baseline as well as at several different post-transplantation time points (7 d, 15 d, 1st month, 2nd month, 3rd month, 6th month and 1st year). Formal consent was obtained from both patients and healthy controls, with approval of the study protocol obtained by the institutional ethical committee. Pediatric, re-transplant and combined transplant patients were excluded. The inclusion criteria included primary liver and kidney transplantation, ABO compatibility and HIV negativity. The primary study outcome was the occurrence of overall OI, which took into consideration the following etiologies: Bacterial, fungal and viral post-transplant infection (including CMV disease, either viral syndrome or end-organ disease). The post-transplant follow-up period of 1 year was divided into three different intervals: early post-transplant period (up to the 1st month), intermediate (from the 1st to the 6th month) and long-term (from the 6th month to the 1st year). All post-transplant recipients were assessed on a regular basis by the consultant specialist in their respective outpatient transplant clinics, with a sample (urine or blood) taken for microbiological and biochemistry assessment. Based on laboratory findings, LTr and KTr were classified into two different study groups, with [INF; 60% of LTr (n = 18) and 61.3% of KTr (n = 19)] and without [NoINF; 40% of LTr (n = 12) and 38.7% of KTr (n = 12)] post-transplant OI.
Cefuroxime (1500 mg/iv per 8 h) was administered to all methicillin-resistant Staphylococcus negative recipients, whereas Teicoplanin (200 mg/iv per 12 h) was given to patients positive for methicillin-resistant Staphylococcus. Oral Nystatin (5 cc/8 h) was also provided as Candida sp prophylaxis. Trimethoprim-sulfamethoxazole (160/800 mg/iv per 24 h) was given, over six months, as Pneumocystis jiroveci pneumonia (PJP) prophylaxis. Oral Itraconazole (200 mg/24 h) was also given over three months to prevent Aspergillus sp. infection. Oral Pyrimethamine (25 mg/24 h) + folic acid was given as prophylaxis against Toxoplasma sp, with treatment extended up to six months in cases where serology was positive. In patients CMV seropositive, Ganciclovir (5 mg/kg per 12 h) or Valganciclovir (900 mg/kg per 12 h) were given as induction prophylaxis treatment. CMV prophylaxis induction with iv-Ganciclovir or oral-Valganciclovir for 2 wk followed up by oral-Valganciclovir for 3 mo. In those cases of a CMV seronegative recipient and CMV seropositive donor, the induction treatment was extended for 4 wk and maintained up to 6 mo. Post-transplantation CMV infections were treated with iv-Ganciclovir for 2 or 3 wk in both types of transplant, and oral-Valganciclovir was maintained for 3 mo. Finally, BK viral infection was treated by the administration of oral leflunomide (100 mg/24 h) over five days.
Initial immunosuppressive therapy consisted of oral Tacrolimus (TRL) 1 mg (6 mg/24 h) or oral Mycophenolic acid (MMF) 500 mg (1 g/24 h for KTr or 1.5 g/24 h for LTr) with Prednisone 20 mg/d with progressing tapering. The average drug level achieved for TRL was 2.6-17.3 ng/mL. The average drug level achieved for MMF was 0.40-4.15 μg/mL. The initial dose was modified in case of adverse side effects, such as diarrhea or leucopenia. In case of AR, the rescue therapy provided was based on the administration of steroid boluses (500–1000 mg methylprednisolone/24 h) for 3 d. In case of chronic rejection (CR), the rescue therapy provided was based on the administration of oral TRL (FK506; 0.1 mg/kg per 24 h).
Induction therapy was based on the administration of either thymoglobulin (1-1.5 mg iv/kg; Genzyme Polyclonals S.A.S) or basiliximab (anti-CD25, 0.5-2 mg iv/kg; Simultec®, Novartis Farma), with 3 (10%) LTr and 23 (74.2%) KTr receiving basiliximab and 4 (12.9%) KTr receiving thymoglobulin.
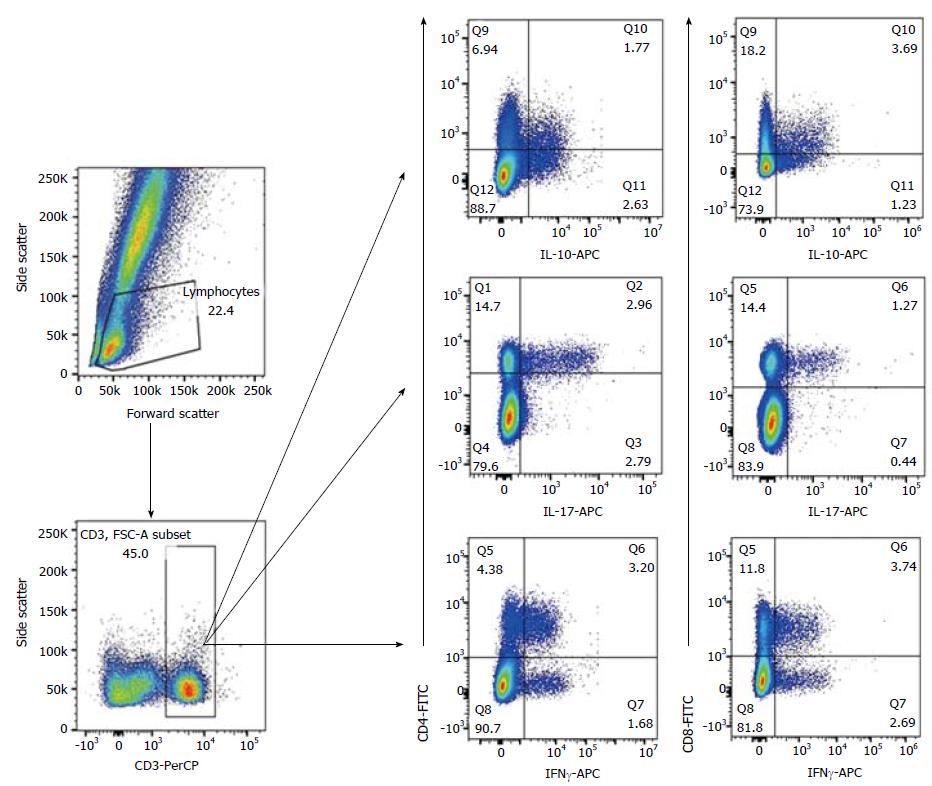
The percentage of CD4+CD69+IFNγ+, CD8+CD69+IFNγ+, CD4+CD69+IL-17+, CD4+CD69+IL-10+ and CD8+CD69+IL-10+ in individual samples was determined by flow cytometry following intracellular staining with anti-cytokine monoclonal antibodies. Upon venipuncture, whole peripheral blood was incubated with Ionomycin (Io) and Phorbol Myristate Acetate (PMA) for 4 h. Following activation, whole peripheral blood was stained with FITC-conjugated anti-human CD8, PE-conjugated anti-human CD69, and PerCP-conjugated anti-human CD3 for 30 min; then fixed and permeabilised using BD FACSTM Permeabilizing Solution (BD Biosciences), followed by intracellular staining with APC-conjugated anti-human IL-10, APC-conjugated anti-human IL-17A and APC-conjugated anti-human IFNγ. All monoclonal antibodies were supplied by Becton Dickinson (San Jose, CA, United States). The FACS CANTO II cytometer (Becton Dickinson, San Jose, CA, USA) was used to acquire at least 20000 with data analysed on BD FACSDiva™ 6.0 Software. Figure 1 shows the gating strategy to quantify the intracellular cytokine production (IFNγ, IL-17 and IL-10) on peripheral CD4+ and CD8+ T lymphocytes.
The primary study outcome was the occurrence of overall OI during the 1st year post-transplantation. To the purpose of this study we took into consideration the occurrence of overall OI episode as the incident of any clinical event including all viral infection (Citomegalovirus, CMV and non-CMV infections, such as Herpes-Zoster Virus, HZV; Herpes Simplex Virus, HSV; Epstein-Bar Virus, EBV and BK virus), as well as bacterial, fungal and parasite infections as a whole. Table 1 summarises all opportunistic agents diagnosed in both LTr and KTr during the 1st year post-transplantation. Bacterial infection was diagnosed in those patients with a positive test in bloodstream and/or urine samples. Microbiological cultures were used to find bacterial microorganisms such as Escherichia coli, Staphylococcus sp., Enterococcus sp., Pseudomonas sp., Serratia sp., Proteus sp. with positivity considered in cases of > 10000 Colonial Forming Units (CFU)/mL. Urine tract infection due to yeast microorganisms was observed in all cases, due to Candida albicans, with diagnosis based on the presence of > 10000 CFU/mL following urine culture. In addition, the rapid test for detecting Clostridium’s toxin was performed to diagnose infection by Clostridium difficile. Viral infection was determined using serological and molecular DNA-based methods. CMV infection was diagnosed by the presence of either IgM or IgG anti-CMV in symptomatic patients. CMV infection was assigned to either anti-CMV IgG antibody level ≥ 0.6 UI/mL or anti-CMV IgM antibody ≥ 30 UA/ml in symptomatic patients. Post-transplant active CMV and BK virus infections were confirmed using real-time polymerase chain reaction (qPCR) in plasma and/or urine samples. The presence of anti-EARLY IgG (≥ 1/10) and/or anti-VCA IgM (> 0.400 DO) in symptomatic patients was considered evidence of EBV infection. Similarly, only the presence of anti-HSV type 1 and 2 IgM (≥ 20 UI/mL) was considered evidence of active herpes virus infection.
| Type of opportunistic microorganism | Liver transplant recipients (n = 30) | Kidney transplant recipients (n = 31) |
| Presence of overall opportunistic infection (Yes/No) | 18 (60)/12 (40) | 19 (61.3)/12 (38.7) |
| Presence of bacterial infection (Yes/No) | 12 (66.7)/6 (33.3) | 17 (84.2)/3 (15.8) |
| Staphylococcus hominis | 2 (16.7)/10 (83.3) | 1 (5.8)/15 (94.2) |
| Staphylococcus epidermidis | 5 (41.7)/7 (58.3) | 5 (29.4)/11 (70.6) |
| Staphylococcus haemolyticus | 2 (16.7)/10 (83.3) | 2 (11.8)/14 (88.2) |
| Enterococcus faecalis | 1 (8.3)/11 (91.7) | 3 (17.6)/13 (82.4) |
| Enterococcus faecium | 0 | 3 (17.6)/13 (82.4) |
| Clostridium difficile | 2 (16.7)/10 (83.3) | 3 (17.6)/13 (82.4) |
| Proteus mirabilis | 1 (8.3)/11 (91.7) | 4 (23.5)/12 (76.5) |
| Pseudomonas aeruginosa | 2 (16.7)/10 (83.3) | 5 (29.4)/11 (70.6) |
| Serratia marcescens | 1 (8.3)/11 (91.7) | 0 |
| Escherichia coli | 2 (16.7)/10 (83.3) | 13 (76.5)/3 (23.5) |
| Treponema pallidum | 1 (8.3)/11 (91.7) | 0 |
| Enterobacter aerogenes | 2 (16.7)/10 (83.3) | 1 (5.8)/15 (94.2) |
| Enterobacter cloacae | 0 | 3 (17.6)/13 (82.4) |
| Streptococcus sp. | 1 (8.3)/11 (91.7) | 0 |
| Citrobacter koseri | 0 | 2 (11.8)/14 (88.2) |
| Morganella morganii | 0 | 1 (5.8)/15 (94.2) |
| Klebsiella oxytoca | 0 | 2 (11.8)/14 (88.2) |
| Klebsiella pneumoniae oxytoca | 0 | 1 (5.8)/15 (94.2) |
| Hafnia alvei | 0 | 1 (5.8)/15 (94.2) |
| Salmonella typhi | 0 | 2 (11.8)/14 (88.2) |
| Presence of yeast infection (Yes/No) | 4 (22.2)/14 (77.8) | 3 (15.8)/16 (84.2) |
| Candida albicans | 4 (100)/0 | 3 (100)/0 |
| Presence of viral infection (Yes/No) | 17 (94.4)/1 (5.6) | 17 (89.5)/2 (10.5) |
| Cytomegalovirus | 11 (64.7)/6 (35.3) | 12 (70.6)/5 (29.4) |
| BK virus | 3 (17.6)/14 (82.4) | 6 (35.3)/11 (64.7) |
| Epstein-Barr virus | 2 (11.8)/15 (88.2) | 3 (17.6)/14 (82.4) |
| Varicella-Zoster virus | 2 (11.8)/15 (88.2) | 0 |
| Presence of parasitic infection (Yes/No) | 3 (16.7)/15 (83.3) | 3 (15.8)/16 (84.2) |
| Toxoplasma gondii | 3 (100)/0 | 2 (66.7)/1 (33.3) |
| Strongyloides stercoralis | 0 | 1 (33.3)/2 (66.7) |
Demographic data and results from our prospective follow-up study were collected and analysed in a unified database (SPSS 22.0, SPSS Inc., Chicago, IL, United States). Qualitative data are expressed as frequency and percentage. Quantitative data are shown as the mean ± SEM. Nonparametric Kolmogorov-Smirnov test was applied to identify whether the data followed a Gaussian distribution. Samples were adjusted to a nonparametric distribution. Nonparametric U Mann-Whitney test was applied to unpaired quantitative continuous variables, whereas nonparametric Wilcoxon test was applied to evaluate the relationship between paired quantitative continuous variables. Optimal biomarker cut-off points to discriminate between patients with and without OI were based on receiver operating characteristic (ROC) curves and calculated with the best Youden index (sensitivity + specificity-1)[21]. Discriminatory capacity was defined by the area under the curve (auROC) measure, with 0.7-0.8 deemed acceptable, 0.8-0.9 excellent and > 0.9 outstanding[22]. The predictive value for the model was assessed with χ2 test. Survival curves for the first episode of OI were plotted using the Kaplan–Meier method, and differences between groups compared with the log-rank test. Recipient and donor factors were entered into univariate Cox model and those factors found to be significant at P < 0.25 level were subsequently entered into multivariate model, using a backward stepping procedure, to find the best model. In addition in this model AR, induction therapy and average drug dose were added as controlled variables. Results were expressed as hazard ratios (HRs) with 95%CIs. All statistical tests were two-tailed, with a P < 0.05 representing statistical significance.
Overall, 60% of LTr and 61% of KTr developed at least one post-transplant OI event during the 1st year post-transplantation. Generally, the infection pattern varied from bacterial, fungal and non-CMV infections following the first weeks post-transplantation to mainly CMV and non-CMV infections, such as HSV or HZV as previously described[15], seen towards the end of the follow-up period. Recipient’s clinical and demographic data found to be significant between infected- and non-infected-LTr and KTr are shown in Table 2.
| Liver recipients (n = 30) | Kidney recipients (n = 31) | |||||
| NoINF (n = 12) | INF (n = 18) | P | NoINF (n = 12) | INF (n = 19) | P | |
| Donor age (yr) | 60.75 ± 3.32 | 58.93 ± 4.71 | 0.905 | 51.21 ± 3.171 | 55.67 ± 3.831 | 0.0171 |
| Recipient age (yr) | 51.25 ± 2.87 | 53.79 ± 2.37 | 0.282 | 51.58 ± 3.25 | 51.42 ± 2.81 | 0.351 |
| Recipient gender (M/F), n (%) | 14 (87.5)/2 (12.5) | 9 (64.3)/5 (35.7) | 0.669 | 10 (41.7)/2 (28.6) | 14 (58.3)/5 (71.4) | 0.087 |
| Total lymphocyte (%) | 15.38 ± 3.63 | 11.39 ± 2.08 | 0.397 | 16.09 ± 2.85 | 11.66 ± 2.33 | 0.768 |
| Total lymphocyte (cells/mm3) | 813.34 ± 163.70 | 750.02 ± 191.84 | 0.711 | 1121.67 ± 173.35 | 1073.16 ± 235.68 | 0.197 |
| Total leukocyte (× 109/L) | 6.29 ± 0.55 | 7.09 ± 1.10 | 0.652 | 8.36 ± 1.151 | 10.98 ± 1.331 | 0.0061 |
| SGOT (U/L) | 187.44 ± 97.28 | 117.16 ± 18.09 | 0.738 | 27.66 ± 4.61 | 19.17 ± 1.09 | 0.669 |
| SGPT (U/L) | 161.69 ± 69.20 | 135.56 ± 14.77 | 0.891 | 44.51 ± 9.81 | 27.06 ± 1.98 | 0.762 |
| SALP (U/L) | 177.47 ± 14.72 | 186.62 ± 14.40 | 0.847 | 106.84 ± 8.231 | 85.36 ± 2.741 | 0.0081 |
| SGGT (U/L) | 197.27 ± 23.651 | 351.28 ± 42.441 | 0.0051 | 109.97 ± 25.34 | 64.36 ± 11.74 | 0.074 |
| Glomerular filtration (mL/min) | 82.82 ± 7.36 | 81.28 ± 6.27 | 0.571 | 45.87 ± 2.901 | 72.42 ± 2.521 | 0.0191 |
| Serum creatinine | 0.93 ± 0.051 | 1.08 ± 0.061 | 0.0271 | 6.22 ± 0.60 | 5.83 ± 0.51 | 0.251 |
| Induction therapy (thymoglobulin/basiliximab) | 0(0 )/0(0) | 0(0)/1(3.3) | 1(8.3)/11(91.7) | 4(21.1)/12(63.2) | 0.161 | |
| Post-transplant therapy (TRL/TRL + MMF), n (%) | 7(58.3)/5(41.7) | 10(55.6)/8(44.4) | 0.880 | 0/0/12(100) | 1(5.3)/2(10.5)/16(84.2) | 0.350 |
| Maintenance therapy (TRL/TRL + MMF), n (%) | 7(58.3)/5(41.7) | 13(72.2)/5(27.8) | 0.461 | 0/0/12(100) | 2(10.5)/2(10.5)/15(78.9) | 0.235 |
| TRL dose (mg/d) | 7.92 ± 0.281 | 6.67 ± 0.391 | < 0.0011 | 13.08 ± 1.51 | 12.35 ± 1.37 | 0.179 |
| MMF dose (mg/d) | 2062.50 ± 73.451 | 1848.57 ± 79.001 | 0.0341 | 1620 ± 164.701 | 1917.89 ± 58.871 | < 0.0011 |
| Cmin TRL (ng/mL) | 10.46 ± 0.69 | 9.61 ± 0.47 | 0.445 | 10.54 ± 1.981 | 6.53 ± 1.971 | 0.0121 |
| Cmin MMF (μg/mL) | 2.91 ± 0.601 | 0.97 ± 0.291 | 0.0161 | 1.07 ± 0.18 | 3.80 ± 1.20 | 0.071 |
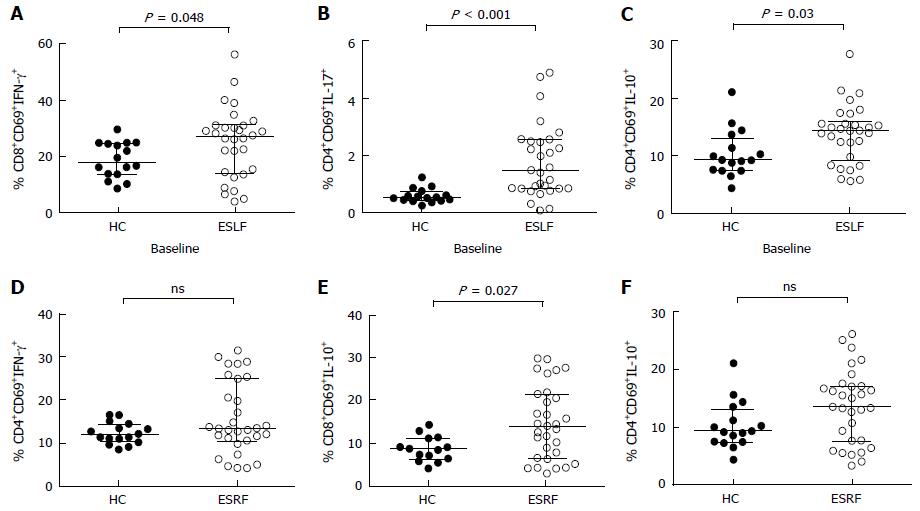
Prior to transplantation, circulating CD4+ and CD8+ T lymphocyte analysis found that, pre-transplant percentages of CD8+CD69+INFγ+, CD4+CD69+IL-17+ and CD4+CD69+IL10+ lymphocytes (Figure 2A-C) in patients with ESLF and CD8+CD69+IL-10+ lymphocytes (Figure 2E) in patients with ESRF were significantly greater compared to HC. In contrast, there were no significant differences in the percentage of CD4+CD69+INFγ+ and CD4+CD69+IL-10+ between ESRF patients and HC.
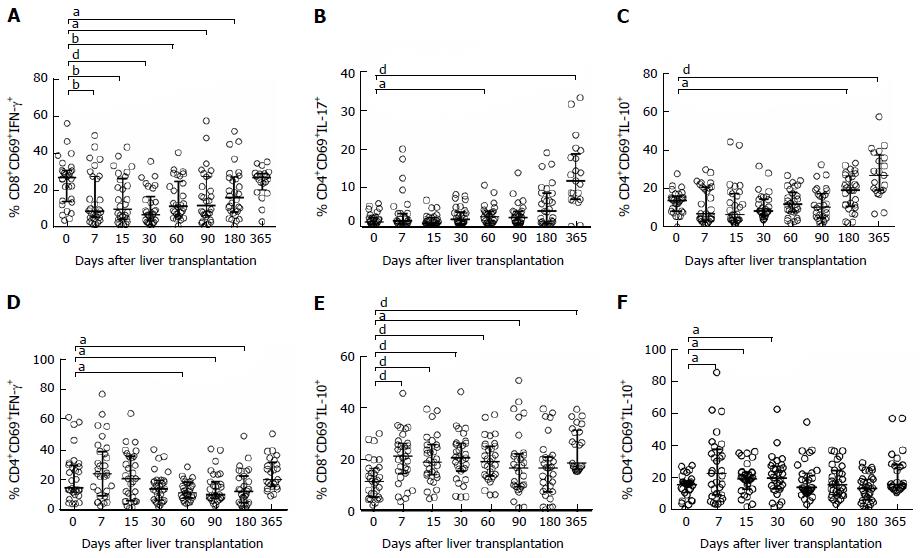
Post-transplantation follow-up analysis of the different CD4+ and CD8+ T lymphocyte subsets showed that amongst LTr the percentage of CD8+CD69+IFNγ+ decreased significantly within the early post-transplantation period, whereas the percentages of CD4+CD69+IL-10+ and CD4+CD69+IL-17+ experienced an up-regulation during the study period. Of particular interest was the IFNγ-producing-CD8+ T lymphocytes, which observed a significant drop during the first weeks following transplant surgery compared to pre-transplant values, which then gradually recovered back to their basal levels (Figure 3A). On the other hand, TH17 (Figure 3B) and TH2 (Figure 3C) lymphocytes were significantly greater at the intermediate and long-term period compared to baseline levels. Likewise, the percentage of CD4+ and CD8+ T lymphocyte subsets amongst KTr also experienced changes during the post-transplantation follow-up period. Particularly, the percentage of CD4+CD69+INFγ+ lymphocytes initially increased upon transplantation; however, levels dropped significantly during the post-transplantation intermediate-term (Figure 3D). On the other hand, percentages of CD4+CD69+IL-10+ (Figure 3E) and CD8+CD69+IL-10+ (Figure 3F) T lymphocyte subsets experienced a significant early up-regulation, which remained constant during the post-transplantation period compared to their basal levels. These data are summarized in supplementary Table 1.
Recipients with post-transplant opportunistic infection had significantly greater IL-17 and IL-10 and lower IFNγ intracellular production capacity on stimulated CD3+CD69+ T lymphocytes
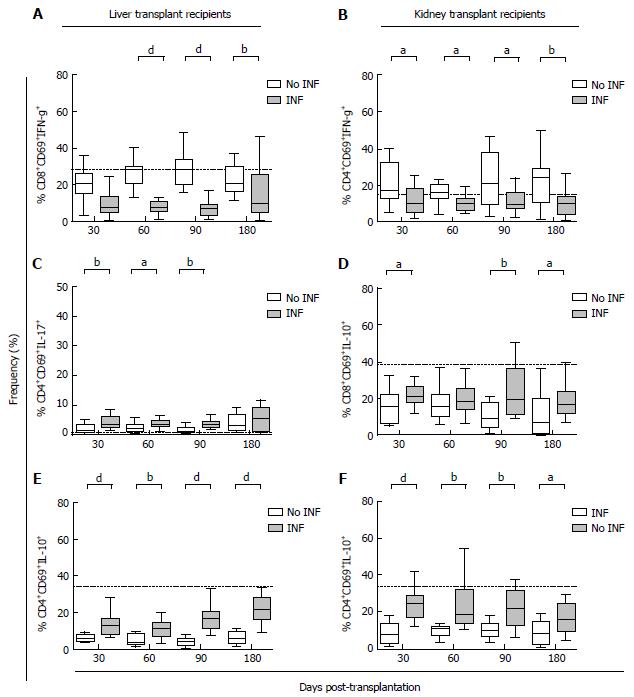
The incidence of OI episodes was found to be higher within intermediate-term in both kinds of transplant recipients; with 54.8% and 64.1% of OI episodes occurring between the 1st and 6th month following LT and KT, respectively. Therefore, the post-transplantation T lymphocyte stratification analysis was focused within this period. In this regard, LTr who developed an OI episode displayed a lower percentage of CD8+CD69+IFNγ+ compared to the OI-free study group at 60 (Figure 4A, P < 0.001), 90 (Figure 4A, P < 0.001) and 180 (Figure 4A, P = 0.01) d post-transplantation. On the other hand, LTr with OI had a significantly higher intracellular IL-10-cytokine production capacity by CD3+CD4+CD69+ T lymphocytes at 30 (Figure 4E, P = 0.0007), 60 (Figure 4E, P = 0.001), 90 (Figure 4E, P < 0.001) and 180 d (Figure 4E, P < 0.001) post-transplantation in comparison with recipients who did not develop OI. In addition, the percentage of CD4+CD69+IL-17+ in LTr with OI was significantly greater at 30 (Figure 4C, P = 0.005), 60 (Figure 4C, P = 0.03) and 90 d (Figure 4C, P = 0.001) post-transplantation.
The T lymphocyte kinetics amongst KTr showed a similar trend for both pro- and anti-inflammatory cytokine production capacities. In particular, KTr who developed an OI episode within the intermediate-term displayed a significantly less intracellular IFNγ production capacity by CD3+CD4+CD69+ T lymphocytes compared to patients free of infection at 30 (Figure 4B, P = 0.035), 60 (Figure 4B, P = 0.02), 90 (Figure 4B, P = 0.028) and 180 d (Figure 4B, P = 0.008) post-transplantation. On the other hand, a higher IL-10-producing T lymphocytes capacity was seen in KTr who subsequently developed an OI episode. Post-transplant percentage of CD8+CD69+IL-10+ in KTr who developed OI was significantly increased at 30 (Figure 4D, P = 0.032), 90 (Figure 4D, P = 0.002) and 180 d (Figure 4D, P = 0.016) post-transplantation. Similarly, a significantly increased percentage of CD4+CD69+IL-10+ T lymphocytes at 30 (Figure 4F, P < 0.001), 60 (Figure 4F, P = 0.002), 90 (Figure 4F, P = 0.001) and 180 d (Figure 4F, P = 0.01) was observed in KTr who developed an OI episode within the intermediate post-transplantation term. These data are shown in supplementary Table 2.
Following the stratification analysis, we wanted to find the potential capability of these T lymphocyte subsets as surrogate biomarkers capable of stratifying both LTr and KTr at high risk of overall post-transplant OI. The post-transplantation percentage of stimulated cytokine-producing CD3+CD69+ T lymphocytes was found to have an impact on OI incidence.
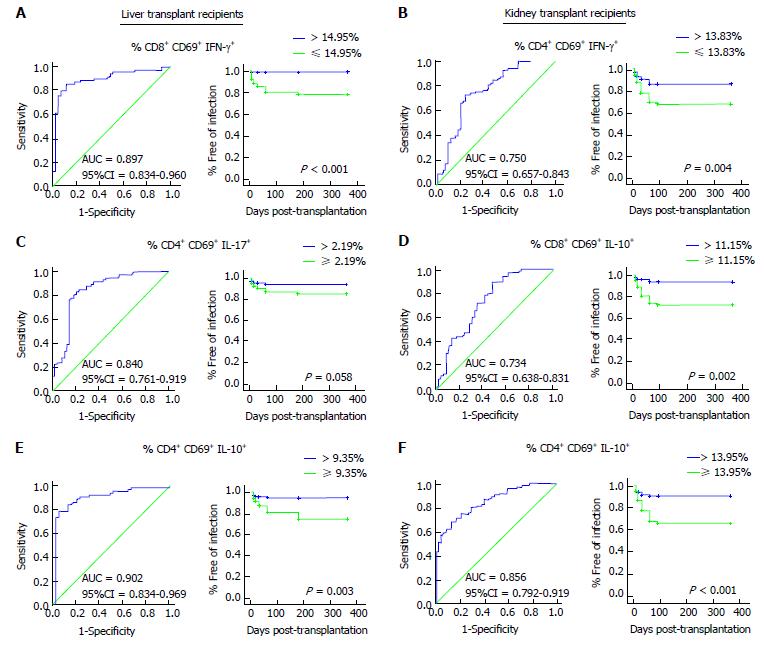
The auROC curve analysis showed that the disparity in T lymphocyte population was distinguishable amongst LTr and KTr at high risk of overall post-transplant OI. Particularly, patients with a percentage of CD8+CD69+IFNγ+ ≤ 14.95% (Figure 5A; AUC = 0.897, 95%CI: 0.834-0.960, P < 0.001) in LTr and a percentage of CD4+CD69+IFNγ+ ≤ 13.83% (Figure 5B; AUC = 0.750, 95%CI: 0.657-0.843, P < 0.001) in KTr were considered to be at a significantly high risk of post-transplant OI. In fact, 92.3% of LTr (n = 16) and 82% of KTr (n = 15) who developed OI displayed post-transplant levels of CD8+CD69+IFNγ+ ≤ 14.95% and CD4+CD69+IFNγ+ ≤ 13.83%, respectively at any time point between the 1st and 6th month post-transplantation. The Kaplan-Meier curve showed that time free of OI was significantly shorter for those LTr and KTr whose cut-off values were below the threshold (Figure 5A; P < 0.001, Long Rank test and Figure 5B; P = 0.004, Long Rank test).
With regards TH17 subset, a percentage of CD4+CD69+Il-17+ T lymphocytes ≥ 2.19% (Figure 5C; AUC = 0.840, 95%CI: 0.761-0.919, P < 0.001) was also capable to stratify LTr at high risk of post-transplant OI. 55.8% of LTr were classified at high risk of overall post-transplant OI with a percentage of TH17 above cut-off, of whom 15 out of 18 LTr (86.6%) developed OI and 3 recipients (13.4%) did not develop OI despite being stratified within the high risk group. A percentage of CD4+CD69+IL-17+ ≥ 2.19% in LTr at any time point between the 1st and 6th month post-transplantation resulted in a shorter time free of overall OI near of signification (Figure 5C; P = 0.058, Long Rang test).
Finally, a percentage of CD8+CD69+IL-10+ T lymphocytes ≥ 11.15% (Figure 5D; AUC = 0.734, 95%CI: 0.638-0.831, P < 0.001) in KTr and a percentage of CD4+CD69+IL-10+ T lymphocytes ≥ 9.35% (Figure 5E; AUC = 0.902, 95%CI: 0.834-0.969, P < 0.001) in LTr and ≥ 13.95% (Figure 5F; AUC = 0.856, 95%CI: 0.792-0.919, P < 0.001) in KTr accurately discriminated both cohort of patients at high risk of overall post-transplant OI. KTr with a percentage of CD8+CD69+IL-10+ < 11.15% had significantly reduced overall OI episodes compared to those recipients with values ≥ 11.15% (Figure 5D; P = 0.002, Long Rang test). 23 KTr (73.6%) were shown to be at high risk of OI, of whom 17 (75.3%) developed OI between the 1st and 6th month post-transplantation exhibiting a percentage above cut-off. Considering the intracellular IL-10 production capacity by the CD3+CD4+CD69+ T lymphocyte subpopulation, a percentage ≥ 9.35% in LTr (Figure 5E; P = 0.003, Long Rang test) and ≥ 13.95% in KTr (Figure 5F; P < 0.001, Long Rang test) resulted in a worse outcome with significantly increased overall post-transplantation OI episodes. 50.5% (n = 15) of LTr as well as another 50% (n = 16) of KTr were stratified at high risk of infection showing percentages of TH2 all above the threshold. Indeed, 96.2% of LTr and 87.1% of KTr of those at high risk developed at least one episode of OI between the 1st and the 6th month post-transplantation. Cut-off values, specificities and sensitivities for the surrogate biomarkers of post-transplant OI are shown in Table 3.
| Biomarker | Cut-off | AUC (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) |
| Liver transplant | ||||
| % CD8+CD69+ IFNγ+ T Lymphocytes | 14.95 | 0.897 (0.834-0.960) | 85.71 (75.29-92.93) | 88.37 (74.92-96.11) |
| % CD4+CD69+IL-17+ T Lymphocytes | 2.19 | 0.840 (0.761-0.919) | 80.56 (69.53-88.94) | 81.25 (67.37-91.05) |
| % CD4+CD69+IL-10+ T Lymphocytes | 9.35 | 0.902 (0.834-0.969) | 78.12 (66.03-87.49) | 94.87 (82.68-99.37) |
| Kidney transplant | ||||
| % CD4+CD69+ IFNγ+ T Lymphocytes | 13.83 | 0.750 (0.657-0.843) | 72.37 (60.91-82.01) | 75 (60.40-86.36) |
| % CD4+CD69+IL-10+ T Lymphocytes | 13.95 | 0.856 (0.792-0.919) | 71.05 (59.51-80.89) | 83.33 (69.78-2.52) |
| % CD8+CD69+IL-10+ T Lymphocytes | 11.15 | 0.734 (0.638-0.831) | 89.33 (80.06-95.28) | 52.17 (36.95-67.11) |
We further examined the relationship between different recipient/donor factors and the occurrence of post-transplant OI in LTr and KTr. Following auROC curve analysis; univariate and multivariate Cox regression models were carried out. Results of the univariate and multivariate analysis of recipients and donor factors are shown in Table 4. Several factors were shown to be associated with an increased risk of post-transplant OI in LTr as well as KTr. Amongst them, recipient gender and serum alkaline phosphatase (SALP) enzyme in LTr showed a trend (P = 0.067 and P = 0.078, respectively) to a worse post-transplant primary study point, whereas in KTr, recipient gender in conjunction with SALP, serum creatinine levels and dose of MMF, were significantly observed as independent risk factor of post-transplant OI episodes (P = 0.046, P = 0.016, P = 0.014 and P = 0.035, respectively). In addition to this, the percentage of total lymphocyte and serum Gamma-Glutamyl Transpeptidase (SGGT) (P = 0.022 and P = 0.035, respectively) was observed as the only clinical recipient factor having an impact in the occurrence of OI in LT. Amongst T lymphocyte subsets, LTr with a post-transplant percentage of CD8+CD69+IFNγ+ below cut-off (P = 0.002) and a post-transplant percentage of CD4+CD69+IL-10+ above cut-off (P = 0.006) resulted in a significantly worse outcome leading to an increase number of OI episodes, whereas the post-transplant percentage of CD4+CD69+IL-17+ showed a trend (P = 0.07) towards a shorter time free of OI. Similarly, the post-transplant percentage of CD4+CD69+IFNγ+ (P = 0.006), CD4+CD69+IL-10+ (P = 0.001) and CD8+CD69+IL-10+ (P = 0.007) in KTr also were shown as independent risk factor of OI.
| Predictive factors for OI | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Liver transplant (n = 30) | ||||||
| % CD8+CD69+IFNγ+ T lymphocytes | 22.56 | 3.01-169.56 | 0.002 | 21.12 | 2.80-159.31 | 0.003 |
| % CD4+CD69+IL-10+ T lymphocytes | 3.77 | 1.46-9.75 | 0.006 | 2.84 | 1.09-7.35 | 0.032 |
| % CD4+CD69+IL-17+ T lymphocytes | 2.43 | 0.93-6.35 | 0.07 | 1.37 | 0.45-4.16 | 0.584 |
| Total leukocyte | 1.08 | 0.98-1.18 | 0.111 | 0.95 | 0.78-1.17 | 0.666 |
| Total lymphocytes (%) | 0.94 | 0.90-0.99 | 0.022 | 0.94 | 0.89-0.99 | 0.023 |
| Total lymphocyte (cells/mm3) | 1.24 | 1.18-1.30 | 0.150 | 1.00 | 0.99-1.04 | 0.261 |
| Donor age | 0.98 | 0.96-1.01 | 0.221 | 0.96 | 0.93-0.99 | 0.026 |
| Recipient gender | 2.40 | 0.94-6.09 | 0.067 | 4.56 | 1.46-14.20 | 0.009 |
| TRL dose (mg/d) | 0.96 | 0.87-1.10 | 0.815 | 0.82 | 0.59-1.13 | 0.230 |
| MMF dose (mg/d) | 0.87 | 0.49-1.52 | 0.619 | 0.99 | 0.99-1.01 | 0.454 |
| Induction therapy | 0.92 | 0.33-2.51 | 0.864 | 3.90 | 3.71-4.09 | 0.990 |
| Post-transplant IS | 1.89 | 0.78-4.59 | 0.157 | 1.98 | 0.64-6.15 | 0.238 |
| Acute rejection | 1.51 | 0.63-3.62 | 0.362 | 3.95 | 0.75-20.93 | 0.107 |
| SALP (U/L) | 1.02 | 0.92-1.17 | 0.078 | 1.00 | 0.99-1.05 | 0.706 |
| SGGT (U/L) | 1.19 | 1.13-1.25 | 0.035 | 1.01 | 0.91-1.03 | 0.333 |
| Kindey transplant (n = 31) | ||||||
| % CD4+CD69+IFNγ+ T lymphocytes | 2.36 | 1.28-4.33 | 0.006 | 3.29 | 1.71-6.35 | < 0.001 |
| % CD4+CD69+IL-10+ T lymphocytes | 3.49 | 1.68-7.30 | 0.001 | 4.76 | 2.05-11.08 | 0.003 |
| % CD8+CD69+IL-10+ T lymphocytes | 4.17 | 1.49-11.68 | 0.007 | 2.52 | 0.74-8.57 | 0.139 |
| Recipient gender | 1.91 | 1.01-3.61 | 0.046 | 1.15 | 0.57-2.32 | 0.705 |
| TRL dose (mg/d) | 0.95 | 0.89-1.01 | 0.112 | 0.92 | 0.85-0.99 | 0.037 |
| MMF dose (mg/d) | 1.18 | 1.12-1.24 | 0.035 | 1.01 | 1.00-1.03 | 0.053 |
| Induction therapy | 1.32 | 0.91-1.91 | 0.143 | 1.50 | 0.96-2.35 | 0.076 |
| Post-transplant IS | 0.94 | 0.67-1.32 | 0.723 | 0.63 | 0.19-2.01 | 0.434 |
| Acute rejection | 1.34 | 0.64-2.81 | 0.439 | 1.44 | 0.59-3.48 | 0.417 |
| Serum creatinine | 1.02 | 1.00-1.03 | 0.014 | 1.02 | 1.00-1.04 | 0.013 |
| SALP (U/L) | 0.98 | 0.97-0.99 | 0.016 | 0.98 | 0.97-1.01 | 0.074 |
| SGOT (U/L) | 0.98 | 0.95-1.01 | 0.242 | 0.98 | 0.96-1.01 | 0.195 |
In the multivariate analysis, with regards LT, the only donor factor resulting in a reduction of the risk of post-transplant OI was donor age (HR: 0.96, 95%CI: 0.93-0.99, P = 0.026). Percentage of total lymphocytes remained as a factor for a better outcome (HR: 0.94, 95%CI: 0.89-0.99, P = 0.036). On the other hand, recipient gender (HR: 4.56, 95%CI: 1.46-14.20, P = 0.009) was shown to have a negative impact in the occurrence of OI. Amongst the surrogate biomarkers for risk of infection, the imbalance between the TH1 and TH2 response was shown to be the most significant factor in poor post-transplant outcome (HR: 21.12, 95%CI: 2.80-159.31, P = 0.003 and HR: 2.84, 95%CI: 1.09-7.35, P = 0.032, respectively), resulting in an increased risk of overall post-transplant OI. Amongst KTr, in the multivariate analysis, TRL dose (HR: 0.92, 95%CI: 0.85-0.99, P = 0.037) and serum creatinine levels (HR: 1.02, 95%CI: 1.00-1.04, P = 0.013) were factors associated with post-transplant OI whereas MMF dose only showed a trend towards worse post-transplant outcome. (P = 0.053). The two main recipient factors associated with a worse impact to overall infection were the percentage of CD4+CD69+IFNγ+ (HR: 3.29, 95%CI: 1.71-6.35, P < 0.001) and CD4+CD69+IL-10+ (HR: 4.76, 95%CI: 2.05-11.08, P = 0.003). Post-transplant percentage of CD8+CD69+IL-10+ T lymphocytes between months 1 and 6 was not associated with overall OI (P = 0.139). Further assessment of the presence of AR, as well as the administration of induction therapy in both LTr and KTr, showed an impact on the occurrence of post-transplant OI, in which the administration of induction therapy in KTr (HR: 1.5, 95%CI: 0.96-2.35, P = 0.076) was the only factor showing a trend towards a worse clinical outcome.
This prospective study describes the usefulness of in vitro stimulation of whole peripheral blood to quantify the intracellular cytokine production capacity from two independent cohorts of patients (LTr and KTr) with orthotopic liver and kidney transplantation as predictive biomarkers for overall post-transplant OI. To our knowledge, this report is the first to analyze the impact of TH1, TH2 and TH17 adaptive immune response in post-transplant OI outcome. The main objective was to analyze the occurrence of overall OI after OLT and LT, according to post-transplant percentages of CD4+CD69+IFNγ+, CD8+CD69+IFNγ+, CD4+CD69+IL-17+, CD4+CD69+IL-10+ and CD8+CD69+IL-10+.
Our data show high occurrence of post-transplant OI due to an imbalance between TH1/TH2 adaptive immune response in LTr and KTr between the 1st and 6th month after transplantation. Besides, TH17 adaptive immune response in LTr was also found to have an impact to post-transplant infection. Importantly, we have demonstrated that post-transplant percentages for both pro- and anti-inflammatory cytokine-producing T lymphocytes to be the most significant factors determining the overall OI susceptibility.
Pre-transplant levels of IFNγ, IL-17 and IL-10 producing T lymphocytes in patients with ELSF and ERSF were shown to be significantly different when compared to healthy individuals. Specifically, we found a significantly increased overall percentage of TH1, TH2 and TH17 populations in LTr, however in KTr TH1 and TH2 cells were observed no significant; nevertheless, there was a trend towards higher percentage in ESRF patients compared to healthy individuals. This data are in concordance with previous evidence showing an increased level of TH1 and TH17 cells in patients with ESRF[23].
Overall, the IFNγ-dependent immune response was shown to be significantly reduced in LTr and KTr during the follow-up period compared to basal levels. This reduced IFNγ production capacity was more substantial in liver than kidney recipients but nevertheless, in both cases a significant reduction in IFNγ production capacity was found within the highest OI occurrence in post-transplant period (1st to 6th month). On the other hand, IL-17 as well as IL-10-dependant responses increased gradually from day 1 up until one year after transplantation in both types of transplants and this increase was found significant compared to pre-transplant levels. Erol et al[24] 2017 investigated the intracellular IFNγ and IL-17 levels in a cohort of 50 KTr during 6 mo after transplantation. No significant difference was observed between pre- and post-transplant levels at any time point. However, Loverre et al[25] 2011 investigated a cohort of 72 KTr in which intracellular IFNγ (TH1), IL-4 (TH2) and IL-17 (TH17) production capacity was measured. Overall, they found a significant decrease in IFNγ expressing CD4+ T lymphocytes at 24 mo in patients with delayed graft function (DGF) compared to pre-transplant, whereas TH2 subset significantly increased after transplantation compared to baseline levels as measured by GATA3 protein expression in both patients with DGF as we all as acute tubular damage (ATD). Although our investigation did not compare post-transplant percentages of T lymphocytes with healthy individuals, our results shown concordance with the findings from P.J. van de Berg et al[26] 2012, in which they found a significantly decreased absolute number of CD4+ and CD8+ T cells in patients with stable graft function compared to healthy individuals at 6 mo, but more interestingly towards a differentiated and effector T-cell phenotype, specially observed in those CMV-seropositive recipients.
OI has been found to be more frequent in the first six months after orthotopic liver[27] and kidney[28] transplantation when the immunosuppressant reaches maximum levels. As shown in our results, stratification analysis showed that the percentage of CD8+CD69+IFNγ+ in LTr and CD4+CD69+IFNγ+ in KTr were significantly reduced in patients who developed OI. On the contrary, the IL-17 and IL-10-dependant immune response in both LTr and KTr was significantly augmented in patients who suffered OI during this period. Recently, a prospective study carried out in a cohort of 304 KTr found that recipients who subsequently developed OI had a significantly decreased count in total lymphocytes, CD3+, CD4+ and CD8+ T-cells as well as NK-cells at month 1 post-transplantation[11]. Although, in our cohort of liver and kidney recipients the total count for both percentage and absolute number of peripheral lymphocytes was not statistically significant between both study groups, a trend was observed towards less count of total lymphocytes in patients with OI. The same study also used these T lymphocyte subpopulations as predictive biomarkers for OI, however they only took into consideration the quantitative side of the adaptive immune response against opportunistic pathogens. Thus, we believe that quantitative analysis, although rapid and affordable, could potentially miss beneficial information underlying the overall count of T CD4+ and CD8+. Therefore, qualitative assays which provide functional information should also be performed to monitor transplant recipients. Consequently, intracellular cytokine quantification was carried out in this research as an estimation of the functional adaptive immune response against opportunistic pathogens. Our group had reported the usefulness of quantifying the intracellular cytokine production as a surrogate predictive marker of adverse event, such as acute cellular rejection, in LTr and KTr[9]. Moreover, the intracellular-staining method based on flow cytometry was previously used to monitor patients infected by intracellular[29] and extracellular[30] pathogens as well as to define immune-status in non-infected individuals. In spite of the methodology requiring specific equipment for its implementation and well-trained scientists in cell culture and flow cytometry, the majority of Histocompatibility laboratories already use similar approaches for their phenotypical and functional assays leading us to believe that such methodology should not have a significant cost impact. Where implementation can prove impossible, as in the case of many small laboratories, there would be the option of using a referral laboratory service, therefore reducing any financial impact.
Our data has demonstrated the accuracy of this cytokine-producing T cell functional assay by means of monitoring the susceptibility of post-transplant OI in LTr and KTr. As such, our auROC predictive model showed that LTr with post-transplant percentages of CD8+CD69+INFγ+ ≤ 14.95%, CD4+CD69+IL-17+ ≥ 2.19% and CD4+CD69+IL-10+ ≥ 9.35% were stratified at high risk of OI between months 1 and 6. Similarly, KTr with post-transplant percentages of CD4+CD69+INFγ+ ≤ 13.83%, CD4+CD69+IL-10+ ≥ 13.95% and CD8+CD69+IL-10+ ≥ 11.15% were also significantly found at high risk of OI throughout the same period of time. The deficiency of IFNγ production by stimulated CD8+ T cells in LTr and CD4+ T cells in KTr, in conjunction with an increased production of IL-10 cytokine by CD3+CD4+CD69+ T cells in both types of transplant were significantly associated, confirmed by multivariate model, with a higher occurrence of overall OI and worse impact on patient wellbeing. Although post-transplant percentage of IL-17-producing CD3+CD4+CD69+ T cells in LTr and IL-10-producing CD3+CD8+CD69+ both had some impact on patient’s outcome in univariate analysis, this effect was not seen in multivariate analysis. In line with the findings in this research, the negative effect of the imbalance of cytokine-producing T lymphocytes in the overall post-transplant OI seen at pre-transplant in our LTr and KTr had yet been seen (data not shown).
In addition, we have also reported several recipient and donor factors that still remain critical determinants for morbidity outcome in solid transplant patients. Donor age has been associated with an increased risk of OI infection in both LT and KT[31,32], amongst other donor factors, such as CMV serostatus and deceased donor source. Recipient gender[33] and immunosuppressive therapy, especially MMF[32,34], as well as induction therapy[35], have too been implicated in the susceptibility to some post-transplant OI. Our results confirm previous findings showing that donor age, recipient gender, immunosuppressive therapy with MMF and the administration of polyclonal antithymocyte globulin or basiliximab (anti-IL-2R or anti-CD25) should be taken into consideration as donor/recipient risk factors to post-transplant OI. On the other hand, we have also found serum levels of ALP and GGT in LTr and serum levels of ALP and creatinine in KTr to be associated with post-transplant OI; however, this data should be taken with caution as potential confounder may exist due to our small cohort of patients. Further investigation should be performed to elucidate these recipient factors as risks to post-transplant OI.
Analysis of our data has revealed several potential limitations. Likewise any uni-centre prospective study, the number of patients recruited to this purpose could have resulted as one limiting factor for the primary study outcome. Given that we have demonstrated significant associations in the basis of recipient/donor risk factors for post-transplant OI; these findings must be confirmed in larger (multi-center if possible) prospective study. Despite the limitations of the study.
In conclusion, our results add to the field of transplantation a validated, rapid and affordable CMI assay that provides basic functional information as to the monitoring of CD4+ and CD8+ T lymphocytes throughout the post-transplantation period. Based on these results, and those from recent studies, several post-transplant strategies could be proposed for the management of recipients. Particularly our study could be relevant in the setting of recipients showing an imbalance between the adaptive TH1 and TH2 immune response. Finally, our findings suggest that TH17 adaptive immune response, along with several recipient characteristics and donor age should be not consider in isolation but as a whole based on recipient features.
Nowadays liver and kidney transplant are well-established therapeutic options for patients with end stage liver and kidney diseases. However, the administration of immunosuppressant is not exempt of side effects that ultimately could lead to worse transplant outcome.
Monitoring of adaptive immune response by flow cytometry provides means of further understanding on how T lymphocytes vary throughout the post-transplant period.
In this study, the authors aim to validate the intracellular cytokine production functional assay as means of cell-mediated immunity monitoring of post-transplant patients with opportunistic infection.
A longitudinal study was carried out in two cohorts of transplant recipients where patients were prospectively monitored for one year post-transplantation.
LTr with OI had significantly lower % of CD8+CD69+IFNγ+ T cells at 60, 90 and 180 d post-transplantation. Higher % of CD4+CD69+IL-10+ as well as CD4+CD69+IL-17+ T cells were yet reported at 30, 60 and 90 d. KTr with OI had significantly lower % of CD4+CD69+IFNγ+ T cells at 30, 60, 90 and 180 d post-transplantation whereas IL-10-producing CD4+ and CD8+ T cells were significantly higher at 30, 90 and 180 d.
The quantification of intracellular cytokine production by flow cytometry has been validated as a reliable functional assay that provides trustworthy information to a better management of transplanted patients. The occurrence of opportunistic infection was significantly correlated with an imbalance between TH1, TH2 and TH17 cells in both liver and kidney transplant recipients.
Post-transplant administration of immunosuppressant as well as prophylaxis therapies could be adapted according to the levels of TH1, TH2 TH17 in an individual basis.
We, Dr. Francisco Boix and Dr. Manuel Muro want to pay a special mention to Cristabel Trujillo who kindly reviewed this manuscript, which without her contribution this manuscript would have not been possible.
| 1. | Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1386] [Cited by in RCA: 1372] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 2. | Khurana A, Brennan DC. Current concepts of immunosuppression and side effects. Pathology of Solid Organ Transplantation. Berlin, Germany: Springer-Verlag 2011; 11–30. |
| 3. | Fishman JA; AST Infectious Diseases Community of Practice. Introduction: infection in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S3-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Gelson W, Hoare M, Dawwas MF, Vowler S, Gibbs P, Alexander G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Cooper JE, Wiseman AC. Novel immunosuppressive agents in kidney transplantation. Clin Nephrol. 2010;73:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Brunet M, Shipkova M, van Gelder T, Wieland E, Sommerer C, Budde K, Haufroid V, Christians U, López-Hoyos M, Barten MJ. Barcelona Consensus on Biomarker-Based Immunosuppressive Drugs Management in Solid Organ Transplantation. Ther Drug Monit. 2016;38 Suppl 1:S1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Boix F, Mrowiec A, Muro M. Cytokine Expression Profile as Predictive Surrogate Biomarkers for Clinical Events in the Field of Solid Organ Transplantation. Curr Protein Pept Sci. 2017;18:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Boix F, Bolarín JM, Mrowiec A, Eguía J, Gonzalez-Martinez G, de la Peña J, Galian JA, Alfaro R, Moya-Quiles MR, Legaz I. CD28 biomarker quantification and expression level profiles in CD4+ T-lymphocytes in solid organ transplantation. Transpl Immunol. 2017;42:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Millán O, Rafael-Valdivia L, San Segundo D, Boix F, Castro-Panete MJ, López-Hoyos M, Muro M, Valero-Hervás D, Rimola A, Navasa M. Should IFN-γ, IL-17 and IL-2 be considered predictive biomarkers of acute rejection in liver and kidney transplant? Results of a multicentric study. Clin Immunol. 2014;154:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Boix-Giner F, Millan O, San Segundo D, Muñoz-Cacho P, Mancebo E, Llorente S, Rafael-Valdivia L, Rimola A, Fábrega E, Mrowiec A. High frequency of central memory regulatory T cells allows detection of liver recipients at risk of early acute rejection within the first month after transplantation. Int Immunol. 2016;28:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Fernández-Ruiz M, López-Medrano F, Allende LM, Andrés A, García-Reyne A, Lumbreras C, San-Juan R, Morales JM, Paz-Artal E, Aguado JM. Kinetics of peripheral blood lymphocyte subpopulations predicts the occurrence of opportunistic infection after kidney transplantation. Transpl Int. 2014;27:674-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Fernández-Ruiz M, Parra P, López-Medrano F, Ruiz-Merlo T, González E, Polanco N, Origüen J, San Juan R, Andrés A, Aguado JM. Serum sCD30: A promising biomarker for predicting the risk of bacterial infection after kidney transplantation. Transpl Infect Dis. 2017; 19: Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Fernández-Ruiz M, López-Medrano F, San-Juan R, Aguado JM. Post-transplant hypogammaglobulinemia and risk of infection after kidney transplantation: Magnitude matters. Transpl Infect Dis. 2017; 19: Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Fernández-Ruiz M, Silva JT, López-Medrano F, Allende LM, San Juan R, Cambra F, Justo I, Paz-Artal E, Jiménez C, Aguado JM. Post-transplant monitoring of NK cell counts as a simple approach to predict the occurrence of opportunistic infection in liver transplant recipients. Transpl Infect Dis. 2016;18:552-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Sun HY, Singh N. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin Infect Dis. 2011;53:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 17. | Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 746] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 20. | Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771-5777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1489] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 21. | Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13773] [Cited by in RCA: 12571] [Article Influence: 285.7] [Reference Citation Analysis (1)] |
| 23. | Ma L, Zhang H, Hu K, Lv G, Fu Y, Ayana DA, Zhao P, Jiang Y. The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol. 2015;16:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Erol A, Arpali E, Murat Yelken B, Kocak B, Calıskan YK, Nane I, Turkmen A, Savran Oguz F. Evaluation of TH17 and TH1 Immune Response Profile in Patients After Renal Transplant. Transplant Proc. 2017;49:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Loverre A, Divella C, Castellano G, Tataranni T, Zaza G, Rossini M, Ditonno P, Battaglia M, Palazzo S, Gigante M. T helper 1, 2 and 17 cell subsets in renal transplant patients with delayed graft function. Transpl Int. 2011;24:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | van de Berg PJ, Hoevenaars EC, Yong SL, van Donselaar-van der Pant KA, van Tellingen A, Florquin S, van Lier RA, Bemelman FJ, ten Berge IJ. Circulating lymphocyte subsets in different clinical situations after renal transplantation. Immunology. 2012;136:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Hernandez Mdel P, Martin P, Simkins J. Infectious Complications After Liver Transplantation. Gastroenterol Hepatol (NY). 2015;11:741-753. [PubMed] |
| 28. | Jha V. Post-transplant infections: An ounce of prevention. Indian J Nephrol. 2010;20:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Antas PR, Sales JS, Pereira KC, Oliveira EB, Cunha KS, Sarno EN, Sampaio EP. Patterns of intracellular cytokines in CD4 and CD8 T cells from patients with mycobacterial infections. Braz J Med Biol Res. 2004;37:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Kwak DJ, Augustine NH, Borges WG, Joyner JL, Green WF, Hill HR. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect Immun. 2000;68:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients--an analysis of USRDS data. Am J Transplant. 2007;7:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | van Hoek B, de Rooij BJ, Verspaget HW. Risk factors for infection after liver transplantation. Best Pract Res Clin Gastroenterol. 2012;26:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz). 2011;59:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40:1407-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Kute VB, Lu K, Sugawara Y, Taheri S S- Editor: Wang JL L- Editor: A E- Editor: Li D